Abstract
Background
Bethesda category III and IV thyroid nodules fall in the indeterminate risk of malignancy category. These nodules have been a relatively elusive entity to manage as previous studies have shown a wide variation in malignancy rates in different regions and institutions across the world. However, data from the subcontinent with regards to this is scarce.
Aim and objective
This study aimed to determine the characteristics and malignancy rates of cytology proven Bethesda Category III and IV thyroid nodules and its association with clinical, histopathological and laboratory variables, in the regional population.
Method
A retrospective search was performed on all patients with thyroid nodules who presented to this hospital, from January 2011 to September 2018. Patients who had cytology proven Bethesda category III and IV thyroid nodules that underwent surgery were included in the study.
Results
Malignancy in Bethesda Category III and Bethesda Category IV thyroid nodules was 29.6% and 47.1%, respectively. There was no significant association determined between malignancy rate and various clinical, histopathological, and radiological characteristics.
Conclusion
The malignancy rates in Bethesda category III and IV thyroid nodules in this study are significantly higher than that initially suggested by the Bethesda consensus publication but is comparable to international data present.
Keywords: Bethesda Category III, Bethesda Category IV, Fine Needle Aspiration Cytology (FNAC), Thyroid nodule, Malignancy, Ultrasound thyroid
Introduction
Thyroid nodules are common in adults. Studies using ultrasonography have estimated the prevalence of thyroid nodules to be 20–76% in the adult population [1]. The incidence of malignancy among nodules is approximately 5% [2]. However, there are studies which have suggested that it may be as high as 15% [3]. In the last three decades, the overall incidence of thyroid cancer has increased dramatically, and it is considered as one of the fastest-growing cancers among women [4].
Ultrasound-guided fine-needle aspiration (FNA) is an acknowledged diagnostic modality for evaluating thyroid nodules [5], [6], [7]. Nodules that undergo FNA, their cytology is assessed using the Bethesda System for Reporting Thyroid Cytopathology (BSRTC) and hence further managed accordingly [8]. This system categorizes specimens into six categories (I-VI), each having a different malignant potential. The malignant potential of Bethesda Category II, V, and VI nodules are well- established, and they have set recommendations for management [9]. On the contrary, Bethesda Category III and IV nodules' malignancy potential is variable, and the management depends on the stratification of risk factors such as family history of thyroid cancer, history of radiation therapy to the neck, rapid growth of the nodule, above normal thyroid stimulating hormone (TSH) [10], and ultra-sonographic characteristics (nodule, shape, echogenicity, regularity of borders, calcification and vascularity) [11]. The management can consist of observational clinical follow-up with no intervention, repeating FNA, or surgery (lobectomy/thyroidectomy) [12], [13], [14].
The literature on the malignancy potential of Bethesda Category III and IV nodules is very variable across different institutes and regions. It has been suggested that local malignancy rate should be determined, and management should be tailored, accordingly. Studies from different institutes from various parts of the world show a malignant potential of Bethesda category III nodules to be as variable as from 15.7% to 54.6% and of category IV nodules to be 16.8% to 72.4% [15], [16]. This is much higher than what was initially reported by Cibas et al, where malignancy rate for Bethesda Category III thyroid nodule was estimated to be 5–15% and for Bethesda category IV thyroid nodules was estimated to be 15–30% [12].
Furthermore, data for malignancy rate of Bethesda Category III and IV thyroid nodules in the sub-continent is scarce and limited to a handful of studies. This study aims to determine the characteristics and malignancy rates of cytology proven Bethesda Category III and IV thyroid nodules and its association with clinical, histopathological and laboratory variables, in the regional population.
Materials and methods
Subjects, study design, and clinical setting
We performed a retrospective chart analysis of all patients with cytology proven Bethesda Categories III and IV thyroid nodules presenting to the hospital from January 2011 to September 2018. The study was approved by the local Institutional Review Board.
During the hospital visit, all patients had a thorough clinical, laboratory and radiological evaluation. This consisted of a thorough history and examination, thyroid function tests, and imaging studies consisting of at least one of the following: magnetic resonance imaging (MRI) neck, ultrasound thyroid, or technetium (Tc − 99 m) pertechnetate thyroid scan. All patients underwent ultrasound- guided-FNA.
The cytopathological assessment was done using the Bethesda System of Reporting Thyroid Cytology [12], [17]. The FNA cytology smears were air-dried and stained using Diff Quik stain. Once dried, specimens were fixed in alcohol and stained with Papanicolaou stain. Category III lesions were called follicular lesion of undetermined significance (FLUS)/atypia of undetermined significance (AUS) [18]. Both terminologies were used and depicted similar cytological findings. These included follicular cells with minimal cytological atypia, including slight nuclear irregularity, minimal increase in size, and overlap.
Bethesda Category IV lesions were called follicular neoplasm (FN) [19] and included cellular smears with cells showing nuclear irregularity and atypia and arranged in microfollicle formation. The FNA smears were compared to histology as the gold standard (where available). The tissue was serially sectioned and preserved in 10% buffered formalin. These were then embedded into the paraffin and stained with hematoxylin and eosin and reviewed under a microscope. The histology specimens were assessed for the presence of a capsule and a capsular or vascular invasion, including the number of vessels involved.
Based on the risk factors (>40 years of age, female gender, positive family history, or history of radiation), clinical features (lymphadenopathy, new onset of voice changes, or a sudden increase in the size of growth), radiological features (size, and the number of nodules), and preference of physicians and patients, patients were either placed on observational clinical follow-up with no intervention, underwent a repeat FNA, or had surgery (lobectomy/thyroidectomy) [20].
All males and females who presented to the SKMCH&RC, Lahore, for assessment of thyroid nodules, which were categorized as Bethesda Category III or IV, and underwent surgical excision of the gland were included in the study. Patients with thyroid nodules, of any other Bethesda category, were excluded.
Clinical information
The data of the subjects were deidentified. Electronic medical records were reviewed to collect information regarding demographics, baseline clinical characteristics, laboratory findings, histopathological, and radiological analysis, and treatment modalities. Malignancy rate was determined for subjects that underwent surgery, by correlating the cytopathology report with the histopathological report.
Statistical analysis
The statistical analysis was performed using SPSS software (version 22.0; SPSS, Chicago, IL). Descriptive statistics were computed for each variable. If any study variable was missing for the included subjects, it was excluded in the statistical analysis. Chi-Square and t-tests were used for the analyses between independent and dependent variables. However, if the assumptions for the chi-square test were not met, Fisher exact test was used. A p-value of <0.05 was considered statistically significant.
Results
A total of 66 charts of patients with Bethesda Category III and 107 charts of patients with Bethesda Category IV thyroid nodules were identified and reviewed. Among the 66 patients with Bethesda Category III thyroid nodules, 39 patients were excluded because they were either lost to follow-up (12), had radioactive iodine therapy (RAI) (2), or they were put under clinical observation and follow up, without any intervention (25) (Fig. 1a). On the contrary, of the 107 patients with Bethesda Category IV thyroid nodules, 20 patients were excluded. These patients were lost to follow-up (12) or followed in the clinic without intervention (8) (Fig. 1b).
Fig. 1.
Summary of management strategies of patients with Bethesda Category III & IV thyroid nodules. Patients that subsequently underwent surgery were included in the present investigation.
Of the 27 subjects with Bethesda Category III thyroid nodules, 18 (66.7%) were females. The median age of the cohort was 40 years (range: 18–69 years). The majority of subjects were from Punjab province (70.4%). Neck swelling was reported by 24 (88.9%) subjects at the time of initial presentation, 11.1% (3) had a positive family history for thyroid cancer, and 1 (3.7%) subject had higher than normal levels of thyroid-stimulating hormone (TSH). Ten subjects underwent a Tc-99 m pertechnetate thyroid scan, and among these, 8 (80%) had cold nodules. On the ultrasound thyroid imaging, 50% (8) of the subjects had a solitary nodule, and 14 (87.5%) subjects had a nodule of at least 2 cm or more. Similarly, on MRI of the neck, 85.2% (23) of the subjects had a nodule of at least 2 cm or more. These results have been summarized in Table 1a.
Table 1a.
Baseline characteristics of subjects with Bethesda Category III thyroid nodules.
| Study characteristic | Category | Number (%) |
|---|---|---|
| Age (Years) | Median (Range) | 40 (18–69) |
| Gender | Males | 9 (33.3%) |
| Females | 18 (66.7%) | |
| Demographics | Punjab | 19 (70.4%) |
| Khyber Pakhtunkhwa | 5 (18.5%) | |
| Others (Baluchistan, Afghanistan, Kashmir) | 3 (11.1%) | |
| Presenting symptom | Neck Swelling | 24 (88.9%) |
| Family history | Positive | 3 (11.1%) |
| Radiation exposure | Present | 1 (3.7%) |
| Thyroid function | Euthyroid | 26 (96.3%) |
| Altered (hyperthyroidism or hypothyroidism) | 1 (3.7%) | |
| Thyroid scan | Cold nodule(s) | 8 (80%) |
| Hot nodule(s) | 2 (20%) | |
| Nodularity (ultrasound) | Multinodular goiter | 8 (50%) |
| Solitary nodule | 8 (50%) | |
| Size (ultrasound) | Less than 2 cm | 2 (12.5%) |
| 2 cm or more | 14 (87.5%) | |
| Size (magnetic resonance imaging) | Less than 2 cm | 4 (14.8%) |
| 2 cm or more | 23 (85.2%) |
Among the 87 subjects with Bethesda Category IV thyroid nodules, 65 (74.7%) were females. The median age was 39 years (range: 7–78 years), and most of the subjects were from Punjab province. In 94.3% (82) of the subjects, neck swelling was the presenting symptom. There was a positive family history of thyroid cancer in 12 (13.8%) subjects, 3 (3.4%) subjects had a history of radiation exposure, and in 12.6% (11) of the subjects, the levels of TSH were abnormal. Thirty-six subjects completed the Tc-99 m pertechnetate thyroid scan, and 1 (2.8%) subject had a hot nodule. Ultrasound thyroid imaging indicated that 24 (57.1%) subjects had a multinodular goiter, and 76.2% (32) of the subjects had a nodule of at least 2 cm or more in size. On the MRI of the neck, in 61 (76.3%) subjects, the size of the nodules was at least 2 cm or more (Table 1b).
Table 1b.
Baseline characteristics of subjects with Bethesda Category IV thyroid nodules.
| Study characteristic | Category | Number (%) |
|---|---|---|
| Age (Years) | Median (Range) | 39 (7–78) |
| Gender | Males | 22 (25.3%) |
| Females | 65 (74.7%) | |
| Demographics | Punjab | 59 (67.8%) |
| Khyber Pakhtunkhwa | 18 (20.7%) | |
| Others (Baluchistan, Afghanistan, Kashmir) | 10 (11.5%) | |
| Presenting symptom | Neck Swelling | 82 (94.3%) |
| Family history | Positive | 12 (13.8%) |
| Radiation exposure | Present | 3 (3.4%) |
| Thyroid function | Euthyroid | 76 (87.4%) |
| Altered (hyperthyroidism or hypothyroidism) | 11 (12.6%) | |
| Thyroid scan | Cold nodule(s) | 35 (97.2%) |
| Hot nodule(s) | 1 (2.8%) | |
| Nodularity (ultrasound) | Multinodular goiter | 24 (57.1%) |
| Solitary nodule | 18 (42.9%) | |
| Size (ultrasound) | Less than 2 cm | 10 (23.8%) |
| 2 cm or more | 32 (76.2%) | |
| Size (magnetic resonance imaging) | Less than 2 cm | 19 (23.8%) |
| 2 cm or more | 61 (76.3%) |
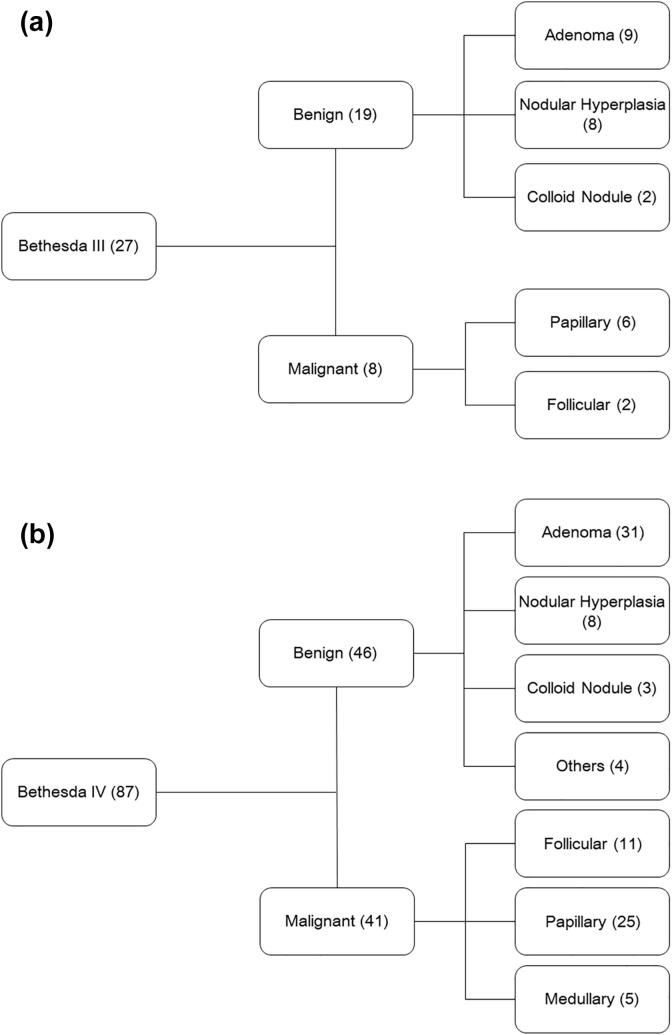
The malignancy rate of Bethesda Category III thyroid nodules was 29.6%, and the most common malignant diagnosis was papillary thyroid carcinoma. On the contrary, among benign nodules, adenoma and nodular hyperplasia were the most common histopathologies (Fig. 2a). There was no significant association present between the malignancy status of the Bethesda Category III thyroid nodules and demographic, clinical, histopathological, and radiological parameters (Table 2a).
Fig. 2.
Histopathological diagnoses of Bethesda Category III & IV thyroid nodules.
Table 2a.
Stratification of the subjects with Bethesda Category III based on malignancy status of the thyroid nodules.
| Study characteristic | Category | Malignant | Not Malignant | P- Value |
|---|---|---|---|---|
| Age (Years) | Median (Range) | 41 (18–58) | 34 (20–69) | 0.99 |
| Gender | Males | 2 (25%) | 7 (36.8%) | 0.68 |
| Females | 6 (75%) | 12 (66.7%) | ||
| Demographics | Punjab | 4 (50%) | 15 (78.9%) | 0.30 |
| Khyber Pakhtunkhwa | 3 (37.5%) | 2 (10.6%) | ||
| Others (Baluchistan, Afghanistan, Kashmir) | 1 (12.5%) | 2 (10.6%) | ||
| Presenting symptom | Neck Swelling | 8 (100%) | 16 (84.2%) | 0.53 |
| Family history | Positive | 1 (12.5%) | 2 (10.5%) | 1.00 |
| Radiation exposure | – | 1 (5.3%) | 1.00 | |
| Thyroid function | Euthyroid | 8 (100%) | 18 (94.7%) | 1.00 |
| Altered (hyperthyroidism or hypothyroidism) | – | 1 (5.3%) | ||
| Thyroid scan | Cold nodule (s) | 2 (100%) | 6 (75%) | 1.00 |
| Hot nodule (s) | – | 2 (25%) | ||
| Nodularity (ultrasound) | Multinodular goiter | 2 (40%) | 6 (54.5%) | 1.00 |
| Solitary nodule | 3 (60%) | 5 (45.5%) | ||
| Size (ultrasound) | Less than 2 cm | 2 (40%) | – | 0.08 |
| 2 cm or more | 3 (60%) | 11 (100%) | ||
| Size (magnetic resonance imaging) | Less than 2 cm | – | 2 (11.1%) | 1.00 |
| 2 cm or more | 4 (100%) | 16 (88.9%) |
The malignancy rate of Bethesda Category IV thyroid nodules was 47.1%. The most common diagnosis among malignant nodules was papillary thyroid carcinoma, and in benign lesions, it was of adenoma (Fig. 2b). The Bethesda Category IV thyroid nodules' malignancy status was found to have no significant associations with any of the demographic, clinical, histopathological, and radiological parameters (Table 2b).
Table 2b.
Stratification of the subjects with Bethesda Category IV based on malignancy status of the thyroid nodules.
| Study characteristic | Category | Malignant | Not Malignant | P-Value |
|---|---|---|---|---|
| Age (Years) | Median (Range) | 39 (17–72) | 39 (7–78) | 0.75 |
| Gender | Males | 11 (26.8%) | 11 (23.9%) | 0.76 |
| Females | 30 (73.2%) | 35 (76.1%) | ||
| Demographics | Punjab | 26 (63.4%) | 33 (71.7%) | 0.15 |
| Khyber Pakhtunkhwa | 10 (24.4%) | 8 (17.4%) | ||
| Others (Baluchistan, Afghanistan, Kashmir) | 5 (12.2%) | 5 (10.9%) | ||
| Presenting symptom | Neck Swelling | 40 (97.6%) | 42 (91.3%) | 0.37 |
| Family history | Positive | 6 (14.6%) | 6 (13%) | 0.83 |
| Radiation exposure | – | 3 (6.5%) | 0.244 | |
| Thyroid function | Euthyroid | 36 (87.8%) | 40 (87%) | 0.91 |
| Altered (hyperthyroidism or hypothyroidism) | 5 (12.2%) | 6 (13%) | ||
| Thyroid scan | Cold nodule (s) | 18 (100%) | 17 (94.4%) | 1.00 |
| Hot nodule (s) | – | 1 (100%) | ||
| Nodularity (ultrasound) | Multinodular goiter | 6 (42.9%) | 18 (64.3%) | 0.19 |
| Solitary nodule | 8 (57.1%) | 10 (35.7%) | ||
| Size (ultrasound) | Less than 2 cm | 2 (14.3%) | 8 (28.6%) | 0.45 |
| 2 cm or more | 12 (85.7%) | 20 (71.4%) | ||
| Size (magnetic resonance imaging) | Less than 2 cm | 7 (17.9%) | 12 (29.3%) | 0.30 |
| 2 cm or more | 32 (82.1%) | 29 (70.7%) |
Discussion
The purpose of this single institutional retrospective analysis was to determine the characteristics and malignancy rates of cytology proven Bethesda Category III and IV thyroid nodules and its association with clinical, histopathological, and laboratory variables in the regional population. The malignancy rate for Bethesda category III and IV thyroid nodules was 29.6% and 47.1%, respectively. No statistically significant associations were identified between malignancy rate and clinical, histopathological, and laboratory characteristics.
The malignancy rate for Bethesda category III and IV thyroid nodules is inconsistent. Prior investigations have reported a 15.7%−54.7% and a 16.8%−72.4% malignancy rate for Bethesda category III and IV thyroid nodules [15], [16], respectively (Table 3). An important reason for this disparity in reporting is the criteria for selecting the patients to undergo surgical intervention. Similarly, there are differences in the study population. Studies that have been conducted on similar populations have comparable malignancy rates. The cohort of the present investigation is closest to the study by Pasha et al. The malignancy rates of both studies for the Bethesda Category III thyroid nodules are analogous.
Table 3.
Malignancy rates in Bethesda Category III and IV thyroid nodules, international and regional comparison.
| Reference | Bethesda III |
Bethesda IV |
||||
|---|---|---|---|---|---|---|
| Cytology(N) | Underwent surgical resection (% of total cohort) | % Malignancy | Cytology(N) | Underwent surgical resection (% of total cohort) | % Malignancy | |
| Ho et al. 2014[21] | 541 | 369 (68.2) | 37.9 | NR | NR | NR |
| Deniwar et al. [22] | 65 | 45 (69) | 34.0 | 42 | 41 (97.7) | 50.0 |
| Godoi Cavalheiro B et al. [15] | 478 | 478 (100) | 15.7 | 137 | 137 (100) | 16.8 |
| Chandra S, et al. [24] | 63 | 31 (49.2) | 28.5 | NR | NR | NR |
| Abbas al-Kurd et al. 2019 [27] | 14 | 14 (100) | 35.7 | 162 | 162 (100) | 64.2 |
| Chirayath et al. [16] | 140 | 75 (53.6) | 54.7 | 36 | 29 (80.6) | 72.4 |
| Yaprak Bayrak, et al. 2020[28] | 510 | 108 (21.2) | 25.0 | 440 | 47 (10.7) | 27.6 |
| Pasha HA, et al. [23] | 81 | 81 (100) | 33.0 | NR | NR | NR |
| Present study | 66 | 27 (40.9) | 29.6 | 107 | 87 (81.3) | 47.1 |
N: No of nodules.
NR: Not reported.
The median age of subjects with Bethesda Category III thyroid nodules was 40 years (18–69 years), and the median age of participants with Bethesda Category IV thyroid nodules was 39 years (7–78 years). This is relatively younger than the average age of the study population reported by others. The exact reason for this difference is unknown and beyond the scope of this study. However, this could be associated with genetic variations. The investigations that have been conducted on similar cohorts have reported comparable mean age at the time of presentation [21], [22], [23], [24]. The most common presenting symptom was neck swelling in the present study.
This is not surprising, as the level of medical awareness and health consciousness is low, and there are limited medical facilities. Patients do not undergo routine medical visits and only present to the hospital after the nodules become symptomatic.
There were no statistically significant risk factors identified for malignancy in the present study. Previously, investigators have reported a positive family history of thyroid cancer, history of radiation therapy to the neck, rapid or sudden growth of nodule, and above normal TSH levels as risk factors for malignancy [25]. Lack of association in the present study is likely due to a smaller number of subjects and limited level of education and history of symptoms, due to which majority of the subjects were unable to provide an adequate history of risk factors. Similarly, in most subjects, detailed reports of ultrasonography of the thyroid gland were not available, as they had been conducted at outside healthcare facilities.
Among the cancer subtypes, papillary thyroid cancer was the most common malignant variant in both Bethesda category III and IV thyroid nodules followed by follicular cancer. At the same time, adenoma was the most common benign histopathology seen in subjects with Bethesda category III and IV thyroid nodules. Other investigators worldwide have reported a similar distribution of histopathology [26].
There were a few limitations in this study. This was a retrospective chart review. Due to the study's inherent nature, it was not possible to account for all clinical, histopathological, and radiological characteristics. Furthermore, due to the lack of randomization and blindness, there is a high likelihood of magnifying or understating associations. Nonetheless, in the present study, data were extracted from the electronic hospital information system, and records from all hospital visits were correlated to reduce the risk of reporting bias. Another limitation of the study was the unavailability of standardized ultrasonography report of the subjects. As indicated earlier, most of the subjects had their scans from outside healthcare facilities, and it was not possible to retrieve the raw files of these studies. Similarly, the total number of participants in the present study was small. However, these are comparable to other investigations. Lastly, molecular testing was not used for stratifying the risk of malignancy in this study. This is because, at the time of writing, this test is unavailable in Pakistan.
Conclusion
To our knowledge this is the first study in Pakistan which has determined the malignancy rates in both Bethesda category III and IV thyroid nodules. The malignancy rate for Bethesda category III thyroid nodules in this study is significantly higher than that initially suggested by the Bethesda consensus publication (5–15%) but is comparable to global data. The malignancy rate for Bethesda category IV thyroid nodules is also significantly higher than estimated by the Bethesda consensus publication (15–30%). Our study implies that Bethesda Category III and IV thyroid nodules in our practice setting have a higher potential of malignancy than traditionally believed. Further, larger studies are needed in this region to determine the malignancy potential of Bethesda category III and IV thyroid nodules to establish guidelines for management of these indeterminate category nodules.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Khalel O.A., Elzeftawy A.A., Elsheweal A.M., Zaitoon M.A. Detection of thyroid malignancy among thyroid swellings in Zagazig University hospitals. J Health Med Nurs. 2017 Feb 14;1(2):27–43. [Google Scholar]
- 2.Tuttle R.M., Ball D.W., Byrd D., Dilawari R.A., Doherty G.M., Duh Q.Y. Thyroid carcinoma. J Natl Compr Canc Netw. 2010 Nov 1;8(11):1228–1274. doi: 10.6004/jnccn.2010.0093. [DOI] [PubMed] [Google Scholar]
- 3.Frates MC, Benson CB, Doubilet PM, Kunreuther E, Contreras M, Cibas ES, Orcutt J, Moore Jr FD, Larsen PR, Marqusee E, Alexander EK. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab 2006 Sep 1;91(9):3411. [DOI] [PubMed]
- 4.Ramírez-Vick M, Nieves-Rodríguez M, Lúgaro-Gómez A, Pérez-Irizarry J. Increasing incidence of thyroid cancer in Puerto Rico, 1985-2004. Puerto Rico Health Sci J 2011 Aug 26;30(3). [PubMed]
- 5.Chung S.R., Baek J.H., Choi Y.J., Sung T.Y., Song D.E., Kim T.Y. The role of core needle biopsy for the evaluation of thyroid nodules with suspicious ultrasound features. Korean J Radiol. 2019 Jan 1;20(1):158–165. doi: 10.3348/kjr.2018.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhartiya R., Mallik M., Kumari N., Prasad B.N. Evaluation of thyroid lesions by fine-needle aspiration cytology based on Bethesda system for reporting thyroid cytopathology classification among the population of South Bihar. Ind J Med Paediatric Oncol Official J Ind Soc Med Paediatr Oncol. 2016 Oct;37(4):265. doi: 10.4103/0971-5851.195742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ronen O., Cohen H., Sela E., Abu M. Differences in cytopathologist thyroid nodule malignancy rate. Cytopathology. 2020 Apr 25 doi: 10.1111/cyt.12841. [DOI] [PubMed] [Google Scholar]
- 8.Park J.H., Yoon S.O., Son E.J., Kim H.M., Nahm J.H., Hong S. Incidence and malignancy rates of diagnoses in the bethesda system for reporting thyroid aspiration cytology: an institutional experience. Korean J Pathol. 2014 Apr;48(2):133. doi: 10.4132/KoreanJPathol.2014.48.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haugen B.R., Alexander E.K., Bible K.C., Doherty G.M., Mandel S.J., Nikiforov Y.E. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016 Jan 1;26(1):1–33. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wartofsky L. Increasing world incidence of thyroid cancer: increased detection or higher radiation exposure? Hormones. 2010 Apr 1;9(2):103–108. doi: 10.14310/horm.2002.1260. [DOI] [PubMed] [Google Scholar]
- 11.Iannuccilli J.D., Cronan J.J., Monchik J.M. Risk for malignancy of thyroid nodules as assessed by sonographic criteria: the need for biopsy. J Ultrasound Med. 2004 Nov;23(11):1455–1464. doi: 10.7863/jum.2004.23.11.1455. [DOI] [PubMed] [Google Scholar]
- 12.Cibas E.S., Ali S.Z. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid. 2017 Nov 1;27(11):1341–1346. doi: 10.1089/thy.2017.0500. [DOI] [PubMed] [Google Scholar]
- 13.Al Dawish M.A., Robert A.A., Muna A., Eyad A., Al Ghamdi A., Al Hajeri K. Bethesda system for reporting thyroid cytopathology: a three-year study at a tertiary care referral center in Saudi Arabia. World J Clin Oncol. 2017 Apr 10;8(2):151. doi: 10.5306/wjco.v8.i2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maity P., Jha A.K., Sengupta M., Basu K., Chatterjee U., Ghosh S. Thyroid bethesda atypia of undetermined significance or follicular lesion of undetermined significance (aus/flus): a heterogenous group. J Cytol. 2019 Oct;36(4):200. doi: 10.4103/JOC.JOC_160_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavalheiro BG, Leite AK, de Matos LL, Miazaki AP, Ientile JM, Kulcsar MA, Cernea CR. Malignancy rates in thyroid nodules classified as Bethesda categories III and IV: retrospective data from a tertiary center. Int J Endocrinol Metab 2018:Jan;16(1). [DOI] [PMC free article] [PubMed]
- 16.Chirayath S.R., Pavithran P.V., Abraham N., Nair V., Bhavani N., Kumar H. Prospective study of Bethesda categories III and IV thyroid nodules: outcomes and predictive value of BRAFV600E mutation. Ind J Endocrinol Metab. 2019 May;23(3):278. doi: 10.4103/ijem.IJEM_635_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bongiovanni M., Spitale A., Faquin W.C., Mazzucchelli L., Baloch Z.W. The Bethesda system for reporting thyroid cytopathology: a meta-analysis. Acta Cytol. 2012;56(4):333–339. doi: 10.1159/000339959. [DOI] [PubMed] [Google Scholar]
- 18.Rosario P.W. Thyroid nodules with atypia or follicular lesions of undetermined significance (Bethesda Category III): importance of ultrasonography and cytological subcategory. Thyroid. 2014 Jul 1;24(7):1115–1120. doi: 10.1089/thy.2013.0650. [DOI] [PubMed] [Google Scholar]
- 19.Deaver K.E., Haugen B.R., Pozdeyev N., Marshall C.B. Outcomes of Bethesda categories III and IV thyroid nodules over 5 years and performance of the Afirma gene expression classifier: a single- institution study. Clin Endocrinol. 2018 Aug;89(2):226–232. doi: 10.1111/cen.13747. [DOI] [PubMed] [Google Scholar]
- 20.Taccaliti A., Palmonella G., Silvetti F., Boscaro M. Thyroid neoplasm. Thyroid and parathyroid diseases-new insights into some old and some new issues. InTech. 2012 Mar;7:45–76. [Google Scholar]
- 21.Ho A.S., Sarti E.E., Jain K.S., Wang H., Nixon I.J., Shaha A.R. Malignancy rate in thyroid nodules classified as Bethesda category III (AUS/FLUS) Thyroid. 2014 May 1;24(5):832–839. doi: 10.1089/thy.2013.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deniwar A., Hambleton C., Thethi T., Moroz K., Kandil E. Examining the Bethesda criteria risk stratification of thyroid nodules. Pathol-Res Pract. 2015 May 1;211(5):345–348. doi: 10.1016/j.prp.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Pasha H.A., Dhanani R., Mughal A., Ahmed K.S., Suhail A. Malignancy rate in thyroid nodules with atypia or follicular lesion of undetermined significance. Int Arch Otorhinolaryngol. 2020 Jun;24(2):227–231. doi: 10.1055/s-0039-1698784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chandra S., Chandra H., Bisht S.S. Malignancy rate in thyroid nodules categorized as atypia of undetermined significance or follicular lesion of undetermined significance-an institutional experience. J Cytol. 2017 Jul;34(3):144. doi: 10.4103/JOC.JOC_234_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiore E., Vitti P. Serum TSH and risk of papillary thyroid cancer in nodular thyroid disease. J Clin Endocrinol Metab. 2012 Apr 1;97(4):1134–1145. doi: 10.1210/jc.2011-2735. [DOI] [PubMed] [Google Scholar]
- 26.Cabanillas M.E., McFadden D.G., Durante C. Thyroid cancer. Lancet. 2016 Dec 3;388(10061):2783. doi: 10.1016/S0140-6736(16)30172-6. [DOI] [PubMed] [Google Scholar]
- 27.Al-Kurd Abbas, Maree A, Mizrahi I, Kaganov K, Weinberger JM, Mali B, Mazeh H, Hirshoren N. An Institutional Analysis of Malignancy Rate in Bethesda III and IV Nodules of the Thyroid. Am. J. Otolaryngol. Head Neck Surg. 2019 [Google Scholar]
- 28.Yaprak Bayrak B, Eruyar AT Malignancy rates for Bethesda III and IV thyroid nodules: a retrospective study of the correlation between fine-needle aspiration cytology and histopathology. BMC endocrine disorders. 2020 doi: 10.1186/s12902-020-0530-9. [DOI] [PMC free article] [PubMed] [Google Scholar]