Abstract
Deprivation of maternal care has been associated with higher pain sensitivity in offspring. In the present study, we hypothesized that the maternal licking/grooming behavior was an important factor for the development of the pain regulatory system. To test this hypothesis, we used male F2 offspring of early-weaned (EW) F1 mother mice that exhibit lower frequency of licking/grooming behavior. The formalin test revealed that F2 offspring of EW F1 dams showed significantly higher pain behavior than F2 offspring of normally-weaned (NW) F1 dams. We found that the mRNA levels of transient receptor potential vanilloid 1 (TRPV1), a nociceptor, were higher in the lumbosacral dorsal root ganglion (DRG) of F2 offspring of EW F1 dams than those of F2 offspring of NW F1 dams, suggesting that the higher pain sensitivity may be attributed to low licking/grooming, which may result in developmental changes in nociceptive neurons. In the DRG, mRNA levels of Mas-related G-protein coupled receptor B4 (MrgprB4), a marker of sensory neurons that detect gentle stroking, was also up-regulated in the F2 offspring of EW F1 dams. Considering that gentle touch alleviates pain, Mrgprb4 up-regulation may reflect a compensatory change. The present findings indicate important implications of maternal licking/grooming behavior in the development of the pain regulatory system.
Keywords: dorsal root ganglion, early weaning, mas-related G-protein coupled receptor B4 (MrgprB4), pain, transient receptor potential vanilloid 1 (TRPV1)
Introduction
Pain sensitivity in adulthood has been thought to be regulated by early-life environments [1,2,3]. Children who experience early-life stress, including physically traumatic events or socially and/or psychologically poor environments, have an increased risk of chronic pain in adulthood [4,5,6,7]. In support of these clinical findings, animal studies have shown that maternal separation throughout the pre-weaning period induces abnormal behavioral changes against nociceptive stimuli to the skin in adulthood [8,9,10]. Furthermore, it has also been shown that maternal separation, depending on the timing, duration, and number of maternal separation episodes, results in variable developmental outcomes [11]. Longer periods (3–6 h during postnatal days 2–14) of maternal separation exaggerated the hypothalamic-pituitary-adrenal axis response to a stressor, whereas brief periods (~15 min) decreased the adrenal reactivity in adult offspring [12,13,14]. However, specific factors in maternal care that are responsible for the appropriate development of pain sensitivity in the offspring remain unknown.
Maternal care is important for the physical and mental development of offspring [14,15,16]. Maternal care provides multiple sensory inputs to offspring through the somatosensory, gustatory, olfactory, auditory, and visual system inputs [17,18,19,20]. For example, maternal touch in children supports neurodevelopmental outcomes [21]. In rodents, the mother provides her pups with licking and grooming (LG) stimulation as a somatosensory input, not only to keep clean but also to help urinate/defecate and regulate body temperature [22]. LG stimulation completely or partially restores the central nervous system (CNS) dysfunction caused by maternal separation [23,24,25,26]. On the other hand, little is known about the developmental effects of LG stimulation in maternal care on the peripheral sensory neurons such as the nociceptive neurons in the dorsal root ganglia (DRGs).
Nociceptive neurons, which have a peripheral branch that innervates the skin and a central branch that carries the somatosensory information to the spinal cord, respond to intense mechanical, thermal, and noxious chemical stimuli, and express specialized molecular receptors in their peripheral terminals capable of pain transduction [27,28,29,30,31]. The central branch synapses with secondary sensory neurons, and the nerve terminals release neurotransmitters causing local neurogenic inflammation and activation of postsynaptic receptors associated with pain perception located in the spinothalamic tract neurons [32]. Cutaneous sensory neurons perceiving tactile stimulation have recently been suggested to be involved in pain perception [33, 34]. C-low-threshold mechanoreceptive (C-LTMR) neurons, which are not involved in direct pain response in non-pathological conditions, perceive somatosensory inputs including LG stimulation [35,36,37]. Activation of the C-LTMR neurons inhibits nociceptive signaling through synaptic integration in the spinal dorsal horn [33, 38,39,40].
Here, we hypothesized that maternal LG stimulation might be an important factor for the appropriate development of the pain sensitivity in offspring. To test this hypothesis, we utilized F2 male offspring of early-weaned (EW) F1 mother mice that show lower LG behavior [41]. A comparison between F2 offspring of normally-weaned F1 mother mice (NW-F2 offspring) and offspring of early-weaned F1 mother mice (EW-F2 offspring), who themselves were normally weaned, is a good model to examine the effect of maternal LG stimulation in the development. We compared the pain-related behavior evoked by formalin injection into the hind paw skin between NW- and EW-F2 offspring. Previously, we have observed higher gene expression levels of MAS-related G-protein coupled receptor B4 (Mrgprb4), a marker of C-LTMR neurons in the thoracolumbar DRGs innervating the trunk region in the EW-F2 offspring [42]. In addition to the pain-related behavior, we also hypothesized that maternal LG stimulation might induce changes in expression of nociceptive molecules in the DRGs projecting to the hindlimb region. To test this, we investigated gene expression changes in the nociceptor channels transient receptor potential vanilloid 1 (Trpv1) and transient receptor potential ankyrin 1 (Trpa1) [27,28,29,30,31, 43, 44], and in the nociceptive neurotransmitters substance P (SP) and calcitonin gene-related peptide (CGRP) [32] in the DRGs. We also analyzed the gene expression of Mrgprb4.
Material and Methods
Animal preparation and procedures
C57BL/6J mice obtained from Japan Clea Co., Ltd. (Yokohama, Japan) were used for the experiments. Male and female mice were pair-housed in cages (175 × 245 × 125 mm) for breeding, and pups were reared by both parents until weaning. Food and water were supplied ad libitum, and the environment was maintained at a constant temperature (24 ± 1°C) and humidity (50 ± 5%) under a 12 h light-dark cycle (lights on at 6 a.m.). All animal experiments were approved by the Ethical Committee of Azabu University (#180316-6).
The procedure to obtain EW mice (F1) was similar to our previous study [45]. Briefly, pregnant female mice were checked daily every morning until parturition. For each litter, the date of birth was designated as postnatal day 0 (PD0). On PD16, half of the litter was separated from each dam and assigned to the EW group. The remaining pups were assigned to the NW group, cared for with standard procedures, and weaned on PD28. The EW mice were fed powdered pellets until PD28. Thereafter, they were fed regular pellets, as were the NW mice after weaning. After weaning, 2 or 3 pups were kept together in cages according to their original group and sex. When both the EW and NW F1 female mice (EW-F1 and NW-F1, respectively) were 8 weeks old, each female was paired with a NW male mouse. All of the F2 litters were NW on PD28 and housed as described above. In the present study, F2 males from EW and NW F1 dams (EW-F2 and NW-F2, respectively) were studied in adulthood, because sex differences in nociception have been reported [46,47,48]. In addition, we utilized 4–8-month old mice in this study, based on the report indicating that there was no significant effect of age in adult mice (2–12-month-old) on pain sensitivity [49].
We first performed the formalin test using NW- and EW-F2 male offspring (34–39-week-old, NW-F2 offspring: n=9; EW-F2 offspring: n=7). Second, we examined gene expression levels in the lumbosacral and thoracolumbar DRGs of NW- and EW-F2 male offspring (22-week-old, NW-F2 offspring: n=6; EW-F2 offspring: n=3) which had not used in the formalin test. The mice were euthanized by cervical dislocation, and the lumbosacral (L3-S1) DRGs were harvested for gene expression studies.
Maternal behavior observations
To confirm the lower LG behavior of EW-F1 dams, maternal behaviors of all F1 dams used in the present study (EW-F1: n=3; NW-F1: n=3) were digitally videotaped during six observation periods (60 min each) on the 7 day after postpartum. In addition to EW mice, the lower LG behavior until the first 10 days postpartum has been confirmed in the rat model of nongenomic transmission across the generation of the low maternal behavior [50]. All observation periods were performed during the light cycle (6 a.m.–7 a.m., 8 a.m.–9 a.m., 10 a.m.–11 a.m., 12 p.m.–1 p.m., 2 p.m.–3 p.m., 4 p.m.–5 p.m.). Within each observation period, the presence or absence of each behavior in 3 min segments were scored by a well-trained observer (20 observations/period × six periods=120 observations per dam). The observed maternal behaviors were LG, hovering and nursing, and no interaction with pups.
Formalin test
Adult male NW- and EW-F2 offspring were habituated for 60 min in an individual transparent Plexiglas cylinder. Thereafter, 2% formalin was subcutaneously injected into the plantar left hind paw (10 µl volume) of each mouse, and the behavior of each mouse was digitally videotaped for 30 min. The presence or absence of left hind paw licking/biting and self-grooming during periods of 5-s at 1-min intervals was scored using the Observer software (Noldus, Leesburg, VA). The early (acute) phase of the formalin test was defined as 0–5 min post-injection, and the late (tonic) phase as 10–30 min post-injection. The early phase of the formalin response is thought to be due to direct effects of formalin on nociceptive fibers, and the late phase is ascribed to inflammation [51, 52]. Data are presented as the percentage of licking/biting behavior exhibited in each phase by each mouse.
Analysis of mRNA levels in the trunk skin and DRGs
The lumbosacral (L3-S1) and thoracolumbar (T2-L2) DRGs were dissected for analysis of gene expression levels. The lumbosacral DRGs innervate hindlimb regions, and thoracolumbar DRGs innervate trunk regions [53]. Total RNA was isolated with the RNeasy Plus Micro Kit (QIAGEN, Venlo, Netherlands). Concentration and purity were assessed with a NanoDrop-1000 (Thermo Scientific Inc., MA, USA). Total RNA was reverse-transcribed and amplified by using a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific Inc., MA, USA). The amount of messenger ribonucleic acid (mRNA) was quantitatively analyzed with the TaqMan gene expression assays, TaqMan Master Mix, and the 7500 Fast Real-time PCR system (Thermo Fisher Scientific Inc.). The TaqMan gene expression assays Mm00725448_s1 (Ribosomal protein P0, Rplp0), Mm01246302_m1 (Transient receptor potential vanilloid 1, Trpv1), Mm01227437_m1 (Transient receptor potential ankyrin 1, Trpa1), Mm01166996_m1 (Substance P, SP), Mm00801463_g1 (Calcitonin gene-related peptide, CGRP), and Mm01701887_g1 (Mas-related G-protein coupled receptor B4, Mrgprb4) were used. The amount of mRNA was normalized with that of Rplp0 in individual samples [54,55,56]. For comparison between the NW- and EW-F2 offspring, data are shown as ratios relative to NW-F2 offspring values.
Statistical analysis
Results are expressed as means ± SE. All statistical analyses were performed using GraphPad Prism version 6.0 (GraphPad Software Inc., CA, USA). A two-way repeated measures analysis of variance (ANOVA) with Bonferroni post hoc test was performed to compare multiple groups. An unpaired t-test with two-tailed distribution was used to assess statistical significance where two group was compared. Spearman’s correlation coefficient was used to assess associations. A P value <0.05 was considered statistically significant.
Results
Maternal behavior of normally-weaned or early-weaned F1 dams
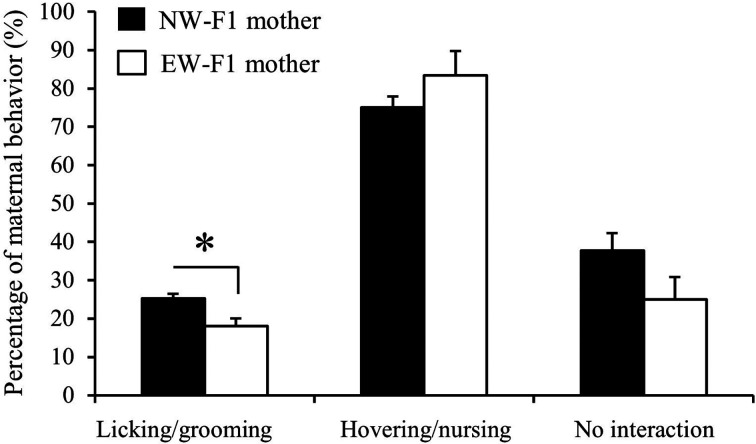
We analyzed the maternal behavior of NW- and EW-F1 dams. As shown in Fig. 1, the percentage of LG behavior of EW-F1 dams was significantly lower than that of NW-F1 dams (P<0.05, EW-F1 dam: 18.07 ± 2.00%; NW-F1 dam: 25.28 ± 1.21%). However, there were no significant differences in the percentage of hovering and nursing behavior (P=0.32, EW-F1 dam: 83.46 ± 6.35%; NW-F1 dam: 75.01 ± 2.92%) and no interaction with pups (P=0.16, EW-F1 dam: 25.00 ± 5.77%; NW-F1 dam: 37.79 ± 4.48%) between the two groups. The F2 male offspring of these F1 dams were used in the subsequent experiments.
Fig. 1.
Maternal behavior in normally-weaned (NW)- or early-weaned (EW)-F1 dams. The percentage of behavior of licking/grooming, hovering and nursing, and no interaction with pups in NW- (black) or EW-F1 dams (white). Data are presented as the mean ± SE (n=3) and were analyzed using an unpaired t-test with two-tailed distribution (*: P<0.05).
Paw licking behavior evoked by formalin injection in adult F2 offspring of normally or early-weaned F1 dams
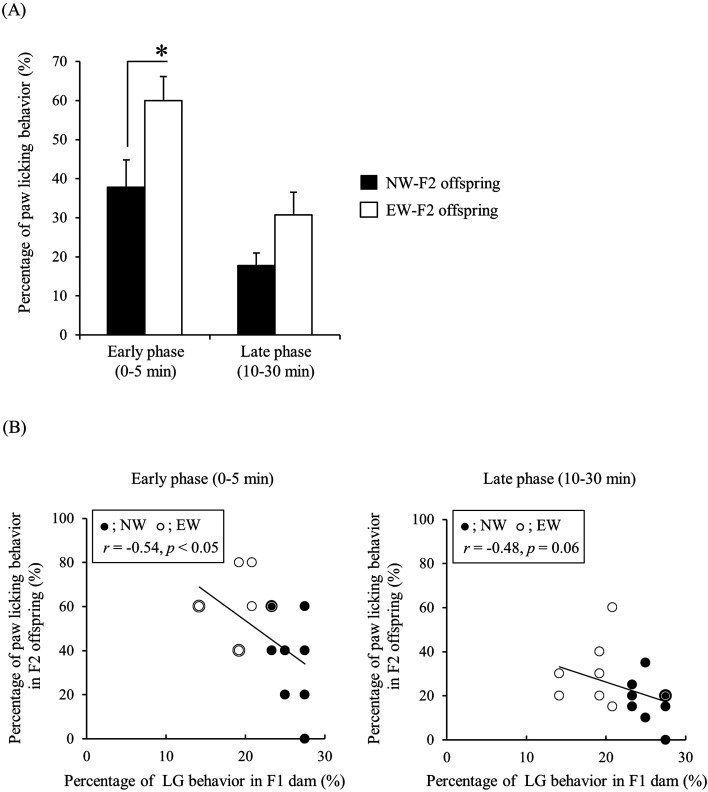
NW- and EW-F2 male offspring showed biphasic pain behavior evoked by formalin injection in the hind paw in agreement with previously published results [57]. A two-way repeated measures ANOVA detected a significant main effect of maternal care and phase (maternal care: F (1, 14)=7.372, P<0.05; phase: F (1, 14)=24.70, P<0.05), but there was no significant interaction between maternal care and phase (F (1, 14)=0.8767, P=0.37). Bonferroni post hoc analysis revealed that the percentage of paw licking behavior in EW-F2 offspring was significantly higher than that in NW-F2 offspring in the early phase (P<0.05, EW-F2 offspring: 60.00 ± 6.17%; NW-F2 offspring: 37.78 ± 7.03%, Fig. 2A). However, the difference between groups was not significant in the late phase (P=0.25, EW-F2 offspring: 30.71 ± 5.82%; NW-F2 offspring: 17.78 ± 3.24%, Fig. 2A). The correlation between percentage of LG behavior in F1 dam and percentage of paw licking behavior in F2 offspring was significant in the early phase (r=−0.54, P<0.05), but approached significance in the late phase (r=−0.48, P=0.06, Fig. 2B).
Fig. 2.
Paw licking behavior of normally-weaned (NW)- or early-weaned (EW)-F2 offspring in the formalin test. (A) The percentage of paw licking behavior of NW- (black) or EW-F2 offspring (white) in the early and late post-experimental phases. Data are presented as the mean ± SE (n=7–9) and were analyzed using a two-way repeated measures ANOVA with post hoc Bonferroni analysis (*: P<0.05). (B) The correlation between percentage of licking and grooming (LG) behavior in F1 dams and percentage of paw licking behavior in F2 offspring. Black double circles indicate the data of overlapped NW-F2 offspring, and white double circles indicate the data of overlapped EW-F2 offspring.
Expression of mRNA in the lumbosacral and thoracolumbar DRGs in adult male F2 offspring of normally-weaned or early-weaned F1 dams
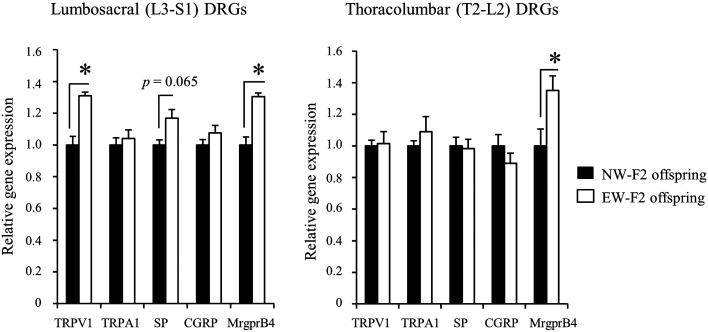
We quantified mRNA levels of genes involved in pain perception in the lumbosacral (L3-S1) DRGs projecting to the hindlimb skin, and thoracolumbar (T2-L2) DRGs innervating to the trunk skin. TRPV1 and TRPA1are major nociceptors of sensory neurons [27,28,29,30,31, 43, 44]. SP and CGRP are released from sensory neurons via activation of nociceptors, and induce neurogenic inflammation and central sensitization [32]. In the lumbosacral DRGs (Fig. 3), Trpv1 mRNA levels in EW-F2 offspring were significantly higher than those in NW-F2 offspring (P<0.05, EW-F2 offspring: 1.31 ± 0.02; NW-F2 offspring: 1.00 ± 0.05). The SP mRNA levels were also higher in EW-F2 offspring than those in NW-F2 offspring, although the difference did not reach levels of significance (P=0.065, EW-F2 offspring: 1.17 ± 0.05; NW-F2 offspring: 1.00 ± 0.03). No significant difference was observed in Trpa1 and CGRP mRNA levels between EW- and NW-F2 offspring (Trpa1 [EW-F2 offspring: 1.04 ± 0.06; NW-F2 offspring: 1.00 ± 0.05], CGRP [EW-F2 offspring: 1.08 ± 0.05; NW-F2 offspring: 1.00 ± 0.03]). We also examined mRNA levels of the sensory neuron marker MrgprB4 in C-LTMR neurons [36] and found that Mrgprb4 mRNA levels in EW-F2 offspring were significantly higher than those in NW-F2 offspring (P<0.05, EW-F2 offspring: 1.30 ± 0.02; NW-F2 offspring: 1.00 ± 0.05). In the thoracolumbar DRGs projecting to the trunk region (Fig. 3), there were no significant changes in Trpv1, Trpa1, SP and CGRP mRNA levels between the two groups (Trpv1 [EW-F2 offspring: 1.02 ± 0.07; NW-F2 offspring: 1.00 ± 0.04], Trpa1 [EW-F2 offspring: 1.09 ± 0.10; NW-F2 offspring: 1.00 ± 0.03], SP [EW-F2 offspring: 1.13 ± 0.07; NW-F2 offspring: 1.00 ± 0.05], CGRP [EW-F2 offspring: 0.89 ± 0.06; NW-F2 offspring: 1.00 ± 0.07]). In contrast, Mrgprb4 mRNA levels in EW-F2 offspring were significantly higher than those in the NW-F2 offspring (P<0.05, EW-F2 offspring: 1.35 ± 0.09; NW-F2 offspring: 1.00 ± 0.11).
Fig. 3.
Gene expression in the lumbosacral and thoracolumbar dorsal root ganglia (DRGs) of normally-weaned (NW)- or early-weaned (EW)-F2 offspring. Gene expression in the lumbosacral (L3-S1) and thoracolumbar (T2-L2) DRGs in NW- (black) or EW-F2 offspring (white). Gene expression data are shown as ratios relative to the mRNA levels in the NW-F2 offspring. Data are presented as the mean ± SE (n=3–6) and were analyzed using an unpaired t-test with two-tailed distribution (*: P<0.05).
Discussion
In this study, we found that higher pain behavior, reflected by an increase in the paw licking behavior in formalin-injected adult male mice, is evoked in F2 offspring of EW-F1 dams, which show lower LG behavior than NW-F1 dams. This suggests that LG stimulation during the pre-weaning period plays an important role in the development of pain responses in male mice. In addition to the behavioral changes, the mRNA levels of TRPV1, a nociceptor in primary sensory neurons in the lumbosacral DRGs, were significantly higher in the offspring of EW-F1 dams. Similarly, mRNA levels of MrgprB4, which is expressed in the C-LTMR neurons perceiving the somatosensory inputs like LG stimuli, were significantly higher in the lumbosacral DRGs of F2 offspring of EW-F1 dams. Although it is not completely deniable that the early weaned-experience in the F1 dams had caused the epigenetic influences of their offspring’s neurons in the DRG during the prenatal period, our results strongly suggest that postnatal LG stimulation has a developmental effect on an offspring’s pain-related behavior and gene expression in the primary sensory neurons.
In the present study, EW-F1 dams showed lower LG behavior than NW-F1 dams, although behavior of hovering and nursing and no interaction with pups was not different between them. This indicates that F2 offspring of EW-F1 dams are an appropriate model to examine the developmental effect of LG stimulation. Nociceptive behavior evoked by formalin injection into the hind paw in EW-F2 offspring was significantly higher than that in NW-F2 offspring in the post-injection early phase (P<0.05), but not in the late phase (P=0.25). Additionally, the correlation between percentage of LG behavior in the F1 dams and percentage of paw licking behavior in the early phase in F2 offspring was significant (r=−0.54, P<0.05). These results suggest that lower LG stimulation in the pre-weaning period induced higher pain responses in adult male mice. It has been demonstrated that naturally-occurring lower LG stimulation induces decreased withdrawal latencies to nociceptive stimulation in rat offspring [58]. These suggest that lower LG stimulation significantly impacts the development of pain sensitivity in rodents. In the formalin test, the early response phase is due to direct effects on nociceptive fibers, and the late phase is ascribed to inflammation [51, 52]. The result of significantly higher pain behavior in EW-F2 than in NW-F2 offspring in the early, but not in the late formalin post-injection phase suggests that lower LG stimulation might induce developmental changes in nociceptive sensory neurons.
Based on previous studies indicating that gene expression changes in nociceptive molecules in the peripheral sensory neurons are involved in altered behavioral nociceptive responses [59, 60], we examined the differences of gene expression in the DRGs between NW- and EW-F2 offspring. The significantly higher expression of Trpv1 (P<0.05) in the lumbosacral DRGs projecting to the hindlimb skin and the higher expression of SP (P=0.065) in the EW-F2 offspring than those in NW-F2 offspring, suggest that LG stimulation may have a developmental impact on the expression of certain subsets of nociceptive molecules in the sensory neurons; the observed changes in gene expression may contribute to the pain hypersensitivity of EW-F2 offspring. In the thoracolumbar DRGs, mRNA levels of Trpv1, SP, Trpa1, and CGRP were not different between the NW-F2 and EW-F2 offspring. This difference in gene expression between lumbosacral and thoracolumbar DRGs could be because the molecules in the lumbosacral DRGs may have a predominant role in transmitting the internal or external harmful stimulation on the lower abdominal or hindlimb region. Interestingly, in the DRG sensory neurons innervating the urinary bladder, differential activations of TRPV1 among DRGs have been suggested based on the observation that the response of bladder lumbosacral DRG neurons to capsaicin was higher than that of the bladder thoracolumbar DRG neurons [61].
The present findings also raise the question of how LG stimulation during the pre-weaning period affects the development of pain signaling. It is thought that gentle stroking, as in LG stimulation, is perceived by peripheral receptors of C-LTMR neurons in the DRG [36, 37]. We currently do not know the precise mechanism for this and hypothesize that the activity of C-LTMR neurons during the pre-weaning period is involved. It has been suggested that in adult mice, increased C-LTMR activity results in elevated release of the chemokine-like secretion protein, TAFA-4, from central nerve terminals of the spinal dorsal horn that suppresses the activity of nociceptive neurons [38,39,40, 62]. It is possible that the activity of nociceptive neurons is higher in EW-F2 than in NW-F2 offspring during the pre-weaning period because of the lower C-LTMR activity produced by lower LG stimulation. An unknown epigenetic mechanism might underlie the frequent activation of nociceptive neurons during the pre-weaning period, which could lead to an increased expression of nociceptive molecules, such as TRPV1, in adulthood, and a subsequent induction of higher pain sensitivity. However, additional investigation is needed to test this hypothesis.
Here we observed that Mrgprb4 expression was higher in the lumbosacral DRGs in EW-F2 offspring than that in NW-F2 offspring. Considering that the activation of C-LTMR neurons suppresses the activity of nociceptive neurons [38,39,40, 62], it is possible that the up-regulation of Mrgprb4 levels in the lumbosacral DRGs of EW-F2 offspring acts as a compensatory change against the higher pain sensitivity. It has been previously shown that in the CNS, the descending pain inhibitory system from specific brain regions to the spinal cord is activated during peripheral pain inflammation [63, 64]. In addition to the CNS, the induction of a pain inhibitory system is also suggested by several reports showing that development of pain during cancer induces increased expression of Kv4.3, a voltage-activated A-type potassium ion channel, which have an inhibitory effect on pain sensitivity in the DRGs [65,66,67]. Although our gene expression data including nociceptive molecules has a potential limitation of a small sample size, Mrgprb4 gene expression levels in the thoracolumbar DRGs were higher in EW-F2 offspring, which is consistent with previous results [42]. Further studies are needed to clarify the reason(s) and mechanism(s) responsible for the Mrgprb4+ neuron increase in EW-F2 offspring. In addition, it has been suggested that many other factors except for MrgprB4 are involved in the perception of tactile stimulation in the DRG neurons [68,69,70]. In future, a detailed analysis of the transcriptome in the DRG in this animal model may delineate new insights into the developing tactile perception.
In summary, we revealed that lower maternal LG stimulation induces higher pain responses and up-regulates the expression levels of pain-related genes in the lumbosacral DRGs. This study has important implications for maternal LG behavior, suggesting an essential role of this behavior in the development of pain sensitivity.
Conflict of Interest
TS is an employee of the Kao Corporation.
Acknowledgments
This work was supported by the Japan Society for the Promotion of Science and Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (#18H02356 to K.M. and #15H02479 to T.K.). TS is an employee of the Kao Corporation. Kao Corporation provided support in the form of salaries for TS, and research materials, but did not have any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sternberg WF, Ridgway CG. Effects of gestational stress and neonatal handling on pain, analgesia, and stress behavior of adult mice. Physiol Behav. 2003; 78: 375–383. doi: 10.1016/S0031-9384(03)00015-5 [DOI] [PubMed] [Google Scholar]
- 2.Butkevich IP, Barr GA, Mikhailenko VA, Otellin VA. Increased formalin-induced pain and expression of fos neurons in the lumbar spinal cord of prenatally stressed infant rats. Neurosci Lett. 2006; 403: 222–226. doi: 10.1016/j.neulet.2006.04.059 [DOI] [PubMed] [Google Scholar]
- 3.Schwaller F, Fitzgerald M. The consequences of pain in early life: injury-induced plasticity in developing pain pathways. Eur J Neurosci. 2014; 39: 344–352. doi: 10.1111/ejn.12414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones GT, Power C, Macfarlane GJ. Adverse events in childhood and chronic widespread pain in adult life: Results from the 1958 British Birth Cohort Study. Pain. 2009; 143: 92–96. doi: 10.1016/j.pain.2009.02.003 [DOI] [PubMed] [Google Scholar]
- 5.Von Korff M, Alonso J, Ormel J, Angermeyer M, Bruffaerts R, Fleiz C, et al. Childhood psychosocial stressors and adult onset arthritis: broad spectrum risk factors and allostatic load. Pain. 2009; 143: 76–83. doi: 10.1016/j.pain.2009.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mills R, Alati R, O’Callaghan M, Najman JM, Williams GM, Bor W, et al. Child abuse and neglect and cognitive function at 14 years of age: findings from a birth cohort. Pediatrics. 2011; 127: 4–10. doi: 10.1542/peds.2009-3479 [DOI] [PubMed] [Google Scholar]
- 7.Perry R, Sullivan RM. Neurobiology of attachment to an abusive caregiver: short-term benefits and long-term costs. Dev Psychobiol. 2014; 56: 1626–1634. doi: 10.1002/dev.21219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Medeiros CB, Fleming AS, Johnston CC, Walker CD. Artificial rearing of rat pups reveals the beneficial effects of mother care on neonatal inflammation and adult sensitivity to pain. Pediatr Res. 2009; 66: 272–277. doi: 10.1203/PDR.0b013e3181b1be06 [DOI] [PubMed] [Google Scholar]
- 9.Takatsuru Y, Yoshitomo M, Nemoto T, Eto K, Nabekura J. Maternal separation decreases the stability of mushroom spines in adult mice somatosensory cortex. Brain Res. 2009; 1294: 45–51. doi: 10.1016/j.brainres.2009.07.092 [DOI] [PubMed] [Google Scholar]
- 10.Vilela FC, Vieira JS, Giusti-Paiva A, Silva MLD. Experiencing early life maternal separation increases pain sensitivity in adult offspring. Int J Dev Neurosci. 2017; 62: 8–14. doi: 10.1016/j.ijdevneu.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 11.Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev. 2007; 31: 3–17. doi: 10.1016/j.neubiorev.2006.05.003 [DOI] [PubMed] [Google Scholar]
- 12.Denenberg VH, Karas GG. Effects of differential infantile handling upon weight gain and mortality in the rat and mouse. Science. 1959; 130: 629–630. doi: 10.1126/science.130.3376.629-a [DOI] [PubMed] [Google Scholar]
- 13.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001; 24: 1161–1192. doi: 10.1146/annurev.neuro.24.1.1161 [DOI] [PubMed] [Google Scholar]
- 14.Bailoo JD, Jordan RL, Garza XJ, Tyler AN. Brief and long periods of maternal separation affect maternal behavior and offspring behavioral development in C57BL/6 mice. Dev Psychobiol. 2014; 56: 674–685. doi: 10.1002/dev.21135 [DOI] [PubMed] [Google Scholar]
- 15.Bebbington PE, Bhugra D, Brugha T, Singleton N, Farrell M, Jenkins R, et al. Psychosis, victimisation and childhood disadvantage: evidence from the second British National Survey of Psychiatric Morbidity. Br J Psychiatry. 2004; 185: 220–226. doi: 10.1192/bjp.185.3.220 [DOI] [PubMed] [Google Scholar]
- 16.Cicchetti D, Rogosch FA, Toth SL. Fostering secure attachment in infants in maltreating families through preventive interventions. Dev Psychopathol. 2006; 18: 623–649. doi: 10.1017/S0954579406060329 [DOI] [PubMed] [Google Scholar]
- 17.Casler L. The effects of extra tactile stimulation on a group of institutionalized infants. Genet Psychol Monogr. 1965; 71: 137–175. [PubMed] [Google Scholar]
- 18.Field TM, Schanberg SM, Scafidi F, Bauer CR, Vega-Lahr N, Garcia R, et al. Tactile/kinesthetic stimulation effects on preterm neonates. Pediatrics. 1986; 77: 654–658. [PubMed] [Google Scholar]
- 19.Ohlsson A, Jacobs SE. NIDCAP: a systematic review and meta-analyses of randomized controlled trials. Pediatrics. 2013; 131: e881–e893. doi: 10.1542/peds.2012-2121 [DOI] [PubMed] [Google Scholar]
- 20.Maitre NL, Key AP, Chorna OD, Slaughter JC, Matusz PJ, Wallace MT, et al. The dual nature of early-life experience on somatosensory processing in the human infant brain. Curr Biol. 2017; 27: 1048–1054. doi: 10.1016/j.cub.2017.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brauer J, Xiao Y, Poulain T, Friederici AD, Schirmer A. Frequency of maternal touch predicts resting activity and connectivity of the developing social brain. Cereb Cortex. 2016; 26: 3544–3552. doi: 10.1093/cercor/bhw137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Champagne FA, Curley JP, Keverne EB, Bateson PP. Natural variations in postpartum maternal care in inbred and outbred mice. Physiol Behav. 2007; 91: 325–334. doi: 10.1016/j.physbeh.2007.03.014 [DOI] [PubMed] [Google Scholar]
- 23.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997; 277: 1659–1662. doi: 10.1126/science.277.5332.1659 [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez A, Lovic V, Ward GR, Wainwright PE, Fleming AS. Intergenerational effects of complete maternal deprivation and replacement stimulation on maternal behavior and emotionality in female rats. Dev Psychobiol. 2001; 38: 11–32. doi: [DOI] [PubMed] [Google Scholar]
- 25.Lovic V, Fleming AS. Artificially-reared female rats show reduced prepulse inhibition and deficits in the attentional set shifting task--reversal of effects with maternal-like licking stimulation. Behav Brain Res. 2004; 148: 209–219. doi: 10.1016/S0166-4328(03)00206-7 [DOI] [PubMed] [Google Scholar]
- 26.Chatterjee D, Chatterjee-Chakraborty M, Rees S, Cauchi J, de Medeiros CB, Fleming AS. Maternal isolation alters the expression of neural proteins during development: ‘Stroking’ stimulation reverses these effects. Brain Res. 2007; 1158: 11–27. doi: 10.1016/j.brainres.2007.04.069 [DOI] [PubMed] [Google Scholar]
- 27.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997; 389: 816–824. doi: 10.1038/39807 [DOI] [PubMed] [Google Scholar]
- 28.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004; 41: 849–857. doi: 10.1016/S0896-6273(04)00150-3 [DOI] [PubMed] [Google Scholar]
- 29.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Högestätt ED, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004; 427: 260–265. doi: 10.1038/nature02282 [DOI] [PubMed] [Google Scholar]
- 30.Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA. 2006; 103: 19564–19568. doi: 10.1073/pnas.0609598103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patwardhan AM, Akopian AN, Ruparel NB, Diogenes A, Weintraub ST, Uhlson C, et al. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. J Clin Invest. 2010; 120: 1617–1626. doi: 10.1172/JCI41678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boadas-Vaello P, Castany S, Homs J, Álvarez-Pérez B, Deulofeu M, Verdú E. Neuroplasticity of ascending and descending pathways after somatosensory system injury: reviewing knowledge to identify neuropathic pain therapeutic targets. Spinal Cord. 2016; 54: 330–340. doi: 10.1038/sc.2015.225 [DOI] [PubMed] [Google Scholar]
- 33.Liljencrantz J, Olausson H. Tactile C fibers and their contributions to pleasant sensations and to tactile allodynia. Front Behav Neurosci. 2014; 8: 37. doi: 10.3389/fnbeh.2014.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mancini F, Nash T, Iannetti GD, Haggard P. Pain relief by touch: a quantitative approach. Pain. 2014; 155: 635–642. doi: 10.1016/j.pain.2013.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, et al. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009; 462: 651–655. doi: 10.1038/nature08505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vrontou S, Wong AM, Rau KK, Koerber HR, Anderson DJ. Genetic identification of C fibres that detect massage-like stroking of hairy skin in vivo. Nature. 2013; 493: 669–673. doi: 10.1038/nature11810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Pichon CE, Chesler AT. The functional and anatomical dissection of somatosensory subpopulations using mouse genetics. Front Neuroanat. 2014; 8: 21. doi: 10.3389/fnana.2014.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Y, Perl ER. A specific inhibitory pathway between substantia gelatinosa neurons receiving direct C-fiber input. J Neurosci. 2003; 23: 8752–8758. doi: 10.1523/JNEUROSCI.23-25-08752.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watanabe N, Piché M, Hotta H. Types of skin afferent fibers and spinal opioid receptors that contribute to touch-induced inhibition of heart rate changes evoked by noxious cutaneous heat stimulation. Mol Pain. 2015; 11: 4. doi: 10.1186/s12990-015-0001-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liljencrantz J, Strigo I, Ellingsen DM, Krämer HH, Lundblad LC, Nagi SS, et al. Slow brushing reduces heat pain in humans. Eur J Pain. 2017; 21: 1173–1185. doi: 10.1002/ejp.1018 [DOI] [PubMed] [Google Scholar]
- 41.Kikusui T, Isaka Y, Mori Y. Early weaning deprives mouse pups of maternal care and decreases their maternal behavior in adulthood. Behav Brain Res. 2005; 162: 200–206. doi: 10.1016/j.bbr.2005.03.013 [DOI] [PubMed] [Google Scholar]
- 42.Sakamoto T, Ishio Y, Ishida Y, Mogi K, Kikusui T. Low maternal care enhances the skin barrier resistance of offspring in mice. PLoS One. 2019; 14: e0219674. doi: 10.1371/journal.pone.0219674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006; 124: 1269–1282. doi: 10.1016/j.cell.2006.02.023 [DOI] [PubMed] [Google Scholar]
- 44.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006; 50: 277–289. doi: 10.1016/j.neuron.2006.03.042 [DOI] [PubMed] [Google Scholar]
- 45.Mogi K, Ishida Y, Nagasawa M, Kikusui T. Early weaning impairs fear extinction and decreases brain-derived neurotrophic factor expression in the prefrontal cortex of adult male C57BL/6 mice. Dev Psychobiol. 2016; 58: 1034–1042. doi: 10.1002/dev.21437 [DOI] [PubMed] [Google Scholar]
- 46.Kim SJ, Calejesan AA, Li P, Wei F, Zhuo M. Sex differences in late behavioral response to subcutaneous formalin injection in mice. Brain Res. 1999; 829: 185–189. doi: 10.1016/S0006-8993(99)01353-0 [DOI] [PubMed] [Google Scholar]
- 47.Gaumond I, Arsenault P, Marchand S. The role of sex hormones on formalin-induced nociceptive responses. Brain Res. 2002; 958: 139–145. doi: 10.1016/S0006-8993(02)03661-2 [DOI] [PubMed] [Google Scholar]
- 48.Chanda ML, Mogil JS. Sex differences in the effects of amiloride on formalin test nociception in mice. Am J Physiol Regul Integr Comp Physiol. 2006; 291: R335–R342. doi: 10.1152/ajpregu.00902.2005 [DOI] [PubMed] [Google Scholar]
- 49.Shoji H, Takao K, Hattori S, Miyakawa T. Age-related changes in behavior in C57BL/6J mice from young adulthood to middle age. Mol Brain. 2016; 9: 11. doi: 10.1186/s13041-016-0191-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999; 286: 1155–1158. doi: 10.1126/science.286.5442.1155 [DOI] [PubMed] [Google Scholar]
- 51.Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992; 51: 5–17. doi: 10.1016/0304-3959(92)90003-T [DOI] [PubMed] [Google Scholar]
- 52.Sawynok J, Liu XJ. The formalin test: characteristics and usefulness of the model. Rev Analg. 2003; 7: 145–163. doi: 10.3727/000000003783992982 [DOI] [Google Scholar]
- 53.Taleghany N, Sarajari S, DonCarlos LL, Gollapudi L, Oblinger MM. Differential expression of estrogen receptor alpha and beta in rat dorsal root ganglion neurons. J Neurosci Res. 1999; 57: 603–615. doi: [DOI] [PubMed] [Google Scholar]
- 54.Christie KJ, Krishnan A, Martinez JA, Purdy K, Singh B, Eaton S, et al. Enhancing adult nerve regeneration through the knockdown of retinoblastoma protein. Nat Commun. 2014; 5: 3670. doi: 10.1038/ncomms4670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng C, Kobayashi M, Martinez JA, Ng H, Moser JJ, Wang X, et al. Evidence for epigenetic regulation of gene expression and function in chronic experimental diabetic neuropathy. J Neuropathol Exp Neurol. 2015; 74: 804–817. doi: 10.1097/NEN.0000000000000219 [DOI] [PubMed] [Google Scholar]
- 56.Okano J, Kojima H, Katagi M, Nakagawa T, Nakae Y, Terashima T, et al. Hyperglycemia Induces Skin Barrier Dysfunctions with Impairment of Epidermal Integrity in Non-Wounded Skin of Type 1 Diabetic Mice. PLoS One. 2016; 11: e0166215. doi: 10.1371/journal.pone.0166215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cibert-Goton V, Yuan G, Battaglia A, Fredriksson S, Henkemeyer M, Sears T, et al. Involvement of EphB1 receptors signalling in models of inflammatory and neuropathic pain. PLoS One. 2013; 8: e53673. doi: 10.1371/journal.pone.0053673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker CD, Xu Z, Rochford J, Johnston CC. Naturally occurring variations in maternal care modulate the effects of repeated neonatal pain on behavioral sensitivity to thermal pain in the adult offspring. Pain. 2008; 140: 167–176. doi: 10.1016/j.pain.2008.08.004 [DOI] [PubMed] [Google Scholar]
- 59.Isensee J, Wenzel C, Buschow R, Weissmann R, Kuss AW, Hucho T. Subgroup-elimination transcriptomics identifies signaling proteins that define subclasses of TRPV1-positive neurons and a novel paracrine circuit. PLoS One. 2014; 9: e115731. doi: 10.1371/journal.pone.0115731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Starobova H, S W A H, Lewis RJ, Vetter I. Transcriptomics in pain research: insights from new and old technologies. Mol Omics. 2018; 14: 389–404. doi: 10.1039/C8MO00181B [DOI] [PubMed] [Google Scholar]
- 61.Dang K, Bielefeldt K, Gebhart GF. Differential responses of bladder lumbosacral and thoracolumbar dorsal root ganglion neurons to purinergic agonists, protons, and capsaicin. J Neurosci. 2005; 25: 3973–3984. doi: 10.1523/JNEUROSCI.5239-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Delfini MC, Mantilleri A, Gaillard S, Hao J, Reynders A, Malapert P, et al. TAFA4, a chemokine-like protein, modulates injury-induced mechanical and chemical pain hypersensitivity in mice. Cell Rep. 2013; 5: 378–388. doi: 10.1016/j.celrep.2013.09.013 [DOI] [PubMed] [Google Scholar]
- 63.Tsuruoka M, Willis WD. Descending modulation from the region of the locus coeruleus on nociceptive sensitivity in a rat model of inflammatory hyperalgesia. Brain Res. 1996; 743: 86–92. doi: 10.1016/S0006-8993(96)01025-6 [DOI] [PubMed] [Google Scholar]
- 64.Maeda M, Tsuruoka M, Hayashi B, Nagasawa I, Inoue T. Descending pathways from activated locus coeruleus/subcoeruleus following unilateral hindpaw inflammation in the rat. Brain Res Bull. 2009; 78: 170–174. doi: 10.1016/j.brainresbull.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 65.Yang EK, Takimoto K, Hayashi Y, de Groat WC, Yoshimura N. Altered expression of potassium channel subunit mRNA and alpha-dendrotoxin sensitivity of potassium currents in rat dorsal root ganglion neurons after axotomy. Neuroscience. 2004; 123: 867–874. doi: 10.1016/j.neuroscience.2003.11.014 [DOI] [PubMed] [Google Scholar]
- 66.Duan KZ, Xu Q, Zhang XM, Zhao ZQ, Mei YA, Zhang YQ. Targeting A-type K(+) channels in primary sensory neurons for bone cancer pain in a rat model. Pain. 2012; 153: 562–574. doi: 10.1016/j.pain.2011.11.020 [DOI] [PubMed] [Google Scholar]
- 67.Zemel BM, Ritter DM, Covarrubias M, Muqeem T. A-Type KV Channels in Dorsal Root Ganglion Neurons: Diversity, Function, and Dysfunction. Front Mol Neurosci. 2018; 11: 253. doi: 10.3389/fnmol.2018.00253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Delmas P, Hao J, Rodat-Despoix L. Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nat Rev Neurosci. 2011; 12: 139–153. doi: 10.1038/nrn2993 [DOI] [PubMed] [Google Scholar]
- 69.Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011; 147: 1615–1627. doi: 10.1016/j.cell.2011.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lou S, Duan B, Vong L, Lowell BB, Ma Q. Runx1 controls terminal morphology and mechanosensitivity of VGLUT3-expressing C-mechanoreceptors. J Neurosci. 2013; 33: 870–882. doi: 10.1523/JNEUROSCI.3942-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]