Abstract
We have created entirely biological tissue-engineered vascular grafts (TEVGs) using sheets of cell-assembled extracellular matrix (CAM) produced by human fibroblasts in vitro. A large animal TEVG would allow long-term pre-clinical studies in a clinically relevant setting (graft size and allogeneic setting). Therefore, canine, porcine, ovine, and human skin fibroblasts were compared for their ability to form CAM sheets. Serum sourcing greatly influenced CAM production in a species-dependent manner. Ovine cells produced the most homogenous and strongest animal CAM sheets but remained ≈3-fold weaker than human sheets despite variations of serum, ascorbate, insulin, or growth factor supplementations. Key differences in cell growth dynamics, tissue development, and tissue architecture and composition were observed between human and ovine. This study demonstrates critical species-to-species differences in fibroblast behavior and how they pose a challenge when attempting to substitute animal cells for human cells during the development of tissue-engineered constructs that require long-term cultures.
Keywords: Skin fibroblasts, large animal, cell-assembled extracellular matrix, vascular tissue-engineering, interspecies differences
Introduction
Skin fibroblasts in long-term cultures lay down a large amount of extracellular matrix (ECM) as a cohesive sheet at the bottom of their culture containers. We have previously produced entirely biological tissue-engineered vascular grafts (TEVGs) by rolling theses sheets of cell-assembled extracellular matrix (CAM) around a mandrel, which were then placed in a bioreactor during a long maturation phase to allow sheet layer fusion.1 Such autologous TEVGs were implanted as hemodialysis access grafts in patients and were functional for up to 20 months.2,3 However, the long maturation phase is associated with a high production cost that can be an important limitation for this approach. Recently, a more rapid and versatile textile-based assembly strategy was developed to eliminate the need for a bioreactor step.4 Indeed, CAM sheets can be cut in long strips (or “ribbons”), that can be further processed by twisting to make threads. Ribbons and threads are then assembled by weaving to produce human woven TEVGs with supraphysiological mechanical properties.
While TEVGs, made from rolled CAM sheets, performed well in clinical trials, and despite the fact that they are made of the same material, the new textile-based TEVGs will require extensive in vivo evaluation before being implanted in a patient. In vivo studies of human TEVGs in immunosuppressed xenogeneic models, such as rats or primates, are possible but these do not reflect the clinical setting and/or can be very limited by ethical and economical concerns. Therefore, our long-term goal is to develop a large animal model for the evaluation of CAM-based tissue-engineered constructs in an autologous or allogeneic setting. Such a model can better reflect the clinical situation by allowing the study of grafts in immunocompetent animals for extended periods and with grafts of relevant dimensions. Interspecies cell behavior differences are well established5,6 but can be especially important in tissue engineering strategies that involve long-term cultures where these differences can have cumulative effects. For example, Ouellet et al.7 used human skin fibroblast cultures to develop, over 21 days, a tissue sheet for bladder and urethral replacement but were unable to produce similar tissues with porcine skin fibroblasts. Another example is the work of Niklason’s8 group who developed bovine TEVGs from smooth muscle cells (SMCs) seeded in a biodegradable polymer matrix and cultured for 56 days. These TEVGs had physiological burst pressures (>2000 mmHg) but the group was not able to obtain similar results with porcine cells9–13 and struggled for 10 years to successfully produce human TEVGs with clinically relevant burst pressures.14–17 These examples highlight the challenge of interspecies translation and the need to study differences in cultured cell behaviors. The goal of this study was to identify, among well-established large animal models for vascular prostheses evaluation (canine, porcine, and ovine),18 which species could yield skin fibroblasts capable of producing CAM sheets similar to that of human.
Materials and methods
Cell isolation and culture
Human skin fibroblasts (HSFs) were isolated from breast skin of a 31-year-old woman undergoing breast reduction surgery (in accordance with article L. 1243-3 of the code of public health and under the agreement DC-2008-412 with the University Hospital Center of Bordeaux, France [update 10/10/2014]) as previously described.19 HSFs were seeded at a density of 1 × 104 cells·cm−2. Cells were cultured in a DMEM/F-12 (Gibco #31331-028) medium supplemented with 20% fetal bovine serum (FBS; Biowest #S1810.500) and 1X Penicillin/Streptomycin (Pen/Strep) antibiotics (Gibco #15140122). Canine skin fibroblasts (CSF; abm #T0269, age and sex of animal unknown) were purchased and seeded at 1 × 104 cells·cm−2 and cultured in the same culture medium. Porcine and ovine groin skin biopsies were collected from post-mortem animals (3-month-old female and 20-month-old female, respectively). Porcine and ovine skin fibroblasts (PSFs and OSFs, respectively) were isolated using an explant culture technique. Dermis fragments of 1 to 2 mm2 were cut from skin samples, placed inside T25 flasks, and cultured in 1 mL of the same culture medium for at least 7 days. Dermis fragments were then removed. Cells were trypsinized, seeded at 1 × 104 cells·cm−2, and cultured in the same medium. Media were changed three times a week. HSFs, CSFs, and PSFs were then used between passages 4–8, and 4–13 for OSFs.
Culture conditions for CAM sheet production
HSFs, CSFs, PSFs, and OSFs were seeded at 1 × 104 cells·cm−2 in 6-well plates. Stainless-steel wire anchors were added in the wells. Cells were cultured in a DMEM/F-12 medium supplemented with 0.50 mM sodium L-ascorbate (Sigma #A4034), 1X Pen/Strep, and 20% FBS. Two serums (Biowest [S1] and Hyclone Fetal Clone III #SH30109 [S2]) were used either pure (S1 20% and S2 20%) or in combinations (S1 5% + 15% S2, S1 10% + 10% S2, and S1 15% + 5% S2 also referred as S1 1:3 S2, S1 1:1 S2, and S1 3:1 S2). CAM sheets were cultured for 8 weeks, unless complete lack of sheet production or sheet detachments from steel wire anchors was observed. Media were changed three times a week.
CAM sheet mechanical properties
A perforation test with a computer-controlled device (Shimadzu Autograph AGS-X series, force transducer 100 N) was performed to evaluate CAM sheet mechanical properties. The center of a circular sheet, mounted on a custom holding device, was loaded with a spherical tip of a probe (Ø = 9.5 mm) at a rate of 20 mm·min−1 until perforation. Data were analyzed with Trapezium-X software.
Cell growth dynamic
HSFs and OSFs were seeded at 1 × 104 cells·cm−2 in 6-well plates and cultured in a DMEM/F-12 medium supplemented with 20% S1. Cells were trypsinized and counted after 1, 3, 5, and 9 days of culture. Media were changed three times a week. Cell culture observations were performed with an inverted microscope Leica DMi1 and phase-contrast micrographs were taken before trypsinization.
Effect of serum concentration on CAM sheet production and time course study
HSFs and OSFs were seeded at 1 × 104 cells·cm−2 in 6-well plates and cultured in a DMEM/F-12 medium supplemented with 0.50 mM sodium L-ascorbate, 1X Pen/Strep, and their respective optimal FBS condition, either S1 1:1 S2 or S2 pure, at different concentrations (10%, 15%, and 20%). Media were changed three times a week and perforation tests were performed on 8-week-old sheets. HSFs and OSFs were then cultured in 20% S1 1:1 S2 and 10% S2 pure, respectively, from results obtained in the previous experiments. Cells were seeded at the same density in 6-well plates and cultured in the same medium. Media were also changed three times a week. The sheet perforation force was evaluated after 4, 5, 6, 7, and 8 weeks in culture. At each time-point, CAM sheet samples were taken using a biopsy punch (Ø = 8 mm), and frozen until needed.
DNA quantity assessment of CAM sheets
Human and ovine CAM punches were thawed at room temperature and DNA quantified using a QIAamp DNA Mini Kit (QIAGEN, #51304) following manufacturer instructions. The absorbance was measured using a nanophotometer P330 (Implen) to quantify DNA (µg·mL−1). The DNA quantity was expressed as a quantity per surface unit (µg·mm−2) to compare human and ovine CAM sheets.
Collagen content of CAM sheets
Human and ovine CAM punches were thawed at room temperature and tested for hydroxyproline content following manufacturer instructions (Biovision #K226-100). The absorbance (OD550) was measured with a multilabel plate reader (VICTOR X3, PerkinElmer). A 13% factor of hydroxyproline and collagen was used to calculate the collagen content of the original CAM sheet, as previously described.20,21 The perforation force (gf) and collagen content (µg·mm−2) ratio was calculated for human and ovine sheets at each time-point.
CAM sheet histology
At each time-point, fresh human and ovine CAM sheets were fixed in 4% paraformaldehyde (PFA) (Microm Microtech, Antigenfix) for 1 h. CAM sheets were rolled, dehydrated, and paraffin-embedded. Histological cross-sections (cut in the full thickness of rolled-CAM sheets) were deparaffinized using toluene for 10 min, rehydrated in ethanol baths of decreasing concentrations, and stained with a Masson’s trichrome coloration (vert lumière).
Effect of ascorbate, insulin, and growth factors supplementation on CAM sheet production
HSFs and OSFs were seeded at 1 × 104 cells·cm−2 in 6-well plates and cultured in a DMEM/F-12 medium supplemented with different concentrations of sodium L-ascorbate (0.25, 0.50, 0.75, and 1.0 mM), 1X Pen/Strep, and FBS, either 20% S1 1:1 S2 or 10% S2 pure, respectively. Media were changed three times a week and perforation tests were performed on 8-week-old sheets. Insulin, from an Insulin (1 g·L−1)—Transferrin (550 mg·L−1)—Selenium (670 µg·L−1) solution (Gibco #41400045), was freshly added at different concentrations (1, 2, and 4 µg·mL−1) prior to medium changes. Also growth factors, such as Epidermal Growth Factor (EGF; Peprotech #AF-100-15), Transforming Growth Factor-β1 (TGF-β1; Peprotech #AF-100-21C), basic-Fibroblast Growth Factor (bFGF; Peprotech #100-18C), and Platelet-Derived Growth Factor-BB (PDGF-BB; Peprotech #100-14B) were freshly added to the culture medium at three different concentrations (1, 3, and 9 ng·mL−1). HSFs and OSFs were seeded at the same density in 6-well plates and cultured in their respective media supplemented with 0.50 mM of sodium L-ascorbate. S2 serum contained approximately 136 ng·mL−1 of Insulin Growth Factor-1 (IGF-1) and 2.7 ng·mL−1 of Transforming Growth Factor-Beta1 (TGF-β1).22 Media were changed three times a week and perforation tests were performed on 8-week-old sheets.
Fabrication of PLA anchors and cell culture
Poly(lactic) acid filament (PLA; ESUN®) was used to fabricate simple wire anchors (width = 515 ± 5 µm and height = 370 ± 10 µm) by fused deposition modeling (FDM) using a custom-made 3D printer (“Technoshop,” Technological Department at the University of Bordeaux (IUT de Bordeaux), France). A reinforced version of PLA anchor was designed with Rhinoceros software, with an outer ring (inner diameter 32 mm) connected to a single inner wire with a 28-mm inner diameter. Both PLA anchors were assessed for CAM sheet production. HSFs and OSFs were seeded at 1 × 104 cells·cm−2 in 6-well plates and cultured for 8 weeks in DMEM/F-12 supplemented with 0.50 mM of sodium L-ascorbate, 1X Pen/Strep, and FBS, and either 20% S1 1:1 S2 or 10% S2 pure, respectively.
Statistical analyses
Data are represented as means ± standard deviation (SD). Statistical analyses (Prism, GraphPad) were performed using unpaired t-tests (two tailed), one-way ANOVA tests with Tukey’s multiple comparisons, or two-way ANOVA tests with multiple comparisons, as mentioned. Slopes of regression lines were compared following a method described in Chapter 18 of J Zar,23 Biostatistical Analysis, fifth edition, Prentice-Hall, 2010. Statistical differences corresponded to a p < 0.05.
Results
Ovine cells produced better CAM sheets than canine and porcine cells but not human cells
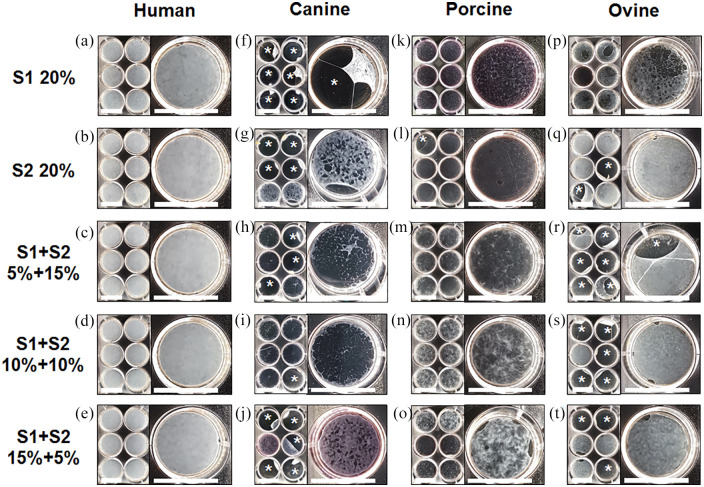
HSFs, CSFs, PSFs, and OSFs were cultured in two serums (S1 and S2) previously identified as suitable for CAM production with HSFs. Serums were used at a final concentration of 20% either pure or mixed in different proportions (1:3, 1:1, and 3:1). Human cells produced opaque and homogeneous sheets in all tested conditions (Figure 1(a)–(e)). While canine cells grew well in all conditions, they did not produce cohesive sheets (Figure 1(f)–(j)). Porcine cells did not produce sheets in pure serum conditions (Figure 1(k) and (l)) but created very thin and heterogeneous sheets in two (S1 1:3 S2 and S1 1:1 S2) out of three combined serum conditions (Figure 1(m)–(o)). In contrast, ovine cells created fairly dense and homogeneous CAM sheets in some conditions (Figure 1(q)–(t)) but not in pure S1 (Figure 1(p)). Unlike human sheets, ovine sheets were often lost because they detached from the peripheral wire anchor. In S1 1:3 S2, S1 1:1 S2, and S1 3:1 S2, detachments occurred in 100%, 83%, and 50% of cases (Figure 1(r)–(t)), while only 33% of sheets detached in S2 pure (Figure 1(q)). Altogether, this data suggests that ovine cells are the most promising candidate for the production of large animal CAM sheets.
Figure 1.
Ovine fibroblasts produced more homogeneous CAM sheets than canine or porcine fibroblasts. Macroscopic views of sheets produced in 6-well plates, and closer views of one well after 8 weeks of culture. CAM sheets were produced in cultures supplemented with 20% of two serums (S1 and S2) used either pure or in combinations (S1 5% + 15% S2, S1 10% + 10% S2, and S1 15% + 5% S2). (a–e) Human cells produced dense CAM sheets in all conditions, (f–j) canine cells produced non-homogeneous sheets that frequently tore and were not recoverable, (k–o) porcine cells produced very thin sheets in some conditions, (p–t) ovine cells produced the best-looking sheets of all animals’ cells.
Detachments (*) of canine (f–j) and ovine (q–t) sheets from the stainless-steel wire anchor were common but did not occur with human sheets (a–e). Scale bars = 35 mm.
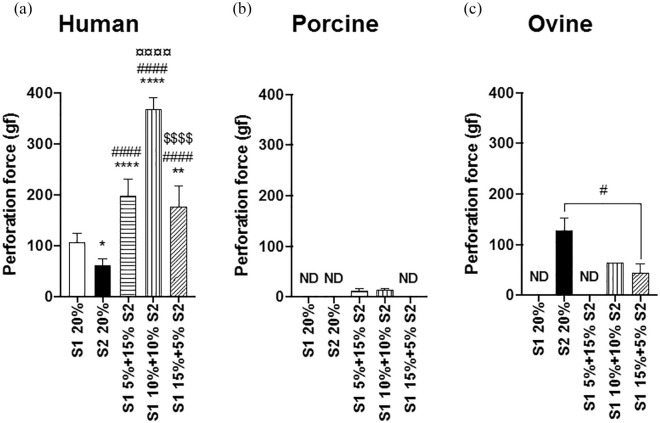
For HSFs, combining serums produced statistically stronger sheets than with pure serums (Figure 2(a)). The strongest human CAM sheets were produced in culture conditions mixing S1 and S2 in equivalent proportions (S1 1:1 S2 = 368 ± 24 gf, n = 6). PSFs also produced better sheets in mixed serums but these were extremely weak sheets (S1 1:3 S2 = 13 ± 5 gf, n = 3 and S1 1:1 S2 = 14 ± 4 gf, n = 3, Figure 2(b)). However, combining serums did not improve the ovine sheet perforation force. In fact, the strongest ovine sheets were produced in S2 pure (S2 20% = 128 ± 24 gf, n = 3, Figure 2(c)), although they were 3-fold weaker than the strongest human sheets.
Figure 2.
Serum influenced the perforation force of CAM sheets. CAM sheets were produced in culture conditions supplemented with 20% of two serums (S1 and S2) used either pure or combined (S1 5% + 15% S2, S1 10% + 10% S2, and S1 15% + 5% S2). Ovine fibroblasts (c) produced much stronger sheets than porcine cells (b), but weaker than human cells (a). Results are expressed as means ± SD, n = 1-6, one-way ANOVA. (c) An unpaired t-test was performed between S2 20% and S1 15% + 5% S2, as S1 10% + 10% S2 (n = 1) was not included in the statistical analysis.
Statistical differences were expressed as followed: *p < 0.05, **p < 0.01, and ****p < 0.0001; * statistically different from S1 20%, # statistically different from S2 20%, ¤ statistically different from S1 5% + 15% S2, and $ statistically different from S1 10% + 10% S2.
ND: no data.
OSFs were more proliferative and contractile than HSFs in standard culture conditions
When cultured for up to 9 days in the same medium, HSFs and OSFs exhibited similar morphologies but displayed different growth dynamics. Microscopic observations showed that human and ovine cells have a typical spindle-shaped morphology, and similar patterns upon confluence (Figure 3(a)–(h)). However, OSFs attained confluence after 5 days of culture (Figure 3(g)), whereas HSFs just reached the same level of confluence after 9 days of culture (Figure 3(d)). In addition, OSF cell layers contracted to form nodules and cell depleted areas after 9 days of culture (Figure 3(h)), which might have influenced cell counts (underestimate). Finally, cell counts confirmed that OSFs were statistically more proliferative than HSFs in the same culture conditions (Figure 3(i)).
Figure 3.

Human and ovine skin fibroblasts showed different growth dynamics. Representative phase contrast micrographs of HSFs and OSFs taken after 1 (a and e), 3 (b and f), 5 (c and g), and 9 (d and h) days of culture. Both species showed a typical spindle-shaped morphology but ovine cells reached confluence faster (e–h) than their human counterparts (a–d). (h) After 9 days, ovine cells contracted to create clusters/nodules (•) alongside cell depleted areas (*). Scale bar = 200 µm. (i) Cell proliferation was quantified by counting. Ovine cells showed a higher proliferation rate and reached a higher density. Counts of OSFs at D9 may have been underestimated because of incomplete cell recovery due to nodule formation. Results are expressed as means ± SD, n = 3, two-way ANOVA (*p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001; * statistically different from day 1, # statistically different from day 3, $ statistically different from day 5, and £ statistical differences between human and ovine cell counts at a specific time-point). One representative experiment out of four.
Serum concentration does not influence ovine CAM perforation force as it does human CAM
HSFs and OSFs were cultured in culture conditions with different concentrations (10, 15, and 20%) of their respective optimal serum condition (S1 1:1 S2 or S2 pure) (Figure 4). The human sheet perforation force increased almost linearly with serum concentration (10%: 49 ± 30 gf, n = 5, 15%: 145 ± 35 gf, n = 6, and 20%: 320 ± 35 gf, n = 6). Conversely, OSFs showed no sensitivity to serum concentration (10%: 99 ± 24 gf, n = 4, 15%: 121 ± 25 gf, n = 6, and 20%: 107 ± 22 gf, n = 6).
Figure 4.

Serum concentration did not influence the perforation force of ovine CAM sheets but has a critical impact on human CAM sheet strength. Human and ovine CAM sheets were produced in their respective optimal serum at various concentrations (10, 15, and 20%). Results are expressed as means ± SD, n = 4–6, one-way ANOVA (***p < 0.001, ****p < 0.0001; * statistically different from 10% and # statistically different from 15%). One representative experiment out of three.
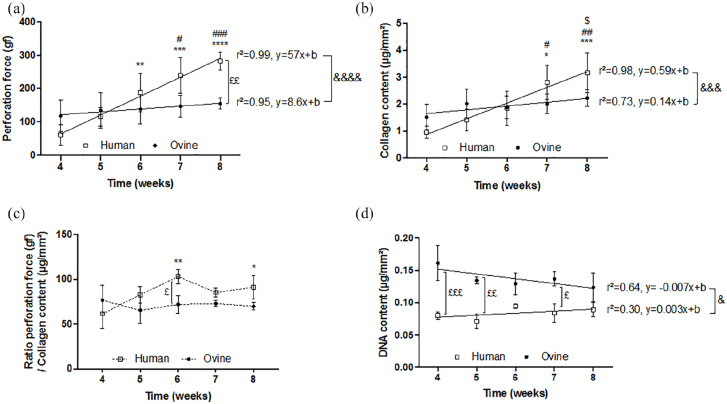
OSFs produced neither more collagen nor stronger CAM sheets with time compared to HSFs
HSFs and OSFs were cultured for 4, 5, 6, 7, and 8 weeks, in their respective optimal culture conditions (20% S1 1:1 S2 or 10% S2 pure). Between 4 and 8 weeks of culture, the human sheet perforation force increased 5-fold (p < 0.0001), while the ovine sheet perforation force did not statistically change (p = 0.8119) (Figure 5(a)). In addition, human and ovine linear regression slopes (r2 = 0.99, y = 57 x + b and r2 = 0.95, y = 8.6 x + b, respectively) were significantly different (p < 0.0001). At 4 weeks of culture, human sheets were 2-fold weaker than ovine sheets (p = 0.4313) but they were 2-fold stronger at 8 weeks (p = 0.0058). Similarly, the collagen content per surface area of human sheets increased almost 3-fold (p = 0.0002) between 4 and 8 weeks of culture, while the ovine sheet collagen content did not statistically change in time (Figure 5(b)). Whereas no statistical differences were observed between human and ovine sheet collagen content at each time-point, slopes of human and ovine linear regressions (r2 = 0.98, y = 0.59 x + b and r2 = 0.73, y = 0.14 x + b, respectively) were significantly different (p = 0.0008). This data supports the idea that the fibrillar collagen network is responsible for the mechanical strength of the CAM. The perforation force to collagen content ratios were similar for human and ovine CAM sheets (Figure 5(c)). However, the human ratio was significantly increased at some later time points while the ovine ratio did not change with time. The human ratio appeared to become higher than the ovine ratio as sheets matured but a statistical difference was only observed at 6 weeks. Finally, the ovine sheet DNA content was almost 2-fold higher than that of human sheets at 4 weeks of culture (p = 0.0003) and was also statistically higher at 5 (p = 0.0028) and 7 (p = 0.0126) weeks (Figure 5(d)). Interestingly, more OSFs did not lead to more collagen deposition or stronger sheets, on the contrary. While the DNA content of both human and ovine CAM sheets did not statistically change between 4 and 8 weeks of culture, human sheets trended upward (1.1-fold increase, p = 0.9783) and ovine sheets trended downward (0.8-fold decrease, p = 0.0682). In fact, linear regression slopes of human and ovine sheet DNA contents were statistically different (p = 0.0465).
Figure 5.
Culture time influenced almost linearly the strength and collagen content of human CAM sheets, unlike ovine sheets. Human and ovine CAM sheets were produced in their respective optimal serum over 4, 5, 6, 7, and 8 weeks. (a) Perforation force as a function of time in culture. Ovine fibroblasts did not produce stronger sheets with time, unlike human fibroblasts (means of three experiments ± SD, n = 3). (b) Collagen content as a function of time in culture. Human cells produced significantly higher amounts of collagen between early and later time points, unlike ovine cells (means of three experiments ± SD, n = 3). (c) Perforation force to collagen content ratio of ovine and human sheets as a function of time in culture. While culture time influenced the human sheet ratio, ovine sheets had a constant ratio (mean ratios ± SD, n = 3). (d) DNA content as a function of time in culture. DNA content in ovine sheets was almost 2-fold higher than in human sheets (means of two (human) and three (ovine) experiments ± SD, n = 2 and n = 3). Two-way ANOVA (*p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001; * statistically different from 4 weeks, # statistically different from 5 weeks, $ statistically different from 6 weeks, ^ statistically different from 7 weeks, and £ statistical differences between human and ovine sheets at a specific time-point). Brackets represent statistical differences between linear regression slopes (&).
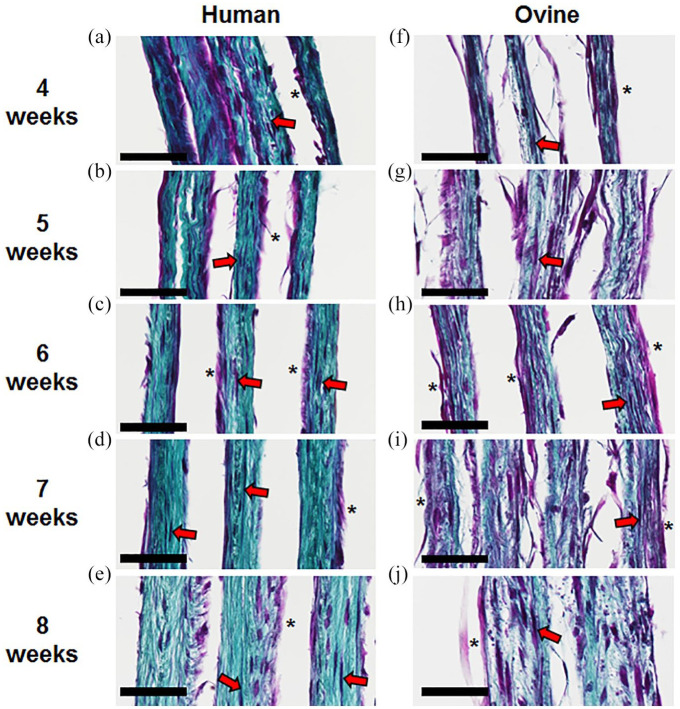
CAM sheet histology confirmed that ovine sheets contained more cells and relatively less collagen in comparison to human sheets
Histological cross-sections of human CAM sheets showed that HSFs produced sheets that became thicker between 4 and 8 weeks of culture (Figure 6(a)–(e)). HSFs were mainly located on the top side of the sheet (*) on a dense collagen network in which some stretched cells were embedded (red arrows). Similarly, ovine sheets became thicker over time and showed a similar cell distribution (Figure 6(f)–(j)). However, they contained more cells and less collagen, which is coherent with the biochemical quantifications.
Figure 6.
Human fibroblasts produced a denser ECM than ovine fibroblasts. Histological cross sections of multiple layers of human (a–e) and ovine (f–j) CAM sheets after 4, 5, 6, 7, and 8 weeks of culture (Masson’s trichrome, cells = purple, collagen = blue/green). Human and ovine cells were distributed throughout the sheet (red arrows) and a clear layer of cells was located on the top side of the sheet (*). Scale bar = 50 µm.
OSFs did not produce stronger CAM sheets when ascorbate concentrations varied or when insulin or growth factors were added
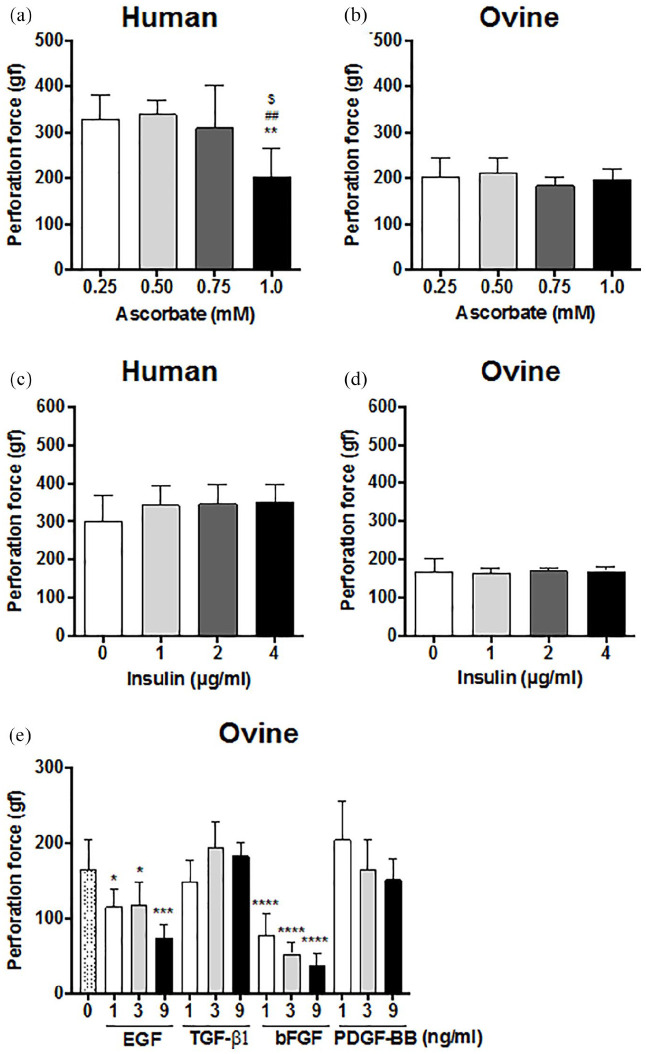
HSFs and OSFs were cultured for 8 weeks in their respective CAM culture media supplemented with different ascorbate concentrations around the “standard” production concentration (0.5 mM). HSFs produced CAM sheets with similar perforation forces between 0.25 mM and 0.75 mM ascorbate (Figure 7(a)). However, human sheets lost almost 40% of their strength when HSFs were cultured with 1 mM ascorbate, suggesting a possible toxicity. Conversely, the ovine sheet perforation force was not affected by variations of ascorbate concentration, even at 1 mM (Figure 7(b)). The influence of insulin and growth factor supplementations, such as EGF, TGF-β1, bFGF, and PDGF-BB were also evaluated. None of the supplements statistically increased the human nor the ovine sheet perforation force (Figure 7(c)–(e)). In fact, OSFs produced weaker sheets in all tested concentrations of EGF or bFGF compared to sheets produced without growth factor supplementations (Figure 7(e)).
Figure 7.
Ascorbate, insulin or growth factor supplementation did not increase human or ovine sheet perforation force. Strength of human (a) and ovine (b) sheets produced over 8 weeks with concentrations of ascorbate different than the “standard” 0.5 mM (means ± SD, n = 3–6). One-way ANOVA (* statistically different from 0.25 mM, # statistically different from 0.50 mM, and $ statistically different from 0.75 mM). One representative experiment out of three. Strength of human (c) and ovine (d) sheets produced with their optimal serum supplemented with insulin (means ± SD, n = 3–6). One representative experiment out of three. (e) Strength of ovine sheets produced with growth factor supplementation (means ± SD, n = 5–6). No strength increases were achieved (* statistically different from 0 ng.mL–1). One-way ANOVA. One representative experiment out of three. Statistical differences were expressed as followed: *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
PLA anchors avoid ovine CAM sheet loss due to detachment
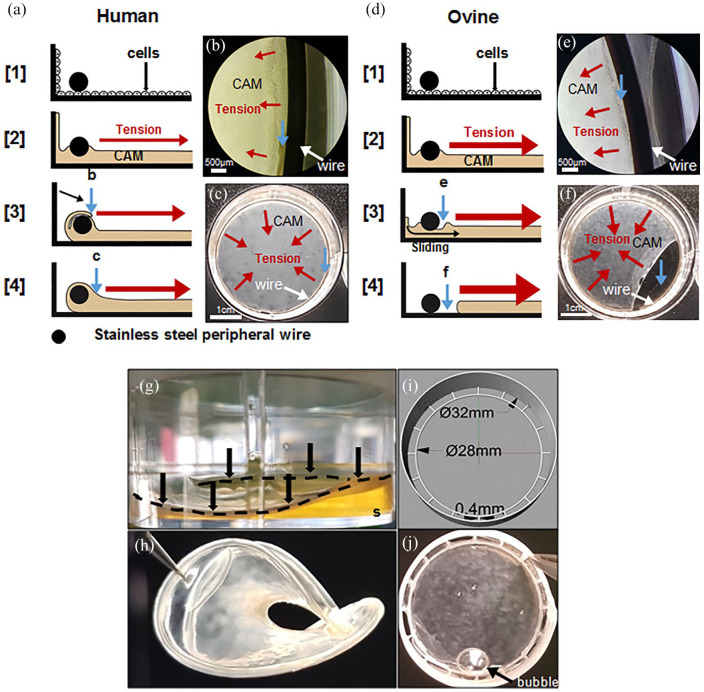
Beside the lower mechanical strength of the ovine CAM sheets, the fact that a large number of sheets were lost because of sheet contraction was problematic (Figure 1). Figure 8 shows a montage illustrating the difference between the development of human and ovine sheet attachment to the stainless-steel peripheral wire anchor (Figure 8(a)–(f)). HSFs and OSFs were cultured in their respective optimum serums in 6-well plates. Human sheets grew on both sides of the peripheral wire anchor and, ultimately, formed a tissue that surrounded the wire anchor (Figure 8(a) steps [1]–[3]). Also, human sheets could detach from the wall of the well due to the tissue tension and fall on the wire surrounding it (Figure 8(a) step [3] and (b)). After 3 weeks, both events prevented human sheet detachment for their entire culture period (Figure 8(a) step [4] and (c)). However, OSFs were more contractile and pulled more than HSFs on the sheet, which led to the sheet sliding under the wire after a week (Figure 8(d) step [3] and (e)) and the ovine sheet detachment a week later (Figure 8(d) step [4] and (f)). We hypothesized that a surface allowing for better cell adhesion would lead to a faster enveloping of the wire, hence we replaced the stainless-steel wire by a PLA wire (Figure 8(g) and (h)). The peripheral wire of PLA allowed the ovine cells to much more rapidly grow on the wire and resulted in a complete envelopment. However, after only 3 weeks, the PLA wire started to bend under the contractile forces of the tissue (Figure 8(g)). A week later, the ovine sheet completely folded while still attached to the PLA wire (Figure 8(h)). In order to solve this issue, a reinforced PLA anchor was designed and 3D printed (Figure 8(i)). While the inner ring allowed the ovine sheet to envelop the PLA wire, the multi-layered outer ring provided sufficient mechanical strength to prevent it from bending or folding throughout the culture (Figure 8(j)).
Figure 8.
Ovine CAM sheets often detached from stainless steel wire anchors but strongly attached to PLA anchors. (a–f) montage illustrating the difference between the development of human and ovine sheet attachment to the steel peripheral wire anchor. Cells grew to confluence under the wire (step [1]) and then started depositing the ECM to form the CAM sheet (step [2]). Red arrows show the direction of the force generated by tissue tension. (a) Human cells grew on and CAM accumulated near the wire slowly enveloping it, meanwhile, the CAM detached from the wall and folded over the wire enveloping it (steps [3] and [4]). (b) Phase-contrast micrograph showing folding over the anchor (at 3 weeks). (c) Macroscopic view of a well-attached human CAM sheet at the bottom of a 6-well plate (at 8 weeks). Blue arrows with lowercase letters show the same region shown by the blue arrows of the matching lowercase figure. White arrows point to the anchor. (d) Ovine cells were more contractile and developed earlier/larger tissue tension pulling away the CAM accumulating against the wire before it could envelop it (step [3]). Without a strong attachment, the sheets slipped under the wire and contracted (step [4]). (e) Phase-contrast micrograph of an ovine sheet showing tissue separation from the anchor and a lack of folding after 1 week. (f) Macroscopic view showing sheet detachment a week later. (g) Ovine sheets produced with a PLA peripheral wire anchor (black arrows and discontinued black line) in a 6-well plate after 3 weeks. PLA anchors allow strong attachment of ovine sheets but tissue tension was strong enough to bend the PLA leading to the lifting of the sheet from the culture substrate (s). (h) After 4 weeks of culture, the structure is completely folded and unusable. (i and j) PLA anchorage devices were 3D-printed with a reinforced outer ring connected to an inner wire allowing strong ovine sheet attachments and withstood tissue contractile forces. A medium bubble can be seen on the sheet (black arrow).
Discussion
In the field of tissue-engineering, working with human cells is a good strategic choice because it can allow rapid translation to the clinic to perform pioneering studies.1,2 However, returning to an animal model after first-in-man studies is often necessary to refine the design or understand the remodeling mechanism of a construct.24,25 Xenografting a human CAM-based construct in an animal model would lead to immune responses that would interfere with the evaluation of its long-term potential. Indeed, both xenogeneic fibroblasts and ECM can trigger the immune system.26–29 Another option would be to study the human construct in immunosuppressed animal and/or primate models but these can be very limited by animal size, costs, and ethical concerns. Finally, one can endeavor to recreate the construct using cells from a relevant animal model. While species-to-species differences in cell behavior can appear minimal in short-term cultures or in molecular studies, these differences become critical in complex, long-term tissue cultures and it can take many years to find strategies to compensate for these differences.8–17 In this study, we showed that CAM production is very species-dependent. We also identified serum sourcing as a key species-dependent factor. In the conditions tested, ovine skin fibroblasts produced CAM that more closely resembled human CAM than other large animal cells did. Our long-term goal is to develop a large animal model to evaluate a woven CAM-based TEVG. The objective of this study was to develop a method to produce animal CAM that would be sufficiently robust to be turned into yarn that can be woven using a circular loom.4
Our results showed that the source of serum influenced the production of ECM by cells in long-term cultures in a clearly species-dependent manner. Similarly, Chabaud et al.30 looked at the influence of eight different serum sources on the ECM production by human and rabbit dermal fibroblasts cultured for 4 weeks. Both species showed high serum-source dependency when final tissue thickness was evaluated. Clear species-to-species variations were also observed since the best and worst serums were different between species. Finally, they reported that human cells produced more than 2-fold thicker sheets than rabbit cells regardless of the serum selected suggesting other non-serum-dependent species-specific differences. Niklason’s8 group also observed species-dependent ECM synthesis when producing TEVGs.9–17 While bovine TEVGs displayed burst pressure higher than 2000 mmHg,8 their burst pressure dropped about 30-fold when the model was produced using human cells.14 In our study, we showed the critical role of serum in tissue-engineering strategies that depend on in vitro ECM synthesis. Indeed, the right serum can make the difference between producing a cohesive CAM sheet and not having a sheet at all. In the conditions tested in this study, the ovine cells produced CAM sheets that were much superior to canine and porcine sheets. However, the fact that ovine sheets remained 3-fold weaker than human sheets suggests that other culture conditions may play an important role in CAM production.
We have repeatedly observed that OSFs were more proliferative and less elongated than HSFs when cultured in the same medium. Other groups also reported differences in fibroblast growth dynamics between human and small (e.g. rat, rabbit, and dunnart) and large (e.g. kangaroo, and porcine) animal models.7,31,32 However, to our knowledge, this study is the first to report that ovine and human skin fibroblasts behave differently in short or in long culture periods. This difference in cell behavior is important since ovine models have become very popular as pre-clinical models for tissue engineering studies and/or the validation of medical devices.
HSFs produced denser and stronger sheets when cultured with higher serum concentrations (15% or 20%). This is consistent with results reported by Tan et al.,33 who showed that human dermal fibroblasts exposed for 24 h to high serum concentrations (up to 60%) produced more ECM proteins than with lower serum concentrations (0% or 10%). Furthermore, they showed that the largest proportional increase was observed with 20% serum concentration and that HSFs seemed to reach a plateau of metabolic activity between serum concentrations of 40% and 60%. In addition, Lee et al.34 obtained thicker and denser tissue sheets when culturing human skin fibroblasts during 3 weeks using 25% or 50% serum in comparison to 10% serum. They also did not observe significant differences between 25% and 50% serum concentrations. Surprisingly, OSFs produced sheets of similar strength independently of the serum concentration (between 10% and 20%). A similar behavior has been observed with bovine corneal fibroblasts cultured in 1% or 10% FBS, which produced sheets of similar thickness after 3 weeks in culture.35 We hypothesize that OSFs might have reached a plateau of metabolic activity with only 10% of the serum we selected.
Human sheet strength and collagen content increased linearly with culture time. This supports the idea that the fibrillar collagen network is the mechanical backbone of the CAM sheet. This strength increase is consistent with results we have published recently and with our extensive experience in growing human CAM sheets.19 In fact, this time-dependent strength increase can be used to target a specific sheet strength when using cell lines from different donors that can have different rate of strength increase.4 Surprisingly, the culture time had practically no effect on ovine sheet strength or collagen content after 4 weeks. Unlike the ovine CAM, the force to collagen ratio was significantly higher in more mature human CAM which suggests increased crosslinking of the collagen matrix. Histological analysis indicated that the ovine collagen network appeared less dense than the human matrix. This striking difference in sheet growth dynamics could be due to the ovine CAM composition. Indeed, we hypothesize that, after 4 weeks of culture, the ECM produced by OSFs provided a negative feedback or was otherwise not conductive to the production or assembly of collagen. Supplementary studies are needed to understand the mechanistic differences between human and ovine CAM production by investigating the structure and nature of the ECM.
We tried to increase collagen production by OSFs in order to bring up the mechanical strength of ovine CAM to human levels. As ascorbate is essential to stimulate collagen production in skin fibroblasts,36–39 we investigated the influence of different concentrations in the culture medium. OSFs were insensitive to the ascorbate concentration while HSFs produced weaker sheets when the concentration exceeded 0.75 mM. This is consistent with the results of Switzer and Summer38 who also observed ascorbate toxicity when HSFs were exposed to concentrations higher than 0.75 mM. We also investigated the influence of insulin supplementation to increase collagen production, as Tranquillo’s group showed an almost 2-fold increase of the strength of their TEVG that relied on ECM production by HSFs in fibrin gels.40 However, our results showed that insulin in similar concentrations did not influence human nor ovine sheet perforation force. This could suggest that our serum supplementation fully stimulated the insulin-like growth factor I (IGF-1) receptors responsible for the insulin-induced collagen synthesis.41 Other growth factors have been used to stimulate cells to produce more ECM.8–10,42–45 Our results showed that OSFs did not produce stronger sheets with any growth factor supplementations in our culture conditions. Interestingly, EGF or bFGF supplementations statistically decreased the ovine CAM strength, which is consistent with some studies who showed that these growth factors can sometimes inhibit the collagen synthesis by human skin fibroblasts.46–49 Overall, our findings showed that, among evaluated factors, none were able to increase collagen production by OSFs or HSFs in our specific CAM culture conditions. This suggests that the combination of serum (at 10% or 20% for ovine and human cells, respectively) and ascorbate supplementation overshadowed the effects of additives that are well-known for stimulating the ECM production. Further studies could investigate macromolecular crowding, which was reported to increase collagen synthesis in short (from 2 to 6 days) and in longer (up to 14 days) fibroblast cultures by Zeugolis’ group.50,51 This group, among others, has also reported that hypoxia can strongly enhance collagen synthesis,40,50 a condition that might be tested in future experiments but would be challenging to integrate into a manufacturing process.
Ovine sheet detachments from stainless steel anchors was a major cause of sheet loss during CAM production but it was not a problem with human CAM. We hypothesize that this difference was due to a faster and higher tension development in the ovine sheet. This is consistent with the almost 2-fold higher cell concentration in ovine sheets. Based on previous work in our laboratory, which showed great cell adherence on PLA structures produced by FDM,52,53 we created reinforced PLA anchors to promote early cell attachment in order to resist ovine sheet tensions. These improved anchors allowed the same production success rate observed with steel wires and human sheets (100%).
The anatomical source of skin fibroblasts was different between human and animal (breast vs upper inner thigh, respectively). While we cannot dismiss the potential influence of this variable on the results, Sacco et al.54 have shown that human skin fibroblasts from the upper inner thigh are less proliferative than fibroblasts from breast. In the present study, the opposite was observed. Although the observations of Sacco et al.54 may be specific to humans, they can also suggest that species differences overshadowed anatomic origin effects. In addition, the influence of the age of the donor was not evaluated in this study.
In conclusion, this study demonstrates the important species-to-species variability of ECM synthesis in long-term cultures. Nonetheless, we identified culture conditions where ovine cells reliably produced homogeneous, dense, and robust CAM sheets. While ovine CAM sheets were not as strong as human sheets, this relative weakness can be offset by using wider ribbons to produce a yarn with a similar breaking strength (Supplemental Figure 1) suggesting that they can be assembled into robust yet fully biological ovine textiles. The next step in this project will be to produce small-diameter woven TEVGs and to evaluate their long-term efficacy in an allogeneic ovine model to mimic the clinical setting in terms of size and immune environment.
Supplemental Material
Supplemental material, sj-pptx-1-tej-10.1177_2041731420978327 for Cell-assembled extracellular matrix (CAM) sheet production: Translation from using human to large animal cells by Yoann Torres, Maude Gluais, Nicolas Da Silva, Sylvie Rey, Agathe Grémare, Laure Magnan, Fabien Kawecki and Nicolas L’Heureux in Journal of Tissue Engineering
Acknowledgments
We thank Robin Siadous for its technical support. We also thank Dr Géraldine Cassiat-Morisset, David Gonthier, and Virginie Loyer from L’Institut de Rythmologie et Modélisation Cardiaque de Bordeaux (Liryc) for the collect of ovine skin biopsies. We thank the DETERCA laboratory for the collect of porcine skin biopsy.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Ministère de la Recherche et de l’Enseignement Supérieur, the Chaire Senior de l’Initiative d’Excellence de l’Université de Bordeaux (IdEx Bordeaux), the Agence Nationale de la Recherche (ANR-16-CE18-0024-01), the Région Nouvelle Aquitaine (2016-1R30402), and the European Research Council (Advanced Grant # 785908).
ORCID iDs: Yoann Torres  https://orcid.org/0000-0001-9139-1024
https://orcid.org/0000-0001-9139-1024
Fabien Kawecki  https://orcid.org/0000-0002-1409-623X
https://orcid.org/0000-0002-1409-623X
Supplemental material: Supplemental material for this article is available online.
References
- 1. L’Heureux N, Dusserre N, Konig G, et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med 2006; 12(3): 361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. L’Heureux N, McAllister TN, De La Fuente LM. Tissue-engineered blood vessel for adult arterial revascularization. N Engl J Med 2007; 357(14): 1451–1453. [DOI] [PubMed] [Google Scholar]
- 3. McAllister TN, Maruszewski M, Garrido SA, et al. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet 2009; 373(9673): 1440–1446. [DOI] [PubMed] [Google Scholar]
- 4. Magnan L, Labrunie G, Fénelon M, et al. Human textiles: a cell-synthesized yarn as a truly “bio” material for tissue engineering applications. Acta Biomater 2020; 105: 111–120. [DOI] [PubMed] [Google Scholar]
- 5. Burgess-Herbert SL, Euling SY. Use of comparative genomics approaches to characterize interspecies differences in response to environmental chemicals: challenges, opportunities, and research needs. Toxicol Appl Pharmacol 2013; 271(3): 372–385. [DOI] [PubMed] [Google Scholar]
- 6. Eizirik DL, Pipeleers DG, Ling Z, et al. Major species differences between humans and rodents in the susceptibility to pancreatic β-cell injury. Proc Natl Acad Sci U S A 1994; 91(20): 9253–9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ouellet G, Dubé J, Gauvin R, et al. Production of an optimized tissue-engineered pig connective tissue for the reconstruction of the urinary tract. Tissue Eng Part A 2011; 17(11–12): 1625–1633. [DOI] [PubMed] [Google Scholar]
- 8. Niklason LE, Gao J, Abbott WM, et al. Functional arteries grown in vitro. Science 1999; 284(5413): 489–493. [DOI] [PubMed] [Google Scholar]
- 9. Prabhakar V, Grinstaff M, Moats A, et al. Engineering porcine arteries: effects of scaffold modification. J Biomed Mater Res 2002; 67(A): 303–311. [DOI] [PubMed] [Google Scholar]
- 10. Dahl SLM, Rucker RB, Niklason LE. Effects of copper and cross-linking on the extracellular matrix of tissue-engineered arteries. Cell Transplant 2005; 14(6): 367–374. [DOI] [PubMed] [Google Scholar]
- 11. Dahl SLM, Rhim C, Song YC, et al. Mechanical properties and compositions of tissue engineered and native arteries. Ann Biomed Eng 2007; 35(3): 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Solan A, Dahl SLM, Niklason LE. Effects of mechanical stretch on collagen and cross-linking in engineered blood vessels. Cell Transplant 2009; 18(8): 915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Quint C, Kondo Y, Manson RJ, et al. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc Natl Acad Sci U S A 2011; 108(22): 9214–9219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McKee JA, Banik SSR, Boyer MJ, et al. Human arteries engineered in vitro. EMBO Rep 2003; 4(6): 633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Poh M, Boyer M, Solan A, et al. Blood vessels engineered from human cells. Lancet 2005; 365: 2122–2124. [DOI] [PubMed] [Google Scholar]
- 16. Gong Z, Niklason LE. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs). FASEB J 2008; 22(6): 1635–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dahl SLM, Kypson AP, Lawson JH, et al. Readily available tissue-engineered vascular grafts. Sci Transl Med 2011; 3(68): 68ra9. [DOI] [PubMed] [Google Scholar]
- 18. Byrom MJ, Bannon PG, White GH, et al. Animal models for the assessment of novel vascular conduits. J Vasc Surg 2010; 52(1): 176–195. [DOI] [PubMed] [Google Scholar]
- 19. Magnan L, Labrunie G, Marais S, et al. Characterization of a cell-assembled extracellular matrix and the effect of the devitalization process. Acta Biomater 2018; 82: 56–67. [DOI] [PubMed] [Google Scholar]
- 20. Neuman RE, Logan MA. The determination of hydroxyproline. J Biol Chem. 1950; 184(1): 299–306. [PubMed] [Google Scholar]
- 21. Edwards CA, O’Brien WD. Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta. 1980; 104(2): 161–167. [DOI] [PubMed] [Google Scholar]
- 22. Thermo Fisher Scientific. Growth Factors in Thermo Scientific Hyclone Cell Culture Serum [Internet]. Art to Science, p.2 https://static.thermoscientific.com/images/D22225~.pdf (2013, accessed 15 June 2020).
- 23. Zar JH. Biostatistical analysis. 5th ed. Englewood Cliffs, NJ: Prentice-Hall, 2010, p.960. [Google Scholar]
- 24. Dean EW, Udelsman B, Breuer CK. Current advances in the translation of vascular tissue engineering to the treatment of pediatric congenital heart disease. Yale J Biol Med 2012; 85(2): 229–238. [PMC free article] [PubMed] [Google Scholar]
- 25. Shin’oka T, Imai Y. Transplantation of a tissue-engineered pulmonary artery. N Engl J Med 2001; 344(7): 532–533. [DOI] [PubMed] [Google Scholar]
- 26. Sher SE, Hull BE, Rosen S, et al. Acceptance of allogeneic fibroblasts in skin equivalent transplants. Transplantation 1983; 36(5): 552–557. [DOI] [PubMed] [Google Scholar]
- 27. Liang C, Xu Y, Zheng D, et al. RNAi-mediated silencing of HLA A2 suppressed acute rejection against human fibroblast xenografts in the striatum of 6-OHDA lesioned rats. J Neuroimmunol 2016; 297: 28–37. [DOI] [PubMed] [Google Scholar]
- 28. Allaire E, Bruneval P, Mandel C, et al. The immunogenicity of the extracellular matrix in arterial xenografts. Surgery 1997; 122(1): 73–81. [DOI] [PubMed] [Google Scholar]
- 29. Allaire E, Mandet C, Bruneval P, et al. Cell and extracellar matrix rejection in arterial concordant and discordant xenografts in the rat. Transplantation 1996; 62: 794–803. [DOI] [PubMed] [Google Scholar]
- 30. Chabaud S, Simard M, Gendreau I, et al. Origin of serum affects quality of engineered tissues produced by the self-assembly approach. Scientifica (Cairo). 2016; 2016: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stanley JF, Pye D, MacGregor A. Comparison of doubling numbers attained by cultured animal cells with life span of species. Nature 1975; 255: 158–159. [DOI] [PubMed] [Google Scholar]
- 32. Ohtsubo M, Roberts JM. Cyclin-dependent regulation of G1 in mammalian fibroblasts. Science 1993; 259: 1908–1911. [DOI] [PubMed] [Google Scholar]
- 33. Tan EML, Uitto J, Bauer EA, et al. Human skin fibroblasts in culture: procollagen synthesis in the presence of sera from normal human subjects and from patients with dermal fibroses. J Invest Dermatol 1981; 76(6): 462–467. [DOI] [PubMed] [Google Scholar]
- 34. Lee DY, Yang JM, Baek MK. A dermal equivalent can be developed from fibroblast culture by means of a high concentration of serum. Br J Dermatol 2011; 164(5): 1109–1111. [DOI] [PubMed] [Google Scholar]
- 35. Bueno EM, Saeidi N, Melotti S, et al. Effect of serum and insulin modulation on the organization and morphology of matrix synthesized by bovine corneal stromal cells. Tissue Eng Part A 2009; 15(11): 3559–3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. L’Heureux N, Pâquet S, Labbé R, et al. A completely biological tissue-engineered human blood vessel. FASEB J 1998; 12(1): 47–56. [DOI] [PubMed] [Google Scholar]
- 37. Syedain ZH, Meier LA, Bjork JW, et al. Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring. Biomaterials 2011; 32(3): 714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Switzer BR, Summer GK. Collagen synthesis in human skin fibroblasts: effects of ascorbate, α-ketoglutarate and ferrous ion on proline hydroxylation. J Nutr 1972; 102(6): 721–728. [DOI] [PubMed] [Google Scholar]
- 39. Phillips CL, Combs SB, Pinnell SR. Effects of ascorbic acid on proliferation and collagen synthesis in relation to the donor age of human dermal fibroblasts. J Investig Dermatol 1994; 103(2): 228–232. [DOI] [PubMed] [Google Scholar]
- 40. Bjork JW, Meier LA, Johnson SL, et al. Hypoxic culture and insulin yield improvements to fibrin-based engineered tissue. Tissue Eng Part A 2012; 18(7–8): 785–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goldstein RH, Hpoliks CF, Pilch PF, et al. Stimulation of collagen formation by insulin and insulin-like growth factor I in cultures of human lung fibroblasts*. Endocrinology 1989; 124(2): 964–970. [DOI] [PubMed] [Google Scholar]
- 42. Weidenhamer NK, Tranquillo RT. Influence of cyclic mechanical stretch and tissue constraints on cellular and collagen alignment in fibroblast-derived cell sheets. Tissue Eng Part C 2013; 19(5): 386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Neidert MR, Lee ES, Oegema TR, et al. Enhanced fibrin remodeling in vitro with TGF-β1, insulin and plasmin for improved tissue-equivalents. Biomaterials 2002; 23(17): 3717–3731. [DOI] [PubMed] [Google Scholar]
- 44. Lee DY, Yang JM, Park KH. A dermal equivalent developed from fibroblast culture alone: effect of EGF and insulin. Wound Repair Regen 2007; 15(6): 936–939. [DOI] [PubMed] [Google Scholar]
- 45. Reed MAYJ, Vernon RB, Abrass IB, et al. TGF-β1 induces the expression of type I collagen and SPARC, and enhances contraction of collagen gels, by fibroblasts from young and aged donors. J Cell Physiol 1994; 158: 169–179. [DOI] [PubMed] [Google Scholar]
- 46. Colige A, Nusgens B, Lapiere CM. Response to epidermal growth factor of skin fibroblasts from donors of varying age is modulated by the extracellular matrix. J Cell Physiol 1990; 145(3): 450–457. [DOI] [PubMed] [Google Scholar]
- 47. Hata R, Sunada H, Arai K, et al. Regulation of collagen metabolism and cell growth by epidermal growth factor and ascorbate in cultured human skin fibroblasts. Eur J Biochem 1988; 173(2): 261–267. [DOI] [PubMed] [Google Scholar]
- 48. Song R, Bian HN, Lai W, et al. Normal skin and hypertrophic scar fibroblasts differentially regulate collagen and fibronectin expression as well as mitochondrial membrane potential in response to basic fibroblast growth factor. Brazilian J Med Biol Res 2011; 44(5): 402–410. [DOI] [PubMed] [Google Scholar]
- 49. Shi HX, Lin C, Lin BB, et al. The anti-scar effects of basic fibroblast growth factor on the wound repair in vitro and in vivo. PLoS One 2013; 8(4): e59966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Satyam A, Kumar P, Cigognini D, et al. Low, but not too low, oxygen tension and macromolecular crowding accelerate extracellular matrix deposition in human dermal fibroblast culture. Acta Biomater. 2016; 44: 221–231. [DOI] [PubMed] [Google Scholar]
- 51. Kumar P, Satyam A, Fan X, et al. Macromolecularly crowded in vitro microenvironments accelerate the production of extracellular matrix-rich supramolecular assemblies. Sci Rep 2015; 5: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Grémare A, Guduric V, Bareille R, et al. Characterization of printed PLA scaffolds for bone tissue engineering. J Biomed Mater Res A 2018; 106(4): 887–894. [DOI] [PubMed] [Google Scholar]
- 53. Guduric V, Metz C, Siadous R, et al. Layer-by-layer bioassembly of cellularized polylactic acid porous membranes for bone tissue engineering. J Mater Sci Mater Med 2017; 28(5): 78. [DOI] [PubMed] [Google Scholar]
- 54. Sacco AM, Belviso I, Romano V, et al. Diversity of dermal fibroblasts as major determinant of variability in cell reprogramming. J Cell Mol Med 2019; 23(6): 4256–4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pptx-1-tej-10.1177_2041731420978327 for Cell-assembled extracellular matrix (CAM) sheet production: Translation from using human to large animal cells by Yoann Torres, Maude Gluais, Nicolas Da Silva, Sylvie Rey, Agathe Grémare, Laure Magnan, Fabien Kawecki and Nicolas L’Heureux in Journal of Tissue Engineering