Abstract
Background
The non-inferiority of combined breast conservation surgery (BCS) and radiotherapy (breast conservation therapy or BCT) compared to mastectomy in sporadic breast cancer cases is well recognised. Uncertainty remains regarding optimal surgical practice in BRCA mutation carriers.
Aims
To evaluate the oncological safety of combined BCT versus mastectomy in BRCA mutation carriers following breast cancer diagnosis.
Methods
A systematic review was performed as per PRISMA and MOOSE guidelines. Observational studies comparing BCS and mastectomy in BRCA carriers were identified. Dichotomous variables were pooled as odds ratios (OR) using the Mantel–Haenszel method. Log hazard ratios (lnHR) for locoregional recurrence (LRR), contralateral breast cancer, disease-free and overall survival and their standard errors were calculated from Kaplan-Meier or cox-regression analyses and pooled using the inverse variance method.
Results
Twenty three studies of 3807 patients met inclusion criteria; 2200 (57.7%) were BRCA1 and 1212 (31.8%) were BRCA2 carriers. Median age at diagnosis was 41 years with 96 months follow up. BCS was performed on 2157 (56.7%) while 1408 (41.5%) underwent mastectomy. An increased risk of LRR was observed in patients treated with BCS (HR:4.54, 95% Confidence Interval: 2.77–7.42, P < 0.001, heterogeneity (I2) = 0%). However, the risks of contralateral breast cancer (HR:1.51, 95%CI: 0.44–5.11, P = 0.510, I2 = 80%), disease recurrence (HR:1.16, 95%CI: 0.78–1.72, P = 0.470, I2 = 44%), disease-specific recurrence (HR:1.58, 95%CI: 0.79–3.15, P = 0.200, I2 = 38%) and death (HR:1.10, 95%CI: 0.72–1.69, P = 0.660, I2 = 38%) were equivalent for combined BCT and mastectomy.
Conclusions
Survival outcomes following combined BCT is comparable to mastectomy in BRCA carriers. However, the risk of LRR is increased. Patient counselling should be tailored to incorporate these findings.
Keywords: Breast cancer, Genetics, Surgical oncology, BRCA mutations
Highlights
-
•
BRCA carriers see similar survival after breast conservation therapy and mastectomy.
-
•
Locoregional recurrence rates are increased following breast conservation.
-
•
Surgical counselling may reflect these findings for BRCA mutation carriers.
1. Introduction
In the western world, the lifetime risk of developing sporadic breast cancer is 12.4% [1]. Estimations suggest that 10% of breast cancer is due to hereditary predisposition, with family history amplifying the likelihood of developing the disease [2]. Moreover, this risk is exponentially increased in those harbouring breast cancer gene (BRCA) mutations. These genes are highly penetrant; there is a reported cumulative risk of almost 80% for female breast cancers in BRCA1 mutations, as well as a 50% risk for BRCA2 mutation carriers [[3], [4], [5]].
Surgical resection is the primary curative strategy for breast cancer. In cases of sporadic breast cancers this frequently involves breast conservation therapy (BCT) in the form of combined breast conservation surgery (BCS) and adjuvant radiotherapy, provided clear margins of resection are achieved [6]. Risks of locoregional recurrence (LRR) have been overestimated in the past, and in recent times, the paradigm has shifted such that omitting adjuvant radiotherapy may even be considered in some select patients with breast cancer, given their predicted risk of LRR being low following BCS [7].
However, in patients possessing BRCA-mutations, clinical outcomes such as locoregional control, oncological outcomes, and expected prognoses are less certain [8]; the international perception is that the risk of new primary cancers is so high as to make breast conservation a dangerous therapeutic strategy, and there is a vogue towards bilateral mastectomy being the most appropriate strategy for surgical management of newly diagnosed breast cancer in BRCA mutation carriers [6,9,10]. Currently, there are unanswered questions concerning survival advantage including those related to the underlying breast cancer subtype and prognostic criteria related to the index cancer; (eg. BRCA1 mutation carriers have the tendency to develop triple negative breast cancers) [11].
Surgical strategy also impacts the requirement for second operations, while risk reduction strategies are beneficial for BRCA mutation carriers yet to develop cancers [12]; in those diagnosed with primary cancer, the impact of local field radiotherapy on the index breast may have significant impact on future tumours, and indeed survival. Currently, ethical considerations limit the practicality of performing a prospective randomised-controlled trial on this topic, adding further uncertainty regarding optimal surgical oncological practice for those with BRCA mutations.
Given this uncertainty, the primary aim of the current study was to evaluate the oncological safety of BCT versus mastectomy with respect to LRR, new primary breast cancers, and survival in BRCA mutation carriers.
2. Materials and methods
2.1. Search strategy
A systematic review was performed in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist and Meta-Analyses Of Observational Studies in Epidemiology (MOOSE) guidelines [13,14]. The first and second authors conducted a comprehensive electronic search of the EMBASE, SCOPUS and PUBMED databases for studies to be considered in this analysis, the latest of which occurred on the 30th of November 2020. The search terms “BRCA” AND “lumpectomy” OR “BRCA” AND “breast conserving surgery” OR “BRCA” AND “breast conserving therapy” OR “hereditary breast and ovarian syndrome” AND “lumpectomy” OR “hereditary breast and ovarian syndrome” AND “breast conserving surgery” OR “hereditary breast and ovarian syndrome” AND “breast conserving therapy”. Secondary referencing was conducted by manually reviewing reference lists of potentially eligible studies. Manuscripts published in languages other than English were excluded. Studies were not restricted based on year of publication. All titles were initially screened, and studies deemed appropriate had their abstracts and full texts reviewed. In studies with data derived from the same patient sample, studies providing the most relevant survival data were included for analyses.
2.2. Inclusion and exclusion criteria
Studies meeting the following inclusion criteria were considered for inclusion in this analysis [1]: included patients carrying BRCA mutations with histopathological confirmation of breast cancer diagnoses [2], patients underwent surgical resection of their breast carcinoma [3], patient clinicopathological, immunohistochemical, treatment and survival data were available. Studies meeting the following criteria were excluded from this analysis [1]: assessed the oncological safety of prophylactic or bilateral mastectomies only [2], review articles [3], studies including less than 5 patients in their series or case reports [4], editorial articles, and [5] conference abstracts.
2.3. Data extraction and quality assessment
A formal literature search was performed by the first and second authors (M.G.D and C.M.D) using a predefined search strategy. Duplicate studies were removed. Both reviewers assessed the retrieved manuscripts for the above inclusion and exclusion criteria, before extracting [1]: First author name [2], year of publication [3], nature of study [4], country [5], number of patients in the series [6], number of BRCA 1 and BRCA 2 mutations carriers in each series [7], surgeries performed [8], clinicopathological data [9], treatment modalities and characteristics, and [10] survival data. Data specific to clinicopathological and treatment characteristics (expressed as risk ratios (RR), 95% confidence intervals (95% CI) and P-values) and clinical outcomes and survival (expressed as hazards ratios (HR), 95% CI, and P-values) were directly extracted from study manuscripts. Risk of bias and methodology quality assessment was conducted in concordance to the Newcastle-Ottawa scale [15]. In case of discrepancies in opinion between both reviewers, a third reviewer was asked to arbitrate (É.J.R). Studies reporting data from the same centre were evaluated for the duplication of patients data; studies determined to possess data overlapping with different studies were removed.
3. Results
3.1. Literature search
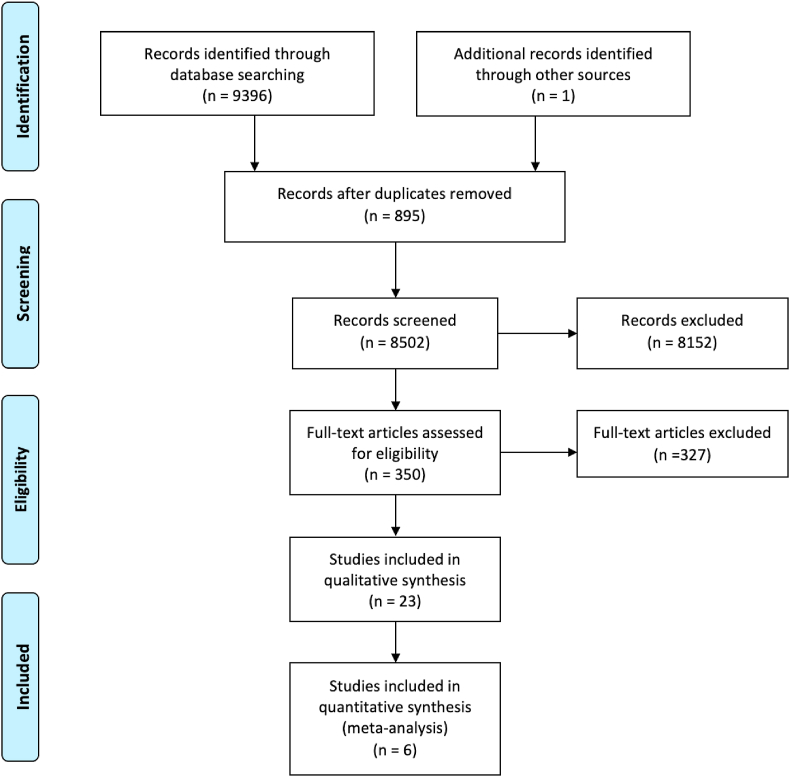
The initial literature search yielded a total of 9397 studies. Following removal of 895 duplicate studies, 8502 studies were screened for relevance for inclusion, of which 350 studies had their abstracts reviewed. Sixty four studies had their full manuscripts screened, before 23 studies were included for meta-analysis (Fig. 1) [[16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39]]. The included studies are illustrated in Table 1.
Fig. 1.
PRISMA flow diagram detailing the systematic search process.
Table 1.
Details regarding the 22 independent patient cohorts included in this systematic review.
| Author | Year | Study Type (LOE) | Country | BRCA (N) | BRCA1 | BRCA2 | BCS (N) | Mastectomy (N) | Median age (years) | Follow up (months) | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Robson | 2005 | RC (III) | US | 87 | 62 | 25 | 87 | – | 43 | 76 | 6 |
| Turner | 1999 | RC (III) | US | 8 | 5 | 3 | 8 | – | 36 | 174 | 5 |
| Garcia-Etienne | 2009 | RC (III) | Italy | 54 | 26 | 28 | 54 | – | 36 | 48 | 6 |
| Yoon | 2019 | RC (III) | Korea | 51 | 33 | 18 | 51 | – | 44 | 85 | 6 |
| Nilsson | 2014 | RC (III) | Sweden | 162 | – | – | 45 | 118 | 43 | 155 | 7 |
| Chiba | 2016 | RC (III) | US | 173 | 100 | 73 | 60 | 109 | 46 | 41 | 6 |
| Haffty | 2002 | RC (III) | US | 22 | 15 | 7 | 22 | – | 34 | 168 | 6 |
| Ye | 2020 | RC (III) | China | 63 | – | – | 63 | – | 41 | 61 | 5 |
| Van den Broek | 2018 | RC (III) | Netherlands | 261 | 190 | 71 | 125 | 136 | 39 | 40 | 6 |
| Robson | 1995 | RC (III) | US | 35 | 27 | 9 | 35 | – | – | 124 | 5 |
| Drooger | 2015 | RC (III) | Netherlands | 691 | 517 | 174 | 349 | 337 | – | 109 | 7 |
| Huang | 2020 | RC (III) | China | 176 | – | – | 44 | 132 | – | 48 | 5 |
| Pierce | 2010 | RC (III) | US | 655 | 394 | 261 | 302 | 353 | 42 | 103 | 6 |
| Kirova | 2010 | RC (III) | France | 27 | 19 | 8 | – | – | 43 | 167 | 5 |
| Pierce | 2016 | RC (III) | US | 160 | 123 | 37 | 160 | – | 40 | 95 | 6 |
| Metcalfe | 2011 | RC (III) | Canada | 396 | 259 | 142 | 396 | – | 42 | 126 | 6 |
| Brekelmens | 2007 | RC (III) | Netherlands | 260 | 90 | 170 | 111 | 135 | 43 | 52 | 6 |
| Eccles | 2001 | RC (III) | UK | 75 | 75 | – | 36 | 39 | 38 | 107 | 7 |
| El Tamer | 2004 | RC (III) | US | 51 | 30 | 21 | 21 | 30 | 48 | 79 | 6 |
| Robson | 1998 | RC (III) | US | 30 | 23 | 7 | 9 | 19 | 36 | 66 | 6 |
| Cao | 2019 | RC (III) | China | 103 | 31 | 72 | 103 | – | 45 | 80 | 6 |
| Foulkes | 1997 | RC (III) | US | 12 | 12 | – | 11 | – | 45 | 37 | 5 |
| Bernstein-Mohlo | 2020 | RC (III) | Israel | 255 | 169 | 86 | 127 | 128 | 44 | 71 | 7 |
LOE; level of evidence, N; number, BCS; breast conservation surgery, NOS; Newcastle-Ottawa Scale, RC; retrospective cohort, US; United States, UK; United Kingdom.
3.2. Study characteristics
This review included 3807 patients from 10 countries. Of these, 2200 possessed BRCA1 mutations (57.7%) and 1212 possessed BRCA2 mutations (31.8%). The median age at the time of breast cancer diagnosis was 41 years and median follow up was 96 months. Two thousand and one hundred and fifty seven patients underwent BCS (56.7%), while 1579 underwent mastectomy (41.5%).
3.3. Histopathological and immunohistochemical data
Invasive ductal carcinoma histopathological subtype (P < 0.001), pathological tumour stages 1–2 (P < 0.001) and lymph node negativity (P < 0.001) were all associated with undergoing BCS (all Fisher’s exact test, †). Data not associated with surgical approach are demonstrated in Table 2.
Table 2.
Associations of histopathological and immunohistochemical data and breast conservation and mastectomy.
| Parameter | BCT | Mastectomy | P-value |
|---|---|---|---|
| T1/2 | 1589 | 812 | <0.001ba |
| T3 | 12 | 71 | |
| IDC | 1078 | 230 | <0.001ba |
| Not IDC | 299 | 186 | |
| Grade 1/2 | 291 | 135 | 0.562a |
| Grade 3 | 741 | 320 | |
| LN negative | 1206 | 592 | <0.001ba |
| LN positive | 599 | 426 | |
| ER positive | 655 | 390 | 0.141a |
| ER negative | 1027 | 541 | |
| PgR positive | 167 | 122 | 0.131a |
| PgR negative | 359 | 210 | |
| HER2 positive | 32 | 25 | 0.750a |
| HER2 negative | 524 | 375 |
BCT; breast conservation therapy, T; tumour stage, IDC; invasive ductal carcinoma histological subtype, LN; lymph node, ER; estrogen receptor, PgR; progesterone receptor, HER2; human epidermal growth factor receptor-2.
Denotes statistical significance.
Fisher’s exact test.
3.4. Treatment characteristics
Neoadjuvant chemotherapy (NAC) prescription was reported in two analyses; 42.6% of patients underwent NAC in the BCS group (203/477) vs. 51.7% in the mastectomy group (240/464) (P = 0.005, †). In the BCS group, 28.5% of patients with ER + disease received adjuvant endocrine therapies (431/1510–12 studies), while 31.4% did so in the mastectomy group (338/1076–5 studies) (P = 0.116, †). Of patients undergoing BCS, 68.7% received adjuvant chemotherapy (AC) (885/1289–12 studies) vs. 60.7% in those undergoing mastectomy (526/866–4 studies) (P < 0.001, †). In the BCS group, 97.3% underwent adjuvant radiotherapy (1337/1374–9 studies), as did 41.8% in the mastectomy group (363/867–5 studies) (P < 0.001, †). In those undergoing BCS, 52.7% underwent prophylactic oophorectomy (736/1396–7 studies) vs. 50.7% in the mastectomy group (544/1072–5 studies) (P = 0.155, †). No included studies outlined data concerning surgical management of the axilla (Table 3).
Table 3.
Associations of treatment characteristics and breast conservation surgery and mastectomy.
| Treatment parameter | BCS | Mastectomy | P-value |
|---|---|---|---|
| NAC | 203 | 240 | 0.005ba |
| No NAC | 274 | 224 | |
| EHT | 431 | 338 | 0.116a |
| No EHT | 1079 | 738 | |
| AC | 885 | 526 | <0.001ba |
| No AC | 404 | 340 | |
| ALND No ALND |
N/R | N/R | N/C |
| XRT | 1337 | 363 | <0.001ba |
| No XRT | 32 | 504 | |
| Oophorectomy | 763 | 544 | 0.155a |
| No oophorectomy | 660 | 528 |
BCS; breast conservation surgery, NAC; neoadjuvant chemotherapy, EHT; Endocrine hormonal therapy, AC; Adjuvant systemic chemotherapy, ALND; axillary lymph node dissection, XRT; adjuvant radiotherapy, N/R; not reported, N/C; not calculatable.
Denotes statistical significance.
Fisher’s exact test.
3.5. Locoregional recurrence
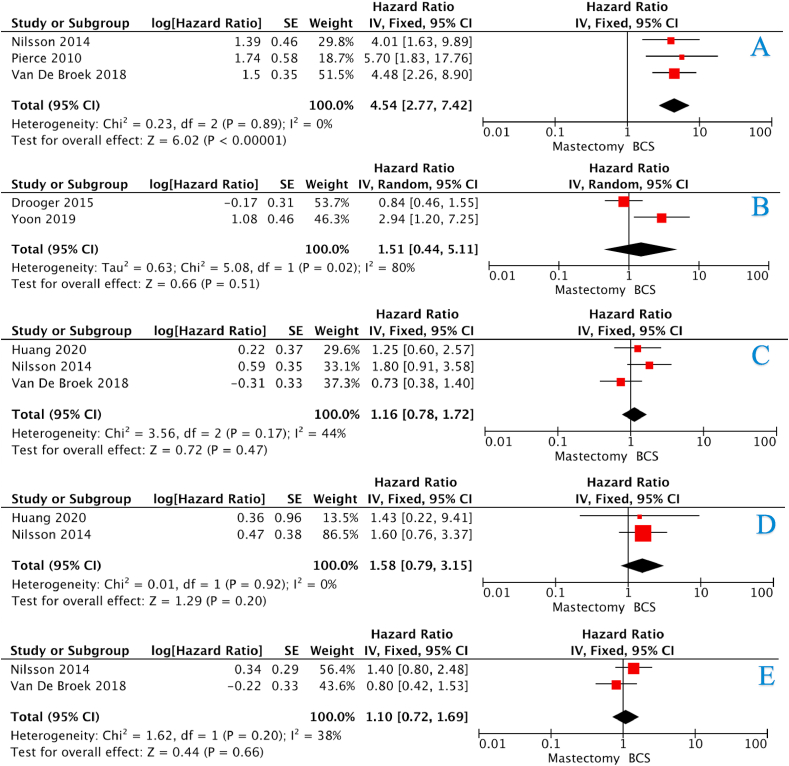
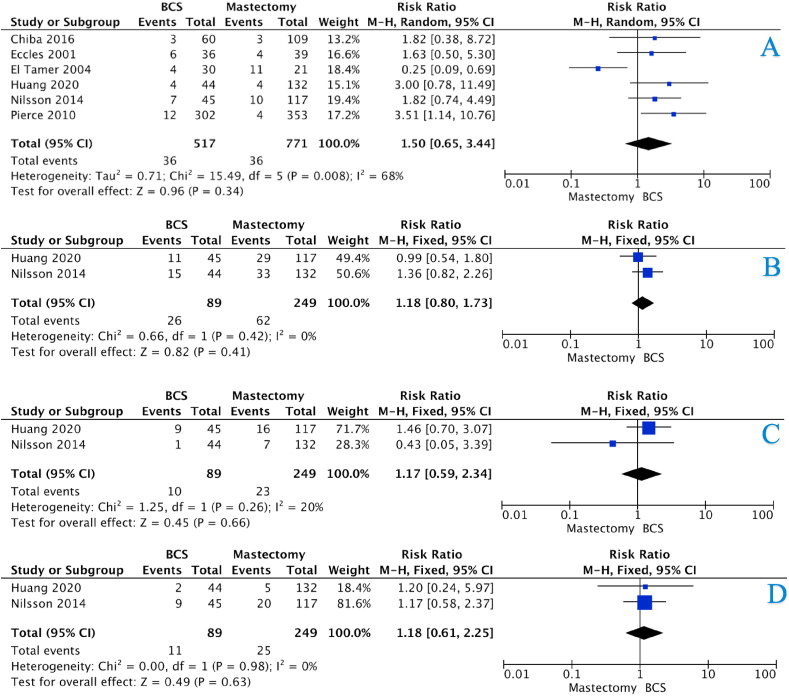
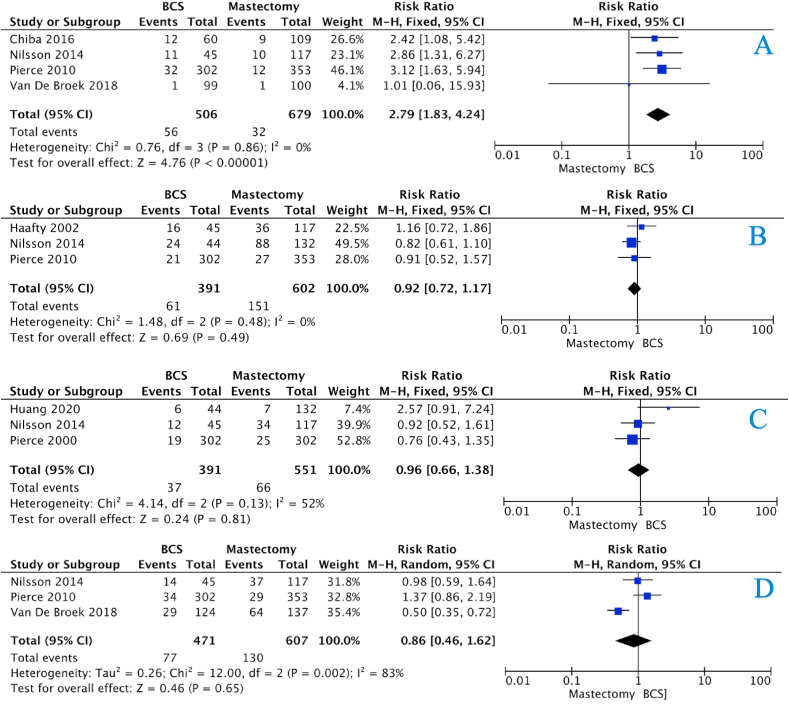
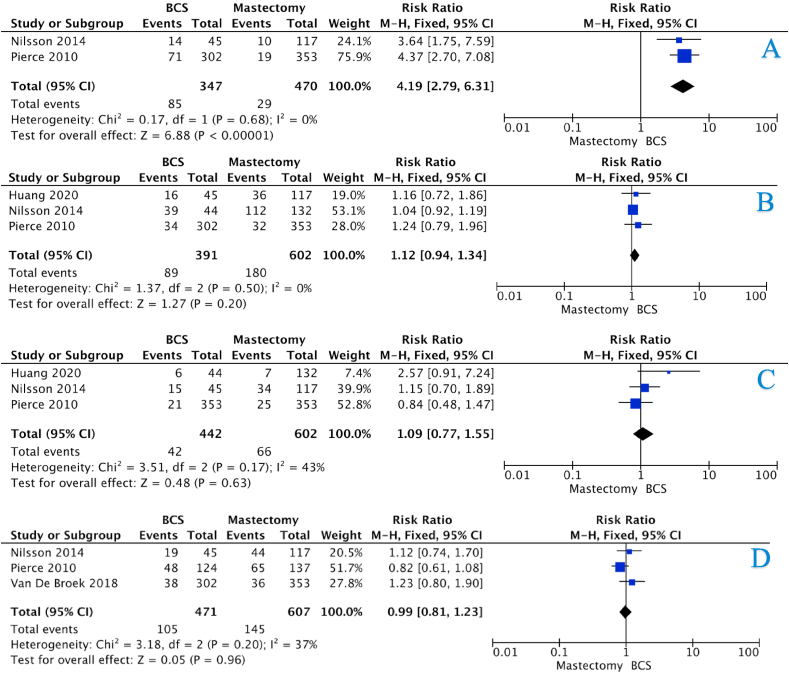
The incidence of LRR for the BCS and mastectomy groups at 5-years were 14.7% vs. 4.8%, 15.5% vs. 4.7% at 10-years, and 27.5% vs. 6.2% at 15-years (all P < 0.001, †) (Table 4). There was an overall increased risk of LRR in those who underwent BCS (HR:4.54, 95%CI: 2.77–7.42, P < 0.001, heterogeneity (I2) = 0%) (Fig. 2A – 3 studies). The risk of LRR was equivocal in those who underwent BCS and mastectomy at 5-years (HR:1.50, 95%CI: 0.65–3.44, P = 0.420, I2 = 68%) (Fig. 3A – 6 studies). However, the risk of LRR increased at 10-years and 15-years respectively ([HR:2.79, 95%CI: 1.83–4.24, P < 0.001, I2 = 0%] & [HR:4.19, 95%CI: 2.79–6.31, P < 0.001, I2 = 0%]) (Fig. 4, Fig. 5A – 4 & 2 studies respectively).
Table 4.
Recurrence and survival data for those undergoing breast conservation surgery and mastectomy at 5-years, 10-years and 15-years.
| Parameter | BCS | Mastectomy | P-value |
|---|---|---|---|
| 5-Year Recurrence and Survival | |||
| LRR | 262 | 36 | <0.001ba |
| Free of recurrence | 1519 | 735 | |
| CLBC | 74 | N/R | N/R |
| Free of CLBC | 552 | N/R | |
| Recurrence | 133 | 62 | 0.031ba |
| Free of recurrence | 585 | 187 | |
| DSS | 85 | 23 | 0.036ba |
| Free of DSS | 498 | 226 | |
| Dead | 101 | 27 | <0.001ba |
| Alive | 602 | 350 | |
| 10-Year Recurrence and Survival | |||
| LRR | 284 | 32 | <0.001ba |
| Free of recurrence | 1545 | 647 | |
| CLBC | 200 | N/R | N/R |
| Free of CLBC | 483 | N/R | |
| Recurrence | 241 | 151 | 0.881a |
| Free of recurrence | 707 | 451 | |
| DSS | 144 | 66 | 0.002ba |
| Free of DSS | 638 | 485 | |
| Dead | 309 | 135 | 0.141a |
| Alive | 1160 | 600 | |
| 15-Year Recurrence and Survival | |||
| LRR | 310 | 29 | <0.001ba |
| Free of recurrence | 817 | 441 | |
| CLBC | 125 | N/R | N/R |
| Free of CLBC | 159 | N/R | |
| Recurrence | 90 | 180 | 0.016ba |
| Free of recurrence | 302 | 422 | |
| DSS | 42 | 66 | 0.444a |
| Free of DSS | 400 | 536 | |
| Dead | 111 | 145 | 0.507a |
| Alive | 389 | 462 | |
LRR; locoregional recurrence, CLBC; contralateral breast cancer, DFS; disease-free survival, DSS; disease-specific survival, N/R; not reported Please note: Data from each study was not available to contribute to each outcome (e.g.: DFS, OS, etc.). Crude numbers are calculated from available data only.
Denotes statistical significance.
Fisher’s exact test.
Fig. 2.
Forest plots illustrating (A) locoregional recurrence, (B) contralateral breast cancer, (C) disease-free survival, (D) disease-specific survival, and (E) overall survival for those undergoing BCS and mastectomy.
Fig. 3.
Forest plots illustrating 5-year (A) locoregional recurrence, (B) disease-free survival, (C) disease-specific survival, and (D) overall survival for those undergoing BCS and mastectomy.
Fig. 4.
Forest plots illustrating 10-year (A) locoregional recurrence, (B) disease-free survival, (C) disease-specific survival, and (D) overall survival for those undergoing BCS and mastectomy.
Fig. 5.
Forest plots illustrating 15-year (A) locoregional recurrence, (B) disease-free survival, (C) disease-specific survival, and (D) overall survival for those undergoing BCS and mastectomy.
3.6. Contralateral breast cancer
Overall, the incidence of contralateral breast cancer (CLBC) for those treated with BCS was 11.8% at 5-years, 29.3% at 10-years, and 45.6% at 15-years (Table 4). The risk of contralateral breast cancer development was equivalent for BCS or mastectomy (HR:1.51, 95%CI: 0.44–5.11, P = 0.510, I2 = 80%) (Figs. 3B–2 studies).
3.7. Disease-free survival
The incidence of disease recurrence for the BCS and mastectomy groups at 5-years were 15.5% vs. 24.9% (P = 0.031, †), 25.4% vs. 25.1% at 10-years (P = 0.881, †), and 23.0% vs. 29.9% at 15-years (P = 0.016, †) (Table 4). The risk of disease recurrence was equivocal for the BCS and mastectomy groups (HR:1.16, 95%CI: 0.78–1.72, P = 0.470, I2 = 44%) (Figs. 2C–3 studies), with no increased risk of disease recurrence after 5-years, 10-years or 15-years (Fig. 3, Fig. 4, Fig. 5B).
3.8. Disease-specific survival
The incidence of disease-specific recurrence for the BCS and mastectomy groups at 5-years were 14.3% vs. 9.2% (P = 0.036, †), 18.4% vs. 12.0% at 10-years (P = 0.002, †), and 9.5% vs. 11.0% at 15-years (P = 0.444, †) (Table 4). The risk of disease-specific recurrence was equivalent for the BCS and mastectomy groups (HR:1.58, 95%CI: 0.79–3.15, P = 0.200, I2 = 0%) (Fig. 2D– studies), with no increased risk of disease recurrence after 5-years, 10-years or 15-years (Fig. 3, Fig. 4, Fig. 5C).
3.9. Overall survival
The incidence of mortality for the BCS and mastectomy groups at 5-years were 14.4% vs. 9.2% (P < 0.001, †), 21.0% vs. 18.4% at 10-years (P = 0.141, †), and 22.2% vs. 23.9% at 15-years (P = 0.507, †) (Table 4). The risk of mortality was equivalent for the BCS and mastectomy groups (HR:1.10, 95%CI: 0.72–1.69, P = 0.660, I2 = 38%) (Fig. 2E– studies), with no increased risk of mortality after 5-years, 10-years or 15-years (Fig. 3, Fig. 4, Fig. 5D).
4. Discussion
In cases of sporadic breast cancer, benchmark practice recommends BCS as the “gold standard” procedure for tumour resection where feasible [40]. The evidence regarding BRCA mutation carriers with breast cancer is less certain. To our knowledge, this comprehensive systematic review and meta-analysis is the largest study evaluating the oncological safety profile of combined BCT versus mastectomy for BRCA mutation carriers following breast cancer diagnosis. The most important finding in this pooled analyses of over 3800 BRCA mutation carriers treated surgically with either BCS or mastectomy was the non-inferior survival analyses with respect to disease-free (DFS), disease-specific (DSS) and overall survival (OS) following up to a decade and a half of follow up. However, evidence of worse locoregional control following BCS overtime was also demonstrated, with a 4.5 times increased risk of LRR in the BCT group. This supports BCS as a valid option to be offered as a primary surgical strategy within BRCA mutation carriers, provided patients are appropriately counselled pre-operatively.
In patients with a family history, current international accepted practice for breast cancer management is to address the risk based on expression of key genes; in those with BRCA1/2 abnormalities, the current advice is to encourage bilateral mastectomy at diagnosis [41]. The data from this meta-analysis demonstrates BCS to have comparable oncological safety to mastectomy in BRCA mutation carriers with regards to survival. There was no survival disadvantage in a strategy encompassing BCT of the index case. Consequently, combined BCT should be discussed as a potential strategy allowing breast conservation following breast cancer diagnosis in BRCA mutation carriers, should the patient prefer such an approach. This is especially true where appropriate screening and surveillance is available as risks of LRR and new cancers are low at approximately 2.18% per year [42].
Oncological resection operates through zero-order kinetics, such that 100% of excised tumour cells are killed [43]. This concept remains true for both BCS and mastectomy, once surgeons successfully achieve clear margins in the setting of BCS. Although the inherent risk of mutation-related breast cancer occurring on the residual breast tissue spared by BCS remains, this analysis suggests this risk to be almost negligible when compared to outcomes following mastectomy. The authors wish to acknowledge that in certain clinical scenarios, such as those of increased tumour burden, diffuse in-situ disease, aggressive pathological characteristics, and in smaller breasts, mastectomy should remain the oncological procedure of choice. Moreover, counselling towards contralateral risk-reducing mastectomy should also be given to BRCA mutation carriers regardless of the patient’s initial choice regarding surgery for their cancer [44].
While seminal work by Fisher et al., demonstrated that long-term survival outcomes for sporadic breast cancer were non-inferior following combined BCT vs. mastectomy [6], this analysis demonstrates inferiority concerning LRR in BRCA patients treated with BCS, and an overt risk of LRR at intermediate- and long-term follow-up. These results are in agreement with a recent review by Co et al. [45] and refute any similar assertion concerning BCS for local control in BRCA mutation carriers. Eight of the included studies reported worse LRR outcomes following BCS relative to mastectomy, despite greater than 97% of patients receiving adjuvant radiotherapy. Valachis et al. previously indicated that combined BCT was initially equivocal in BRCA mutation carriers and sporadic breast cancer patients; however, the former were at an increased risk of LRR 7-years post resection [46], while Biglia et al. also highlighted increased LRR risk at greater than 15 years [47]. Observations from this study are congruent with both reports; and suggest that the clinical utility of mastectomy in achieving long-term locoregional control in BRCA carriers is explicit when compared to combined BCT. Increased LRR rates following BCS within BRCA carriers seems pertinent irrespective of adjuvant radiotherapy prescription, and the authors recommend patient counselling to be tailored as such.
An integral component of BRCA germline mutations is their pivotal role in driving oncogenesis within the younger patient - the median age of breast cancer diagnosis in this pooled analysis was only 41 years. Surgical decision making surrounding hereditary breast cancer must incorporate a myriad of personal, familial and cosmetic patient factors, the essence of which are of greater importance to the younger women [48]. These factors prove crucial in therapeutic and prophylactic surgical decision making. With almost half of the women included in this analyses being aged less than 40-years old, we wish to highlight the overall implication of the low risk of LRR at 10-years and 15-years following BCS. For these women, the incidence of LRR during this interval 5-to-15 years post resection is likely to occur within their 5th or 6th decade of life, long before index diagnoses of several other epithelial cancers including lung, colorectal and oesophageal carcinomas, all of which typically present in the 7th to 8th decades of life [[49], [50], [51]]. For the oncologist, relaying such implications to BRCA mutation carriers at primary diagnoses is of the utmost importance to ensure an accurate timeline of the potential clinical outcomes for the patient in the decades ahead.
4.1. Limitations
This systematic review and meta-analysis suffers from a number of inert limitations; studies included were limited to those published in the English language. Given the ethical implications surrounding BRCA testing and surgical best practice, there were no prospective studies for inclusion evaluating surgical outcomes in BRCA mutation carriers. Consequentially, all included studies were retrospective in nature indicating low-to-moderate levels of evidence. Moreover, patients included in this review underwent surgical resection during 8 decades, with patients recruited to their respective studies from 1959 to 2020; oncological practice has evolved in concordance with our understanding of the molecular properties of breast cancer in this period, which could limit the conclusions drawn from this analysis [52]. Overall, a limited number of studies met inclusion in our meta-analyses. Bilateral mastectomy has not been evaluated as a surgical option in cases of index cancers in this analysis. Difficulty differentiating possible data from studies published from two centres in the United States and the Netherlands may have affected outcomes, however ambiguity concerning this remains.
5. Conclusion
This study contradicts prevailing attitudes that suggest combined BCT confers worse survival when used as an approach to managing breast cancer in BRCA mutation carriers. While, combined BCT is associated with an increased risk of locoregional recurrence compared to mastectomy, irrespective of adjuvant radiotherapy prescription, these recurrences do not appear to adversely impact on survival when managed appropriately. This supports BCS as a valid option to be discussed as a primary surgical strategy allowing breast conservation for BRCA mutation carriers should the patient prefer such an approach. BRCA mutation carriers should be appropriately counselled regarding all surgical options following breast cancer diagnoses and this may allow them achieve the best outcome in an era of personalised medicine.
References
- 1.DeSantis C.E., Ma J., Goding Sauer A., Newman L.A., Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA A Cancer J Clin. 2017;67(6):439–448. doi: 10.3322/caac.21412. [DOI] [PubMed] [Google Scholar]
- 2.Mahdavi M., Nassiri M., Kooshyar M.M., Vakili-Azghandi M., Avan A., Sandry R. Hereditary breast cancer; Genetic penetrance and current status with BRCA. J Cell Physiol. 2019;234(5):5741–5750. doi: 10.1002/jcp.27464. [DOI] [PubMed] [Google Scholar]
- 3.Kuchenbaecker K.B., Hopper J.L., Barnes D.R., Phillips K.A., Mooij T.M., Roos-Blom M.J. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. Jama. 2017;317(23):2402–2416. doi: 10.1001/jama.2017.7112. [DOI] [PubMed] [Google Scholar]
- 4.Gabai-Kapara E., Lahad A., Kaufman B., Friedman E., Segev S., Renbaum P. Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc Natl Acad Sci U S A. 2014;111(39):14205–14210. doi: 10.1073/pnas.1415979111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antoniou A., Pharoah P.D., Narod S., Risch H.A., Eyfjord J.E., Hopper J.L. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72(5):1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher B., Anderson S., Bryant J., Margolese R.G., Deutsch M., Fisher E.R. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 7.Franco P., De Rose F., De Santis M.C., Pasinetti N., Lancellotta V., Meduri B. Omission of postoperative radiation after breast conserving surgery: a progressive paradigm shift towards precision medicine. Clin Transl Radiat Oncol. 2020;21:112–119. doi: 10.1016/j.ctro.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huszno J., Kołosza Z., Grzybowska E. BRCA1 mutation in breast cancer patients: analysis of prognostic factors and survival. Oncol Lett. 2019;17(2):1986–1995. doi: 10.3892/ol.2018.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao J.Q., Olson R.A., Tyldesley S.K. Comparison of recurrence and survival rates after breast-conserving therapy and mastectomy in young women with breast cancer. Curr Oncol. 2013;20(6):e593–e601. doi: 10.3747/co.20.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu K.-D., Li S., Shao Z.-M. Different annual recurrence pattern between lumpectomy and mastectomy: implication for breast cancer surveillance after breast-conserving surgery. Oncol. 2011;16(8):1101–1110. doi: 10.1634/theoncologist.2010-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zumsteg Z.S., Morrow M., Arnold B., Zheng J., Zhang Z., Robson M. Breast-conserving therapy achieves locoregional outcomes comparable to mastectomy in women with T1-2N0 triple-negative breast cancer. Ann Surg Oncol. 2013;20(11):3469–3476. doi: 10.1245/s10434-013-3011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaas R., Verhoef S., Wesseling J., Rookus M.A., Oldenburg H.S., Peeters M.J. Prophylactic mastectomy in BRCA1 and BRCA2 mutation carriers: very low risk for subsequent breast cancer. Ann Surg. 2010;251(3):488–492. doi: 10.1097/SLA.0b013e3181c3c36d. [DOI] [PubMed] [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. Jama. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 15.Ga Wells B.S., O’Connell D., Peterson J., Welch V., Losos M., Tugwell P. 2019. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Google Scholar]
- 16.Brekelmans C.T., Tilanus-Linthorst M.M., Seynaeve C., vd Ouweland A., Menke-Pluymers M.B., Bartels C.C. Tumour characteristics, survival and prognostic factors of hereditary breast cancer from BRCA2-, BRCA1- and non-BRCA1/2 families as compared to sporadic breast cancer cases. Eur J Canc. 2007;43(5):867–876. doi: 10.1016/j.ejca.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Cao W., Xie Y., He Y., Li J., Wang T., Fan Z. Risk of ipsilateral breast tumor recurrence in primary invasive breast cancer following breast-conserving surgery with BRCA1 and BRCA2 mutation in China. Breast Canc Res Treat. 2019;175(3):749–754. doi: 10.1007/s10549-019-05199-8. [DOI] [PubMed] [Google Scholar]
- 18.Chiba A., Hoskin T.L., Hallberg E.J., Cogswell J.A., Heins C.N., Couch F.J. Impact that timing of genetic mutation diagnosis has on surgical decision making and outcome for BRCA1/BRCA2 mutation carriers with breast cancer. Ann Surg Oncol. 2016;23(10):3232–3238. doi: 10.1245/s10434-016-5328-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drooger J., Akdeniz D., Pignol J.P., Koppert L.B., McCool D., Seynaeve C.M. Adjuvant radiotherapy for primary breast cancer in BRCA1 and BRCA2 mutation carriers and risk of contralateral breast cancer with special attention to patients irradiated at younger age. Breast Canc Res Treat. 2015;154(1):171–180. doi: 10.1007/s10549-015-3597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eccles D., Simmonds P., Goddard J., Coultas M., Hodgson S., Lalloo F. Familial breast cancer: an investigation into the outcome of treatment for early stage disease. Fam Cancer. 2001;1(2):65–72. doi: 10.1023/a:1013867917101. [DOI] [PubMed] [Google Scholar]
- 21.Foulkes W.D., Wong N., Brunet J.S., Bégin L.R., Zhang J.C., Martinez J.J. Germ-line BRCA1 mutation is an adverse prognostic factor in Ashkenazi Jewish women with breast cancer. Clin Canc Res. 1997;3(12):2465. [PubMed] [Google Scholar]
- 22.Garcia-Etienne C.A., Barile M., Gentilini O.D., Botteri E., Rotmensz N., Sagona A. Breast-conserving surgery in BRCA1/2 mutation carriers: are we approaching an answer? Ann Surg Oncol. 2009;16(12):3380–3387. doi: 10.1245/s10434-009-0638-7. [DOI] [PubMed] [Google Scholar]
- 23.Haffty B.G., Harrold E., Khan A.J., Pathare P., Smith T.E., Turner B.C. Outcome of conservatively managed early-onset breast cancer by BRCA1/2 status. Lancet. 2002;359(9316):1471–1477. doi: 10.1016/S0140-6736(02)08434-9. [DOI] [PubMed] [Google Scholar]
- 24.Huang X., Cai X.-Y., Liu J.-Q., Hao W.-W., Zhou Y.-D., Wang X. Breast-conserving therapy is safe both within BRCA1/2 mutation carriers and noncarriers with breast cancer in the Chinese population. Gland Surg. 2020;9(3):775–787. doi: 10.21037/gs-20-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirova Y.M., Savignoni A., Sigal-Zafrani B., de La Rochefordiere A., Salmon R.J., This P. Is the breast-conserving treatment with radiotherapy appropriate in BRCA1/2 mutation carriers? Long-term results and review of the literature. Breast Canc Res Treat. 2010;120(1):119–126. doi: 10.1007/s10549-009-0685-6. [DOI] [PubMed] [Google Scholar]
- 26.Metcalfe K., Lynch H.T., Ghadirian P., Tung N., Kim-Sing C., Olopade O.I. Risk of ipsilateral breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Canc Res Treat. 2011;127(1):287–296. doi: 10.1007/s10549-010-1336-7. [DOI] [PubMed] [Google Scholar]
- 27.Nilsson M.P., Hartman L., Kristoffersson U., Johannsson O.T., Borg A., Henriksson K. High risk of in-breast tumor recurrence after BRCA1/2-associated breast cancer. Breast Canc Res Treat. 2014;147(3):571–578. doi: 10.1007/s10549-014-3115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pierce L.J., Strawderman M., Narod S.A., Oliviotto I., Eisen A., Dawson L. Effect of radiotherapy after breast-conserving treatment in women with breast cancer and germline BRCA1/2 mutations. J Clin Oncol. 2000;18(19):3360–3369. doi: 10.1200/JCO.2000.18.19.3360. [DOI] [PubMed] [Google Scholar]
- 29.Pierce L.J., Levin A.M., Rebbeck T.R., Ben-David M.A., Friedman E., Solin L.J. Ten-year multi-institutional results of breast-conserving surgery and radiotherapy in BRCA1/2-associated stage I/II breast cancer. J Clin Oncol. 2006;24(16):2437–2443. doi: 10.1200/JCO.2005.02.7888. [DOI] [PubMed] [Google Scholar]
- 30.Pierce L.J., Phillips K.A., Griffith K.A., Buys S., Gaffney D.K., Moran M.S. Local therapy in BRCA1 and BRCA2 mutation carriers with operable breast cancer: comparison of breast conservation and mastectomy. Breast Canc Res Treat. 2010;121(2):389–398. doi: 10.1007/s10549-010-0894-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robson M., Gilewski T., Haas B., Levin D., Borgen P., Rajan P. BRCA-associated breast cancer in young women. J Clin Oncol. 1998;16(5):1642–1649. doi: 10.1200/JCO.1998.16.5.1642. [DOI] [PubMed] [Google Scholar]
- 32.Robson M., Levin D., Federici M., Satagopan J., Bogolminy F., Heerdt A. Breast conservation therapy for invasive breast cancer in ashkenazi women with BRCA gene founder mutations. J Natl Cancer Inst: J Natl Cancer Inst. 1999;91(24):2112–2117. doi: 10.1093/jnci/91.24.2112. [DOI] [PubMed] [Google Scholar]
- 33.Robson M., Svahn T., McCormick B., Borgen P., Hudis C.A., Norton L. Appropriateness of breast-conserving treatment of breast carcinoma in women with germline mutations in BRCA1 or BRCA2: a clinic-based series. Cancer. 2005;103(1):44–51. doi: 10.1002/cncr.20728. [DOI] [PubMed] [Google Scholar]
- 34.Turner B.C., Harrold E., Matloff E., Smith T., Gumbs A.A., Beinfield M. BRCA1/BRCA2 germline mutations in locally recurrent breast cancer patients after lumpectomy and radiation therapy: implications for breast-conserving management in patients with BRCA1/BRCA2 mutations. J Clin Oncol. 1999;17(10):3017–3024. doi: 10.1200/JCO.1999.17.10.3017. [DOI] [PubMed] [Google Scholar]
- 35.van den Broek A.J., Schmidt M.K., van ’t Veer L.J., Oldenburg H.S.A., Rutgers E.J., Russell N.S. Prognostic impact of breast-conserving therapy versus mastectomy of BRCA1/2 mutation carriers compared with noncarriers in a consecutive series of young breast cancer patients. Ann Surg. 2019;270(2):364–372. doi: 10.1097/SLA.0000000000002804. [DOI] [PubMed] [Google Scholar]
- 36.Yamauchi H., Okawa M., Yokoyama S., Nakagawa C., Yoshida R., Suzuki K. High rate of occult cancer found in prophylactic mastectomy specimens despite thorough presurgical assessment with MRI and ultrasound: findings from the Hereditary Breast and Ovarian Cancer Registration 2016 in Japan. Breast Canc Res Treat. 2018;172(3):679–687. doi: 10.1007/s10549-018-4953-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye F., Huang L., Lang G., Hu X., Di G., Shao Z. Outcomes and risk of subsequent breast events in breast-conserving surgery patients with BRCA1 and BRCA2 mutation. Cancer Med. 2020;9(5):1903–1910. doi: 10.1002/cam4.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoon K.-H., Chae S., Kang E., Shin H.-C., Kim J.H., Kim I.A. Contralateral breast cancer and ipsilateral breast tumor recurrence in BRCA1/2 carriers and non-carriers at high-risk of hereditary breast cancer. J Breast Cancer. 2019;22(4):587–598. doi: 10.4048/jbc.2019.22.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bernstein-Molho R, Laitman Y, Galper S, Jacobson G, Boursi B, Gal-Yam EN, et al. Locoregional treatments and ipsilateral breast cancer recurrence rates in <em>BRCA1/2</em> mutation carriers. Int J Radiat Oncol Biol Phys. [DOI] [PubMed]
- 40.Cardoso F., Kyriakides S., Ohno S., Penault-Llorca F., Poortmans P., Rubio I.T. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30(8) doi: 10.1093/annonc/mdz173. 1194-220, et al. [DOI] [PubMed] [Google Scholar]
- 41.Balman ODe J., Rubio I.T., Cardoso F. BRCA in breast cancer: ESMO clinical practice guidelines. Ann Oncol. 2011;22(31–34) doi: 10.1093/annonc/mdr373. [DOI] [PubMed] [Google Scholar]
- 42.Kotsopoulos J. BRCA mutations and breast cancer prevention. Cancers. 2018;10(12):524. doi: 10.3390/cancers10120524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pollock R.E.M.D. Hamilton; 2003. Principles of surgical Oncology.https://www.ncbi.nlm.nih.gov/books/NBK13204/ Available from: [Google Scholar]
- 44.Trimboli R.M., Schiaffino S., Sardanelli F. In BRCA mutation carriers breast conserving surgery may not be the best choice. Breast Canc Res Treat. 2019;178(1):245–246. doi: 10.1007/s10549-019-05373-y. [DOI] [PubMed] [Google Scholar]
- 45.Co M., Liu T., Leung J., Li C.H., Tse T., Wong M. Breast conserving surgery for BRCA mutation carriers—a systematic review. Clin Breast Canc. 2020;20(3):e244–e250. doi: 10.1016/j.clbc.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 46.Valachis A., Nearchou A.D., Lind P. Surgical management of breast cancer in BRCA-mutation carriers: a systematic review and meta-analysis. Breast Canc Res Treat. 2014;144(3):443–455. doi: 10.1007/s10549-014-2890-1. [DOI] [PubMed] [Google Scholar]
- 47.Biglia N., D’Alonzo M., Sgro L.G., Tomasi Cont N., Bounous V., Robba E. Breast cancer treatment in mutation carriers: surgical treatment. Minerva Ginecol. 2016;68(5):548–556. [PubMed] [Google Scholar]
- 48.Mau C., Untch M. Prophylactic surgery: for whom, when and how? Breast Care. 2017;12(6):379–384. doi: 10.1159/000485830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torre L.A., Siegel R.L., Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1–19. doi: 10.1007/978-3-319-24223-1_1. [DOI] [PubMed] [Google Scholar]
- 50.Center M.M., Jemal A., Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomark Prev. 2009;18(6):1688–1694. doi: 10.1158/1055-9965.EPI-09-0090. [DOI] [PubMed] [Google Scholar]
- 51.Wang Q.-L., Xie S.-H., Wahlin K., Lagergren J. Global time trends in the incidence of esophageal squamous cell carcinoma. Clin Epidemiol. 2018;10:717–728. doi: 10.2147/CLEP.S166078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ades F., Tryfonidis K., Zardavas D. The past and future of breast cancer treatment-from the papyrus to individualised treatment approaches. Ecancermedicalscience. 2017;11:746. doi: 10.3332/ecancer.2017.746. [DOI] [PMC free article] [PubMed] [Google Scholar]