Abstract
Purpose
To report the resolution of subretinal drusenoid deposits (SDDs) and the changes in macular thickness at various segmentation levels in a patient who was treated for vitamin A deficiency-related retinopathy.
Observations
A 67-year-old man with cirrhosis secondary to hepatitis C was referred for unexplained vision decline and nyctalopia. He was diagnosed with vitamin A deficiency after he was found to have yellow-white drusen-like deposits in the posterior pole and midperiphery, which corresponded to SDDs on optical coherence tomography (OCT). Treatment with vitamin A supplementation was initiated, and, over the course of eight months, the symptoms improved, the SDDs resolved, and retinal thickness generally increased, although the rate and pattern of change differed depending on the macular quadrant and the segmentation layer being analyzed.
Conclusions and importance
Vitamin A deficiency should be considered in patients with liver disease who present with drusen-like deposits in the macula and midperiphery. Prompt recognition and treatment may improve symptoms and reverse some retinal pathology, including the presence of SDDs. Vitamin A supplementation in these patients seems to affect the inner retina and outer retina differently.
Keywords: Vitamin A deficiency retinopathy, Subretinal drusenoid deposits, Retinal thickness, Optical coherence tomography, Cirrhosis
1. Introduction
In mammals, vitamin A (all-trans-retinol) is an obligate dietary micronutrient—it cannot be synthesized. Low retinol levels can occur in a variety of conditions and diseases but are perhaps most frequently seen in patients who have had bariatric or gastrointestinal surgery.1,2 However, low retinol levels are also commonly found in patients with liver diseases, which can be due to malnutrition, problems with intestinal absorption or liver storage of vitamin A, and/or disturbed hepatic production of retinol transport proteins.3
Vitamin A plays an essential role in outer retinal functioning during phototransduction—its aldehyde derivative, the chromophore called retinal, is covalently bound to opsin in the transmembrane portion of photoreceptor outer segment discs of both rods and cones, and its isomerization triggers a chain of events that allows for the transmission of light signals in the visual cycle.4 One of the earliest and most common visual presentations of vitamin A deficiency is nyctalopia. The following case serves to illustrate some of the reversible features of vitamin A deficiency retinopathy in a patient with cirrhosis.
2. Case report
A 67-year-old African American man was referred by his optometrist due to gradual, unexplained vision loss. He had a history of intravenous drug use with heroin, alcohol abuse, cirrhosis secondary to hepatitis C, and chronic thrombocytopenia secondary to hypersplenism.
He endorsed nyctalopia but denied photosensitivity. Review of systems was otherwise negative. Pinhole visual acuity (VA) was 20/60 in the right eye (OD) and 20/40 in the left eye (OS). Anterior segment exam was unremarkable. Indirect ophthalmoscopy revealed macular mottling and a dense collection of intermediate-sized, yellow-white drusen-like deposits in a midperipheral ring pattern in both eyes. These deposits were mostly hypoautofluorescent and hyperfluorescent in mid-phases of the fluorescein angiogram (Fig. 1). On near infrared reflectance (NIR) imaging, the lesions were mostly hyporeflective, though a subtle hyperreflective rim could be appreciated. Macular enhanced-depth imaging optical coherence tomography (EDI-OCT) revealed diffuse ellipsoid zone (EZ) layer disruption and subretinal drusenoid deposits (SDDs) in both eyes (Fig. 2). Macular thickness for each eye at each visit was measured within the zones whose borders were the 1-, 3-, and 6-mm diameter Early Treatment Diabetic Retinopathy Study (ETDRS) circles (corresponding to the fovea, parafovea, and perifovea, respectively) at four segmentation layers—total retina, outer nuclear layer (ONL), inner retina, and outer retina (Fig. 3).
Fig. 1.
Blue-light fundus autofluorescence (A) and mid-recirculation phase fluorescein angiogram (B) of the right eye at presentation (Heidelberg Spectralis, Heidelberg Engineering Inc, Franklin, MA, USA). Two separate subretinal drusenoid deposits (SDDs) are indicated by yellow and orange arrows in each image, the locations of which correspond to the arrows of the same colors in Fig. 2. Note that some SDDs are hypoautofluorescent (A; yellow arrow), while others are hyperautofluorescent with a subtle hypoautofluorescent rim (A; orange arrow). The corresponding lesions are subtler on fluorescein angiogram but appear to have the inverse pattern (B; punctate hyperfluorescence with hypofluorescent rim). Left eye is not shown but had similar findings. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
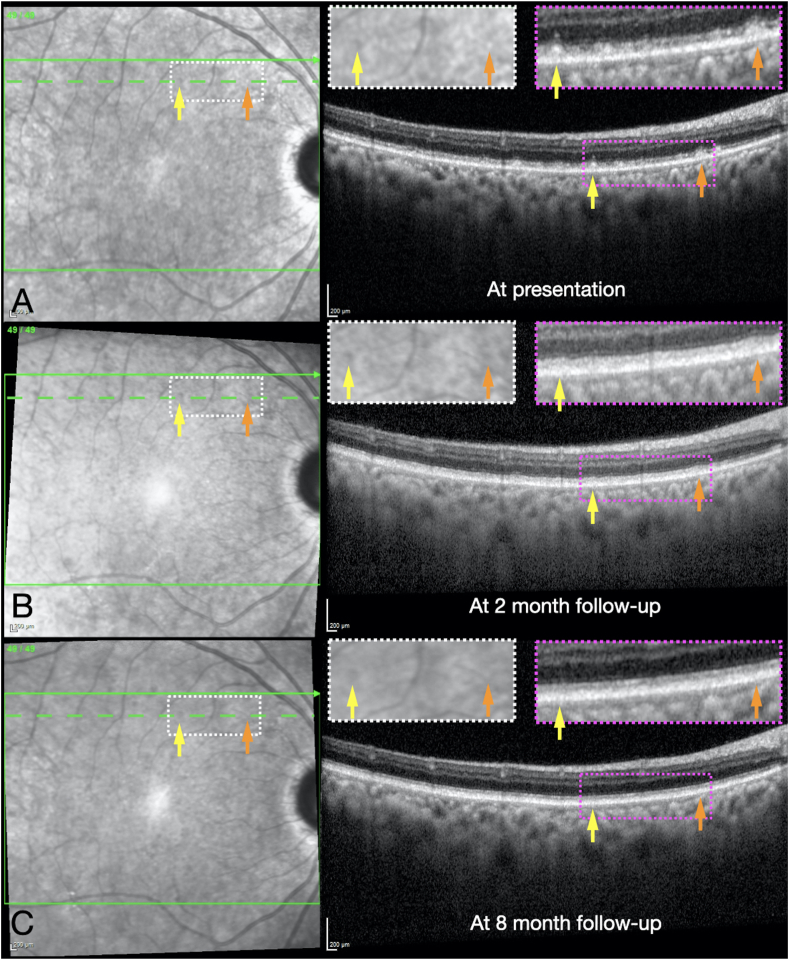
Fig. 2.
Serial near infrared reflectance (NIR) imaging and macular enhanced-depth imaging optical coherence tomography (EDI-OCT; Heidelberg Spectralis) b scans of the right eye at presentation (A), at the 2-month follow-up (B), and at the 8-month follow-up (C). The dashed green horizontal line on each NIR image indicates the location of the OCT b scan. Two separate SDDs are indicated by yellow and orange arrows in each image, the locations of which correspond to the arrows of the same colors in Fig. 1. Magnified insets of NIR images (white dotted rectangles) and OCT b scan images (magenta dotted rectangles) are shown. Note that the SDDs demonstrate a subtle “target” appearance with central hyperreflectivity and a rim of hyporeflectivity with NIR imaging, and are located between the ellipsoid zone (EZ) layer and retinal pigment epithelium (RPE) on the OCT b scans. Also note the gradual resorption of the SDDs and reconstitution of EZ layer at each follow-up (B and C). Left eye is not shown but had similar findings. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
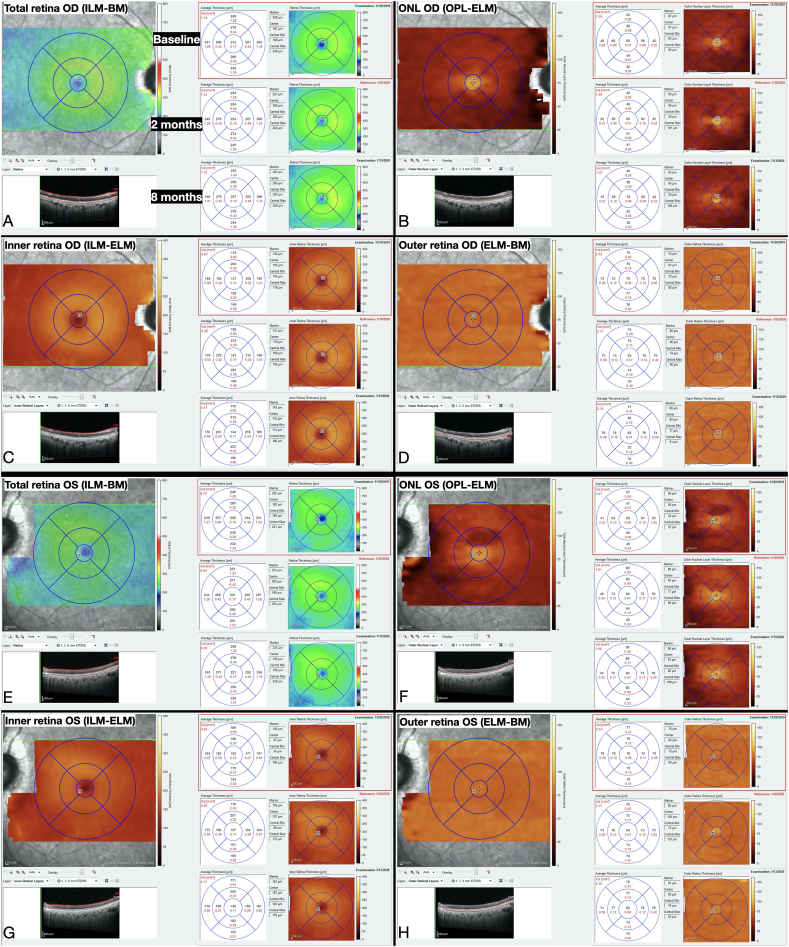
Fig. 3.
Average macular thicknesses measured from EDI-OCT b scans using the proprietary Heidelberg Eye Explorer program at the three zones demarcated by the Early Treatment Diabetic Retinopathy Study (ETDRS) circles of 1-, 3-, and 6-mm diameters, representing the fovea, parafovea, and perifovea, respectively. Segmentation was achieved by applying Heidelberg's automated segmentation for each of the 49 b scans for the right eye at each encounter, and then manually adjusting the retinal segmentation lines to correct for artifactual errors (e.g. due to the presence of blood vessels). Afterward, four different segmentation layers were chosen from Heidelberg's auto-generated list: (A) total retina (from ILM to BM), (B) ONL (from OPL to ELM), (C) inner retina (from ILM to ELM), and (D) outer retina (from ELM to BM). The same steps were performed for the left eye (E–H).
BM: Bruch's membrane; ELM: external limiting membrane; ILM: internal limiting membrane; ONL: outer nuclear layer; OPL: outer plexiform layer.
The patient underwent serum testing for syphilis (negative) and vitamin A levels (<2.5 μg/dL; normal range: 22.0–69.5 μg/dL). Full-field electroretinogram (ffERG) was ordered, but could not be performed due the patient's insurance status. He was diagnosed with severe vitamin A deficiency-related retinopathy and supplementation was coordinated by his hepatologist, which initially was in the form of reduced-dose intramuscular injections (1,000 IU daily due to cirrhosis) and was later switched to oral. At the first follow-up visit two months after starting supplementation, the patient reported improvement in VA and nyctalopia and his serum vitamin A level had increased to 19.4 μg/dL. At the final follow-up visit 6 months later (vitamin A levels were not available for this visit), pinhole VA had improved slightly to 20/50 OD and 20/30 OS, the fundus lesions and SDDs were noted to be dramatically reduced, and the EZ had undergone substantial reconstitution. Average macular thickness changed to different extents and in different directions, depending on the segmentation layer, macular quadrant, and distance from the fovea. Generally, at the fovea, retinal thickness increased at all segmentation layers throughout the entire follow-up period for both eyes (except for the ONL OD). Within the perifovea, the greatest changes were observed in the superior and temporal quadrants, and the mildest changes were noted in the inferior and nasal quadrants in both eyes (Fig. 4).
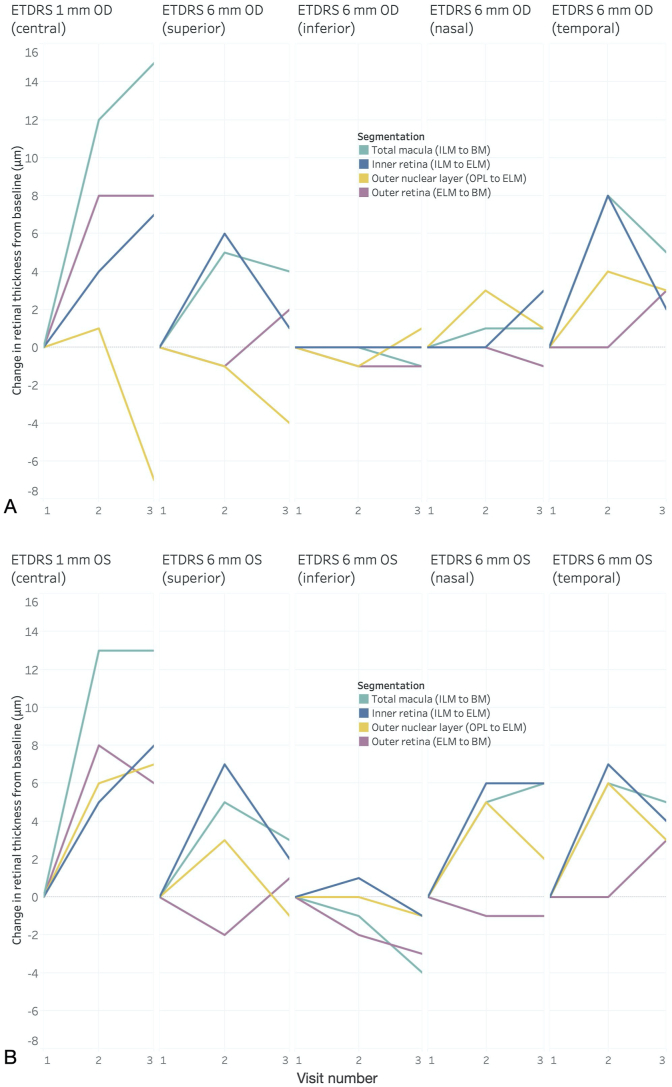
Fig. 4.
Average change in retinal thickness from baseline measured on the Heidelberg Spectralis at the fovea (1-mm ETDRS circle) and the perifovea (6-mm ETDRS circle) at each of the four segmentation layers described in Fig. 3 for the right eye (A) and left eye (B). Vitamin A supplementation was initiated immediately after the first visit (Visit 1; November 2019) and was continued throughout the entire follow-up period (Visits 2 and 3). All changes in retinal thickness were calculated with respect to the values from the baseline visit. At the fovea, average retinal thickness increased most rapidly between the first two visits at all segmentation layers for both eyes and remained net positive until the end of the follow-up period, except for the ONL thickness of the right eye (A; yellow line), which ended up net negative. At the perifovea, retinal thickness changed to varying degrees and in varying directions, depending on the quadrant being analyzed, with the greatest changes observed in the superior and temporal perifovea, and the most modest changes noted in the inferior and nasal perifovea in both eyes. Generally, in all perifoveal quadrants, inner retinal thickness (blue lines) either remained stationary or had a net increase in thickness, whereas outer retinal thickness (magenta lines) initially either decreased or stayed constant, followed by a net increase in both eyes. The change in thickness of the inner versus the outer retina in the perifovea demonstrated a somewhat inverse relationship, such that a rise in one was associated with a decline in the other. Data visualization and graphs were done in Tableau Desktop Professional Edition (version 2020.2.1). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3. Discussion
To the author's knowledge, this is the first published case to describe changes in retinal thickness in different macular areas and to demonstrate resolution of SDDs with OCT b scans in a patient treated for vitamin A deficiency-related retinopathy, though partial EZ and SDD improvement have been reported.1,2 Disruption of the EZ layer and midperipheral retinal white/yellow dots have been well-described in vitamin A deficiency, the latter having been found to localize to the subretinal space—anterior to the retinal pigment epithelium (RPE)—with OCT b scans, similar to the SDDs (i.e. reticular pseudodrusen [RPD]) that have been described as part of the age-related macular degeneration (AMD) spectrum.5 While the term “subretinal drusenoid deposits” is commonly used in the context of AMD, this finding has been reported in a number of retinal disorders.5, 6, 7 However, exactly what these entities are remains unknown. One common hypothesis was that they represent shed photoreceptor outer segments that accumulate in the subretinal space due to reduced RPE phagocytosis.5,7 However, immunoreactivity and electron microscopy studies concluded that the subretinal aggregates were distinct from photoreceptors.7 Additionally, photoreceptor fluorophores are autofluorescent, whereas the majority of SDDs are hypoautofluorescent, though this can vary by SDD morphology.7 Similar to most other reports, the present study found the SDDs to be hypoautofluorescent.
With regard to composition, histopathologic studies of AMD eyes have found that sub-RPE-basal laminar (BL) drusen and SDDs have similar protein contents, but differ in their lipid properties, including the absence of esterified cholesterol and mineralization in SDDs.7 One hypothesis suggested a break-down in bidirectional outer retinal lipid recycling between the RPE and Müller cells/photoreceptor inner segments (possibly due to RPE cell dysfunction), resulting in accumulation of material in the subretinal space.7 SDDs are known to have a life cycle, which may include their regression in later stages, resulting in ONL thinning, EZ disruption, and thinning of the underlying choroid.7 In fact, OCT angiography has been used to demonstrate reduced vessel density and blood flow at the level of the choriocapillaris (CC) in eyes with RPD, possibly implicating CC ischemia in their pathogenesis, though that paper did not specify the underlying disease process(es) in the patients with RPD.6 To date, there is no evidence of CC ischemia in vitamin A deficient patients.
SDDs are generally preferentially found in the superior macula, an area that corresponds to the region of the highest density of rods in the human retina.7, 8, 9 Furthermore, SDDs are known to affect rod function more than cone function.6,7 Mean rod density in the human retina at an eccentricity of 3 mm from the center of the fovea (corresponding to the perifoveal maps shown in Fig. 4) varies by perifoveal quadrant such that superior > inferior > nasal ≈ temporal. Interestingly, the same relative pattern exists for the rod:cone ratio at an eccentricity of 3 mm.9 The present study found that the highest amplitude changes in retinal thickness after vitamin A supplementation occurred in the fovea, followed by the superior and temporal perifovea. The fact that the patient's fovea was the site of the greatest increase in retinal thickness across most segmentation layers is not surprising since the fovea contains only cones and no rods. Rods are disproportionately more affected than cones in cases of vitamin A deficiency, as demonstrated by the characteristic ffERG findings in these cases of absent rod response with only mildly decreased amplitude or increased implicit time of the cone response.2 Rods consume the highest amount of energy among all cell types in the human body in order to maintain a continuous depolarized state in darkness (so-called “dark current”), allowing constant neurotransmitter release to activate second-order neurons in the visual pathway,10 a fact which may explain why they are affected more than cones in certain nutritional disorders, including vitamin A deficiency. The pattern of change in retinal thickness in the present study somewhat parallels the average human rod density map. However, whether the changes in retinal thickness occurred due to the natural life cycle of SDDs, as a result of restoration of photoreceptor functioning after vitamin A supplementation, or a combination of both cannot be determined by the present study.
A notable finding in this study was that changes in perifoveal retinal thickness at the levels of the inner and outer retina seemed to be inversely related. Perifoveal inner retinal thickness tended to increase early followed by a decrease, whereas outer retinal thickness followed the opposite pattern. One possible explanation is that anatomical restoration after repletion of vitamin A occurred first in the inner retinal layers, and only later in the outer retinal layers. While its role in outer retina and RPE is well-established, the role of vitamin A on the functioning of the inner retina, including amacrine and ganglion cells, is still not clear. An ERG study of vitamin A deficient rats did find evidence of reduced oscillatory potentials (OPs), which were reversible with vitamin A supplementation, leading the authors to conclude that OPs are the most sensitive and early marker of vitamin A deficiency-associated visual impairment.11 Conversely, in their case report of a patient with vitamin A deficiency secondary to bariatric surgery, Saenz-de-Viteri and Sádaba found that while macular thickness did increase after vitamin A supplementation, ganglion cell layer thickness did not vary significantly.1
Limitations of this study include the solo patient sample size, the lack of vitamin A level at the final follow-up, the lack of a ffERG, and the possibility of incorrect retinal layer segmentation, which may, in turn, have led to inaccurate thickness measurements of the four segmentation layers. For example, it is not exactly clear why the ONL thickness in the right eye of the patient decreased to such a degree between visits two and three, primarily in the fovea, but also in nearly all quadrants of the perifovea (Fig. 4). Since ONL thickness was defined as the hyporeflective space comprising the distance between the outer plexiform layer (OPL) and the external limiting membrane (ELM), one possible explanation is that there was artifactual variability in presumed ONL thickness as a result of changes in the reflectivity of Henle's fiber layer (HFL) due to non-uniform OCT pupil light entry across patient visits, which may have led to incorrect OPL segmentations. HFL represents the obliquely oriented, unmyelinated axons of rods and cones, which synapse in the OPL. While HFL is part of histologic OPL, it frequently appears hyporeflective on OCT b scans and gets bundled together with the ONL, though it can also appear hyperreflective with certain pupil light entry orientations due its property of birefringence.12,13 One study found that HFL may constitute up to 50% of the hyporeflective thickness between the ELM and OPL on OCT b scans, underscoring the importance of properly identifying HFL when attempting to measure “true ONL thickness.”12 In the present study, the horizontal Heidelberg OCT at each visit was done as a “follow-up” to the previous, a process which links the b scans and may also automatically rotate them to match the orientation of the prior scan. Thus, other clues may be used to identify image tilt due to a decentered pupil light entry during the acquisition of a horizontal OCT b scan, including the angle of posterior shadowing of various chorioretinal structures (e.g. retinal blood vessels or pigment epithelial detachments),12 differences in reflectivity of the interdigitation zone in nasal versus temporal macula (due to a phenomenon known as the optical Stiles-Crawford effect),14 or the presence of a black triangle at the top or bottom of the b scan.15 Some of these clues of image tilt are clearly visible on some of the horizontal b scans from the present study (Fig. 5). However, it is important to note that this study analyzed the changes in retinal thickness across different time points, not the absolute values of the thickness. Therefore, as long as the OCT b scans were uniformly decentered across the different patient visits, which they appeared to be, the alterations in segmentation layer thicknesses should have little-to-no bearing on the results.
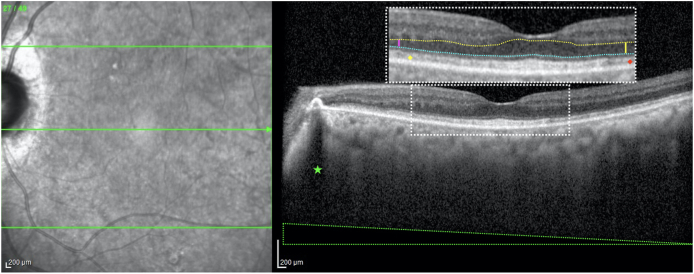
Fig. 5.
Nasal or temporal decentration of OCT light entry on the pupil can affect the reflectivity of various retinal layers and alter thickness measurements on horizontal b scans. This b scan of the left eye from the patient's first visit demonstrates how a temporal pupil entry position causes image tilt (evidenced by the black triangle demarcated by the dotted green lines at the bottom) despite the apparently vertical posterior shadowing from the nasal pigment epithelial detachment (green star). A decentered scan also results in differences in reflectivity of certain retinal layers, including Henle's fiber layer (HFL) and the interdigitation zone (IZ). The colored dotted lines in the magnified inset denote the segmentations of the outer plexiform layer (OPL; yellow) and the external limiting membrane (ELM; cyan) used for the purposes of measuring the thickness of the outer nuclear layer (ONL), which was taken to be the hyporeflective space between the OPL and the ELM. Note how a temporal pupil light entry makes the ONL appear thicker in the temporal macula (yellow vertical line) than in the nasal macula (magenta vertical line). This is due to the optical “unmasking” of HFL nasally (resulting in the hyperreflectivity just posterior to true OPL), whereas temporally it gets erroneously bundled in with ONL. A similar phenomenon, called the optical Stiles-Crawford effect, occurs at the level of the IZ, causing this band to appear slightly more hyperreflective nasally (yellow diamond) than temporally (red diamond). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Conclusions
Patients with liver disease who are visually symptomatic and are found to have drusen-like deposits in the posterior pole and midperiphery should be promptly tested for vitamin A deficiency. Early supplementation can lead to improvements in vision and can also reverse fundus findings, including EZ disruption and SDDs. Vitamin A supplementation in these patients may act first at the level of the inner retina, followed by the outer retina. More studies with larger sample sizes are needed to validate these findings.
Patient consent
Written consent to publish this case has not been obtained. This report does not contain any personal identifying information.
Funding
No funding or grant support.
Declaration of competing interestCOI
The following authors have no financial disclosures: L.Z.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
None.
References
- 1.Saenz-de-Viteri M., Sádaba L. Optical coherence tomography assessment before and after vitamin supplementation in a patient with vitamin A deficiency. Medicine (Baltim) 2016;95(6) doi: 10.1097/MD.0000000000002680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lima de Carvalho J.R., Jr., Tsang S.H., Sparrow J.R. Vitamin A deficiency monitored by quantitative short wavelength fundus autofluorescence in a case of bariatric surgery. Retin Cases Brief Rep. 2019 doi: 10.1097/ICB.0000000000000931. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janczewska I., Ericzon B., Eriksson L. Influence of orthotopic liver transplantation on serum vitamin A levels in patients with chronic liver disease. Scand J Gastroenterol. 1995;30(1):68–71. doi: 10.3109/00365529509093238. [DOI] [PubMed] [Google Scholar]
- 4.Shichida Y., Matsuyama T. Evolution of opsins and phototransduction. Philos Trans R Soc Lond B Biol Sci. 2009;364(1531):2881–2895. doi: 10.1098/rstb.2009.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aleman T., Garrity S., Brucker A. Retinal structure in vitamin A deficiency as explored with multimodal imaging. Doc Ophthalmol. 2013;127(3):239–243. doi: 10.1007/s10633-013-9403-0. [DOI] [PubMed] [Google Scholar]
- 6.Alten F., Heiduschka P., Clemens C., Eter N. Exploring choriocapillaris under reticular pseudodrusen using OCT-Angiography. Graefes Arch Clin Exp Ophthalmol. 2016;254(11):2165–2173. doi: 10.1007/s00417-016-3375-1. [DOI] [PubMed] [Google Scholar]
- 7.Spaide R., Ooto S., Curcio C. Subretinal drusenoid deposits AKA pseudodrusen. Surv Ophthalmol. 2018;63(6):782–815. doi: 10.1016/j.survophthal.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Curcio C.A., Messinger J.D., Sloan K.R., McGwin G., Medeiros N.E., Spaide R.F. Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina. 2013;33(2):265–276. doi: 10.1097/IAE.0b013e31827e25e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curcio C.A., Sloan K.R., Kalina R.E., Hendrickson A.E. Human photoreceptor topography. J Comp Neurol. 1990 Feb 22;292(4):497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- 10.Lin M.K., Kim S.H., Zhang L., Tsai Y.T., Tsang S.H. Rod metabolic demand drives progression in retinopathies. Taiwan J Ophthalmol. 2015;5(3):105–108. doi: 10.1016/j.tjo.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kakiuchi D., Uehara T., Shiotani M. Oscillatory potentials in electroretinogram as an early marker of visual abnormalities in vitamin A deficiency. Mol Med Rep. 2015;11(2):995–1003. doi: 10.3892/mmr.2014.2852. [DOI] [PubMed] [Google Scholar]
- 12.Lujan B., Roorda A., Knighton R., Carroll J. Revealing Henle's fiber layer using spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(3):1486–1492. doi: 10.1167/iovs.10-5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vajzovic L., Hendrickson A.E., O'Connell R.V. Maturation of the human fovea: correlation of spectral-domain optical coherence tomography findings with histology. Am J Ophthalmol. 2012;154(5):779–789. doi: 10.1016/j.ajo.2012.05.004. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao W., Cense B., Zhang Y., Jonnal R.S., Miller D.T. Measuring retinal contributions to the optical Stiles-Crawford effect with optical coherence tomography. Optic Express. 2008;16(9):6486–6501. doi: 10.1364/OE.16.006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park D.W., Lujan B.J. Normal interdigitation zone loss by motion-tracked OCT. Ophthalmol Retina. 2017;1(5):394. doi: 10.1016/j.oret.2017.05.005. [DOI] [PubMed] [Google Scholar]