Abstract
Folic acid (FA), is a group B vitamin, has high reactive oxygen radicals quenching ability, resulting in protection against oxidative damage in aerobic cell. Acetaminophen (N-acetyl-p-aminophenol, APAP) is a nonsteroidal anti-inflammatory drug, and can promote oxidative damage in liver and kidney tissues. The aim of this study was to investigate whether folic acid has protective effects on oxidative liver and kidney injury caused by experimental APAP toxication. Forty female Sprague dawley rats were divided into 5 groups; control, APAP, FA, APAP+FA, and APAP+N-acetylcysteine (NAC) groups. APAP toxication was induced by oral gavage (3 g/kg bodyweight). FA (20 mg/kg bodyweight) and NAC (150 mg/kg bodyweight) were given by oral gavage to the specified groups. Oxidant and antioxidant parameter were determined in liver and kidney tissues. In addition, the liver and kidney tissues were histological evaluated. When compared with APAP group, superoxide dismutase (SOD) and catalase activities and glutathione levels were statistically higher, malondialdehyde (MDA) level and myeloperoxidase activity (except liver tissue) were statistically lower in both APAP+FA and APAP+NAC. Liver and kidney MDA level and kidney SOD activity were significantly lower in APAP+NAC group compared with APAP+FA group. Co-administration of NAC with APAP was found to provide protection, but hepatic cords were defective in some places and some glomerular tubules also had dilatation. Necrotic areas was reduced in the liver and the glomerular structure was in good condition in the APAP+FA group. As a result, FA might have a protective effect against APAP-induced hepato-nephrotoxicity and oxidative stress in rat.
Keywords: acetaminophen, folic acid, oxidative stress, rat, toxicity
Introduction
Acetaminophen (paracetamol) or N-acetyl-p-aminophenol (APAP) is an antipyretic and analgesic drug. Although it is safe to use in clinical doses in human medicine, the studies shows that toxicity caused by overdose APAP may cause liver and kidney damage and even deaths may develop [1, 2]. Because paracetamol is one of the commonest drugs taken in overdose and involved in deliberate self-poisoning in many countries [3,4,5]. At therapeutic doses, the majority of APAP is metabolized by sulphation (20–30%) and glucuronidation (45–55%), while 5–9% is converted to N-acetyl-para-benzoqinonimine (NAPQI), a highly reactive metabolite by cytochrome p450 (CYP 450) enzymes [6]. NAPQI is responsible for APAP induced hepato-nephrotoxicity and converted to nontoxic active form by reduced glutathione (GSH) [7]. However, since the pathways of glucuronidation and sulphation are saturated at APAP overdose, they are largely metabolized to NAPQI by the CYP2E1 enzyme system [8]. NAPQI eventually results in the depletion of intracellular GSH. Once GSH depots have been depleted by approximately 70%, NAPQI binds to cellular proteins and leads to cell injury because of increasing endonuclease G and apoptosis inducing factor. Thus, APAP application causes mitochondrial oxidative stress [9].
The damage caused by the oxidative stress is due to the generation of reactive oxygen species (ROS). When the balance between the generated ROS (such as superoxide radical-O2•, hydroxyl radical-OH•, singlet oxygen-1O2 and nitric oxide-NO•) and antioxidants is disrupted, the free radicals can then interact with macromolecules resulting in alteration of cellular functions or excessive free radical production is common in tissues [10]. Therefore, antioxidant plays important role in this respect. Cellular antioxidant systems, such as hepatic GSH, are very important in protecting against cell damage in APAP hepatotoxicity. NAC is currently recognized worldwide as an antidote to APAP poisoning. Because NAC promotes GSH synthesis in the liver and renews GSH stores. Many compounds such as vitamins [11, 12] and antioxidants [13] have been used for their protective effects against APAP toxicity. Therefore, new treatment modalities in APAP poisoning cases are still of interest.
Folic acid (FA), commonly referred to as Vitamin B9, is a considerable vitamin for numerous metabolic pathways of living organisms. It plays an important role especially in nucleic acid synthesis and protection of DNA structure [14]. Studies show that FA is effective against ROS [15, 16]. Additionally, FA is an important effector in mitochondrial redox homeostasis that regulates GSH biosynthesis and affects the GSH transport system [17].
We planned to establish experimental toxicity model in rats as a result of high dose of APAP. Although there is study showing that effect of FA on experimentally induced APAP toxicity in rats were evaluated biochemical and histological only in liver tissue [18, 19], no studies have been conducted on the oxidative stress of FA in the APAP induced toxicity on both liver and kidney. Consequently, we designed the study to determine the protective effect of FA against APAP-induced hepato-nephrotoxicity in rats. Specifically, we evaluated their impacts on the oxidative stress biomarkers and histopathological evaluation in the exposed rats.
Materials and Methods
Animals
A total of 40 female Sprague dawley rats weighing 291–307 g were obtained from Aydin Adnan Menderes University Experimental Animal Unite (Aydin, Turkey). Rats were kept in transparent cages for 12 h in bright and 12 h in darkness at room temperature of 22–24°C and rats were given standard rat feed (Optima Food Co., Izmir, Turkey) and tap water ad libitum during the study. The Animal Experiments Local Ethics Committee of the University of Aydin Adnan Menderes approved this study (2017/041). We have reported this study consistent with the Animals in Research: Reporting In Vivo Experiments (ARRIVE) guidelines [20].
Chemical substances
APAP (code; A7085), FA (code; F7876), NAC (code; A7250) and the other chemicals were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). The chemicals were prepared daily by dissolving it in distilled water. Assay kit for the determination of total protein was purchased from Archem Diagnostic Ind. Ltd., (Istanbul, Turkey).
Experimental procedure
The experimental animals were randomly divided into five groups (eight in each group); control group, APAP group (3,000 mg/kg bodyweight), FA group (20 mg/kg bodyweight), APAP+FA group and APAP+NAC group (150 mg/kg bodyweight). The doses and treatment periods of APAP, FA and NAC in the present study were selected based on reports of stated studies [15, 21,22,23]. NAC and FA in the groups were administered 1.5 h after APAP administration. The study duration was 1 day and the substances were administered by oral gavage. Control group received 0.9% NaCl to compare the effects of experimental APAP toxicity in liver and kidney in other groups. Rats were euthanized by cervical dislocation under 5 mg/kg bodyweight xylazine (Alfazyne 2%, Alfasan, Turkey) and 50 mg/kg bodyweight ketamine (Alfamine 10%, AtaFen, Izmir, Turkey) anesthesia 24 h after drug administration. Animals were dissected for oxidant and antioxidant parameter and histological analysis in liver and kidney tissue samples. Tissue samples were stored at −80°C until analysis (NU 9668, Nuaire, Fernbrook Lane Plymouth, USA).
Tissue homogenization
Liver and kidney tissue samples were homogenized in a 10% of 150 mM phosphate buffer solution (pH 7.4), using a teflon-head homogenizer (IKA Overhead Stirrer, Staufen, Germany). Tissue homogenates were then centrifuged (Hettich Zentrifugen, Mikro 200 R, Tuttlingen, Germany) at 12,000 rpm at 4°C for 10 min. Supernatants were collected and analyzed within the same day. Potassium phosphate (0.05% 50 mM) buffer (pH 6.0) was used for myeloperoxidase (MPO) analysis by the same method.
Determination of antioxidant/oxidant status in blood and tissue
Superoxide dismutase (SOD) estimation was based on the inhibition of nitro blue tetrazolium reduction using the xanthine:xanthine oxidase system as a superoxide generator. SOD activity in tissue was measured at 560 nm according to the method of [24]. Results obtained were expressed as U/mg protein. The catalase (CAT) activity was determined by measuring the decomposition of hydrogen peroxide (H2O2) at 240 nm, and it was expressed as k/mg tissue protein for tissue [25]. GSH analysis in the tissues were performed according to the method of Tietze [26] at 412 nm and results was calculated as mg/g protein. Malondialdehyde (MDA) analysis was based on measuring the optical density at 532 nm of the color produced by MDA in thiobarbituric acid according to [27] and the results were calculated as nmol/mg protein. MPO enzyme activity was measured according to the method of [28] and the results were expressed as mmol/min/mg protein tissue. Total protein was used to calculate oxidant/antioxidant parameters in supernatants. For this purpose, the biuret method using commercially available kits (Archem Diagnostic Ind. Ltd., Istanbul, Turkey) was used and the results were calculated as mg/ml protein. All these parameters measured using spectrophotometer (Shimadzu UV-1601, Kyoto, Japan).
Histological evaluation
Liver and kidneys were taken in 10% neutral buffered formalin and fixed for 24 h. Following the histological processing, the tissues were embedded in paraffin and serial cross sections (6 µm thickness) were taken at intervals of 50 µm. Paraffin sections were stained with Hematoxylin-Eosin (H&E) and Masson’s trichrome stain for general evaluations. Periodic acid Schiff was used to show glycogen in hepatocytes [29]. Sections were examined with Olympus BX43 research microscope and photographed. Characteristics of cell damage and lesions in the liver and kidney tissues were scored on a semi-quantitative scale: [+, mild (less than 25% of the tissues were affected); ++, moderate (25–50% of the tissues were affected); +++, severe (50–75% of the tissues were affected); very severe (more than 75% of the tissues were affected)] [30].
Statistical analysis
We used SPSS (Statistical Package for the Social Sciences; SPSS Inc., Chicago, IL, USA) for Windows Version 22 for all analyses. The suitability of the data for normal distribution was evaluated using the Shapiro Wilk test and for homogeneity of variance with Levene’s test. Differences between initial and final body weights for each group were analyzed using paired samples t-test. Kruskal-Wallis oneway analysis of variance and a post hoc multiple comparison tests (Mann-Whitney U test with Bonferroni correction) were used to analyze antioxidant/oxidant parameters in tissues [31]. Differences were considered statistically significant if P<0.05. All data were given as mean and ± SE.
Results
We found no statistically significant differences in live weight among the experimental groups at the beginning (P=0.776) and after the study (P=0.470). Only in the APAP and APAP+FA groups were the body weight significantly decreased at the final of the study when compared at the start of the study (Table 1). The body weights before study of the APAP group was 307.50 ± 6.54 g, it decreased to 293.50 ± 7.03 g at the final of the study (P=0.012). Likewise, the body weights before study of the APAP+FA group was 300.00 ± 8.81 g, it decreased to 289.75 ± 9.70 g at the final of the study (P=0.012).
Table 1. Average body weights of experimental groups before and after the study.
| Groups | Body weights before study | Body weights after study | P |
|---|---|---|---|
| Control | 305.62 ± 7.98 | 306.75 ± 8.44 | NS |
| APAP | 307.50 ± 6.54 | 293.50 ± 7.03 | 0.012 |
| FA | 306.25 ± 16.76 | 307.50 ± 17.26 | NS |
| APAP+FA | 300.00 ± 8.81 | 289.75 ± 9.70 | 0.012 |
| APAP+NAC | 291.25 ± 6.66 | 285.25 ± 6.88 | NS |
| P | NS | NS | |
APAP: acetaminophen, FA: folic acid, APAP+FA: acetaminophen+ folic acid, APAP+NAC: acetaminophen+ N-acetylcysteine. NS: not significant (t-test).
The antioxidant parameters of the liver tissue were significantly higher and oxidant parameters were significantly lower in the control group compared to the APAP group (Table 2). The antioxidant parameters were significantly increased and MDA level was decreased in both APAP+FA and APAP+NAC groups compared to the APAP group. Whereas MPO activity was higher in the APAP group compared to the control group (P=0.001), but there was no difference between APAP, APAP+FA and APAP+NAC groups (P<0.05).
Table 2. The antioxidant and oxidant parameter levels of liver tissue in experimental groups.
| Groups | Parameters | ||||
|---|---|---|---|---|---|
| SOD | GSH | CAT | MDA | MPO | |
| Control | 3.68 ± 0.16 | 15.25 ± 0.87 | 7.06 ± 1.05 | 103.33 ± 1.73 | 76.94 ± 5.59 |
| APAP | 1.40 ± 0.12a | 3.94 ± 0.79a | 4.62 ± 0.42c | 134.25 ± 3.66a | 115.28 ± 15.47c |
| FA | 2.01 ± 0.09a,** | 18.05 ± 2.57*** | 8.51 ± 0.49*** | 106.39 ± 2.74*** | 62.09 ± 3.62c, *** |
| APAP+FA | 5.00 ± 0.19a, *** | 13.72 ± 0.94*** | 7.32 ± 0.89* | 113.51 ± 3.64c, ** | 102.98 ± 7.76c |
| APAP+NAC | 5.05 ± 0.14a, *** | 14.72 ± 0.86*** | 8.15 ± 1.23** | 91.62 ± 2.84b, *** | 82.87 ± 5.21 |
| P | 0.001 | 0.001 | 0.026 | 0.001 | 0.001 |
APAP: acetaminophen, FA: folic acid, APAP+FA: acetaminophen+ folic acid, APAP+NAC: acetaminophen+ N-acetylcysteine. SOD (Superoxide dismutase, U/mg protein), GSH (Reduced glutathione, mg/g protein), CAT (Catalase, k/mg protein), MDA (Malondialdehyde, nmol/mg protein), MPO (Myeloperoxidase, mmol/min/mg protein). a: P<0.001, b: P<0.01 and c: P<0.05 significantly different from control group. ***: P<0.001, **: P<0.01 and *: P<0.05 significantly different from APAP group (Bonferroni test).
In the kidney tissues SOD and CAT activities and GSH levels were significantly low and MDA level and MPO activities were significantly high in the APAP group, compared to the control group (Table 3). Antioxidant parameters were significantly higher in both APAP+NAC and APAP+FA groups compared to APAP group, and there was no difference between control and APAP+FA groups (except GSH level) (P>0.05). When compared with APAP group, MDA level and MPO activity were lower in other experimental groups (P=0.001).
Table 3. The antioxidant and oxidant parameter levels of kidney tissue in experimental groups.
| Groups | Parameters | ||||
|---|---|---|---|---|---|
| SOD | GSH | CAT | MDA | MPO | |
| Control | 5.77 ± 0.15 | 13.74 ± 0.53 | 2.12 ± 0.17 | 88.60 ± 5.03 | 40.37 ± 1.57 |
| APAP | 4.59 ± 0.08a | 3.85 ± 0.48a | 0.97 ± 0.06b | 138.46 ± 7.04a | 61.19 ± 6.26c |
| FA | 7.59 ± 0.26 a, *** | 12.38 ± 1.06*** | 4.91 ± 1.02b, *** | 94.69 ± 5.70*** | 31.50 ± 2.09b, *** |
| APAP+FA | 6.23 ± 0.22*** | 6.35 ± 0.58a, * | 2.90 ± 0.26*** | 111.53 ± 2.91a, ** | 31.29 ± 2.33b, *** |
| APAP+NAC | 5.22 ± 0.16b, ** | 9.67 ± 0.81a, *** | 2.95 ± 0.26c, *** | 90.83 ± 2.28*** | 34.60 ± 4.01c, *** |
| P | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
APAP: acetaminophen, FA: folic acid, APAP+FA: acetaminophen+ folic acid, APAP+NAC: acetaminophen+ N-acetylcysteine. SOD (Superoxide dismutase, U/mg protein), GSH (Reduced glutathione, mg/g protein), CAT (Catalase, k/mg protein), MDA (Malondialdehyde, nmol/mg protein), MPO (Myeloperoxidase, mmol/min/mg protein). a: P<0.001, b: P<0.01 and c: P<0.05 significantly different from control group. ***: P<0.001, **: P<0.01 and *: P<0.05 significantly different from APAP group (Bonferroni test).
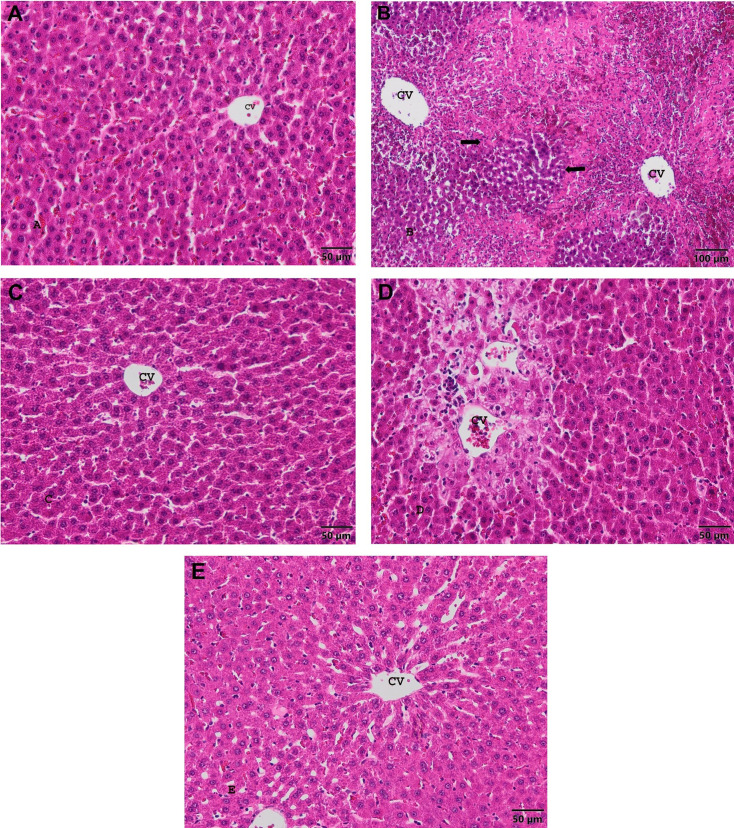
When liver sections of the control group were examined, no liver damage was observed, normal hepatic cells arranged radially around the central veins, normal sinusoidal spaces between the cords and normal liver structure were observed (Fig. 1A). Congestion has been observed in hepatic sinusoids of APAP group. Hepatic cords were enlarged and their order was distorted. Necrosis were generally seen around the central veins. In some cases, necrosis with widespread bleeding were located zonal (Fig 1B). Hepatic parenchyma lost glycogen storage ability. In the FA group, the liver structure was similar to normal (Fig. 1C). The administration of FA with APAP reduced necrotic areas in the liver (Fig. 1D). However, in some sections, cord structure were determined to be impaired in places. In the APAP+NAC group, the liver structure was similar to normal (Fig. 1E), but hepatic cords were defective in some places, deterioration of hepatocytes structure. Microscopic findings of the liver was summarized in Table 4.
Fig. 1.
Liver central veins (CV) and parenchyma image of experimental groups. A. Control group. Bar: 50 µm. H&E stain. B. APAP group. Bar: 100 µm. Necrotic areas (arrows) H&E stain. C. FA group. Bar: 50 µm. H&E stain. D. APAP+FA group. Bar: 50 µm. H&E stain. E. APAP+NAC group. Bar: 50 µm. H&E stain.
Table 4. Effect of FA on the histological lesions induced by treatment of rats with APAP.
| Tissue | Groups | |||||
|---|---|---|---|---|---|---|
| Control | APAP | FA | APAP+FA | APAP+NAC | ||
| Liver | Disruption in the structure of hepatic cords | - | ++++ | + | ++ | ++ |
| Enlargement of the central veins | - | ++++ | + | ++ | ++ | |
| Edema | - | ++++ | + | ++ | ++ | |
| Hemorrhage | - | ++++ | + | ++ | ++ | |
| Necrosis | - | ++++ | - | ++ | + | |
| Vacuolar degeneration | - | +++ | + | ++ | ++ | |
| Glycogen storage ability | ++++ | - | ++ | ++ | ++ | |
| Kidney | Congestion | - | +++ | + | ++ | + |
| Tubular dilatation | - | +++ | + | ++ | + | |
| Enlarged Bowman spacing | - | +++ | + | ++ | + | |
| Tubular necrosis/degeneration | - | +++ | - | + | + | |
APAP: acetaminophen, FA: folic acid, APAP+FA: acetaminophen+folic acid, APAP+NAC: acetaminophen+N-acetylcysteine. (−): no pathological change, (+): mild (++): moderate, (+++): severe, (++++): very severe.
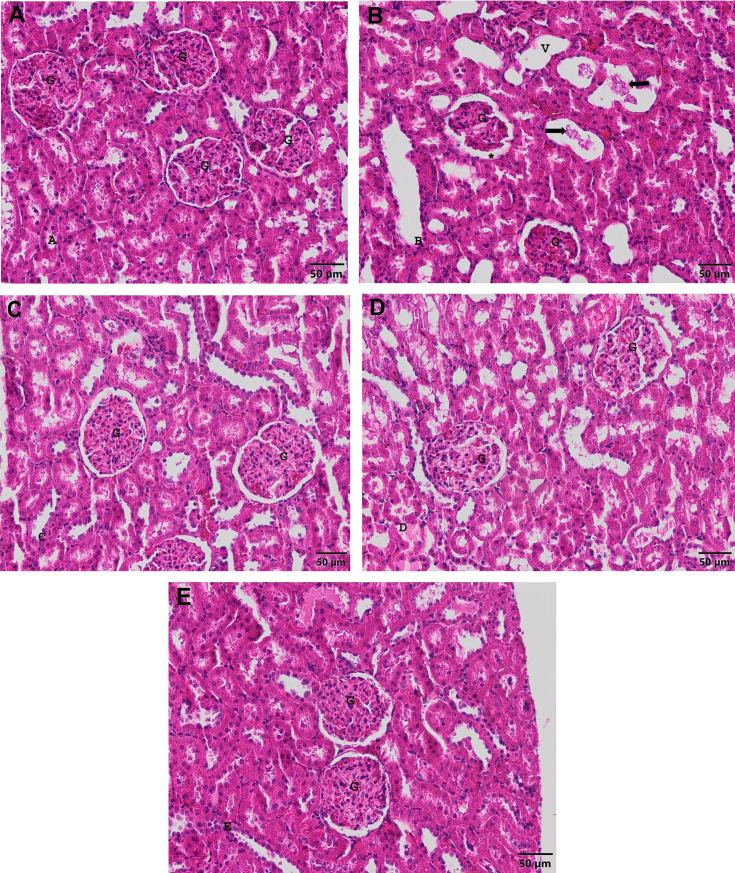
Glomerulus, Bowman capsule, Bowman interval, renal tubules and blood vessels were found to have normal histological structure (Fig. 2A). In the APAP group, the vessels were hyperemic. Dilatations were quite evident in the tubules. Disruptions were common in tubulus epithelium. Hyaline cast were seen in some tubules (Fig. 2B). In the FA group, normal histological kidney structure was observed (Fig. 2C). In the APAP+FA group, the glomerular structure was in good condition, but there was still slight enlargement in the Bowman range, and dilatation in some tubules (Fig. 2D). In the APAP+NAC group, some tubules also had dilatation (Fig. 2E). The degree of histological findings in kidney tissue is shown in Table 4.
Fig. 2.
Kidney image of experimental groups. G: Glomerulus. A. Control group. Bar: 50 µm. H&E stain. B. APAP group. *: Enlarged Bowman spacing. V: Dilated tubules. Hyaline cast (arrows) Bar: 50 µm. H&E stain. C. FA group. Bar: 50 µm. H&E stain. D. APAP+FA group. Bar: 50 µm. H&E stain. E. APAP+NAC group. Bar: 50 µm. H&E stain.
Discussion
Oxidative stress induces the production of free oxygen radicals, an undesirable by-product. It is the main factor in APAP induced liver and renal toxicity and can exacerbate free radical chain reactions [8]. The increase in free oxygen radicals leads to lipid peroxidation (LPO) in the membranes, causing damage or death to cells and tissues in association with impairments to cellular structures and functions [32]. Enzymatic antioxidant and non-enzymatic systems are natural preservatives against free radicals. If the oxidant-antioxidant balance is disturbed excessive production of free radicals causes oxidative damage to cellular molecules such as lipids, carbohydrates, proteins, RNA and DNA [33].
The number of drugs used during pregnancy in humans and animals are limited. APAP has become the most commonly recommended safe analgesic during pregnancy. But, APAP has been demonstrated to cross the placenta and in toxic doses may harm the fetal and maternal hepatocytes [34]. Therefore, female rat was preferred in our study.
When compared with APAP group, APAP+FA group had significantly high levels of SOD activity in both tissues. The protective effect of FA against APAP induced liver and kidney damage may be due to inhibition of excess ROS production and protection of cellular antioxidant defense mechanisms. Because of rapid production of O2•- in tissues the SOD activity might have increased to protection the cells. SOD converts O2•- into H2O2, which then decomposes into water via CAT and GPx. Therefore, the important production of non-radical H2O2 in biological systems occur by SOD. Studies show that SOD and CAT activities significantly decreased due experimental APAP caused toxicity in liver and kidney [21, 35]. In these studies protective effects of antioxidants (such as quercetin and curcumin) were evaluated and successfully results were obtained. The reason for decreased CAT activity in both tissue may cause accumulation of free oxygen radicals in APAP group (Table 1 and 2). CAT is one of the enzymatic antioxidants that protect tissues against H2O2. Found in high amounts in liver and erythrocytes and reduces H2O2 to molecular O2. The reason for decreased CAT activity may cause accumulation of free oxygen radicals in APAP group. Our study showed FA administration reduced the response in the liver (P=0.026) and kidney (P=0.001) tissues. Our findings were in accordance with the results of previous study. Uysal et al. [12] showed that thiamine, another B group vitamin, administration ameliorated the CAT activity in APAP-induced rat liver tissue.
GSH is thiol-containing compounds and one of the most important defence systems present in the mammals cell. GSH levels in liver and kidney homogenizes were significantly higher in the APAP+FA group than in the APAP group. The decreased GSH level in APAP group may be due to excessive NAPQI and O2•- production or peroxides due to APAP administration [36]. The GSH level statistically decreased under exposure to APAP in our study. The decrease in the GSH level might suggest that enzyme activity of Glutathione peroxidase (GPx) that might have decreased during the oxidative process. GPx is a cytosolic enzyme that reduces H2O2 to molecular H2O and oxidized glutathione (GSSG) using GSH, also plays a role in the reduction of hydroperoxides and prevents the onset and development of LPO [37].
The oxidant marker of LPO due to oxidative stress is MDA and MDA has mutagenic, genotoxic, and carcinogenic effects [38]. In the present study, APAP-treated rats showed a significant increase in levels of MDA, in the rat liver and kidney tissues which was reduced by co-treatment of animals by FA significantly. Dallak et al. [13] suggested a relationship between APAP toxicity and increased MDA level and decreased GSH level. In our study, MDA levels in liver and kidney tissues were found to be significantly highest in APAP group compared to the other experimental groups. In another study, APAP given to rats at a dose of 3 g/kg/day has caused a significant increase in LPO levels and a significant decrease in GSH levels compared to the control group [22]. In our study, elevated MDA levels in tissues may be due to an increase in APAP induced ROS production (particularly OH• radicals) resulting by oxidative stress. The OH• radical, which may a genotoxic effect, interacts with purine and pyrimidine bases resulting by DNA damage.
MPO activity is a source of free radicals. This enzyme is a marker for acute inflammation and polymorphonuclear cell infiltration [39]. According to the data obtained, MPO activity in liver tissue was statistically higher in APAP, APAP+FA and APAP+NAC groups compared to control group. High levels of MPO activity in these groups are indicators of established oxidative liver damage. Unlike these results, MPO activity was significantly lover by APAP+FA and APAP+NAC group compared with the APAP group in kidney tissue. The present study of kidney tissue results are concordant with Uysal et al. [12]. Our study clearly demonstrate hepato-nephrotoxicity may occur due to an increase in the intracellular levels of ROS, which are toxic at high levels and can interact with macromolecules. The present study demonstrated the influence of APAP on some non-enzymatic and enzymatic biomarkers of oxidative stress in liver and kidney of rats and its amelioration by pre-treatment by antioxidant, FA.
Studies indicated that APAP causes severe liver damage such as massive neutrophilic and lymphocytic infiltration, intense coagulative necrosis, cytoplasmic vacuoles, vacuolar degeneration in hepatocytes, congestion of central vein and portal vein in the liver [23, 40]. In our study, APAP group showed dilatation of the central veins, centrilobular necrosis, congestion and vacuolar degeneration, as well as loss of glycogen storage ability of hepatic parenchyma. FA have been shown to reduce necrosis of the centrilobular zone in this study. This suggests that FA may play a protective role as an antioxidant in APAP toxicity. It is reported that different doses of APAP administration causes kidney damage such as tubular vacuolization, lymphocyte infiltration and tubular dilatation [40,41,42]. In our study, hyperemia in vessels, dilatation in tubules and disruptions in tubular epithelium were observed in APAP group. In the APAP+FA group, there was a slight enlargement in the Bowman interval, and dilatation in some tubules (Fig. 2).
In conclusion, this study indicate that FA has a preventative effect on the oxidative damage and histologically against APAP induced liver and kidney tissues. This effect may be due to the folic acid inhibiting intracellular ROS production and contributing to cellular GSH synthesis. NAC has been used for the treatment of numerous disorders such as APAP intoxication, cardiovascular diseases, respiratory diseases, heavy metal toxicity and some psychiatric disorders. NAC has various biological effects including, protecting cytoskeleton structure, elevating cellular GSH levels, scavenging of ROS, immuno-modulatory activity, antimutagenic and antineoplastic activities [43]. Although it is currently recognized worldwide as an antidote to APAP poisoning, the use of this drug has many adverse effects like allergy, anaphylaxis, angioedema, bronchospasm [19]. Since FA cannot be stored in the human body it’s deficiency is very important for healthy living [14]. It’s deficiency may lead megaloblastic anaemia, diabetes, neural tube defects in developing foetuses, cancer and cardiovascular diseases, and Alzheimer’s disease [17]. Only folic acid in excess (greater than 1 mg/day) can mask the vitamin B12 deficiency symptoms which may lead to other health risks such as nerve damage [44]. Considering these, the results obtained can contribute to the development of a new alternative pharmacological drug model against APAP toxication, which is not harmful to human and animal health.
Acknowledgments
This research article was summarized from the first author’s master thesis. Additionally, the authors would like to acknowledge Prof. Dr. Hamdi Avci, Department of Pathology, Faculty of Veterinary Medicine, Aydin Adnan Menderes University, for evaluation of the histopathological findings.
References
- 1.Waring WS, Jamie H, Leggett GE. Delayed onset of acute renal failure after significant paracetamol overdose: A case series. Hum Exp Toxicol. 2010; 29: 63–68. doi: 10.1177/0960327109350799 [DOI] [PubMed] [Google Scholar]
- 2.Guggenheimer J, Moore PA. The therapeutic applications of and risks associated with acetaminophen use: a review and update. J Am Dent Assoc. 2011; 142: 38–44. doi: 10.14219/jada.archive.2011.0026 [DOI] [PubMed] [Google Scholar]
- 3.Chiew AL, Isbister GK, Kirby KA, Page CB, Chan BSH, Buckley NA. Massive paracetamol overdose: an observational study of the effect of activated charcoal and increased acetylcysteine dose (ATOM-2). Clin Toxicol (Phila). 2017; 55: 1055–1065. doi: 10.1080/15563650.2017.1334915 [DOI] [PubMed] [Google Scholar]
- 4.Marks DJB, Dargan PI, Archer JRH, Davies CL, Dines AM, Wood DM, et al. Outcomes from massive paracetamol overdose: a retrospective observational study. Br J Clin Pharmacol. 2017; 83: 1263–1272. doi: 10.1111/bcp.13214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salmonson H, Sjöberg G, Brogren J. The standard treatment protocol for paracetamol poisoning may be inadequate following overdose with modified release formulation: a pharmacokinetic and clinical analysis of 53 cases. Clin Toxicol (Phila). 2018; 56: 63–68. doi: 10.1080/15563650.2017.1339887 [DOI] [PubMed] [Google Scholar]
- 6.Moyer AM, Fridley BL, Jenkins GD, Batzler AJ, Pelleymounter LL, Kalari KR, et al. Acetaminophen-NAPQI hepatotoxicity: a cell line model system genome-wide association study. Toxicol Sci. 2011; 120: 33–41. doi: 10.1093/toxsci/kfq375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bessems JGM, Vermeulen NPE. Paracetamol (acetaminophen)-induced toxicity: molecular and biochemical mechanisms, analogues and protective approaches. Crit Rev Toxicol. 2001; 31: 55–138. doi: 10.1080/20014091111677 [DOI] [PubMed] [Google Scholar]
- 8.Parikh H, Pandita N, Khanna A. Phytoextract of Indian mustard seeds acts by suppressing the generation of ROS against acetaminophen-induced hepatotoxicity in HepG2 cells. Pharm Biol. 2015; 53: 975–984. doi: 10.3109/13880209.2014.950675 [DOI] [PubMed] [Google Scholar]
- 9.McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012; 122: 1574–1583. doi: 10.1172/JCI59755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halliwell B, Gutteridge JMC. Lipid peroxidation, oxygen radicals, cell damage, and antioxidant therapy. Lancet. 1984; 323: 1396–1397. doi: 10.1016/S0140-6736(84)91886-5 [DOI] [PubMed] [Google Scholar]
- 11.Matić MM, Paunović MG, Milošević MD, Ognjanović BI, Saičić ZS. Hematoprotective effects and antioxidant properties of β-glucan and vitamin C against acetaminophen-induced toxicity: an experimental study in rats. Drug Chem Toxicol. 2019; 0: 1–8. doi: 10.1080/01480545.2019.1587451 [DOI] [PubMed] [Google Scholar]
- 12.Uysal HB, Dağlı B, Yılmaz M, Kahyaoğlu F, Gökçimen A, Ömürlü IK, et al. Biochemical and Histological Effects of Thiamine Pyrophosphate against Acetaminophen-Induced Hepatotoxicity. Basic Clin Pharmacol Toxicol. 2016; 118: 70–76. doi: 10.1111/bcpt.12496 [DOI] [PubMed] [Google Scholar]
- 13.Dallak M, Dawood AF, Haidara MA, Abdel Kader DH, Eid RA, Kamar SS, et al. Suppression of glomerular damage and apoptosis and biomarkers of acute kidney injury induced by acetaminophen toxicity using a combination of resveratrol and quercetin. Drug Chem Toxicol. 2020; 0: 1–7. doi: 10.1080/01480545.2020.1722156 [DOI] [PubMed] [Google Scholar]
- 14.Akbar S, Anwar A, Kanwal Q. Electrochemical determination of folic acid: A short review. Anal Biochem. 2016; 510: 98–105. doi: 10.1016/j.ab.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 15.Mohammadi A, Omrani L, Omrani LR, Kiani F, Eshraghian A, Azizi Z, et al. Protective effect of folic acid on cyclosporine-induced bone loss in rats. Transpl Int. 2012; 25: 127–133. doi: 10.1111/j.1432-2277.2011.01375.x [DOI] [PubMed] [Google Scholar]
- 16.Singh R, Kanwar SS, Sood PK, Nehru B. Beneficial effects of folic acid on enhancement of memory and antioxidant status in aged rat brain. Cell Mol Neurobiol. 2011; 31: 83–91. doi: 10.1007/s10571-010-9557-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai KG, Chen CF, Ho CT, Liu JJ, Liu TZ, Chern CL. Novel roles of folic acid as redox regulator: Modulation of reactive oxygen species sinker protein expression and maintenance of mitochondrial redox homeostasis on hepatocellular carcinoma. Tumour Biol. 2017; 39: 1010428317702649. doi: 10.1177/1010428317702649 [DOI] [PubMed] [Google Scholar]
- 18.Al-Sowyan NS. Efficacy and safety of folic acid during toxic hepatitis induced by acute overdose of paracetamol. Int J Pharmacol. 2009; 5: 208–214. doi: 10.3923/ijp.2009.208.214 [DOI] [Google Scholar]
- 19.Saravanan T, Shanmugapriya S, Sumitra G, Dhayananth RS, Saravanan A, Manicka Vasuki AK. Hepato-protective effect of folic acid and vitamin B12 in comparison to n-acetylcysteine in experimentally induced acetaminophen toxicity in rats. Biomed Pharmacol J. 2017; 10: 549–555. doi: 10.13005/bpj/1140 [DOI] [Google Scholar]
- 20.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010; 8: e1000412. doi: 10.1371/journal.pbio.1000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yousef MI, Omar SAM, El-Guendi MI, Abdelmegid LA. Potential protective effects of quercetin and curcumin on paracetamol-induced histological changes, oxidative stress, impaired liver and kidney functions and haematotoxicity in rat. Food Chem Toxicol. 2010; 48: 3246–3261. doi: 10.1016/j.fct.2010.08.034 [DOI] [PubMed] [Google Scholar]
- 22.Sudheesh NP, Ajith TA, Janardhanan KK. Hepatoprotective effects of DL-α-lipoic acid and α-Tocopherol through amelioration of the mitochondrial oxidative stress in acetaminophen challenged rats. Toxicol Mech Methods. 2013; 23: 368–376. doi: 10.3109/15376516.2013.769289 [DOI] [PubMed] [Google Scholar]
- 23.Mahmoud YI, Mahmoud AA. Role of nicotinamide (vitamin B3) in acetaminophen-induced changes in rat liver: Nicotinamide effect in acetaminophen-damged liver. Exp Toxicol Pathol. 2016; 68: 345–354. doi: 10.1016/j.etp.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 24.Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988; 34: 497–500. doi: 10.1093/clinchem/34.3.497 [DOI] [PubMed] [Google Scholar]
- 25.Aebi H. Catalase in vitro. Methods Enzymol. 1984; 105: 121–126. doi: 10.1016/S0076-6879(84)05016-3 [DOI] [PubMed] [Google Scholar]
- 26.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969; 27: 502–522. doi: 10.1016/0003-2697(69)90064-5 [DOI] [PubMed] [Google Scholar]
- 27.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95: 351–358. doi: 10.1016/0003-2697(79)90738-3 [DOI] [PubMed] [Google Scholar]
- 28.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982; 78: 206–209. doi: 10.1111/1523-1747.ep12506462 [DOI] [PubMed] [Google Scholar]
- 29.Crossmon G. A modification of Mallory’s connective tissue stain with a discussion of the principles involved. Anat Rec. 1937; 69: 33–38. doi: 10.1002/ar.1090690105 [DOI] [Google Scholar]
- 30.Abdel-Zaher AO, Abdel-Hady RH, Mahmoud MM, Farrag MMY. The potential protective role of alpha-lipoic acid against acetaminophen-induced hepatic and renal damage. Toxicology. 2008; 243: 261–270. doi: 10.1016/j.tox.2007.10.010 [DOI] [PubMed] [Google Scholar]
- 31.Conover WJ. Practical Nonparametric Statistics. 3rd ed. New York: John Wiley & Sons; 1999. p. 272–288. [Google Scholar]
- 32.Imlay JA. Pathways of oxidative damage. Annu Rev Microbiol. 2003; 57: 395–418. doi: 10.1146/annurev.micro.57.030502.090938 [DOI] [PubMed] [Google Scholar]
- 33.Dizdaroglu M. Oxidatively induced DNA damage and its repair in cancer. Mutat Res Rev Mutat Res. 2015; 763: 212–245. doi: 10.1016/j.mrrev.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 34.Wilkes JM, Clark LE, Herrera JL. Acetaminophen overdose in pregnancy. South Med J. 2005; 98: 1118–1122. doi: 10.1097/01.smj.0000184792.15407.51 [DOI] [PubMed] [Google Scholar]
- 35.Karaali HF, Fahmi RR, Borjac JM. Effect of Ocimum basilicum leaves extract on acetaminophen-induced nephrotoxicity in BALB/c mice. J Complement Integr Med. 2018; 16: 1–15. [DOI] [PubMed] [Google Scholar]
- 36.Akakpo JY, Ramachandran A, Kandel SE, Ni HM, Kumer SC, Rumack BH, et al. 4-Methylpyrazole protects against acetaminophen hepatotoxicity in mice and in primary human hepatocytes. Hum Exp Toxicol. 2018; 37: 1310–1322. doi: 10.1177/0960327118774902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fang YZ, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition. 2002; 18: 872–879. doi: 10.1016/S0899-9007(02)00916-4 [DOI] [PubMed] [Google Scholar]
- 38.Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004; 266: 37–56. doi: 10.1023/B:MCBI.0000049134.69131.89 [DOI] [PubMed] [Google Scholar]
- 39.Kettle AJ, Winterbourn CC. Myeloperoxidase: a key regulator of neutrophil oxidant production. Redox Rep. 1997; 3: 3–15. doi: 10.1080/13510002.1997.11747085 [DOI] [PubMed] [Google Scholar]
- 40.Murad HAS, Habib H, Kamel Y, Alsayed S, Shakweer M, Elshal M. Thearubigins protect against acetaminophen-induced hepatic and renal injury in mice: biochemical, histopathological, immunohistochemical, and flow cytometry study. Drug Chem Toxicol. 2016; 39: 190–198. doi: 10.3109/01480545.2015.1070170 [DOI] [PubMed] [Google Scholar]
- 41.Abdeen A, Abdelkader A, Abdo M, Wareth G, Aboubakr M, Aleya L, et al. Protective effect of cinnamon against acetaminophen-mediated cellular damage and apoptosis in renal tissue. Environ Sci Pollut Res Int. 2019; 26: 240–249. doi: 10.1007/s11356-018-3553-2 [DOI] [PubMed] [Google Scholar]
- 42.Ozatik FY, Teksen Y, Kadioglu E, Ozatik O, Bayat Z. Effects of hydrogen sulfide on acetaminophen-induced acute renal toxicity in rats. Int Urol Nephrol. 2019; 51: 745–754. doi: 10.1007/s11255-018-2053-0 [DOI] [PubMed] [Google Scholar]
- 43.Samuni Y, Goldstein S, Dean OM, Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim Biophys Acta. 2013; 1830: 4117–4129. doi: 10.1016/j.bbagen.2013.04.016 [DOI] [PubMed] [Google Scholar]
- 44.Talaulikar V, Arulkumaran S. Folic acid in pregnancy. Obstetrics, Gynaecol Reprod Med. 2013; 23: 286–288. doi: 10.1016/j.ogrm.2013.06.007 [DOI] [Google Scholar]