Abstract
The tumor stroma plays a relevant role in the initiation and evolution of solid tumors. Tumor-stroma ratio (TSR) is a histological feature that expresses the proportion of the stromal component that surrounds cancer cells. In different studies, the TSR represents a potential prognostic factor: a rich stroma in tumor tissue can promote invasion and aggressiveness. The aim of this study was to evaluate the reproducibility and determine the interobserver agreement in the TSR score. The stromal estimate was evaluated in patients diagnosed with colorectal adenocarcinoma (CRA), who underwent surgical resection. We also evaluated age, gender, and other anatomopathological features. Tumor-stroma ratio was calculated based on the slide used in routine diagnostic pathology to determine the T-status. Stromal percentages were separated into 2 categories: ⩽50%—low stroma and >50%—high stroma. The interobserver agreement in the TSR scoring was evaluated among 4 pathologists at different stages of professional experience, using 2 different ways to learn the scoring system. In total, 98 patients were included in this study; 54.1% were male, with a mean age of 61.9 years. Localized disease was diagnosed in 60.2% of patients. Stromal-poor CRA was predominant. The concordance between the TSR percentages of the 4 pathologists was substantial (Kappa > 0.6). There was greater agreement among pathologists for stromal-poor tumors. Substantial agreement and high reproducibility were observed in the determination of TSR score. The TSR score is feasible, suggesting that the presented methodology can be used to facilitate the determination of the stromal proportion of potential prognostic factor.
Keywords: Tumor-stroma ratio, interobserver variability, agreement, colorectal cancer, prognostic factor
Introduction
The tumor, node, metastasis (TNM) staging system is the current and most widely used method for classifying the anatomical characteristics of solid tumor propagation.1-3 It is considered the most important prognostic factor, also guiding the choice of the ideal cancer treatment.4,5
Current research is increasingly focusing on establishing new prognostic factors, investigating their relationship with aggressive cancer phenotypes already known, with consequent more effective therapeutic strategies. Over the last decade, recent models include the tumor-host interface and the role of the stroma tissue.6-8 The tumor microenvironment and the tumor-host interaction are represented in an environment that includes cancer cells and the stroma tissue, which is composed of different types of cells, such as fibroblasts, myofibroblasts, endothelial cells, immune cells, and extracellular matrix.9
The tumor stroma has been identified as an important determinant of initiation and progression in many solid cancers.10,11 The stroma facilitates the survival and proliferation of neoplastic cells and promotes epithelial-mesenchymal transition (EMT), and local and metastatic dissemination.12,13
Tumor-stroma ratio (TSR) is a histological feature that expresses the value of the stromal component that surrounds cancer cells, based on the morphological evaluation of tissue sections, stained with hematoxylin and eosin (H&E).14,15 Tumor-stroma ratio has been shown to be a prognostic factor in several types of malignant epithelial neoplasms, including colon,6,13,16,17 breast,18-20 and esophageal cancers.21 Epithelial malignant neoplasms from patients with adverse prognosis have been documented to show a high proportion of stroma (>50% stroma = high stroma), whereas tumors with abundant carcinoma tissue (⩽50% stroma = low stroma) are associated with a better prognosis.7,9,10,13,14,16,18,20
These data suggest that TSR may be an important and independent prognostic factor. For the incorporation of the stromal estimation into the clinical practice, the TSR quantification needs to be standardized. Various independent groups have used a similar method for scoring the TSR.12,22–30
The present study aimed to evaluate the reproducibility and to determine the interobserver agreement of the TSR assessment using the proposed methods by the international working groups.
Materials and Methods
The study was approved by the local Ethics Committee (registration: 03283218.6.0000.5183) of the Lauro Wanderley University Hospital of the Federal University of Paraíba.
The stromal estimate was evaluated in patients diagnosed with colorectal adenocarcinomas (CRAs) from patients who underwent surgical resection, in an oncology hospital, in the state of Paraíba, Brazil, from 2017 to 2018. Patients undergoing neoadjuvant pretreatment were excluded.
Epidemiological variables corresponded to the patient’s age and gender, which were collected from the medical records. The anatomopathological variables were obtained from the reanalysis of the histological slides of the surgical specimen, as well as collected from the anatomopathological report, including topography; histological type; histological grade; depth of neoplastic invasion (T-status); presence of tumor budding; and perineural invasion, angiolymphatic invasion, lymph node metastasis, and distant metastasis.
The interobserver agreement in the estimation of the TSR was assessed among 4 pathologists who had clinical experience varying from 1 to 20 years. Two pathologists had more than 15 years of professional work time (senior pathologist 1 [S.P.1] and senior pathologist 2 [S.P.2]), 1 pathologist had 2 years of professional activity (beginner pathologist [B.P.]), and 1 pathologist had 5 years of professional activity (trained pathologist [T.P.]). The T.P. was trained and certified by e-learning as part of the “Uniform Noting for International Application of the Tumor-Stroma Ratio as Easy Diagnostic Tool” study. S.P.1/S.P.2 and B.P. participated in a brief session detailing the proposed methodology for stromal estimation scoring. Each pathologist then independently reviewed each slide in a blinded manner and scored the TSR.
Tumor-stroma ratio
Tumor-stroma ratio was calculated based on the slide used in routine diagnostic pathology to determine the T-status. Hematoxylin and eosin stained tissue sections from the primary tumor with 4 µm thickness were analyzed by conventional microscopy.
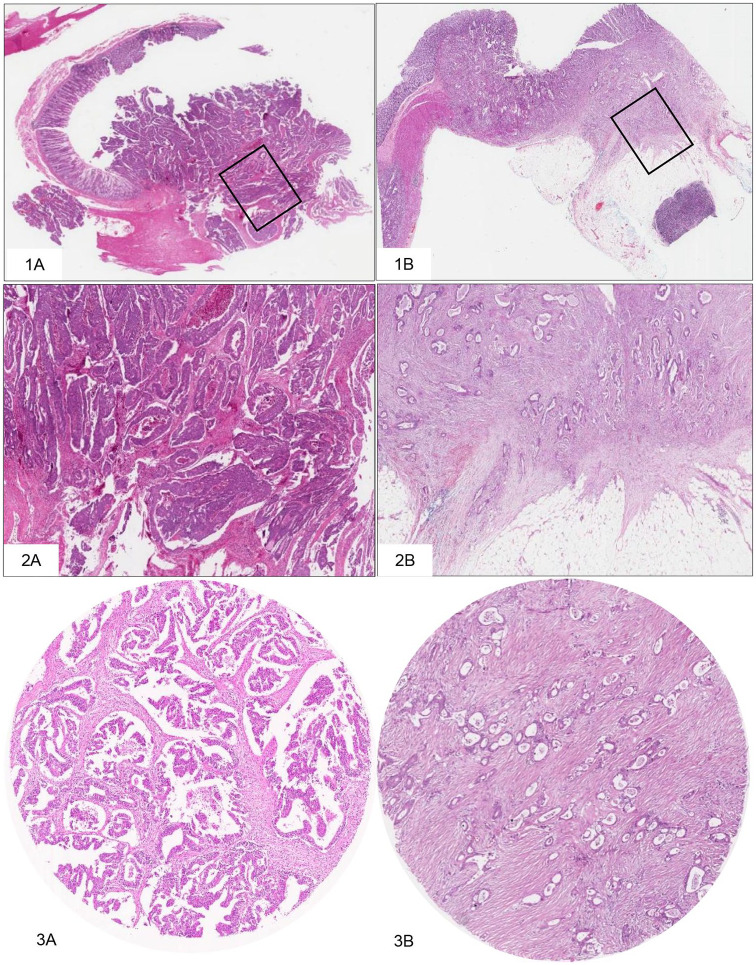
Using a magnification of 2.5× to 5×, regions with a greater number of visible stroma were selected. One area with both tumor and stromal tissues within this vision site was selected using a 10× objective. The tumor cells should be visible on all 4 sides of the selected image field. The amount of stroma tissue was estimated per 10% increment (10%, 20%, 30%, etc) per image field. For statistical analysis, stromal ratio groups were divided into stroma-high and stroma-low groups. Stroma-high tumors were defined as those with >50% stromal area, and stroma-low with ⩽50% stromal area in the histological section.6,14,15 (Figure 1).
Figure 1.
Scoring the TSR.
1. Histological slide of greater depth of invasion—1A: Case 21, 1B: Case 8. 2. Selection of the area with the highest amount of stroma—2A: Case 21, 2B: Case 8. 3. Estimation of stromal percentage, with tumor cells present at all borders of the image field—3A: Case 21, TSR ⩽ 50%, stroma-low; 3B: Case 8, TSR > 50%, stroma-high. Images displaying the microscopic view: H&E 2× objective, 4× objective, and 10× objective. H&E indicates hematoxylin and eosin; TSR, tumor-stroma ratio.
Even if there was only 1 image field with a stroma-high score, this image field was decisive for the classification. In the presence of a doubtful area of high stroma, the total composition of the entire tissue section, using a 2.5× to 5× objective, was considered for the classification of the case.14
Stromal cells in areas with crushing, necrosis, and inflammation artifacts were not scored. In tumors with a mucinous component, the area with mucin was visually excluded from the score, as well as major vascular structures and smooth muscle tissue. Nerves, minor vascular structures, and lymphocytic infiltration were not excluded from the stromal compartment.14
The interobserver agreement for TSR assessment, reported as categorical data, was determined using the Kappa concordance index and intraclass correlation coefficient (ICC).
Results
The study involved 98 patients with a mean age of 61.9 years, and 54.1% were male. The distal colon (including descending colon, sigmoid, and rectal colon) was the most common topography (75.5%) and 92.3% of adenocarcinomas had a moderately differentiated histological grade. T3 status was found in 75.5% of the cases. Perineural invasion was observed in 41%, angiolymphatic invasion in 32%, and lymph node metastasis was present in 41% of the cases.
The distribution in the prognostic stage groups was as follows: Stage 0: 0%, Stage 1: 11.2%, Stage 2: 49%, Stage 3: 34.7%, and Stage 4: 5.1%. Therefore, localized stage was diagnosed in 60.2% and advanced disease (regional and distant) in 39.81% of the patients.
Stromal percentages were separated into 2 categories: stromal percentage ⩽50%—stroma low, stromal percentage >50%—stroma high. The number of cases in each category by pathologist is shown in Table 1.
Table 1.
Number of cases by category of stromal tumor infiltrating.
| Pathologists | TSR ⩽ 50% | TSR > 50% | ||
|---|---|---|---|---|
| n | % | n | % | |
| T.P. | 54 | 55.1 | 44 | 44.9 |
| S.P.1 | 57 | 58.2 | 41 | 41.8 |
| S.P.2 | 66 | 67.3 | 32 | 32.7 |
| B.P. | 50 | 51.0 | 48 | 49.0 |
Abbreviations: B.P., beginner pathologist; S.P.1, senior pathologist 1; S.P.2, senior pathologist 2; T.P., trained pathologist; TSR, tumor-stroma ratio.
The agreement among the pathologists ranged from substantial to almost perfect (Kappa values: 0.67-0.81), with a greater agreement between the T.P. and the pathologists with more professional work time. The ICC value for consistency and the ICC value for agreement were above 0.8.
Comparing stromal estimates between T.P. and S.P.1, there was an agreement of 94.4% for the registration of TSR ⩽ 50% and 86.4% in cases of TSR > 50%. Between T.P. and S.P.2, there was 100% agreement in the TSR record ⩽ 50% and 72.7% agreement in the cases of TSR > 50%. Finally, between B.P. and T.P., there was 81.5% agreement with TSR ⩽ 50% and 86.4% agreement with TSR > 50%. Overall, there was greater agreement among pathologists for stroma-low tumors (Table 2).
Table 2.
Absolute and relative frequency of stromal estimation and interobserver variation.
| Pathologists | T.P. | Total | Kappa | ICC consistencya | ICC agreementb | ||||
|---|---|---|---|---|---|---|---|---|---|
| ⩽50% | >50% | ||||||||
| n | % | n | % | n | % | ||||
| S.P.1 | |||||||||
| ⩽50% | 51 | 94.4 | 6 | 13.6 | 57 | 58.2 | 0.813 | 0.882 (0.823-0.921)* |
0.875 (0.808-0.918)* |
| >50% | 3 | 5.6 | 38 | 86.4 | 41 | 41.8 | |||
| S.P.2 | |||||||||
| ⩽50% | 54 | 100 | 12 | 27.3 | 66 | 67.3 | 0.746 | 0.877 (0.816-0.917)* |
0.823 (0.471-0.919)* |
| >50% | 0 | 0 | 32 | 72.7 | 32 | 32.7 | |||
| B.P. | |||||||||
| ⩽50% | 44 | 81.5 | 6 | 13.6 | 50 | 51 | 0.673 | 0.848 (0.773-0.898)* |
0.840 (0.755-0.895)* |
| >50% | 10 | 18.5 | 38 | 86.4 | 48 | 49 | |||
| Total | 54 | 100 | 44 | 100 | 98 | 100 | |||
Abbreviations: .B.P., beginner pathologist; ICC, intraclass correlation coefficient; S.P.1, senior pathologist 1; S.P.2, senior pathologist 2; T.P., trained pathologist.
ICC for consistency.
ICC for agreement.
Confidence Interval 95%
Discussion
The current study evaluated the interobserver variability among pathologists assessing TSR in CRA using the same methodology proposed by international working groups.12,13,16–21,23,24,26–29,31,32 For evaluating TSR, the Kappa statistic 0.67 to 0.81 can be interpreted as substantial to almost perfect agreement, according to the criteria of Landis and Koch. These criteria categorize a score of 0 as poor, 0 to 0.2 slight, 0.2 to 0.4 fair, 0.4 to 0.6 moderate, 0.6 to 0.8 substantial, and 0.8 to 1.0 almost perfect.33
In the TSR assessment, the ICC value for consistency and the ICC value for agreement were above 0.8, indicating that pathologists agreed both with themselves (ie, were internally consistent) and with each other. There was greater agreement among pathologists in stromal estimation for stromal-poor tumors. Taken together, these results suggest that the proposed methodology can be reliably used to evaluate TSR.
Traditional pathological staging systems are still the most important tool for therapeutic decisions in solid tumors.13 In colorectal cancer (CRC), survival is mainly correlated to the extent of the disease at the time of diagnosis. Much of the recent research into optimizing patient management has focused on identifying prognostic markers that allow the determination of which patient may benefit from adjuvant therapy, as well as predictive markers for the response of individual patients to specific therapeutic regimens.11,12,22,34 A growing body of literature demonstrates the prognostic and predictive significance of TSR.7,9,10,12,13,18,20,22–32
An important aspect in using the stromal estimate is the evaluation of TSR based on routine histological material without the need for special techniques. As indicated by other international studies, we confirmed it is a fast method and without extra costs.14 In addition, the proposed methodology is easy to understand, proving to be reproducible, suggesting that it can be used to facilitate the determination of stromal estimation as a potential prognostic factor.
The low interobserver variation found in the present study was obtained with the use of different ways of learning the TSR score method, from brief participation in an educational session to specific training and certification. The participation of a greater number of pathologists (first study with 4 pathologists), with evaluation and discrimination among the distinct stages of professional experience, constitutes a relevant aspect of the present study, denoting the high reproducible of the method.
Conclusions
The TSR scoring technique proved to be highly reproducible, with a substantial interobserver agreement. Substantial agreement was observed with the use of different ways of learning the TSR score method and among professionals with different stages of professional experience. Simply and reliably, the scoring TSR is a strong method, well suited and economical, and should be implemented in the routine of pathologists in the diagnosis of neoplasms.
Acknowledgments
The authors thank Unit Pathology Laboratory of the Lauro Wanderley University Hospital and the Pathology Laboratory of the Cancer Hospital Napoleão Laureano.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: R.M.S.d.S. helped in the conception and design, acquisition of data, analysis and interpretation of data, drafting the article, critical revision of the article, and approval of the final version to be published. E.M.Q., A.R.P., and F.F.P.N. contributed to the analysis and interpretation of data, and approval of the final version to be published. K.S.C. and E.P.D. helped in the revision of the final version of the manuscript and approval of the version to be published.
Consent for Publication: All authors read, approved the manuscript, and have given their consent for publication of this article.
Data Access Statements: All data are provided in full in the “Results” section of this paper.
Ethical Approval: The execution of the present study was authorized by the Ethics and Research Committee of the Lauro Wanderley University Hospital of the Federal University of Paraíba, under the registration 03283218.6.0000.5183.
ORCID iD: Ricella M Souza da Silva  https://orcid.org/0000-0002-3860-6660
https://orcid.org/0000-0002-3860-6660
References
- 1. Amin MB, American Joint Committee on Cancer and American Cancer Society (eds). AJCC cancer staging manual. 8th ed (editor-in-chief, Mahul B. Amin, MD, FCAP; editors, Stephen B. Edge, MD, FACS [and 16 others]; Donna M. Gress, RHIT, CTR-Technical editor; Laura R. Meyer, CAPM-Managing editor). Berlin: American Joint Committee on Cancer, Springer, 2017. [Google Scholar]
- 2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3. Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. [DOI] [PubMed] [Google Scholar]
- 5. Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356-387. [DOI] [PubMed] [Google Scholar]
- 6. Mesker WE, Junggeburt JMC, Szuhai K, et al. The carcinoma-stromal ratio of colon carcinoma is an independent factor for survival compared to lymph node status and tumor stage. Cell Oncol. 2007;29:387-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zunder SM, Gelderblom H, Tollenaar RA, Mesker WE. The significance of stromal collagen organization in cancer tissue: an in-depth discussion of literature. Crit Rev Oncol Hematol. 2020;151:102907. [DOI] [PubMed] [Google Scholar]
- 8. Ribeiro Franco PI, Rodrigues AP, de Menezes LB, Pacheco Miguel M. Tumor microenvironment components: allies of cancer progression. Pathol Res Pract. 2020;216:152729. [DOI] [PubMed] [Google Scholar]
- 9. Scheer R, Baidoshvili A, Zoidze S, et al. Tumor-stroma ratio as prognostic factor for survival in rectal adenocarcinoma: a retrospective cohort study. World J Gastrointest Oncol. 2017;9:466-474. doi: 10.4251/wjgo.v9.i12.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu J, Liang C, Chen M, Su W. Association between tumor-stroma ratio and prognosis in solid tumor patients: a systematic review and meta-analysis. Oncotarget. 2016;7:68954-68965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ao T, Kajiwara Y, Yonemura K, et al. Morphological consistency of desmoplastic reactions between the primary colorectal cancer lesion and associated metastatic lesions. Virchows Arch. 2020;477:47-55. [DOI] [PubMed] [Google Scholar]
- 12. Park JH, Richards CH, McMillan DC, Horgan PG, Roxburgh CSD. The relationship between tumour stroma percentage, the tumour microenvironment and survival in patients with primary operable colorectal cancer. Ann Oncol. 2014;25:644-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huijbers A, Tollenaar RAEM, Pelt GWV, et al. The proportion of tumor-stroma as a strong prognosticator for stage II and III colon cancer patients: validation in the VICTOR trial. Ann Oncol. 2013;24:179-185. doi: 10.1093/annonc/mds246. [DOI] [PubMed] [Google Scholar]
- 14. van Pelt GW, Kjær-Frifeldt S, van Krieken JHJM, et al. Scoring the tumor-stroma ratio in colon cancer: procedure and recommendations. Virchows Arch. 2018;473:405-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abstracts: 31st European Congress of Pathology. Virchows Arch. 2019;475:1-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mesker WE, Liefers G-J, Junggeburt JMC, et al. Presence of a high amount of stroma and downregulation of SMAD4 predict for worse survival for stage I-II colon cancer patients. Cell Oncol. 2009;31:169-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Pelt GW, Hansen TF, Bastiaannet E, et al. Stroma-high lymph node involvement predicts poor survival more accurately for patients with stage III colon cancer. J Med Surg Pathol. 2016;1:1000116. doi: 10.4172/2472-4971.1000116. [DOI] [Google Scholar]
- 18. de Kruijf EM, van Nes JG, van de Velde CJ, et al. Tumor-stroma ratio in the primary tumor is a prognostic factor in early breast cancer patients, especially in triple-negative carcinoma patients. Breast Cancer Res Treat. 2011;125:687-696. [DOI] [PubMed] [Google Scholar]
- 19. Dekker TJA, van de Velde CJ, van Pelt GW, et al. Prognostic significance of the tumor-stroma ratio: validation study in node-negative premenopausal breast cancer patients from the EORTC perioperative chemotherapy (POP) trial (10854). Breast Cancer Res Treat. 2013;139:371-379. [DOI] [PubMed] [Google Scholar]
- 20. Roeke T, Sobral-Leite M, Dekker TJA, et al. The prognostic value of the tumour-stroma ratio in primary operable invasive cancer of the breast: a validation study. Breast Cancer Res Treat. 2017;166:435-445. [DOI] [PubMed] [Google Scholar]
- 21. Courrech Staal EF, Wouters MW, van Sandick JW, et al. The stromal part of adenocarcinomas of the oesophagus: does it conceal targets for therapy? Eur J Cancer. 2010;46:720-728. [DOI] [PubMed] [Google Scholar]
- 22. West NP, Dattani M, McShane P, et al. The proportion of tumour cells is an independent predictor for survival in colorectal cancer patients. Br J Cancer. 2010;102:1519-1523. doi: 10.1038/sj.bjc.6605674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang K, Ma W, Wang J, et al. Tumor-stroma ratio is an independent predictor for survival in esophageal squamous cell carcinoma. J Thorac Oncol. 2012;7:1457-1461. [DOI] [PubMed] [Google Scholar]
- 24. Moorman AM, Vink R, Heijmans HJ, van der Palen J, Kouwenhoven EA. The prognostic value of tumour-stroma ratio in triple-negative breast cancer. Eur J Surg Oncol. 2012;38:307-313. doi: 10.1016/j.ejso.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 25. Wang Z, Liu H, Zhao R, Zhang H, Liu C, Song Y. [Tumor-stroma ratio is an independent prognostic factor of non-small cell lung cancer]. Zhongguo Fei Ai Za Zhi. 2013;16:191-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang X-L, Jiang C, Zhang Z-X, Liu F, Zhang F, Cheng Y-F. The tumor-stroma ratio is an independent predictor for survival in nasopharyngeal cancer. Oncol Res Treat. 2014;37:480-484. [DOI] [PubMed] [Google Scholar]
- 27. Gujam FJA, Edwards J, Mohammed ZMA, Going JJ, McMillan DC. The relationship between the tumour stroma percentage, clinicopathological characteristics and outcome in patients with operable ductal breast cancer. Br J Cancer. 2014;111:157-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu J, Liu J, Li J, et al. Tumor-stroma ratio is an independent predictor for survival in early cervical carcinoma. Gynecol Oncol. 2014;132:81-86. [DOI] [PubMed] [Google Scholar]
- 29. Lv Z, Cai X, Weng X, et al. Tumor-stroma ratio is a prognostic factor for survival in hepatocellular carcinoma patients after liver resection or transplantation. Surgery. 2015;158:142-150. [DOI] [PubMed] [Google Scholar]
- 30. Chen Y, Zhang L, Liu W, Liu X. Prognostic significance of the tumor-stroma ratio in epithelial ovarian cancer. Biomed Res Int. 2015;2015:589301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pongsuvareeyakul T, Khunamornpong S, Settakorn J, et al. Prognostic evaluation of tumor-stroma ratio in patients with early stage cervical adenocarcinoma treated by surgery. Asian Pac J Cancer Prev. 2015;16:4363-4368. [DOI] [PubMed] [Google Scholar]
- 32. Li H, Yuan SL, Han ZZ, et al. Prognostic significance of the tumor-stroma ratio in gallbladder cancer. Neoplasma. 2017;64:588-593. [DOI] [PubMed] [Google Scholar]
- 33. Landis JR, Koch GG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics. 1977;33:363-374. [PubMed] [Google Scholar]
- 34. Hansen TF, Kjær-Frifeldt S, Lindebjerg J, et al. Tumor-stroma ratio predicts recurrence in patients with colon cancer treated with neoadjuvant chemotherapy. Acta Oncol. 2018;57:528-533. [DOI] [PubMed] [Google Scholar]