Graphical abstract
Keywords: Septal hematoma, Mitral valve repair surgery, Veterinary medicine
Highlights
-
•
IVSH may occur in dogs following surgical MVR.
-
•
The cause of IVSH in dogs undergoing MVR remains unknown.
-
•
Mortality attributable to IVSH in these four dogs was 50%.
Introduction
We present a case series of four dogs that developed postoperative interventricular septal hematoma (IVSH) following surgical mitral valve repair (MVR) under cardiopulmonary bypass (CPB). This potentially fatal complication has not been previously reported in dogs, although there are reports in humans following open heart surgery under CPB. In humans, IVSH is most commonly seen following surgical repair of a ventricular septal defect (VSD) and is speculated to be a result of an injury to the septal perforating artery.1 The cause of IVSH in dogs following MVR remains unknown. We report both invasive and conservative management of this rare complication in dogs, describe outcomes, and discuss possible mechanisms.
Case Presentations
Case 1
An 8-year-old, male, neutered, 4.6-kg Pomeranian cross dog was presented with a 5-month history of congestive heart failure (CHF) secondary to severe myxomatous mitral valve disease (MMVD), American College of Veterinary Internal Medicine (ACVIM) stage C.2 The dog underwent surgical MVR under CPB, with routine heparin anticoagulation. Immediate postrepair transesophageal echocardiography (TEE) suggested successful repair with mild residual mitral regurgitation. Protamine was administered after the dog was weaned off CPB uneventfully. Total CPB time was 111 min, and cross-clamp time was 70 min. Myocardial quiescence was achieved by the infusion of cardioplegia solution every 20 min, allowing electrochemical cardiac arrest. Approximately 6 hours postoperatively, acute hypotension was noted with a mean arterial blood pressure (BP) of 50 mm Hg. Bedside transthoracic echocardiography (TTE) revealed abnormal thickening of the interventricular septum (IVS), which measured 18 mm in diastole compared with 7 mm preoperatively. Multiple small (8 × 4 mm) oval-shaped anechoic mass lesions were evident diffusely throughout the IVS, compatible with IVSH (Figures 1A and 1B). The IVS was hypokinetic and thickened. There was also evidence of mild pleural effusion.
Figure 1.
TTE 6 hours after MVR, during period of hypotension. (A) Right parasternal long-axis view and (B) right parasternal short-axis view revealing multiple small IVSHs (arrow). LA, Left atrium; LV, left ventricle.
TTE 9 hours postoperatively showed progressive pleural fluid, which was hemorrhagic and consistent with hemorrhage from the surgical site. There was no change in the IVSH. Approximately 20 hours postoperatively, the dog developed acute dyspnea and hypoxemia and arrested several minutes later. Attempts at cardiopulmonary resuscitation were unsuccessful.
Case 2
An 11-year-old, female, neutered, 4.4-kg Maltese-Poodle cross dog was presented with a 5-month history of CHF secondary to MMVD, ACVIM stage C, and underwent MVR under CPB.2 Total CPB time was 173 min, and cross-clamp time was 84 min. Postrepair TEE revealed a large (20 × 25 mm) oval-shaped anechoic mass in the IVS. A thin membrane (1.4 mm) was protruding into the left ventricular lumen, presumed to be obstructing left ventricular inflow and outflow (Figures 2A and 2B, Video 1).
Figure 2.
(A) Intraoperative TEE showing a large IVSH. (B) Color Doppler in the area of the narrowed left ventricular outflow tract. Ao, Aorta; LA, left atrium; LV, left ventricle.
Because of its size and mechanical effects (systolic BP 60 mm Hg), it was decided to attempt to decompress the hematoma. The aortic cross-clamp was reapplied, and a further dose of cardioplegia solution was delivered. The hematoma was immediately visible upon inspection as a darkened segment of the left ventricular endocardial surface of the IVS, which was bulging into the left ventricle and displacing the anterior mitral valve leaflet dorsally toward the left atrium (Video 2). The hematoma was incised with a number 11 blade longitudinally to evacuate it, with the incision left open to prevent further reaccumulation of blood. Postevacuation TEE showed successful reduction in the size of the IVSH and improvement in left ventricular filling (Figure 3, Video 3). Systolic BP improved to 100 to 115 mm Hg for 35 min but suddenly reduced to 50 mm Hg after closure of the thoracic cavity and continued to decline despite fluid resuscitation and use of vasopressors. Intracardiac resuscitation and CPB were reinitiated but were unsuccessful.
Figure 3.

TEE after surgical incision showing the reduced size of the IVSH. LA, Left atrium; LV, left ventricle.
Case 3
An 8-year 10-month, female, neutered, 5.3-kg Pomeranian dog was presented with a 6-month history of CHF secondary to MMVD, ACVIM stage D, and underwent MVR under CPB.2 Total CPB time was 123 min, and cross-clamp time was 67 min. Postrepair TEE revealed a mass with a homogenous echo signal surrounded by a thick-walled ring (3.8 mm) measuring 23 × 15 mm in the IVS (Figure 4A). The dog was maintained on CPB for 20 min, during which time adequate BP was maintained while the IVSH remained stable in size without apparent obstruction of the outflow tract. It was decided to monitor the hematoma and to separate the dog from CPB with conservative management of the IVSH. Repeat TEE 50 min later showed organization of the hematoma, and the dog remained stable without any further hemodynamic consequences (Figure 4B).
Figure 4.
(A) Intraoperative TEE showing a large IVSH and (B) 50 min later showing organization of the IVSH into a mixed sonic mass. LA, Left atrium; LV, left ventricle.
The dog recovered uneventfully from surgery and underwent serial TTE to monitor the repair and IVSH. The dog was discharged from the hospital 9 days postoperatively. Eleven-day postoperative TTE showed markedly increased interventricular septal wall thickness (15.3 mm in diastole) although the hematoma had greatly reduced in size (14 × 9 mm; Figure 5). The dog remained asymptomatic, with no arrhythmias noted on recheck examinations. One-month postoperative TTE showed complete resolution of the IVSH (Figure 6). The ejection fraction (50%) and fractional shortening (25.1%) remained adequate at 1-month TTE. The animal remains well 13 months following surgery, with no evidence of postoperative complications.
Figure 5.
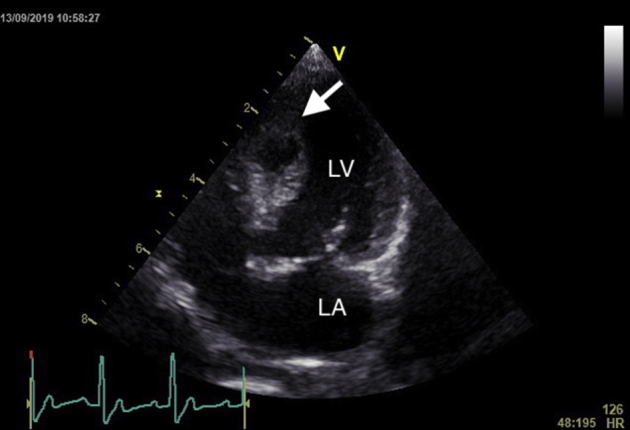
Left apical view of the heart showing reduced size of the IVSH (arrow) 11 days postoperatively. LA, Left atrium; LV, left ventricle.
Figure 6.

Left apical view 1 month postoperatively. LA, Left atrium; LV, left ventricle.
Case 4
An 11-year 7-month, male, neutered, 8.6-kg Cavalier King Charles Spaniel dog was presented with a 5-month history of CHF secondary to MMVD, ACVIM stage C.2 Similar to the previous cases, the dog underwent MVR under CPB. Total CPB time was 130 min, and cross-clamp time was 88 min. Postrepair TEE showed good coaptation of the mitral valve leaflets with minimal residual regurgitation. The dog recovered uneventfully in the intensive care unit. TTE 2 days postoperatively revealed a 25 × 13 mm IVSH (Figures 7A and 7B) and a thickened IVS (23 mm).
Figure 7.
(A) Right parasternal long-axis view showing an incidental IVSH (arrow). (B) Right parasternal short-axis view at the level of the papillary muscles. LA, Left atrium; LV, left ventricle.
The dog had frequent ventricular premature complexes and short paroxysms of ventricular tachycardia during day 2 postoperative TTE and was started on oral sotalol (2.2 mg/kg every 12 hours). The arrhythmia was alleviated and the hematoma regressed over the course of hospitalization. The dog was discharged at day 9 postoperatively. Similar to case 3, the IVS remained mildly thickened (20 mm) at the time of discharge. The IVSH was not visible on 1-month postoperative TTE (Figures 8A and 8B), and there was significant reduction of the previously reported IVS thickening (13 mm). The ejection fraction (45%) and fractional shortening (35%) were adequate. Normal sinus rhythm was confirmed throughout echocardiography, and the dog was maintained on sotalol. Sotalol was discontinued 5 months postoperatively, with no evidence of ventricular arrhythmias. The dog remains well 11 months following surgery.
Figure 8.
(A) Right parasternal long-axis view and (B) right parasternal short-axis view at the level of the papillary muscles 1 month postoperatively showing resolution of the IVSH. LA, Left atrium; LV, left ventricle.
Discussion
An IVSH is a rare complication associated with MVR in our hospital, with an incidence of 3.8% (four of 105) since we first started performing MVR in 2013. To date, IVSH has resulted in an overall mortality rate of 50% (two of four).
In a recent article, Jegatheeswaran et al.1 described their experience in 12 human patients over a 12-year period, which is the largest single-institution review to date.1 They reported an overall mortality rate of 33%. There are <30 human case reports of IVSH currently available in the medical literature, and to our knowledge, there are currently no reports of IVSH in veterinary medicine. In humans, IVSH has been more commonly associated with VSD repair. Although the underlying cause remains unclear, in VSD repair, it is suspected to be a consequence of damage to the septal perforator branch of the posterior descending or left anterior descending coronary artery causing subendocardial bleeding during VSD patch closure.3,4 There are a handful of reports of hematoma formation following mitral valve surgery in humans, in both the free wall and the IVS.5,6 The etiology is likely different from VSD repair, as surgical damage of the septum at the ventricular level seems less likely. In MVR, during artificial chord placement in dogs, the needle enters the tip of the papillary muscle but was not intentionally inserted into the IVS in any of the cases reported here, although damage to the IVS as the needle is manipulated inside the ventricle could not be ruled out. In these reports of IVSH following MVR in humans, suspected etiologies included damage to the left atrium during retraction, damage to the interatrial or IVS during venous cannulation, and damage to the right trigone during mitral annuloplasty.5 This type of interoperative damage (which occurred during robotic surgery with no tactile sensation) seems unlikely in the dogs reported here. There was careful manipulation of the cardiac structures during retraction, and venous cannulation was performed in the right auricle, with care taken to prevent damage to the surrounding area. Injury of the right trigone during mitral annuloplasty was also suspected and remains a possibility in these dogs.5 Damage to the coronary artery (i.e., septal branch in dogs) could also occur as a result of excessive cardioplegia perfusion pressure, inadequate myocardial protection, or reperfusion injury.5,7 Cardioplegia pressures were unremarkable during these cases, and there was successful cardiac quiescence with a standard cardiac ischemic time (67–88 min), making these causes also unlikely. Despite review of the human literature, it is therefore not possible at this stage to hypothesize the exact cause of the development of IVSH in dogs undergoing MVR.
In people, as the septal perforating artery supplies blood to the interventricular conduction system, damage can result in ventricular dysfunction, ventricular arrhythmias, or both.8 Although we do not know the etiology of the IVSH in dogs, complex ventricular arrhythmias were seen in case 4, requiring antiarrhythmic therapy. Ventricular dysfunction and thickening of the IVS after surgery were seen in cases 3 and 4 but subsequently improved after 1 month. Therefore, we suspect that the artery that was damaged mostly likely supplied blood to the interventricular conduction system.
Diagnosis of IVSH can be made during intraoperative TEE, although in one case it was only discovered on TTE 2 days after surgery as an incidental finding. It is suspected that a small IVSH may not have been identified during TEE and may have enlarged as the dog was anticoagulated with parenteral low–molecular weight heparin, oral clopidogrel, and aspirin (our standard anticoagulation protocol) when identified 2 days later. This is similar to a recent report in humans in which only 55% (six of 11 cases) were discovered during intraoperative TEE.1
As IVSH is a rare finding in veterinary medicine, an established treatment protocol is not available. It is also rarely diagnosed in humans, so a consensus management strategy is also lacking. Although surgical VSD repair is very rare in our patient population (n = 5 at our institution), we have seen the development of IVSH only after MVR to date.
Reported strategies in the human literature for management include evacuation (i.e., incisional drainage) or conservative management with close monitoring.1 In the report by Jegatheeswaran et al.,1 two patients (17%) underwent evacuation of the IVSH and one patient died despite undergoing extracorporeal membrane oxygenation. There are individual case reports in humans describing incisional drainage of IVSH with good outcomes.3,9 Similarly, there are reports of success with conservatively managed IVSH.10,11 Management depends on the hemodynamic consequences of IVSH, which can be variable. If the hematoma is large, it can protrude into either ventricular cavity, causing obstruction of the outflow tract and impaired diastolic function, resulting in hemodynamic instability. This therefore may necessitate incisional drainage, but unfortunately, despite initial stabilization of BP and successful separation from CPB after evacuation in case 2 reported here, the patient progressed to refractory hypotension a short time later with progressive myocardial dysfunction. Extracorporeal membrane oxygenation might have been beneficial in this case.1 In cases 3 and 4, conservative management was successful, but this was likely related to the smaller size of the IVSH compared with case 2, which therefore did not cause any immediate hemodynamic consequences. Case 1 was unique, as the IVSH was much smaller in size and was not visualized at the time of intraoperative TEE, although it is possible that it was missed initially. The hemodynamic consequences that developed were severe despite no change in the IVSH. The patient became acutely dyspneic and hypotensive 12 hours after surgery and arrested, but resuscitation was unsuccessful. The role of the IVSH is unknown in this case, and we suspected other mechanisms that may have been related to the dog's ultimate death.
Because of the concern for thrombosis following CPB and MVR, both cases 3 and 4 remained on our standard anticoagulant protocol. The IVSH in both dogs reduced in size despite therapy with aspirin, clopidogrel, and low–molecular weight heparin, but careful consideration needs to be given to anticoagulation once a diagnosis of an IVSH has been made, as further hemorrhage as a result of anticoagulation could certainly turn a relatively innocuous IVSH into one leading to severe complications.9 Although IVSHs in our current MVR program are rare, our current preferred option for hemodynamically stable patients is conservative management with close monitoring, particularly of invasive arterial BP. TTE and TEE are the mainstays of monitoring progression and resolution of IVSH. In the two dogs that survived, we saw IVSH resolution at 1-month reassessment.
Conclusion
We report the first case series of IVSH in veterinary patients after MVR under CPB. Additionally, we describe management of IVSH by incisional drainage and conservative management with variable results. Close monitoring of IVSH in hemodynamically stable patients is a viable option in dogs. Because of the rarity of IVSH, recommendations for the best management practices in dogs continue to be a challenge, and further evaluation of this condition is necessary.
Acknowledgments
We acknowledge the many veterinarians and nurses involved in the care of the four dogs in this report. In particular, we would like to acknowledge the cardiologists at Coast to Coast Cardiology for sharing the echocardiogram from their patient.
Footnotes
Conflicts of interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2020.11.001.
Supplementary Data
Intraoperative TEE showing a large IVSH.
Intraoperative video showing the large IVSH bulging into the left ventricle and displacing the anterior mitral valve leaflet dorsally toward the left atrium. Incisional drainage was performed showing drainage from the IVSH.
Intraoperative TEE after surgical incision showing the reduced size of the IVSH.
References
- 1.Jegatheeswaran A., Cohen M.S., Gaynor J.W., Mascio C.E., Spray T.L., Fuller S. Extracorporeal membrane oxygenation as a novel management strategy for interventricular septal hematoma following ventricular septal defect repair. J Thorac Cardiovasc Surg. 2020;159:1936–1940. doi: 10.1016/j.jtcvs.2019.09.150. [DOI] [PubMed] [Google Scholar]
- 2.Keene B.W., Atkins C.E., Bonagura J.D., Fox P.R., Haggstrom J., Fuentes V.L. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J Vet Intern Med. 2019;33:1127–1140. doi: 10.1111/jvim.15488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drago M., Butera G., Giamberti A., Lucente M., Frigiola A. Interventricular septal hematoma in ventricular septal defect patch closure. Ann Thorac Surg. 2005;79:1764–1765. doi: 10.1016/j.athoracsur.2003.10.123. [DOI] [PubMed] [Google Scholar]
- 4.Yoneyama F., Matsubara M., Sakamoto H., Hiramatsu Y. Interventricular septal hematoma associated with congenital heart surgery: a case report and literature review. J Thorac Cardiovasc Surg. 2017;153:e55–e57. doi: 10.1016/j.jtcvs.2016.10.053. [DOI] [PubMed] [Google Scholar]
- 5.McGrath T., Ushukumari D., Canale L., Gillinov M. Dissecting intramyocardial hematoma after robotic mitral valve repair. Ann Thorac Surg. 2015;99:1048–1051. doi: 10.1016/j.athoracsur.2014.04.134. [DOI] [PubMed] [Google Scholar]
- 6.Pontone G., Bertella E., Andreini D., Pepi M., Polvani G. Postoperative dissecting ventricular haematoma: a conservative strategy with a cardiac magnetic resonance imaging follow-up. Eur Heart J Cardiovasc Imaging. 2014;15:1151. doi: 10.1093/ehjci/jeu079. [DOI] [PubMed] [Google Scholar]
- 7.Vargas-Barron J., Roldan F.J., Romero-Cardenas A., Vazquez-Antona C.A. Intramyocardial dissecting hematoma and postinfarction cardiac rupture. Echocardiography. 2013;30:106–113. doi: 10.1111/echo.12017. [DOI] [PubMed] [Google Scholar]
- 8.James T.N., Burch G.E. Blood supply of the human interventricular septum. Circulation. 1958;17:391–396. doi: 10.1161/01.cir.17.3.391. [DOI] [PubMed] [Google Scholar]
- 9.Mart C.R., Kaza A.K. Postoperative dissecting ventricular septal hematoma: recognition and treatment. ISRN Pediatr. 2011;2011:534940. doi: 10.5402/2011/534940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suteu C.C., Muntean I., Benedek T., Toganel R. Giant dissecting ventricular septal haematoma associated with critical congenital heart disease. Interact Cardiovasc Thorac Surg. 2016;23:837–838. doi: 10.1093/icvts/ivw223. [DOI] [PubMed] [Google Scholar]
- 11.Eyileten Z., Aliyev A., Ciftci O., Ucar T., Odek C., Kendirli T. An extremely rare complication of congenital heart surgery: interventricular septal hematoma. Turk J Pediatr. 2013;55:662–664. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intraoperative TEE showing a large IVSH.
Intraoperative video showing the large IVSH bulging into the left ventricle and displacing the anterior mitral valve leaflet dorsally toward the left atrium. Incisional drainage was performed showing drainage from the IVSH.
Intraoperative TEE after surgical incision showing the reduced size of the IVSH.








