Abstract
A 53-year-old male patient was presented to our institution with the clinical picture of biventricular failure. The echocardiogram revealed congenitally corrected transposition of the great arteries, dextrocardia with situs solitus, atrioventricular discordance and ventriculoatrial discordance, severe systemic and mitral valves regurgitation, and severe pulmonary hypertension (mean pulmonary artery pressure: 51 mm Hg). He underwent heart–lung transplant. He was discharged on postoperative day 25 with left ventricular ejection fraction of 60%–65%, and with oxygen independency.
Keywords: Biventricular failure, congenitally corrected transposition of the great arteries, dextrocardia, heart–lung transplant
Introduction
Congenitally corrected transposition of the great arteries (cc-TGA) is a rare type of congenital heart disease with an incidence of less than 1% of infants with congenital heart disease.1 Other terms have been used to describe this congenital anomaly such as L-TGA, double discordance, and ventricular inversion. The first heart–lung transplantation (HLTx) was performed at Stanford University in 1981.2 Pulmonary vascular disease and cystic fibrosis were the main indications for HLTx initially. However, in the recent years, the indication has been expanded to include congenital heart disease and idiopathic pulmonary artery hypertension.3 We report a rare case of cc-TGA with dextrocardia presenting with biventricular failure and underwent heart–lung transplant (HLTx).
Case description
A 53-year-old male patient presented to our institution with the clinical picture of biventricular failure. The echocardiogram (video) revealed cc-TGA, dextrocardia with situs solitus, atrioventricular discordance and ventriculoatrial discordance (Figure 1(a) and (b)), severe systemic and mitral valves regurgitation, and severe pulmonary hypertension (PH) (mean pulmonary artery pressure: 51 mm Hg). His past medical history was remarkable for complete heart block mandating pacemaker implantation, and atrial fibrillation. The patient was evaluated and was deemed a candidate for HLTx. A 25-year-old male donor who died of hemorrhagic stroke became available and the en bloc heart–lung was recovered by an experienced team (Figure 2(a)).
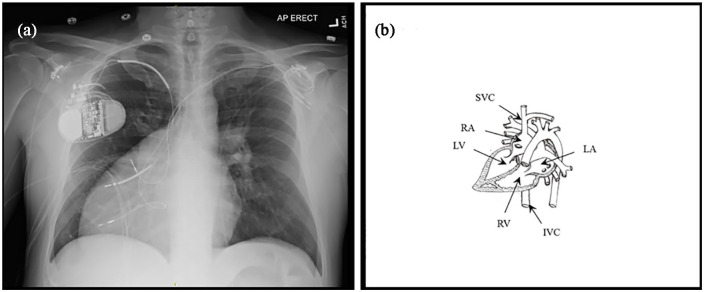
Figure 1.
(a) Preoperative chest radiograph showing the dextrocardia anomaly and (b) diagram showing anatomy of the congenitally corrected transposition of the great arteries with dextrocardia and situs solitus. RV: right ventricle, LV: left ventricle, RA: right atrium, LA: left atrium, IVC: inferior vena cava, SVC: superior vena cava.
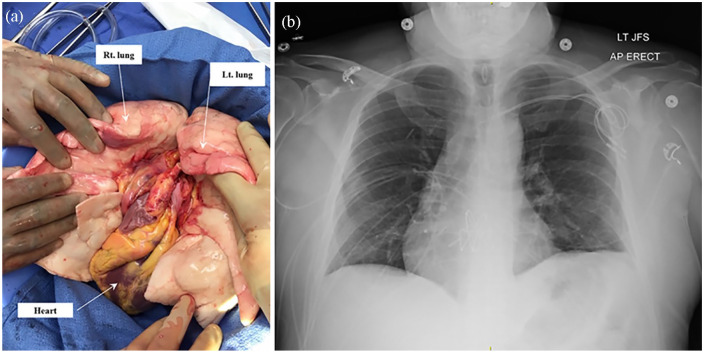
Figure 2.
(a) Intraoperative photo showing the en bloc heart and lung after recovery and (b) postoperative chest radiograph showing the transplanted heart and lungs in the proper position.
The patient underwent HLTx via a clamshell incision. Because of hemodynamic instability, we commenced cardiopulmonary bypass (CPB) with an aortic cannula and a dual-stage venous cannula placed in the right atrium. After the heart was decompressed, the venous cannulation was converted from atrial into bi-caval configuration.
Cardiectomy was performed, including complete removal of right and left atria, leaving a stump of atrial tissue on the inferior vena cava (IVC). The recipient trachea was resected one tracheal ring above the carina. The left recurrent laryngeal and the vagus nerves were identified and preserved during dissection. The two phrenic nerves were identified and protected by leaving pericardial flaps on each side. Tracheal anastomosis was performed first followed by the IVC, aorta and then the superior vena cava (SVC). The aortic cross-clamp was removed and the patient was safely weaned from the CPB. Inotropic support was initiated using dobutamine at 5 μg/kg/min and epinephrine at 0.05 μg/kg/min. At the end of the procedure, the patient was taken to the intensive care unit in a stable condition.
The ischemic time was 296 min and the CPB time was 280 min.
The standard immunosuppression protocol was initiated:
Methylprednisone 1 g IV intraoperatively, then 125 mg bid for the first day, then 1 mg/kg/day in divided doses for 3 days, then prednisone 20 mg daily for 4 weeks, then 10 mg daily.
Mycophenolate 1 g IV intraoperatively, followed by 500 mg bid. Dose was temporarily increased to 1000 mg bid on postoparative day (POD) 18 with suspicion of humoral rejection.
Single dose induction with alemtuzumab 30 mg IV administered on POD 1.
Tacrolimus 0.15 mg/kg/day dose titrated to achieve level of 6–10 μg/L.
On POD 2, the patient was extubated and ambulation was initiated. The patient progressed steadily and ready for discharge; however, on POD 18, he developed pleural effusion and lung infiltrates. Transbronchial lung biopsy revealed focal vasculitis. The diagnosis of possible humoral rejection was considered and the patient received a single dose of 500 mg methylprednisone, and five daily sessions of plasma exchange. Also he developed gastroparesis which completely resolved before discharge. Postoperative chest radiograph showed the heart and lungs in the proper position (Figure 2(b)). The patient was discharged on POD 25 with left ventricular ejection fraction (LVEF) of 60%–65% and with oxygen independency.
Discussion
Over the past 20 years, the number of HLTx has been decreasing from 145 in 1999 to 45 in 2019 and gradually replaced with bilateral lung transplant (BLTx).4 It has been an ongoing debate on whether to perform HLTs or BLTx for patients with PH.5 HLTx is recommended to patients with congenital heart disease and Eisenmenger syndrome, severe right ventricular (RV) dysfunction, and severe left ventricular dysfunction. On the other hand, BLTx provides comparable outcomes; moreover, it offers the advantage of better organ sharing. Given the long waiting time, patients with PH and simple cardiac anomalies such as atrial septal defect, patent ductus arteriosus, or perimembranous ventricular septal defect can be successfully managed with a combination of BLTx and cardiac repair. The cut-off values for BLTx can be approximated at 10%–25% right ventricular ejection fraction (RVEF) and 32%–55% LVEF. For values less than this cut-off, HLTx is recommended.5
We are reporting a successful HLTx in a patient with cc-TGA and dextrocardia. Our patient was presented with advanced cardiac problems including severe PH, severe systemic and mitral valves regurgitation, complete heart block, and biventricular failure. In our patient, the RV was the systemic ventricle and its failure is attributed to the severely incompetent systemic valve and the afterload systemic pressure. Hence, BLTx will not improve the condition of the failing systemic RV and the HLTx was deemed the suitable option. Based on the aforementioned findings, the multidisciplinary team recommended HLTx procedure. HLTx is technically a well-established surgical procedure; however, our case demonstrated unique surgical challenges due to the complexity of the congenital cardiac anomaly (cc-TGA and dextrocardia) of the recipient. The combination of the cc-TGA and dextrocardia is extremely rare. To the best of our knowledge, only one case has been reported by Takemoto et al.6 in the literature describing a similar complexity. This combination posed challenges for both the surgeon and anesthesiologist during the preoperative preparation and during the transplant procedure. The strategy of the approach, cannulation, and the technique was quite different from the standard HLTx. Giving the fact that both the SVC and IVC were presented posteriorly, venous cannulation was initiated via dual-stage venous cannula placed in the right atrium transitioned to bi-caval configuration after decompressing the heart.
Management of HLTx recipients postoperatively is sometimes a challenge to the transplant team due to the combined heat and lung transplant. Postoperative care is more or less the same after HLTx as after lung transplant (LTx). Most of the postoperative complications such as infections and acute and chronic rejection are mainly attributed to the lungs and not the heart.7
The immunosuppression protocol after HLTx is similar to that after LTx. The follow-up of HLTx recipients includes lung function tests, chest imaging, and outpatient clinical assessments. The optimal interval between surveillance bronchoscopies after HLTx is still debatable, and the majority of the centers usually apply their protocol designed for lung transplant recipients. In our patient, we followed the immunosuppression protocol in lung transplant. The protocol was given in details in the case report section. According to the 2016 ISHLT report included the data of patients underwent HLTx in 2004–2014, the survival rates at 1, 2, 5, and 10 years are 63%, 52%, 45%, and 32%, respectively, and a median survival of 5.8 years.8 As of today, 15 months postoperatively, the patient is doing well.
Conclusion
This case highlights the complexity of preoperative and intraoperative management of cc-TGA; moreover, it demonstrates the challenges which may face the surgeons and anesthesiologists during the procedure of HLTx.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient for his anonymized information to be published in this article.
ORCID iD: Magdy M El-Sayed Ahmed  https://orcid.org/0000-0002-0729-8829
https://orcid.org/0000-0002-0729-8829
Supplemental material: Supplemental material for this article is available online.
References
- 1. Bjarke BB, Kidd BSL. Congenitally corrected transposition of the great arteries: a clinical study of 101 cases. Acta Paediatr Scand 1976; 65: 153–160. [DOI] [PubMed] [Google Scholar]
- 2. Reitz BA, Wallwork JL, Hunt SA, et al. Heart-lung transplantation: successful therapy for patients with pulmonary vascular disease. N Engl J Med 1982; 306: 557–564. [DOI] [PubMed] [Google Scholar]
- 3. Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the international society for heart and lung transplantation: thirty-first adult lung and heart-lung transplant report–2014; focus theme: retransplantation. J Heart Lung Transplant 2014; 33(10): 1009–1024. [DOI] [PubMed] [Google Scholar]
- 4. Hill C, Maxwell B, Boulate D, et al. Heart-lung vs. double-lung transplantation for idiopathic pulmonary arterial hypertension. Clin Transplant 2015; 29(12): 1067–1075. [DOI] [PubMed] [Google Scholar]
- 5. Olland A, Falcoz P, Canuet M, et al. Should we perform bilateral-lung or heart-lung transplantation for patients with pulmonary hypertension? Interact Cardiovasc Thorac Surg 2013; 17: 166–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takemoto M, Aoki K, Noma M, et al. Congenitally corrected transposition of the great arteries with dextrocardia. Clin Cardiol 2006; 29(1): 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Idrees JJ, Pettersson GB. State of the art of combined heart-lung transplantation for advanced cardiac and pulmonary dysfunction. Curr Cardiol Rep 2016; 18(4): 36. [DOI] [PubMed] [Google Scholar]
- 8. Yusen RD, Edwards LB, Dipchand AI, et al. The registry of the international society for heart and lung transplantation: thirty-third adult lung and heart–lung transplant report—2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant 2016; 35: 1170–1184. [DOI] [PubMed] [Google Scholar]