Abstract
This study aimed to investigate distinct neurometabolites in the anterior cingulate cortex (ACC), right and left thalamus, and insula of patients with fibromyalgia (FM) compared with healthy controls using proton magnetic resonance spectroscopy (MRS). Levels of N-acetylaspartate (NAA), N-acetylaspartylglutamate (NAAG), total NAA (tNAA = NAA + NAAG), myo-inositol (ml), glutamine (Gln), glutamate (Glu), Glx (Glu + Gln), glycerophosphocholine (GPC), total choline (tCho = GPC + phosphocholine) and glutathione (GSH) levels relative to total creatine (tCr) levels including creatine (Cr) and phosphocreatine (PCr) and relative to Cr levels were determined in the ACC, right and left thalamus, and insula in 12 patients with FM and 13 healthy controls using MRS. In the ACC, NAA/tCr (P = 0.028) and tCho/tCr (P = 0.047) were higher in patients with FM. In the right and left insula, tNAA/tCr (P = 0.019, P = 0.007, respectively) was lower in patients with FM. Patients with FM showed lower levels of ml/Cr (P = 0.037) in the right insula than healthy controls. These findings are paramount to understand decisive pathophysiological mechanisms related to abnormal features in the brain and parasympathetic nervous systems in FM. We suggest that the results presented herein may be essential to understand hidden pathological mechanisms and also life system potential as protective and recovering metabolic strategies in patients with FM.
Keywords: fibromyalgia, magnetic resonance spectroscopy, neurometabolites, anterior cingulate cortex, thalamus, insula
Introduction
Fibromyalgia (FM) is the currently accepted term for chronic widespread musculoskeletal pain.1 Symptoms commonly observed are fatigue, memory problems, and sleep disturbances.2 The clinical features of FM are associated with various psychological symptoms related to stress and post-traumatic stress disorder (PTSD).3,4 In addition, the enhanced nociception was observed in stress-induced chronic widespread pain.5 Peripheral and central abnormalities of nociception are associated with peripheral and central sensitization in patients with FM.6 Peripheral, central, cognitive-emotional, and interpersonal sensitization may be responsible for heightened pain sensitivity in patients with FM.7 Central sensitization in patients with FM is revealed using brain imaging, indicating a decreased functional connectivity in the descending pain-modulating system and an increased activity in the pain matrix related to central sensitization.8
Localized proton magnetic resonance spectroscopy (1H-MRS) is a noninvasive tool for measuring in vivo neurochemical information, frequently referred to as metabolites in the human brain. Investigating abnormal neurometabolites using MRS may be useful for understanding the neuropathology and metabolic dysfunctions in the specific brain areas in patients with FM. A significant increase was found in the Glx (glutamate [Glu] + glutamine [Gln]) levels in the posterior cingulate cortex (PCC) and right amygdala for patients with FM.9,10 Glx/creatine (Cr) and Glu/Cr ratios within the ventrolateral prefrontal cortex bilaterally were significantly higher in patients with FM.11 FM patients had significantly higher levels of Glx/Cr ratios in the posterior gyrus and lower levels of myo-inositol (ml) in the right and left hippocampi.12 In addition, decreased ml/Cr ratios were found in the left sensorimotor area and left hippocampus, and lower levels of choline (Cho) and total NAA (tNAA) levels including N-acetylaspartate (NAA) + N-acetylaspartylglutamate (NAAG) levels were found in the left hippocampus in FM.12 In contrast, Cho levels in the right hippocampus were higher in FM.13 Briefly, previous studies using MRS for FM have mainly reported increases in cerebral Glu levels and reductions in ml and NAA in the hippocampus.12,14,15
In this study, we investigated the chemical biomarkers associated with FM. Among a variety of MRS-detectable metabolites, we focused on levels of NAA, NAAG, tNAA (NAA+NAAG), Gln, Glu, Glx (Glu + Gln), ml, glycerophosphocholine (GPC), total Cho (tCho) (GPC + phosphocholine), and glutathione (GSH) levels relative to total creatine (tCr) levels including Cr and phosphocreatine (PCr) and relative to Cr levels in the anterior cingulate cortex (ACC), bilateral thalamus, and insula.
While previous reports using MRS have mainly focused on the hippocampus, amygdala and PCC, we focused on the ACC, bilateral thalamus, and insula in FM. Thus, this study aimed to investigate neurochemical biomarkers and abnormal neuromatabolites associated with FM compared with healthy controls. Furthermore, we investigated the relationships of abnormal neurometabolites levels with pain and psychiatric symptoms in patients with FM.
Methods
Participants
The present study included 12 patients fulfilling the 2010 American College of Rheumatology criteria for FM, recruited from the Seoul National University Hospital, while 15 individuals of comparable age and gender, exhibiting no pain or neurological symptoms were recruited using online advertisements. However, MRS data were missing for two patients; thus, 13 subjects were used as healthy controls. The subjects used as the healthy controls were the same used in a previous complex regional pain syndrome study,16,17 while two additional participants were included in this study. Subjects who exhibited high levels of high-sensitivity C-reactive protein (hs-CRP) or leukocytosis were excluded. The inclusion criteria for FM subjects were as follows: diagnosis of FM; age between 21 and 63 years; and either benzodiazepine not administered or discontinued 2 weeks before the study. Individuals with a major neuropsychiatric disorder before the diagnosis of FM, neurological disease (cerebrovascular disease or brain tumor), history of brain trauma, high levels of hs-CRP or leukocytosis, as well as those who could not undergo magnetic resonance imaging were excluded. Sensory and affective dimensions of current pain were assessed using the McGill pain Questionnaire Short-Form, comprising 11 McGill Pain Questionnaire-Sensory and four McGill Pain Questionnaire-Affective pain items.18 Moreover, visual analogue scale (VAS) was used for measurement of pain intensity. Stress levels in patients with FM were assessed using the stress response inventor, consisting of 39 items (score range: 0–156) categorized into seven factors: fatigue, tension, frustration, anger, depression, somatization, and aggression.19 The PTSD checklist assessed the development of PTSD in respondents after exposure to a traumatic event.20 This study was approved by the Institutional Review Board at the Seoul National University Hospital (Seoul, Korea). All data were obtained under written informed consent granted by all subjects after a full explanation of the experimental methods.
1H-MRS data acquisition and processing
All magnetic resonance (MR) data were collected with a 3.0-T human MR scanner, using a 16-channel head and neck coil (Siemens Trio system; Siemens Medical Solutions, Erlangen, Germany). For 1H-MRS volume localization, anatomical images were collected using a T2-weighted fast spin echo sequence along the axial (axi), sagittal (sag), and coronal (cor) directions (repetition time [TR] = 6090 ms [axi] and, 5910 ms [sag and cor], echo time [TE] = 89 ms, flip angle = 90°/130°, field of view = 220 × 199 mm2 [axi and cor] and 220 × 220 mm2 [sag], matrix size = 256 × 180, echo train length = 5, echo spacing = 9.93 ms, receiver bandwidth [BW] = 271 Hz/pixel, number of slices = 30 [no gap], slice thickness = 5 mm, number of signal averages [NSA] = 128).
Based on scout images, five volumes of interest (VOIs) were selected in the ACC (2 × 2 × 2 cm3), right and left thalamus (2 × 2 × 1.5 cm3) and right and left insula (2 × 1.5 × 2 cm3) for each subject. The VOI in the right and left thalamus was placed along the axis of the thalamus in order to cover the maximum volume. Following auto shimming over the VOI, 1H-MRS data were acquired using a point-resolved spectroscopy pulse sequence (PRESS)21 with TR/TE = 2000/30 ms, 2048 data points, BW = 2500 Hz, NSA = 128, four dummy scans, and four-step phase cycling. The main PRESS sequence was preceded by water and outer-volume suppression modules. The carrier frequency was adjusted by -2.3 ppm from the water resonance to minimize voxel displacement.
The 1H-MRS data were analyzed using LC Model22 software (ver. 6.3–1J) in the range of 4.2 to 1.0 ppm. The metabolite content was normalized to that of tCr and Cr levels. The final data analysis included only those metabolites with a Cramer–Rao lower bound (CRLB) < 30%. Owing to the selection based on the CRLB < 30%, the number of the samples used in each metabolite was different in each result.
Statistical analysis
Statistical analyses were performed using SPSS Statistics 21.0 (SPSS, Chicago, IL). The differences in MRS between patients with FM and healthy controls were assessed using a two-tailed student’s t-test. After the data were tested for normality, a non-parametric test, the Mann-Whitney U test were used for data that were not normally distributed. The Pearson’s correlation analysis was used to evaluate the association between psychological test scores and neurometabolites using MRS. P-values < 0.05 were considered statistically significant without correction for multiple comparisons.
Results
Study participants and basic information
A total of 12 FM and 13 healthy control subjects completed the study procedures. Demographic and clinical characteristics of the subjects are presented in Supplementary Table 1. Age, gender ratio, and education levels were not significantly different between the two groups. The average pain duration in patients with FM was 4.4 years.
Comparison of neurometabolites in ACC, right and left thalamus, and insula between FM patients and healthy control subjects
We investigated neurometabolites levels in the ACC, right and left thalamus, and insula in patients with FM compared with healthy controls using internal references of tCr (Cr + PCr), and Cr for calculating metabolite ratios.
Abnormal levels of neurometabolites related to microstructural features in ACC and bilateral insula in FM patients
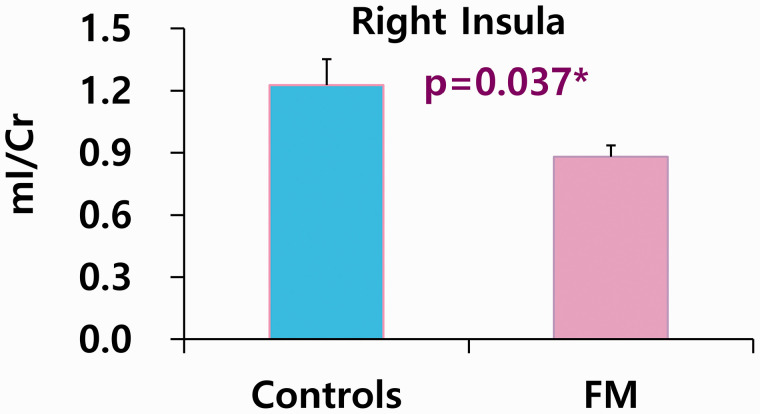
In the ACC, NAA/tCr (P = 0.028) was higher in patients with FM than in healthy controls (Table 1). In the right and left insula, tNAA/tCr (P = 0.019; P = 0.007, respectively) was lower in FM patients (Table 2). However, there were not found any significant results between FM and healthy controls in the right and left thalamus (Table 3). When the reference marker, Cr levels, was used, patients with FM had lower levels of ml/Cr (P = 0.037) in the right insula than healthy controls (Figure 1).
Table 1.
Comparison of neurometabolites in anterior cingulate cortex between fibromyalgia patients and healthy controls.
|
Anterior cingulate cortex | |||||
|---|---|---|---|---|---|
| Metabolites | Groups | N | mean | SD | p-value |
| Gln/tCr | CON | 11 | 0.503 | 0.227 | 0.957 |
| FM | 12 | 0.508 | 0.150 | ||
| Glu/tCr | CON | 13 | 1.379 | 0.099 | 0.354 |
| FM | 12 | 1.431 | 0.170 | ||
| GPC/tCr | CON | 12 | 0.220 | 0.030 | 0.194 |
| FM | 12 | 0.233 | 0.046 | ||
| GSH/tCr | CON | 13 | 0.299 | 0.078 | 0.870 |
| FM | 12 | 0.300 | 0.058 | ||
| mI/tCr | CON | 13 | 0.781 | 0.080 | 0.128 |
| FM | 12 | 0.824 | 0.074 | ||
| NAA/tCr | CON | 13 | 0.822 | 0.225 | 0.028 |
| FM | 12 | 1.029 | 0.216 | ||
| tCho/tCr | CON | 13 | 0.219 | 0.029 | 0.047 |
| FM | 12 | 0.244 | 0.030 | ||
| tNAA/tCr | CON | 13 | 1.201 | 0.130 | 0.575 |
| FM | 12 | 1.171 | 0.132 | ||
| Glx/tCr | CON | 13 | 1.835 | 0.239 | 0.189 |
| FM | 12 | 1.939 | 0.117 | ||
Gray color: Mann-Whitney U test, White background: Student t-test, bold letter: significant results.
CON; healthy controls, FM; fibromyalgia.
Table 2.
Comparison of neurometabolites in right and left insula between fibromyalgia patients and healthy controls.
|
Right insula |
Left insula |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabolites | Groups | N | mean | SD | p-value | Metabolites | Groups | N | mean | SD | p-value |
| Gln/tCr | CON | 6 | 0.354 | 0.060 | 0.837 | Gln/tCr | CON | 5 | 0.496 | 0.183 | 0.219 |
| FM | 9 | 0.344 | 0.093 | FM | 5 | 0.376 | 0.030 | ||||
| Glu/tCr | CON | 11 | 1.205 | 0.181 | 0.123 | Glu/tCr | CON | 12 | 1.109 | 0.204 | 0.784 |
| FM | 12 | 1.296 | 0.075 | FM | 12 | 1.129 | 0.152 | ||||
| GPC/tCr | CON | 11 | 0.244 | 0.037 | 0.380 | GPC/tCr | CON | 10 | 0.268 | 0.049 | 0.157 |
| FM | 12 | 0.256 | 0.024 | FM | 12 | 0.243 | 0.021 | ||||
| GSH/tCr | CON | 11 | 0.269 | 0.053 | 0.140 | GSH/tCr | CON | 10 | 0.301 | 0.069 | 0.445 |
| FM | 12 | 0.298 | 0.041 | FM | 11 | 0.278 | 0.062 | ||||
| mI/tCr | CON | 11 | 0.641 | 0.100 | 0.626 | mI/tCr | CON | 11 | 0.701 | 0.112 | 0.095 |
| FM | 12 | 0.660 | 0.091 | FM | 12 | 0.793 | 0.137 | ||||
| NAA/tCr | CON | 11 | 0.993 | 0.192 | 0.987 | NAA/tCr | CON | 10 | 0.808 | 0.317 | 0.742 |
| FM | 12 | 0.992 | 0.190 | FM | 12 | 0.871 | 0.264 | ||||
| NAAG/tCr | CON | 8 | 0.355 | 0.153 | 0.751 | NAAG/tCr | CON | 9 | 0.662 | 0.242 | 0.553 |
| FM | 3 | 0.388 | 0.121 | FM | 3 | 0.571 | 0.118 | ||||
| tCho/tCr | CON | 11 | 0.265 | 0.054 | 0.607 | tCho/tCr | CON | 12 | 0.276 | 0.063 | 0.110 |
| FM | 12 | 0.256 | 0.024 | FM | 12 | 0.243 | 0.021 | ||||
| tNAA/tCr | CON | 11 | 1.275 | 0.105 | 0.019 | tNAA/tCr | CON | 12 | 1.199 | 0.137 | 0.007 |
| FM | 12 | 1.159 | 0.111 | FM | 12 | 1.044 | 0.115 | ||||
| Glx/tCr | CON | 11 | 1.473 | 0.257 | 0.085 | Glx/tCr | CON | 12 | 1.436 | 0.307 | 0.782 |
| FM | 12 | 1.611 | 0.111 | FM | 12 | 1.403 | 0.271 | ||||
Gray color: Mann-Whitney U test, White background: Student t-test, bold letter: significant results.
CON; healthy controls, FM; fibromyalgia.
Table 3.
Comparison of neurometabolites in right and left thalamus between fibromyalgia patients and healthy controls.
|
Right thalamus |
Left thalamus |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabolites | Groups | N | mean | SD | p-value | Metabolites | Groups | N | mean | SD | p-value |
| Gln/tCr | CON | 7 | 0.709 | 0.390 | 0.410 | Gln/tCr | CON | 10 | 0.604 | 0.120 | 0.572 |
| FM | 6 | 0.554 | 0.227 | FM | 4 | 0.567 | 0.154 | ||||
| Glu/tCr | CON | 12 | 1.122 | 0.259 | 0.976 | Glu/tCr | CON | 13 | 1.153 | 0.138 | 0.067 |
| FM | 12 | 1.125 | 0.081 | FM | 12 | 1.250 | 0.112 | ||||
| GPC/tCr | CON | 12 | 0.236 | 0.037 | 0.264 | GPC/tCr | CON | 13 | 0.257 | 0.022 | 0.123 |
| FM | 12 | 0.258 | 0.054 | FM | 12 | 0.274 | 0.028 | ||||
| GSH/tCr | CON | 11 | 0.357 | 0.176 | 0.466 | GSH/tCr | CON | 12 | 0.343 | 0.111 | 0.298 |
| FM | 12 | 0.316 | 0.079 | FM | 12 | 0.371 | 0.076 | ||||
| mI/tCr | CON | 12 | 0.793 | 0.208 | 0.887 | mI/tCr | CON | 12 | 0.687 | 0.119 | 0.509 |
| FM | 12 | 0.746 | 0.077 | FM | 12 | 0.720 | 0.126 | ||||
| NAA/tCr | CON | 8 | 0.731 | 0.187 | 0.343 | NAA/tCr | CON | 12 | 0.797 | 0.208 | 0.308 |
| FM | 12 | 0.836 | 0.275 | FM | 12 | 0.898 | 0.260 | ||||
| NAAG/tCr | CON | 12 | 0.840 | 0.162 | 0.074 | NAAG/tCr | CON | 13 | 0.692 | 0.186 | 0.293 |
| FM | 8 | 0.663 | 0.257 | FM | 10 | 0.597 | 0.236 | ||||
| tNAA/tCr | CON | 13 | 1.335 | 0.216 | 0.730 | tNAA/tCr | CON | 13 | 1.442 | 0.150 | 0.914 |
| FM | 12 | 1.362 | 0.159 | FM | 12 | 1.436 | 0.124 | ||||
| Glx/tCr | CON | 13 | 1.642 | 0.557 | 0.747 | Glx/tCr | CON | 13 | 1.685 | 0.213 | 0.824 |
| FM | 12 | 1.586 | 0.203 | FM | 12 | 1.667 | 0.164 | ||||
| tCho/tCr | CON | 12 | 0.236 | 0.037 | 0.264 | tCho/tCr | CON | 13 | 0.262 | 0.031 | 0.340 |
| FM | 12 | 0.258 | 0.054 | FM | 12 | 0.274 | 0.028 | ||||
Gray color: Mann-Whitney U test, White background: Student t-test, bold letter: significant results.
CON; healthy controls, FM; fibromyalgia.
Figure 1.
The decreased levels of mI using internal reference marker Cr in right insula of fibromyalgia. Used analysis; Student’s t-test. Controls (n = 6), FM; fibromyalgia (n = 12), mI; myo-inositol, Cr; creatine.
Higher levels of tCho/tCr in ACC related to parasympathetic nervous system in FM patients
In the ACC, tCho/tCr was higher (P = 0.047) in patients with FM than in healthy controls (Table 1), which may be related to parasympathetic nervous system.
Correlations between neurometabolite levels and pain severity, PTSD, and stress levels in FM patients
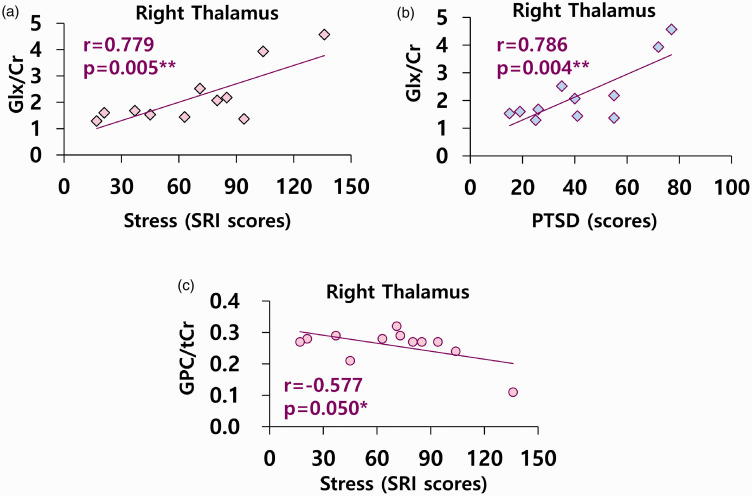
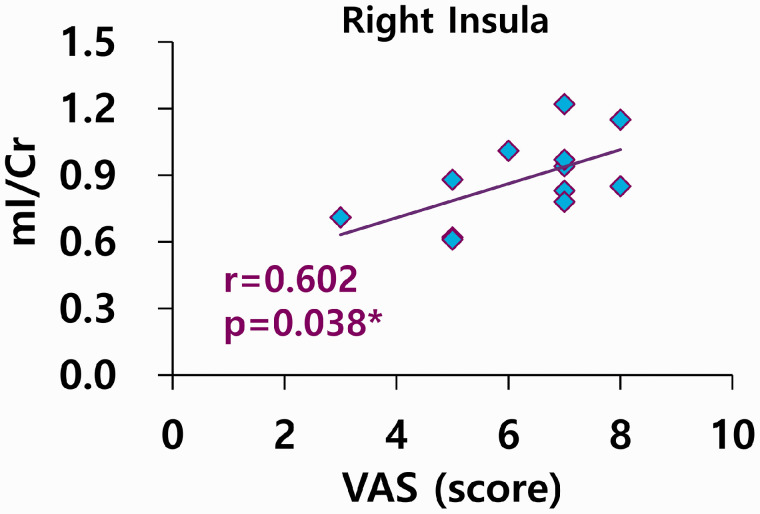
There were positive correlations between Glx/Cr and stress (r = 0.779, P = 0.005) and PTSD (r = 0.786, P = 0.004); however, there was a negative correlation between GPC/tCr and stress levels (r = –0.577, P = 0.050) in the right thalamus in patients with FM (Figure 2). There were positive correlations between mI/Cr and VAS pain scores (r = 0.602, P = 0.038) in the right insula in patients with FM (Figure 3).
Figure 2.
Correlations between neurometabolites and stress and PTSD in right thalamus of fibromyalgia. (a, b) n = 11, Glx; glutamine (Gln) + glutamate (Glu), Cr; creatine. (b) PTSD; post-traumatic stress disorder. (c) n = 12, GPC; glycerophosphocholine, tCr; total creatine levels including creatine (Cr) and phosphocreatine (PCr). (a, c) SRI; Stress Response Inventory.
Figure 3.
Correlations between mI levels using internal reference marker Cr and VAS in right insula of fibromyalgia. n = 12, VAS; Visual Analogue Scale, mI; myo-inositol, Cr; creatine.
Discussion
This study revealed abnormal levels of neurometabolites related to microstructural features and associated with the parasympathetic nervous system in ACC and bilateral insula in patients with FM compared with healthy controls. First, we found abnormal neurometabolites in NAA levels used as a neuronal marker23 and mI levels used as glial marker,24 which may indicate microstructural abnormalities in ACC and bilateral insula of FM. Secondly, tCho/tCr were higher in the ACC, which may be associated with recovering life system in the parasympathetic nervous system in patients with FM. This study elucidated comprehensive pathophysiological mechanisms related to microstructural abnormalities and protective and recovering strategies as life system potential in the brain of patients with FM. Additionally, these pathological abnormalities or recovering strategies were closely associated with stress, PTSD, and pain levels in patients with FM. We emphasized that these abnormalities in the brain metabolites may also exhibit life system potential as protective and recovering metabolic strategies in FM.
The increase of tCho/tCr in ACC may be associated with protective strategies to recover parasympathetic dysfunction in FM. GPC is converted to choline, which is the precursor for the neurotransmitter acetylcholine,25,26 involved in many functions including cognitive function, memory, and muscle control.25,27,28 In the parasympathetic division, neurons are cholinergic, and acetylcholine is the primary neurotransmitter responsible for communication between the neurons on the parasympathetic pathway.29 In addition, the anterior/mid cingulate cortex is critically involved in the response inhibition network in the pain system,30 and ACC links the processes of cognitive interference and parasympathetic modulation with activation in the ACC, a structure critical for the interface between cognition and emotion.31 Fortunately, the increase of tCho/tCr in ACC may be associated with protective mechanisms for recovering parasympathetic regulation in patients with FM. However, high stress in patients with FM affects the brain’s recovery potential for protecting body disorder, considering that the higher stress levels reduces GPC/tCr levels in the right thalamus in FM in this study. On the other hand, FM is associated with decreased connectivity between pain and the sensorimotor brain areas.32 Therefore, the increase in tCho/tCr in the ACC may contribute to the pain regulation and protective mechanisms for emotion and cognition in patients with FM. Moreover, the tCho are biosynthetic precursors of acetylcoline for parasympathetic function. Therefore, we can suggest that the increase in tCho/tCr in FM may be more associated with emotional and cognitive regulation for pain modulation in ACC, compensating parasympathetic dysfunction in the body and brain as the brain’s protective and recovering strategies.
In addition to the increase in tCho/tCr in the ACC, NAA/tCr levels in ACC increased in patients with FM. Not only is ACC related to parasympathetic modulation,31 but also the anterior/mid cingulate cortex are related to the response inhibition network in the pain system.30 Furthermore, NAA is detected in the adult brain in neurons as the neuronal marker.23 Thus, the increase in NAA/tCr in the ACC may contribute to the increase of neuron and pain inhibition and modulation in FM, showing compensating potential for the brain’s effective recovering system. In addition, as the ACC is critical for the interface between cognition and emotion,31 the increase in NAA/tCr in ACC may develop cognitive regulation mechanisms for pain inhibition and modulation, enhancing structural recovery by using the increase of neuronal cell in FM. Moreover, reactive neurogenesis occurs in response to naturally occurring apoptosis as well as injury-induced neuronal death in an adult brain.33 Neurogenesis and morphogenesis were observed through migration and lamination in the regions of the cingulate cortex.34 Therefore, the increase in the NAA/tCr in ACC may be associated with neurogenesis and migration for natural therapeutic strategies in FM.
The insula is associated with sympathetic dysfunctions and pain perception and processing.35,36 In addition, mI/Cr levels, known as a glial marker,24 were lower in the right insula in FM, which may be associated with the structural deficits in the glial cells. Considering that the sympathetic nervous system was involved in trauma stress-induced immune alterations via a mechanism of apoptotic cell death,37 PTSD and stress may induce glial cell death in the insula of patients with FM. Similarly, these reduced levels of mI/tCr may affect lower pain sensitivity in FM, considering that higher pain intensity (VAS) levels were associated with higher ml/Cr levels in this study. Moreover, these lower levels of ml in the right insula may be related to pathological dysfunction in pain perception and processing35 and sympathetic dysfunction.36 Thus, structural abnormalities related to NAA-related neuronal and mI-related glial cells may be a critical pathological factor associated with pain processing in the brain of patients with FM35 or protective and recovering metabolic strategies. Additionally, considering that abnormal levels of GPC are involved in acetylcholine-related parasympathetic dysfunction,38 the increased levels of tCho in the ACC may be associated with parasympathetic dysfunction or protective and recovering mechanisms in FM.
The clinical features of FM are associated with various psychological factors, and stress is a necessary link in the pathway between significant psychological factors and key FM symptoms.3 In addition, FM patients had more PTSD symptoms than controls.4 Additionally, considering high levels of stress and PTSD scores in patients with FM, long-time stress and longer period of pain may contribute to the severity on pain pathology or stress-induced analgesia with protective and recovering potential of FM. Stress-induced analgesia seems to activate the brain networks involved in sensory, affective, and cognitive modulatory circuits.39
This study had a few limitations. Our study examined only a small number of patients with FM. Thus, future studies with larger sample sizes will be required to generalize these findings to such patients.
Altogether, the increase in NAA/tCr, the decrease in mI/Cr, and the increase in tCho/tCr in the ACC and insula may be associated with pathological mechanisms or life system potential for protective and recovering strategies in chronic pain in patients with FM. Moreover, it may be related to stress-induced analgesia, which may contribute to the endogenous protective modulation to regulate stress and pain processing in patients with FM. These findings can suggest extensive and inclusive pathology including structural abnormality, which may affect pathological pain processing and abnormal cognitive and emotional functions. Additionally, these abnormal metabolites in the brain may be associated with protective and recovering strategies as life system potential in chronic pain in patients with FM.
Supplemental Material
Supplemental material, sj-pdf-1-mpx-10.1177_1744806921990946 for Abnormal neurometabolites in fibromyalgia patients: Magnetic resonance spectroscopy study by Ye-Ha Jung, Hyeonjin Kim, Dasom Lee, Jae-Yeon Lee, Won Joon Lee, Jee Youn Moon, Soo-Hee Choi and Do-Hyung Kang in Molecular Pain
Acknowledgments
The authors wish to thank all the participants for their valuable time in being involved in this research.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF-2018R1A2B6001806).
ORCID iD: Do-Hyung Kang https://orcid.org/0000-0002-8741-5748
Supplemental material: Supplemental material for this article is available online.
References
- 1.Sluka KA, Clauw DJ. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience 2016; 338: 114–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clauw DJ. Fibromyalgia: a clinical review. J Am Med Assoc 2014; 311: 1547–1555. [DOI] [PubMed] [Google Scholar]
- 3.Malin K, Littlejohn GO. Psychological factors mediate key symptoms of fibromyalgia through their influence on stress. Clin Rheumatol 2016; 35: 2353–2357. [DOI] [PubMed] [Google Scholar]
- 4.Toussaint LL, Whipple MO, Vincent A. Post-traumatic stress disorder symptoms may explain poor mental health in patients with fibromyalgia. J Health Psychol 2017; 22: 697–706. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Green PG, Levine JD. Stress enhances muscle nociceptor activity in the rat. Neuroscience 2011; 185: 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staud R, Smitherman ML. Peripheral and central sensitization in fibromyalgia: pathogenetic role. Curr Pain Headache Rep 2002; 6: 259–266. [DOI] [PubMed] [Google Scholar]
- 7.English B. Neural and psychosocial mechanisms of pain sensitivity in fibromyalgia. Pain Manag Nurs 2014; 15: 530–538. [DOI] [PubMed] [Google Scholar]
- 8.Cagnie B, Coppieters I, Denecker S, et al. Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Semin Arthritis Rheum 2014; 44: 68–75. [DOI] [PubMed] [Google Scholar]
- 9.Valdés M, Collado A, Bargalló N, et al. Increased glutamate/glutamine compounds in the brains of patients with fibromyalgia: a magnetic resonance spectroscopy study. Arthritis Rheum 2010; 62: 1829–1836. [DOI] [PubMed] [Google Scholar]
- 10.Fayed N, Andres E, Rojas G, et al. Brain dysfunction in fibromyalgia and somatization disorder using proton magnetic resonance spectroscopy: a controlled study. Acta Psychiatr Scand 2012; 126: 115–125. [DOI] [PubMed] [Google Scholar]
- 11.Feraco P, Bacci A, Pedrabissi F, et al. Metabolic abnormalities in pain-processing regions of patients with fibromyalgia: a 3T MR spectroscopy study. AJNR Am J Neuroradiol 2011; 32: 1585–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fayed N, Garcia-Campayo J, Magallón R, et al. Localized 1H-NMR spectroscopy in patients with fibromyalgia: a controlled study of changes in cerebral glutamate/glutamine, inositol, choline, and N-acetylaspartate. Arthritis Res Ther 2010; 12: R134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emad Y, Ragab Y, Zeinhom F, et al. Hippocampus dysfunction may explain symptoms of fibromyalgia syndrome. A study with single-voxel magnetic resonance spectroscopy. J Rheumatol 2008; 35: 1371–1377. [PubMed] [Google Scholar]
- 14.Aoki Y, Inokuchi R, Suwa H. Reduced N-acetylaspartate in the hippocampus in patients with fibromyalgia: a meta-analysis. Psychiatry Res 2013; 213: 242–248. [DOI] [PubMed] [Google Scholar]
- 15.Pyke TL, Osmotherly PG, Baines S. Measuring glutamate levels in the brains of fibromyalgia patients and a potential role for glutamate in the pathophysiology of fibromyalgia symptoms: a systematic review. Clin J Pain 2017; 33: 944–954. [DOI] [PubMed] [Google Scholar]
- 16.Jeon SY, Seo S, Lee JS, et al. [11C]-(R)-PK11195 positron emission tomography in patients with complex regional pain syndrome: a pilot study. Medicine (Baltimore ) 2017; 96: e5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung YH, Kim H, Jeon SY, et al. Neurometabolite changes in patients with complex regional pain syndrome using magnetic resonance spectroscopy: a pilot study. Neuroreport 2019; 30: 108–112. [DOI] [PubMed] [Google Scholar]
- 18.Melzack R. The short-form McGill pain questionnaire. Pain 1987; 30: 191–197. [DOI] [PubMed] [Google Scholar]
- 19.Koh KB, Park JK, Kim CH, et al. Development of the stress response inventory and its application in clinical practice. Psychosom Med 2001; 63: 668–678. [DOI] [PubMed] [Google Scholar]
- 20.Blevins CA, Weathers FW, Davis MT, et al. The posttraumatic stress disorder checklist for DSM‐5 (PCL‐5): development and initial psychometric evaluation. J Traumat Stress 2015; 28: 489–498. [DOI] [PubMed] [Google Scholar]
- 21.Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann NY Acad Sci 1987; 508: 333–348. [DOI] [PubMed] [Google Scholar]
- 22.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993; 30: 672–679. [DOI] [PubMed] [Google Scholar]
- 23.Simmons ML, Frondoza CG, Coyle JT. Immunocytochemical localization of N-acetyl-aspartate with monoclonal antibodies. Neuroscience 1991; 45: 37–45. [DOI] [PubMed] [Google Scholar]
- 24.Chang L, Munsaka SM, Kraft-Terry S, et al. Magnetic resonance spectroscopy to assess neuroinflammation and neuropathic pain. J Neuroimmune Pharmacol 2013; 8: 576–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SH, Choi BY, Kim JH, et al. Late treatment with choline alfoscerate (l-alpha glycerylphosphorylcholine, α-GPC) increases hippocampal neurogenesis and provides protection against seizure-induced neuronal death and cognitive impairment. Brain Res 2017; 1654: 66–76. [DOI] [PubMed] [Google Scholar]
- 26.Lee M, Choi BY, Suh SW. Unexpected effects of acetylcholine precursors on pilocarpine seizure- induced neuronal death. Curr Neuropharmacol 2018; 16: 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fambrough DM. Control of acetylcholine receptors in skeletal muscle. Physiol Rev 1979; 59: 165–227. [DOI] [PubMed] [Google Scholar]
- 28.Tucek S. The synthesis of acetylcholine in skeletal muscles of the rat. J Physiol 1982; 322: 53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wetzel GT, Brown JH. Presynaptic modulation of acetylcholine release from cardiac parasympathetic neurons. Am J Physiol 1985; 248: H33–H39. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt-Wilcke T, Kairys A, Ichesco E, et al. Changes in clinical pain in fibromyalgia patients correlate with changes in brain activation in the cingulate cortex in a response inhibition task. Pain Med 2014; 15: 1346–1358. [DOI] [PubMed] [Google Scholar]
- 31.Matthews SC, Paulus MP, Simmons AN, et al. Functional subdivisions within anterior cingulate cortex and their relationship to autonomic nervous system function. Neuroimage 2004; 22: 1151–1156. [DOI] [PubMed] [Google Scholar]
- 32.Flodin P, Martinsen S, Löfgren M, et al. Fibromyalgia is associated with decreased connectivity between pain- and sensorimotor brain areas. Brain Connect 2014; 4: 587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larson TA, Thatra NM, Lee BH, et al. Reactive neurogenesis in response to naturally occurring apoptosis in an adult brain. J Neurosci 2014; 34: 13066–13076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richter W, Kranz D. Autoradiography of neurogenesis and morphogenesis in the regio cingularis of the rat. III. Migration and lamination in the regions of the cingulate cortex and the area postcentralis. J Hirnforsch 1979; 20: 475–505. [PubMed] [Google Scholar]
- 35.Brown CA, Seymour B, El-Deredy W, Jones AK. Confidence in beliefs about pain predicts expectancy effects on pain perception and anticipatory processing in right anterior insula. Pain 2008; 139: 324–332. [DOI] [PubMed] [Google Scholar]
- 36.Cechetto DF. Cortical control of the autonomic nervous system. Exp Physiol 2014; 99: 326–331. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Sun J, Yu J, et al. Sympathetic nervous system mediates surgical trauma stress-induced splenocyte apoptosis in rats. Eur J Pharmacol 2007; 565: 76–82. [DOI] [PubMed] [Google Scholar]
- 38.Vizi ES. Acetylcholine release from guinea-pig ileum by parasympathetic ganglion stimulants and gastrin-like polypeptides. Br J Pharmacol 1973; 47: 765–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yilmaz P, Diers M, Diener S, et al. Brain correlates of stress-induced analgesia. Pain 2010; 151: 522–529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-mpx-10.1177_1744806921990946 for Abnormal neurometabolites in fibromyalgia patients: Magnetic resonance spectroscopy study by Ye-Ha Jung, Hyeonjin Kim, Dasom Lee, Jae-Yeon Lee, Won Joon Lee, Jee Youn Moon, Soo-Hee Choi and Do-Hyung Kang in Molecular Pain