Abstract
Autoimmune liver disease (AILD) is a series of chronic liver diseases with abnormal immune responses, including autoimmune hepatitis (AIH), primary biliary cholangitis (PBC), and primary sclerosing cholangitis (PSC). The treatment options for AILD remain limited, and the adverse side effects of the drugs that are typically used for treatment frequently lead to a low quality of life for AILD patients. Moreover, AILD patients may have a poor prognosis, especially those with an incomplete response to first-line treatment. Mesenchymal stem cells (MSCs) are pluripotent stem cells with low immunogenicity and can be conveniently harvested. MSC-based therapy is emerging as a promising approach for treating liver diseases based on their advantageous characteristics of immunomodulation, anti-fibrosis effects, and differentiation to hepatocytes, and accumulating evidence has revealed the positive effects of MSC therapy in AILD. In this review, we first summarize the mechanisms, safety, and efficacy of MSC treatment for AILD based on work in animal and clinical studies. We also discuss the challenges of MSC therapy in clinical applications. In summary, although promising data from preclinical studies are now available, MSC therapy is currently far for being applied in clinical practice, thus developing MSC therapy in AILD is still challenging and warrants further research.
Keywords: autoimmune hepatitis, mesenchymal stem cell, primary biliary cholangitis, primary sclerosing cholangitis, therapy
Introduction
Autoimmune liver disease (AILD) is a unique type of chronic liver disease caused by immune dysfunction, which consists of three different types: autoimmune hepatitis (AIH), primary biliary cholangitis (PBC), and primary sclerosing cholangitis (PSC). These three forms of AILD share some common clinical features such as fatigue, loss of appetite, liver discomfort, and icteric sclera, and result in abnormal levels of liver function indicators in a blood test.1 Moreover, in line with the most prominent feature of autoimmune diseases, AILD patients produce autoantibodies. However, the pathogenesis of AILD remains poorly understood. Multiple types of immune cells are recruited into the liver in response to the production of self-antigens, leading to an inflammatory immune reaction.2 Long-term chronic inflammation in the liver leads to liver fibrosis, which can ultimately progress to end-stage liver diseases such as liver cirrhosis and liver failure. Moreover, patients with PBC and AIH are more prone to hepatocellular carcinoma (HCC), whereas patients with PSC have a significantly increased risk of developing cholangiocarcinoma (CCA).3
Although AILD is not very prevalent, if left untreated, the risk of mortality and morbidity increases. Last decades have witnessed the progress in the treatment of AILD which aims to improve clinical symptoms and halt disease progression. For patients with AIH, steroids are used for remission induction and azathioprine (AZA) is often used for remission maintenance. Other available drugs of second- and third-line therapy for AIH such as mycophenolate mofetil and tacrolimus are options for AIH patients with insufficient response or intolerance to the standard therapy. However, these immunosuppressive drugs are associated with many side effects, including Cushingoid features, infections, osteoporosis, and gastrointestinal issues.4 Although treatment with ursodeoxycholic acid (UDCA) is generally considered to be effective in improving biliary function, 25–50% of patients with PBC fail to achieve a complete biochemical response from UDCA treatment.5 An innovative registered drug, farnesoid X receptor (FXR) agonist obeticholic acid (OCA), is a choice for PBC patients with an incomplete response to UDCA therapy. However, OCA always leads to pruritus and is not recommended for patients with decompensated PBC.6 As for patients with PSC, there are no effective treatments at present.7 Liver transplantation is a choice for AILD patients with end-stage liver disease, HCC or CCA, under strict criteria. However, several factors, including the age of transplant recipients, comorbidities, and extrahepatic neoplasms must be taken into consideration when deciding whether offering or not a graft to such patients.8 Besides, the risk of recurrent disease at 10 years is about 20% after liver transplantation.9 Thus, there is an unmet need for treating patients with AILD, and a new therapy is urgently required.
Recent studies have emphasized the broad potential of the clinical application of cell therapy; in particular, mesenchymal stem-cell (MSC) therapy has emerged as a promising treatment owing to its several advantages and has received substantial research attention. MSCs are fibroblast-like plastic-adherent cells with self-renewal and differentiation ability.10 MSCs can be isolated from multiple tissues and expanded massively in vitro, which is a convenient characteristic for clinical use.11 Particularly, the anti-fibrosis, immunoregulation, and hepatocyte differentiation properties make MSCs a promising candidate for AILD treatment.12 In this review, we first focus on recent research highlighting the prospects and underlying mechanisms of MSC therapy in AILD, and address the challenges toward developing this novel treatment for clinical application.
Overview of MSCs
MSCs were first isolated from bone marrow by Friedenstein et al.10 in 1968; later studies found MSCs could derive from many other tissues such as adipose tissue, umbilical cord, dental pulp, amniotic fluid, and placenta.13–17 MSCs have the capacity for self-renewal, proliferation and tri-lineage differentiation towards mesoderm cells, including osteoblasts, adipocytes, and chondroblasts, as well as ectoderm and endoderm cells such as hepatocytes, neurons, and pancreatic islet-β cells, under specific conditions.11,18–20 In addition, accumulating evidence indicates that MSCs can also function by secreting exosomes through which proteins and ribonucleic acids (RNAs) could be delivered to recipient cells and exert specific effects.21
Although MSCs of various origins express identical markers and present the same functions, they have different levels of immunoregulation and differentiation.22 For example, Mattar and Bieback23 summarized that umbilical-cord-derived MSCs (UC-MSCs) have stronger capability to induce regulatory T cells (Tregs) and reduce the endocytic ability of dendritic cells (DCs) than bone-marrow-derived MSCs (BM-MSCs). Another study found that UC-MSCs have a higher rate of proliferation and osteogenic differentiation than BM-MSCs.24
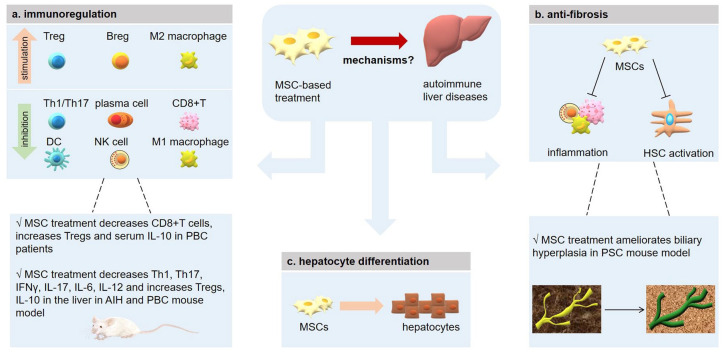
Many studies have demonstrated the safety and feasibility of MSC therapy for different diseases, including graft-versus-host disease (GVHD), cardiovascular disease, cancer, osteoarthritis, diabetes, and liver cirrhosis.25–29 In treating liver diseases, MSCs could migrate to the liver and differentiate into hepatocytes to replace injured cells and restore liver function.30 Moreover, MSCs may inhibit inflammation through their immunoregulatory function to promote hepatocyte survival.31 In addition, MSCs have the ability to attenuate liver fibrosis and slow liver disease progression (Figure 1).32
Figure 1.
Mechanisms of MSC-based treatment in autoimmune liver diseases.
AIH, autoimmune hepatitis; Breg, regulatory B cell; DC, dendritic cell; HSC, hepatic stellate cell; IFN-γ, interferon-γ; IL-10, interleukin-10; MSC, mesenchymal stem cell; NK cell, natural killer cell; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; Th, helper T cell; Treg, regulatory T cell.
MSC function and underlying treatment targets
Roles of MSCs in immunoregulation
Accumulating evidence has highlighted the role of MSCs in immunoregulation. MSCs can interact with immune cells in the liver through several means, including direct cell-to-cell contact or through the secretion of cytokines and other substances.
T cells
The interaction between MSCs and T cells has been intensively investigated. MSCs can inhibit T-cell proliferation through secreting a series of anti-inflammatory molecules, such as nitric oxide, indoleamine 2,3-dioxygenase (IDO), prostaglandin E2 (PGE2), interleukin-10 (IL-10), programmed cell-death 1 ligand 1 (PD-L1), transforming growth factor-β1 (TGF-β1), IL-6, heme oxygenase-1 (HO-1), hepatocyte growth factor (HGF), and galectins.33–38 MSC could inhibit the activation and cytotoxicity capacity of CD8+T cells, as well.39,40 In addition, the suppressive function of MSCs on T cells has been demonstrated in many diseases, including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), GVHD, and liver diseases.41–44 MSCs express high levels of IDO when stimulated by interferon gamma (IFN-γ) and promote the degradation of tryptophan into kynurenine, which could in turn inhibit the proliferation of activated T cells.45 Besides, MSCs can promote T-cell apoptosis via the Fas/FasL pathway.46 MSCs were also known to exert an effect on the differentiation of CD4+ T cells. Specifically, MSCs can inhibit naïve CD4+ T cells from differentiating towards T-helper 1 (Th1) and T-helper 17 (Th17) cells, but can promote the differentiation of CD4+CD25+FOXP3+ (forkhead box P3) regulatory T cell (Treg) and IL-10+ Treg cells.47,48 MSC could secrete TGF-β and activating Smad2 (SMAD family member 2) signaling, which is important for Treg regulation, thus promoting the process of Treg differentiation.49 Evidence showed that MSC therapy in an experimental autoimmune encephalomyelitis model led to an increase in the Treg population and a decrease in the Th17 population, which ultimately resulted in amelioration of the disease.48
B cells
MSCs can also affect B-cell immune responses. Early studies suggested that MSCs could inhibit the proliferation of B cells by arresting the cell cycle at the G0/G1 phase and by engaging programmed cell-death protein 1 (PD-1)/PD-L1 pathway via direct contact.50,51 Moreover, MSCs could suppress the production of immunoglobulin G1 (IgG1) and IgM during B-cell terminal differentiation in mice.52 The underlying mechanisms associated with these effects have also been explored. MSCs could secrete CCL2 (C-C motif chemokine ligand 2), which inhibits signal transducer and activator of transcription 3 (STAT3) activation and promotes paired box 5 (PAX5) expression in plasma cells, thereby suppressing Ig production in B cells.53 IL-1 receptor antagonist (IL-1RA) and olfactory 1/early B-cell factor-associated zinc-finger protein (OAZ) are also important molecules in this immunoregulation process.54,55 Schena et al.56 found that MSCs could inhibit B-cell receptor (BCR)-activated B-cell proliferation and that MSC treatment in SLE mice significantly improved the renal histopathology scores. On the other hand, MSC could induce IL-10-producing CD19+CD24highCD38high regulatory B cells (Breg) in human.57 In line with these findings, Chao et al.58 reported that MSCs ameliorated experimental colitis by strikingly increasing the number of IL-10-producing CD5+ Bregs. Similarly, co-culturing with MSCs could enhance the immunosuppressive activity of B cells by inducing unconventional IL-10-producing CD23+CD43+ Bregs in the same disease mouse model.59
Macrophages
Macrophages specific to the liver are known as Kupffer cells (KCs), which can be classified into two types: the pro-inflammatory type (M1), which undergoes classical activation; and anti-inflammatory type (M2), which undergoes alternative activation. M2 macrophages usually secrete high levels of IL-10 and low levels of IL-6, IL-12, IL-1β and tumor necrosis factor-α (TNF-α), along with higher ability of phagocytosis to exert a negative effect on inflammation.60 Many studies have suggested that MSCs play an important role in the process of macrophage polarization and could promote the differentiation toward the M2 phenotype both in vitro and in vivo. MSC-educated macrophages (MEMs) express more inhibitory molecules such as PD-1/PD-L1 and have a quite different gene profile from that of normal macrophages, which include genes that are positively correlated with anti-inflammatory effects and tissue repair. MEMs were shown to be superior to MSCs in promoting the survival of a GVHD and radiation injury mouse model in vivo.61 These effects of MSCs have also been observed in several other diseases such as RA, wound healing, and acute liver injury.62–64 MSCs can secrete TNF-α-stimulated gene 6 protein (TSG6) to interact with CD44 on the macrophages, and can decrease the TLR2-mediated NF-κB (nuclear factor kappa light chain enhancer of activated B cells) activation of macrophages in a zymosan-induced peritonitis mouse model.65 Moreover, MSCs could mitigate colitis through the upregulation of TGF-β1 expression by recruiting macrophages to the inflammation site.66 In addition, MSC-secreted exosomes could promote a shift in balance to a predominant M2 type by decreasing IL-6 levels and increasing the levels of IL-10 and monocyte chemo-attractant protein-1 (MCP-1), which is a key molecule in macrophage recruitment and activation.67
Other immune cells
Natural killer (NK) cells are key effector immune cells of the innate immune response. MSCs can suppress the proliferation and cytotoxicity of NK cells.68 This effect has been investigated in a liver injury model in which MSC therapy could inhibit the activation of NK cells and improve the liver condition.69 As for dendritic cells (DCs), the most potent antigen-presenting cell (APC), MSCs have been shown to inhibit their maturation, activation, and migration.70,71 MSCs could also induce regulatory DCs to ameliorate disease progression in a fulminant hepatic failure model.44
Summary
MSCs have a great impact on all kinds of immune cells and this property gives them the potential to treat many diseases with an abnormal immune regulation. Notably, the immunosuppressive ability of MSCs is dependent on the strengths and types of inflammatory signals they receive. MSC pre-treating with pro-inflammatory cytokines like IFN-γ and IL-1β can gain a stronger anti-inflammatory ability.72,73 Therefore, this special characteristic should be taken into account when applying MSCs for treatment.
Anti-fibrosis effects of MSCs
Liver fibrosis is a condition characterized by loss of hepatocytes and accumulation of extracellular matrix (ECM), which could result from chronic injury of any etiology. In response to liver injury, many pro-inflammatory cytokines, including TGF-β, IL-4, IL-13, are secreted by infiltrating and resident immune cells. Hepatic inflammation further activates hepatic stellate cells (HSCs) to develop into myofibroblasts, which are the major source of ECM and other matrix proteins responsible for scar formation.74,75 MSC-based treatment in vivo has exhibited therapeutic effects in several liver fibrosis animal models induced by carbon tetrachloride (CCL4) or thioacetamide (TAA), and in several clinical trials.
Inflammation is a strong pathogenic factor in liver fibrosis. Since MSCs have a considerable impact on the immune system, the interaction between MSCs and immune cells has been widely investigated in liver fibrosis. Macrophages can activate fibrogenic myofibroblasts by secreting TGF-β1 and play a pathogenic role in liver fibrosis. Co-culturing MSCs with colony-stimulating factor-1-induced macrophages could induce macrophage development toward the anti-inflammatory M2 phenotype with higher phagocytic activity conferred through elevated expression of PGE2 and TSG-6. Combining MSCs with macrophages was shown to reduce the degree of liver fibrosis more efficiently than MSC monotherapy, and also resulted in higher levels of antifibrotic factors such as matrix metalloproteinases (MMPs) and pro-regenerative factors such as vascular endothelial growth factor.76 MSCs could also induce M2-type macrophages via increasing IL-4 and IL-10 levels, by promoting the mobilization of macrophages both in vitro and in vivo, ultimately alleviating liver fibrosis in rats.77 MSC treatment was also found to potentially promote Treg expansion and to significantly suppress the proliferation of Th17 cells in the liver of CCL4-treated mice via the production of IDO, leading to attenuation of liver fibrosis.78
HSCs play a vital role in the pathogenesis of liver fibrosis. MSCs have been shown to suppress the expression of Delta-like 1 (Dlk1), which is an HSC activator and promotes liver fibrogenesis, thereby ameliorating liver fibrosis.79 Besides, Meier et al.80 showed that conditioned medium from human BM-MSCs could inactivate HSCs in vitro. Furthermore, co-culturing BM-MSCs with HSCs could induce apoptosis and inhibit the proliferation of HSCs.81 Other mechanisms of MSC inhibition on liver fibrosis have also been uncovered. In a TAA-induced cirrhotic rat model, MSC administration significantly decreased the expression of TGF-β1, collagen-1, and α-smooth muscle actin (α-SMA) expression and inhibited Smad3 phosphorylation, which is a downstream effector of the TGF-β1 signaling pathway.82 Another study found that MSCs express high levels of bone morphogenic protein 7 (BMP7) and could mitigate cirrhosis in a CCL4-induced mouse model of liver disease and depletion of BMP7 in MSCs completely abolished their protective effect.83 MSC treatment was also found to reduce the level of collagen deposition by upregulating MMP-13 expression and downregulating TIMP-1 (tissue inhibitor of metalloproteinase-1) expression; overexpression of MMP1 in MSCs further enhanced their anti-fibrotic ability.84
Overall, these studies show that MSCs could ameliorate liver fibrosis via their anti-inflammatory effects, indirectly, and inactivation of HSCs, directly.
Roles of MSCs in hepatocyte differentiation
The immunoregulation and anti-fibrosis properties of MSCs are critical for hepatocyte survival. MSC therapy has been shown to protect the acutely injured liver by directly inhibiting hepatocellular apoptosis and stimulating tissue regeneration.85 Moreover, MSC can repair liver tissue damage by differentiating into hepatocytes and replacing injured cells, thereby restoring liver function.30
Schwartz et al.86 reported that culturing MSCs with fibroblast growth factor-4 (FGF-4) and HGF helped MSCs differentiate into hepatocyte-like cells based on the expression of hepatocyte markers, including nuclear factor-3b (HNF-3b), GATA-binding protein 4 (GATA4), cytokeratin 19 (CK19), transthyretin, a-fetoprotein, CK18, hepatocyte nuclear-factor 4 (HNF-4), HNF-1a, and albumin. Lee et al.20 subsequently designed a novel two-step protocol using HGF and oncostatin M protein to induce the hepatic differentiation of MSCs. Moreover, MSCs can differentiate into hepatocyte-like cells when co-cultured with liver cells or grown in pellet culture.87,88 Based on these in vitro studies, the ability of MSCs to differentiate into hepatocytes has also been investigated in vivo. Studies involving the transfer of human MSCs to liver injury models of rats, mice, and sheep all demonstrated that MSCs consistently differentiated to hepatocyte-like cells.89–93 Moreover, the site of MSC injection is an important consideration, because MSCs preferably distribute at the periportal regions following intraperitoneal injection and can generate hepatocytes more efficiently by intrahepatic injection in sheep.94 These observations provide evidence that MSC treatment for liver disease is feasible owing to their differentiation function; however, this possibility requires obtaining deeper insight into whether MSC-differentiated hepatocytes could provide sufficient metabolic and trophic support in the liver.
MSC-based therapy for AILD
AIH
AIH is a chronic liver disease that affects people of all ages but is more often seen in women and elderly people.95 The prevalence of AIH is 17.44 per 100,000 people worldwide according to a meta-analysis based on 22 studies, and the incidence seemed to double from 1997 to 2015 in an English cohort.96,97 Patients with AIH often manifest elevated serum alanine aminotransferase (ALT), IgG, presence of autoantibodies, and interface hepatitis. AIH can be classified into two types: patients with AIH type 1 (AIH-1) account for 95% of all AIH patients, characterized by positive anti-nuclear antibodies and anti-SMA. Type 2 AIH (AIH-2) is characterized by the presence of anti-liver kidney microsomal type 1 antibodies (anti-LKM1) and/or anti-liver cytosol type 1 (anti-LC1). Most AIH patients require lifelong immunosuppressive treatment; steroids are often used for remission induction, whereas AZA is used for maintenance. However, some patients insufficiently respond to standard therapy or cannot tolerate it.98 Moreover, a study from the Netherlands showed that low doses of corticosteroids could still lead to substantial adverse events such as bone fractures, which contradicted the assumption that administering low doses of corticosteroids could prevent adverse events.99 Thus, AIH patients need to seek alternatives to traditional treatment.
Chen et al.100 established an experimental autoimmune hepatitis (EAH) mouse model induced by liver antigen S100 and treated the EAH mice with 1 × 105 MSCs via the tail vein one to three times on days 21, 28, and 35 according to the different group settings.
One group of EAH mice was administered prednisolone and AZA as a positive control. The EAH mice that received MSCs had attenuated ALT and AST (aspartate aminotransferase) levels, and improved liver histological scores. They also found that the levels of PD-L1 in the liver and serum of EAH mice were higher than those in the normal control mice, and the level of PD-L1 gradually increased with increasing duration of MSC treatment. It is generally believed that an elevated level of PD-L1 plays an anti-inflammatory role in inflammatory diseases, thus, this result indicated that MSCs could increase the PD-L1 level to inhibit inflammation.101 In contrast, the level of the pro-inflammatory cytokine IL-17 in EAH model mice was higher than that in normal control mice, and the use of drugs and MSC treatment reduced IL-17 levels significantly, especially in mice that received multiple doses of MSCs. The role of IL-23 in AIH remains controversial. Some studies suggested that IL-23 can protect against AIH given evidence that IL-23-deficient mice were more susceptible to concanavalin A (ConA)-induced hepatitis.102 In this study, the level of IL-23 in the EAH mouse model decreased but increased after treatment with drugs and MSCs. Therefore, this study supports the MSC treatment efficiency in EAH, and suggested the possible mechanism by which MSCs could ameliorate EAH by upregulating PD-L1 and inhibiting IL-17.
Recently, studies suggest that MSCs can mediate their therapeutic functions in a paracrine rather than a cellular manner, thus, a novel cell-free therapy using MSC-secreted exosomes holds promise for treating many diseases.103 Several studies focused on AIH also investigated the role of MSC-secreted exosomes. Chen et al.104 infected BM-MSCs with pre-miR-223, miR-223 inhibitor, or empty vector, and isolated exosomes from the culture medium that were then intraperitoneally injected into EAH mice. The results showed that both BMSCs-exomiR-223(+) and BMSCs-exomiR-223(null) treatment significantly lowered the levels of ALT and AST, and inflammatory cytokines such as IL-17, TNF-α and IL-1β, and these observations were more obvious in BMSCs-exomiR-223(+) mice. In contrast, mice that received BMSCs-exomiR-223(–) exhibited a more severe condition. The mechanism underlying this effect was considered related to the ability of BMSCs-exomiR-223(+) and BMSCs-exomiR-223(null) to inhibit the downstream NLRP3 [NLR (nucleotide-binding oligomerization domain-like receptors) family pyrin domain containing 3]-caspase-1 pathway through binding between miR-223 and the 3'-untranslated region of NLRP3, which is highly activated in the hepatocytes of EAH mice. Lu et al.105 further revealed a new role of MSC-derived exosomes in EAH mice. Mice treated with MSC-exosomes and MSC-exosomes miR-223-3p(+) showed increased serum IL-10 levels and had normal or even higher Treg/Th17 ratios. Reduction of the expression of STAT3 and p-STAT3 by MSC-exosomes and MSC-exosomes miR-223-3p(+) may explain this effect. Moreover, this effect was validated in a macrophage cell line, in which treatment with both MSC-exosomes and MSC-exosomes miR-223-3p(+) inhibited LPS (lipopolysaccharide)-induced macrophage inflammation, as shown by reduced levels of IL-1β, IL-6, STAT3, and p-STAT3. This study emphasized that MSC-derived exosomes could effectively deliver miR-223-3p to regulate the inflammatory and anti-inflammatory cytokines and upregulate the Treg/Th17 ratio by inhibiting the activation of STAT3. These data indicate that MSC-secreted exosome therapy is effective in treating EAH mice. However, there are some issues with this method for clinical translation. First, exosomes should be modified to target the liver or specific organs. Second, the components of exosomes remain to be identified.
Wang et al.106 modified adipose-tissue-derived MSCs with IL-35 lentivirus and intravenously injected IL-35-MSC or MSC or PBS (phosphate-buffered saline) into ConA-induced AIH mice. Mice in the IL-35-MSC group showed the longest survival and had less liver necrosis. Apoptosis markers such as FasL, and pro-inflammatory cytokines like IFN-γ and IL-17 of liver mononuclear cells (MNCs) in IL-35-MSC mice greatly decreased. Tracing of transplanted IL-35-MSCs suggested that the cells specifically migrated to the injured liver rather than to other organs. In addition, IL-35-MSC treatment enhanced the Janus kinase 1 (JAK1)-STAT1/STAT4 signaling pathway. IL-35 is known as an anti-inflammatory cytokine that is highly expressed by human and mouse Tregs.107 This study demonstrated that introduction of the IL-35 gene into MSCs could help to deliver the anti-inflammatory effect of IL-35 through the homing of MSCs to injury sites to achieve targeted therapy. Overall, this study highlighted that gene-modified MSCs could function better than pure MSCs and exert a stronger impact in treating EAH.
In summary, although some AIH animal studies of MSC therapy have achieved encouraging results, there have been no clinical studies based on MSC therapy conducted to date. Therefore, it is still unknown whether MSCs could have a clinically beneficial effect to improve AIH, and to solve this problem requires further basic and clinical studies.
PBC
PBC is a typical autoimmune disease characterized by non-suppurative inflammation in the small interlobular bile ducts. PBC mainly affects middle-aged women, with a prevalence of 39.2 per 100,000 people.108 Approximately 90% of PBC patients are positive for diagnostic-specific antimitochondrial antibody (AMA), which targets PDC-E2 (the epitope of the E2 subunit of the pyruvate dehydrogenase complex).109 UDCA monotherapy is typically the first-line treatment upon a diagnosis of PBC. However, UDCA can only delay the progression of hepatic fibrosis in the early stage and is not effective in cases of advanced disease.5,110 Besides, 25–50% of PBC patients do not respond to UDCA and are therefore at a higher risk for disease progression. These patients are indicated for second-line drugs such as FXR agonist OCA; however, OCA has some side effects such as dose-dependent pruritus, which occurs in up to 10% of patients and is a major cause of therapy discontinuation.111 Thus, many new therapies for PBC are currently under exploration.
Wang et al.112 first examined the effect of BM-MSC treatment in a PBC mouse model induced by polyinosinic-polycytidylic acid sodium (Poly I:C). Mice were administered 1 × 106 BM-MSCs intravenously and the same volume of PBS was provided to the control group. After 6 weeks of treatment, serum alkaline phosphatase (ALP) levels and AMA titers in the treatment group had decreased markedly. In addition, lymphocytes infiltrating the liver bile duct epithelium also significantly reduced, suggesting that MSCs may inhibit the proliferation and infiltration of immune cells in the liver. Mice in the BM-MSC-treated group also showed attenuated serum levels of inflammatory cytokines such as IFN-γ, which indicates that MSCs may inhibit the Th1 immune response that mediates liver injury. Of note, mice receiving MSC therapy showed increased Tregs in the peripheral blood and lymph nodes, and higher serum levels of TGF-β, which is a cytokine that promotes Treg differentiation. Overall, this study provided the first evidence that MSCs are effective in treating Poly I:C-induced PBC mouse model possibly by involving the interplay between TGF-β and Tregs.
These encouraging results of MSC treatment in PBC animal models herald the prospects and provide biological evidence for clinical research. To date, several clinical studies have explored the efficacy and safety of MSC therapy in PBC patients. A study conducted in China enrolled seven PBC patients with an abnormal ALP level after a minimum of 6 months of adequate UDCA dosage treatment.113 UC-MSCs were infused intravenously into the patients at a concentration of 0.5 × 106 cells/kg body weight once every 4 weeks on three occasions in combination with standard UDCA therapy. None of the patients showed symptoms of short-term adverse effects or long-term complications. Follow-up results showed that serum ALP and gamma-glutamyl transferase (GGT) levels significantly decreased in patients by 48 weeks after receiving UC-MSC treatment. Common clinical symptoms of PBC patients such as fatigue, pruritus, and hypogastric ascites volumes, also improved. This study indicated that UC-MSC transfusion through a peripheral vein is safe and feasible in PBC patients. However, the study was limited by its small sample size and lack of data on liver histological changes. A subsequent study conducted by our group included 10 PBC patients who had an incomplete biochemical response to UDCA for more than 1 year and received 3–5 × 105 cells/kg body weight BM-MSCs by intravenous infusion.114 All patients tolerated the MSC treatment well, and their responses to the PBC-40 questionnaire suggested that they had an improved life quality, especially with respect to the itching, fatigue, and emotional function domains. Blood tests showed that ALT, GGT, and direct bilirubin (DBIL) decreased at 3 and 6 months compared with baseline. The percentage of Tregs in the peripheral blood mononuclear cells of patients significantly increased at 6 months, but total CD4+ T-cell and CD19+ B-cell percentages were not changed. The serum levels of the anti-inflammatory cytokine IL-10 also increased but there was no increase in the level of TGF-β. We also collected two liver biopsies before, and 12 months after, BM-MSC treatment for comparison. Interestingly, no histological progress was observed and there were no significant differences in the frequencies of CD8+ T cells and Tregs, which are important for PBC pathogenesis. This lack of difference may be because of the relatively late time of liver biopsy since our results indicated the therapeutic effect of BM-MSCs reached the peak from 3 to 6 months after MSC infusion.
In summary, both animal experiments and clinical studies have confirmed the safety of MSCs and uncovered their potential for PBC treatment. Nevertheless, it should be noted that both the clinical trials performed to date recruited a small number of patients. Therefore, larger-scale studies with a randomized design are required to offer more strong evidence of the therapeutic use for MSC in PBC.
PSC
PSC is a rare disease with a prevalence of 6–16 per 100,000 people in the general population, and is characterized by damage of the large intra- and extrahepatic bile ducts which leads to stricturing and dilation of the biliary tree, ultimately resulting in finally biliary cirrhosis and portal hypertension.115,116 About 70–80% of patients with PSC have inflammatory bowel disease (IBD), especially ulcerative colitis (UC).117 PSC patients are at a higher risk for several cancers such as CCA and gallbladder adenocarcinoma. PSC patients with IBD are also prone to developing colorectal cancer.3 Previous studies have explored the potential of UDCA in the treatment of PSC given the associated damage of the biliary ducts as in PBC, and these results suggested that although long-term use of UDCA could improve serum liver indicators, it could not improve survival and led to a series of serious adverse events.118 Clinical trials using other immunosuppressive drugs, including corticosteroids, AZA, and cyclosporin, failed to achieve satisfactory results. Biological drugs such as anti-TNF-α-like etanercept are also found to be ineffective for PSC.119 Therefore, there are currently no effective treatments for PSC.7 The estimated median survival of PSC patients from diagnosis to liver transplantation or death ranges from 10 to 21 years, and up to 40% of patients require liver transplantation eventually.115,120 A follow-up study of PSC patients showed that the recurrence rate of PSC at 1, 5, and 10 years after transplantation was 2%, 12%, and 20%, respectively; and the 1-, 5-, and 10-year recurrence-free survival rates were 91%, 76%, and 61%, respectively.121 Thus, PSC patients have a poor prognosis and there is an urgent need for new treatment options for PSC patients.
A major challenge in identifying an effective treatment for PSC is that the pathogenesis of PSC remains poorly understood. Both genetic factors, such as human leukocyte antigen, and environmental factors, such as infection, are suggested to be contributors to the pathogenesis of PSC.122 In addition to traditional etiologies, recent studies have shown that immune dysregulation plays a pathogenic role in PSC. A higher IFN-γ level in PSC mouse models was associated with stronger cytotoxicity of CD8+ T cells and NK cells, and the absence of IFN-γ could decrease the rate of liver cell death, reduce the frequencies of inflammatory macrophages in the liver, and attenuate liver fibrosis.123 High number of M1-type macrophages were found to be recruited by cholangiocytes to the peribiliary region via the CCR2/CCL2 axis in PSC patients and animal models, and depletion of CCR2 could prevent biliary injury and fibrosis.124
To date, only one study has explored the possibility of MSC therapy in a PSC animal model. Sugiura et al.125 induced the development of sclerosing cholangitis in rat using alpha-naphthylisothiocyanate (ANIT), which targets the intrahepatic bile ducts.125 They intravenously injected human amnion-derived MSCs (hAMSCs), conditioned medium (CM) obtained from hAMSCs, or PBS to the rats through the penile vein. Injection of hAMSCs and CM significantly ameliorated biliary hyperplasia, with downregulated CK19 expression and fewer necrotic lesions caused by ANIT; however, fibroblast proliferation was not attenuated. In addition, hAMSCs and CM therapy tended to decrease the levels of peribiliary fibrosis markers such as α-SMA, TGF-β, type I collagen, MMP-2, MMP-9, and TIMP-1. The infiltration of CD68+ KCs in the Glisson’s sheath was found to decrease after hAMSCs and CM therapy. Therefore, this study first demonstrated that hAMSC transplantation and CM administration ameliorated biliary hyperplasia, peribiliary fibrosis, and inflammation in a rat model of PSC. However, the immunoregulatory function of MSCs has not been further explored.
Overall, the therapeutic potential of MSCs in PSC is not well established. The low prevalence of PSC and a lack of well-characterized PSC animal models may delay this investigation process. PSC is mainly characterized by over-activation of the immune system, suggesting that MSCs may exert an immunoregulatory effect. This interaction warrants further investigation to provide more evidence on the safety and efficacy of MSC therapy in PSC.
Summary
The results from several animal and clinical studies are promising and may provide evidence of the efficacy of safety of MSC therapy in AILD (Table 1). However, these results should be interpreted with caution due to a small sample size in each study and a limited number of clinical trials. There are also some ongoing clinical trials of MSC treatment in AILD registered on the Clinical Trial Registry (https://clinicaltrials.gov/): one in PSC [ClinicalTrials.gov identifier: NCT03516006]; one in AIH [ClinicalTrials.gov identifier: NCT01661842], and one in PBC [ClinicalTrials.gov identifier: NCT03668145]. Overall, more data from clinical trials are required.
Table 1.
Characteristics of preclinical and clinical studies of MSC-based treatment in autoimmune liver diseases.
| Study | Condition | Number of patients or animal models | Country | MSC-based treatment | Number of injections | Dosage and delivery routes | Clinical outcomes | Potential mechanisms | |
|---|---|---|---|---|---|---|---|---|---|
| Clinical study | Wang et al.113 | UDCA-resistantPBC | 7 | China | Human UC-MSC | 3 | 0.5 × 106 cells/kg body weight, i.v. | ALP↓, GGT↓, symptoms (fatigue, pruritus) improved, Mayo risk score↑; one self-limiting fever; no short-term or long-term complications | NA |
| Wang et al.114 | UDCA-resistantPBC | 10 | China | Human BM-MSC | 1 | 0.3–0.5 × 106 cells/kg body weight, i.v. | ALT↓, AST↓, GGT↓, DBIL↓, IgM↓, symptoms (fatigue, itchiness, emotional dysfunction) improved; no adverse events were reported | Peripheral CD8+T cells↓, Treg↑; serum IL-10↑ | |
| Animal model study | Chen et al.100 | Hepatic S100-induced AIH mice | 6 in each group | China | Mice BM-MSC | 1/2/3 | 1 × 105 cells, 100 μl, i.v. | ALT↓, AST↓, liver histological score↓ | Liver PD-L1 ↑, IL-17↓, IL-23↑; serum IL-17↓, IL-23↑ |
| Chen et al.104 | Hepatic S100-induced AIH mice | 8 in each group | China | Mice BM-MSC-derived exosomes | 3 | i.p. | ALT↓, AST↓, liver lymphocyte infiltration↓ | Serum TNF-α↓, IL-17↓, IL-1β↓; liver NLRP3 and caspase-1↓ | |
| Wang et al.106 | ConA-induced AIH mice | 15 in each group | China | Mice IL-35-modified-AT-MSC | 1 | i.v. | Longer survival, hepatocyte necrosis and apoptosis↓ | Liver MNC IFNγ↓, IL-17↓, JAK1-STAT1/STAT4 pathway↑ | |
| Lu et al.105 | Hepatic S100-induced AIH mice | 6 in each group | China | Mice MSC-derived exosomes | 2 | 2 µg/g body weight, 200 μl, i.v. | ALT↓, AST↓, liver lymphocyte infiltration↓, improved inflammatory lesions | Serum and liver IL-1β↓, IL-6↓, IL-17↓, IL-10↑; splenic Th17↓, Treg↑, Treg/Th17 ratio↑; liver STAT3 and pSTAT3↓ | |
| Wang et al.112 | Poly I:C-induced PBC mice | 8 in BM-MSC-treated group and 7 in no BM-MSC group | China | Mice BM-MSC | 1 | 1 × 106 cells, i.v. | ALT↓, ALP↓, serum AMA↓, liver lymphocyte infiltration↓ | Peripheral and lymph nodes Treg↑, serum IL-10↑; serum TGF-β1↑, IFNγ↓ | |
| Fan et al.126 | 2OA-BSA-induced PBC mice | 6 in each group | China | Human UC-MSC | 1 | 1 × 106 cells, i.v. | ALT↓, AST↓, ALP↓, GGT↓, anti-PDC-E2 autoantibodies↓, liver histology improved | Th1↓, Th17↓, Th1/Th2 ratio↓ in the liver, spleen and lymph nodes; liver IFNγ, IL-12, IL-17α, IL-23↓; inhibit CD4+T proliferation and Th1 and Th17 differentiation via Gal-9 | |
| Sugiura et al.125 | ANIT-induced PSC rats | 10 in each group | Japan | Human AMSC | 2 | 1 × 106 cells, 200 μl, i.v. | Biliary hyperplasia↓, Kupffer cell infiltration in the Glisson’s sheath↓ | NA | |
2OA-BSA, 2-octynoic acid coupled to bovine serum albumin; AIH, autoimmune hepatitis; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AMA, antimitochondrial antibody; AMSC, amnion-derived mesenchymal stem cell; ANIT, alpha-naphthylisothiocyanate; AST, aspartate aminotransferase; AT-MSC, adipose tissue-derived mesenchymal stem cell; BM-MSC, bone marrow-derived mesenchymal stem cell; CM, conditioned medium; ConA, Concanavalin A; DBIL, direct bilirubin; Gal-9, galectin-9; GGT, gamma glutamyltransferase; IFN-γ, interferon-γ; IL-10, interleukin-10; i.p., intraperitoneally; i.v., intravenously; JAK, Janus kinase; MNC, mononuclear cell; NA, not available; NLRP3, NLR (nucleotide-binding oligomerization domain-like receptors) family pyrin domain containing 3; PBC, primary biliary cholangitis; PDC-E2, E2 subunit of the pyruvate dehydrogenase complex; PSC, primary sclerosing cholangitis; pSTAT3, phospho-signal transducer and activator of transcription 3; TGF- β1, transforming-growth-factor-beta 1; Th, T-helper cell; Treg, regulatory T cell; UC-MSC, umbilical cord-derived mesenchymal stem cell; UDCA, ursodeoxycholic acid.
Challenges of MSC-based treatment in clinical practice
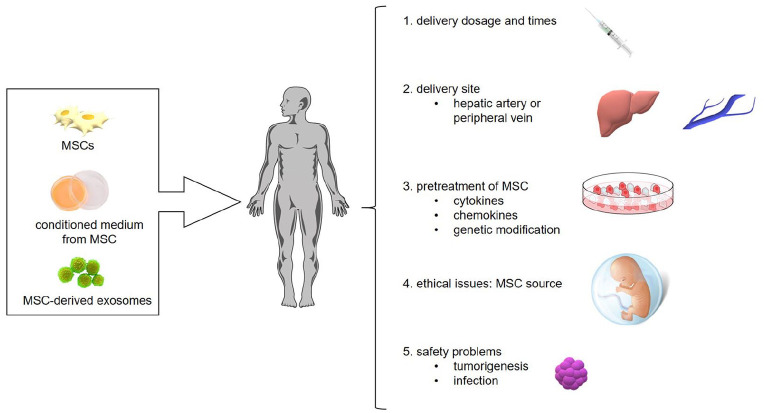
Although, MSC-based therapy has achieved favorable results in animal and clinical studies in AILD, there are still challenges to overcome for the application of this novel treatment (Figure 2).
Figure 2.
Challenges of MSC-based treatment in clinical practice.
MSC, mesenchymal stem cell.
First, MSC therapy is associated with a few safety concerns such as the potential for tumorigenesis, emboli formation, and immune response.127 However, a meta-analysis based on 36 clinical studies found an association between autologous and allogeneic MSC therapy and transient fever, but no relationship with acute infusion toxicity, organ complications, infection, and malignancy.128 More studies with a long follow-up period are required to determine the precise long-term impact on patients.
Second, the heterogeneity of different MSC populations must be taken into consideration for clinical use. MSCs must be expanded through in vitro culture to yield sufficient cell numbers, and culture over several passages can cause the cells to transform and lose their function.129,130 Although this phenomenon is rare in human MSCs, it is vital to analyze the gene components of MSCs and maintain homogeneous MSCs among different infusions for patients. The origin of MSCs is also important. Previous investigations have suggested that tremendous variability exists among MSCs derived from different tissues and different donors.22 And results from a limited number of studies showed that adipose tissue-derived MSCs (AT-MSCs) have similar potential while UC-MSCs are more potent in hepatogenic differentiation when compared with BM-MSCs.131–133 More data are required to illustrate the question that MSCs from which source have the strongest hepatogenic differentiation ability. In addition, the delivery approach, dosages, and frequencies of MSC treatment for AILD patients should be standardized and written into an operation procedure, which would facilitate comparisons of the effectiveness of MSC therapy among studies.
Third, since MSCs showed great potential in treating many diseases, it is important to enhance the therapeutic benefit and make the best use of these cells, which can be achieved through several factors. Evidence suggests that priming MSCs with specific cytokines before infusion into patients is feasible and could enhance the effectiveness of treatment. Exposure to inflammatory cytokines could help MSCs gain immunomodulatory function, whereas they may show a pro-inflammatory phenotype in a quiescent environment. Duijvestein et al.72 showed that pretreatment of MSCs with IFN-γ enhanced their anti-inflammatory ability and resulted in better amelioration of experimental colitis compared with pure MSCs. Moreover, gene-modified MSCs could exert a more powerful therapeutic impact. For example, overexpression of CXCR4 in MSCs by gene editing resulted in greater cell migration and colonization and conferred protection to the damaged liver.134
Conclusions and prospects
Treatment for AILD patients is currently limited, and there is an urgent need for a new therapeutic approach. MSC therapy holds great promise owing to the advantageous properties of the cells, including multipotential for differentiation, anti-fibrosis features, and immunomodulatory functions. Several clinical and animal studies have proven the safety and effectiveness of MSC treatment in AILD; however, there are some issues to be clarified and resolved; in particular, MSC treatment may increase the risk of tumor formation and viral infection; therefore, short-term and long-term adverse events must be monitored closely and dealt with in time. Besides, the standard of clinical use of MSCs should be established. Since modified MSCs appear to have stronger therapeutic efficacy, it is vital to prime or modify MSCs before treatment to facilitate their treatment ability. Only by addressing these concerns will we be able to apply MSC treatment in clinical practice as a mainstream approach, ultimately enhancing the quality of life and improving survival of patients with AILD.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this study was supported by National Key R&D Program of China (2016YFA0101003, 2016YFC0903901); National Natural Science Fund (81771764); CAMS Innovation Fund for Medical Sciences (2017-I2M-3-007).
ORCID iDs: Chengmei He  https://orcid.org/0000-0003-4711-8068
https://orcid.org/0000-0003-4711-8068
Suying Liu  https://orcid.org/0000-0002-4386-9895
https://orcid.org/0000-0002-4386-9895
Contributor Information
Chengmei He, Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China; Key Laboratory of Rheumatology & Clinical Immunology, Ministry of Education, National Clinical Research Center for Dermatologic and Immunologic Diseases, Beijing, China.
Yanlei Yang, Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China; Key Laboratory of Rheumatology & Clinical Immunology, Ministry of Education, National Clinical Research Center for Dermatologic and Immunologic Diseases, Beijing, China.
Kunyu Zheng, Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China; Key Laboratory of Rheumatology & Clinical Immunology, Ministry of Education, National Clinical Research Center for Dermatologic and Immunologic Diseases, Beijing, China.
Yiran Chen, Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China; Key Laboratory of Rheumatology & Clinical Immunology, Ministry of Education, National Clinical Research Center for Dermatologic and Immunologic Diseases, Beijing, China.
Suying Liu, Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China; Key Laboratory of Rheumatology & Clinical Immunology, Ministry of Education, National Clinical Research Center for Dermatologic and Immunologic Diseases, Beijing, China.
Yongzhe Li, Department of Clinical Laboratory, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
Qin Han, Institute of Basic Medical Sciences Chinese Academy of Medical Sciences, School of Basic Medicine Peking Union Medical College, Peking Union Medical College Hospital, Beijing, China; Center of Excellence in Tissue Engineering Chinese Academy of Medical Sciences, Beijing Key Laboratory (No. BZO381), Beijing, China.
Robert Chunhua Zhao, Institute of Basic Medical Sciences Chinese Academy of Medical Sciences, School of Basic Medicine Peking Union Medical College, Peking Union Medical College Hospital, Beijing, China; Center of Excellence in Tissue Engineering Chinese Academy of Medical Sciences, Beijing Key Laboratory (No. BZO381), Beijing, China School of Life Sciences, Shanghai University, Shanghai, China.
Li Wang, Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, 1 Shuaifuyuan, Dongcheng District, Beijing 100730, China.
Fengchun Zhang, Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, 1 Shuaifuyuan, Dongcheng District, Beijing 100730, China.
References
- 1. Doherty DG. Immunity, Tolerance and autoimmunity in the liver: a comprehensive review. J Autoimmun 2016; 66: 60–75. [DOI] [PubMed] [Google Scholar]
- 2. Richardson N, Ng STH, Wraith DC. Antigen-specific immunotherapy for treatment of autoimmune liver diseases. Front Immunol 2020; 11: 1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lleo A, De Boer YS, Liberal R, et al. The risk of liver cancer in autoimmune liver diseases. Ther Adv Med Oncol 2019; 11: 1758835919861914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lohse AW, Sebode M, Jorgensen MH, et al. Second-line and third-line therapy for autoimmune hepatitis: a position statement from the European reference network on hepatological diseases and the international autoimmune hepatitis group. J Hepatol 2020; 73: 1496–1506. [DOI] [PubMed] [Google Scholar]
- 5. Shah RA, Kowdley KV. Current and potential treatments for primary biliary cholangitis. Lancet Gastroenterol Hepatol 2020; 5: 306–315. [DOI] [PubMed] [Google Scholar]
- 6. Lindor KD, Bowlus CL, Boyer J, et al. Primary biliary cholangitis: 2018 practice guidance from the American association for the study of liver diseases. Hepatology 2019; 69: 394–419. [DOI] [PubMed] [Google Scholar]
- 7. Vesterhus M, Karlsen TH. Emerging therapies in primary sclerosing cholangitis: pathophysiological basis and clinical opportunities. J Gastroenterol 2020; 55: 588–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jadlowiec CC, Taner T. Liver transplantation: current status and challenges. World J Gastroenterol 2016; 22: 4438–4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Montano-Loza AJ, Hansen BE, Corpechot C, et al. Factors associated with recurrence of primary biliary cholangitis after liver transplantation and effects on graft and patient survival. Gastroenterology 2019; 156: 96–107.e101. [DOI] [PubMed] [Google Scholar]
- 10. Friedenstein AJ, Petrakova KV, Kurolesova AI, et al. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968; 6: 230–247. [PubMed] [Google Scholar]
- 11. Pittenger MF, Discher DE, Peault BM, et al. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med 2019; 4: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee CW, Chen YF, Wu HH, et al. Historical perspectives and advances in mesenchymal stem cell research for the treatment of liver diseases. Gastroenterology 2018; 154: 46–56. [DOI] [PubMed] [Google Scholar]
- 13. Lee OK, Kuo TK, Chen WM, et al. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 2004; 103: 1669–1675. [DOI] [PubMed] [Google Scholar]
- 14. Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001; 7: 211–228. [DOI] [PubMed] [Google Scholar]
- 15. Fukuchi Y, Nakajima H, Sugiyama D, et al. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells 2004; 22: 649–658. [DOI] [PubMed] [Google Scholar]
- 16. Pierdomenico L, Bonsi L, Calvitti M, et al. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation 2005; 80: 836–842. [DOI] [PubMed] [Google Scholar]
- 17. Antonucci I, Stuppia L, Kaneko Y, et al. Amniotic fluid as a rich source of mesenchymal stromal cells for transplantation therapy. Cell Transplant 2011; 20: 789–795. [DOI] [PubMed] [Google Scholar]
- 18. Chen L-B, Jiang X-B, Yang L. Differentiation of rat marrow mesenchymal stem cells into pancreatic islet beta-cells. World J Gastroenterol 2004; 10: 3016–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Black IB, Woodbury D. Adult rat and human bone marrow stromal stem cells differentiate into neurons. Blood Cells Mol Dis 2001; 27: 632–636. [DOI] [PubMed] [Google Scholar]
- 20. Lee KD, Kuo TK, Whang-Peng J, et al. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology 2004; 40: 1275–1284. [DOI] [PubMed] [Google Scholar]
- 21. Lou G, Chen Z, Zheng M, et al. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med 2017; 49: e346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McLeod CM, Mauck RL. On the origin and impact of mesenchymal stem cell heterogeneity: new insights and emerging tools for single cell analysis. Eur Cell Mater 2017; 34: 217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mattar P, Bieback K. Comparing the immunomodulatory properties of bone marrow, adipose tissue, and birth-associated tissue mesenchymal stromal cells. Front Immunol 2015; 6: 560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 2007; 25: 1384–1392. [DOI] [PubMed] [Google Scholar]
- 25. Boberg E, von Bahr L, Afram G, et al. Treatment of chronic GvHD with mesenchymal stromal cells induces durable responses: a phase II study. Stem Cells Transl Med 2020; 9: 1190–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karantalis V, Hare JM. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res 2015; 116: 1413–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jurewicz M, Yang S, Augello A, et al. Congenic mesenchymal stem cell therapy reverses hyperglycemia in experimental type 1 diabetes. Diabetes 2010; 59: 3139–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. He N, Kong Y, Lei X, et al. MSCs inhibit tumor progression and enhance radiosensitivity of breast cancer cells by down-regulating Stat3 signaling pathway. Cell Death Dis 2018; 9: 1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang Z, Lin H, Shi M, et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol 2012; 27(Suppl. 2): 112–120. [DOI] [PubMed] [Google Scholar]
- 30. Hu C, Zhao L, Wu Z, et al. Transplantation of mesenchymal stem cells and their derivatives effectively promotes liver regeneration to attenuate acetaminophen-induced liver injury. Stem Cell Res Ther 2020; 11: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang M, Yuan Q, Xie L. Mesenchymal stem cell-based immunomodulation: properties and clinical application. Stem Cells Int 2018; 2018: 3057624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hu C, Zhao L, Duan J, et al. Strategies to improve the efficiency of mesenchymal stem cell transplantation for reversal of liver fibrosis. J Cell Mol Med 2019; 23: 1657–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Su J, Chen X, Huang Y, et al. Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell-mediated immunosuppression in mammalian species. Cell Death Differ 2014; 21: 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chabannes D, Hill M, Merieau E, et al. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood 2007; 110: 3691–3694. [DOI] [PubMed] [Google Scholar]
- 35. Németh K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 2009; 15: 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen D, Tang P, Liu L, et al. Bone marrow-derived mesenchymal stem cells promote cell proliferation of multiple myeloma through inhibiting T cell immune responses via PD-1/PD-L1 pathway. Cell Cycle 2018; 17: 858–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sioud M, Mobergslien A, Boudabous A, et al. Evidence for the involvement of galectin-3 in mesenchymal stem cell suppression of allogeneic T-cell proliferation. Scand J Immunol 2010; 71: 267–274. [DOI] [PubMed] [Google Scholar]
- 38. Chen QH, Wu F, Liu L, et al. Mesenchymal stem cells regulate the Th17/Treg cell balance partly through hepatocyte growth factor in vitro. Stem Cell Res Ther 2020; 11: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li M, Sun X, Kuang X, et al. Mesenchymal stem cells suppress CD8+ T cell-mediated activation by suppressing natural killer group 2, member D protein receptor expression and secretion of prostaglandin E2, indoleamine 2, 3-dioxygenase and transforming growth factor-β. Clin Exp Immunol 2014; 178: 516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maccario R, Podestà M, Moretta A, et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica 2005; 90: 516–525. [PubMed] [Google Scholar]
- 41. Zhang Z, Feng R, Niu L, et al. Human umbilical cord mesenchymal stem cells inhibit T follicular helper cell expansion through the activation of iNOS in lupus-prone B6.MRL-Faslpr mice. Cell Transplant 2017; 26: 1031–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zheng ZH, Li XY, Ding J, et al. Allogeneic mesenchymal stem cell and mesenchymal stem cell-differentiated chondrocyte suppress the responses of type II collagen-reactive T cells in rheumatoid arthritis. Rheumatology (Oxford) 2008; 47: 22–30. [DOI] [PubMed] [Google Scholar]
- 43. Tobin LM, Healy ME, English K, et al. Human mesenchymal stem cells suppress donor CD4+ T cell proliferation and reduce pathology in a humanized mouse model of acute graft-versus-host disease. Clin Exp Immunol 2013; 172: 333–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang Y, Cai W, Huang Q, et al. Mesenchymal stem cells alleviate bacteria-induced liver injury in mice by inducing regulatory dendritic cells. Hepatology 2014; 59: 671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meisel R, Zibert A, Laryea M, et al. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 2004; 103: 4619–4621. [DOI] [PubMed] [Google Scholar]
- 46. Akiyama K, Chen C, Wang D, et al. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell 2012; 10: 544–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005; 105: 1815–1822. [DOI] [PubMed] [Google Scholar]
- 48. Luz-Crawford P, Kurte M, Bravo-Alegría J, et al. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther 2013; 4: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tang RJ, Shen SN, Zhao XY, et al. Mesenchymal stem cells-regulated Treg cells suppress colitis-associated colorectal cancer. Stem Cell Res Ther 2015; 6: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood 2006; 107: 367–372. [DOI] [PubMed] [Google Scholar]
- 51. Augello A, Tasso R, Negrini SM, et al. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol 2005; 35: 1482–1490. [DOI] [PubMed] [Google Scholar]
- 52. Asari S, Itakura S, Ferreri K, et al. Mesenchymal stem cells suppress B-cell terminal differentiation. Exp Hematol 2009; 37: 604–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rafei M, Hsieh J, Fortier S, et al. Mesenchymal stromal cell-derived CCL2 suppresses plasma cell immunoglobulin production via STAT3 inactivation and PAX5 induction. Blood 2008; 112: 4991–4998. [DOI] [PubMed] [Google Scholar]
- 54. Luz-Crawford P, Djouad F, Toupet K, et al. Mesenchymal stem cell-derived interleukin 1 receptor antagonist promotes macrophage polarization and inhibits B cell differentiation. Stem Cells 2016; 34: 483–492. [DOI] [PubMed] [Google Scholar]
- 55. Feng X, Che N, Liu Y, et al. Restored immunosuppressive effect of mesenchymal stem cells on B cells after olfactory 1/early B cell factor-associated zinc-finger protein down-regulation in patients with systemic lupus erythematosus. Arthritis Rheumatol 2014; 66: 3413–3423. [DOI] [PubMed] [Google Scholar]
- 56. Schena F, Gambini C, Gregorio A, et al. Interferon-γ-dependent inhibition of B cell activation by bone marrow-derived mesenchymal stem cells in a murine model of systemic lupus erythematosus. Arthritis Rheum 2010; 62: 2776–2786. [DOI] [PubMed] [Google Scholar]
- 57. Franquesa M, Mensah FK, Huizinga R, et al. Human adipose tissue-derived mesenchymal stem cells abrogate plasmablast formation and induce regulatory B cells independently of T helper cells. Stem Cells 2015; 33: 880–891. [DOI] [PubMed] [Google Scholar]
- 58. Chao K, Zhang S, Qiu Y, et al. Human umbilical cord-derived mesenchymal stem cells protect against experimental colitis via CD5+ B regulatory cells. Stem Cell Res Ther 2016; 7: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen X, Cai C, Xu D, et al. Human mesenchymal stem cell-treated regulatory CD23+CD43+ B cells alleviate intestinal inflammation. Theranostics 2019; 9: 4633–4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol 2014; 5: 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bouchlaka MN, Moffitt AB, Kim J, et al. Human mesenchymal stem cell-educated macrophages are a distinct high IL-6-producing subset that confer protection in graft-versus-host-disease and radiation injury models. Biol Blood Marrow Transplant 2017; 23: 897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shin TH, Kim HS, Kang TW, et al. Human umbilical cord blood-stem cells direct macrophage polarization and block inflammasome activation to alleviate rheumatoid arthritis. Cell Death Dis 2016; 7: e2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang Q-Z, Su W-R, Shi S-H, et al. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells 2010; 28: 1856–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lee K-C, Lin H-C, Huang Y-H, et al. Allo-transplantation of mesenchymal stem cells attenuates hepatic injury through IL1Ra dependent macrophage switch in a mouse model of liver disease. J Hepatol 2015; 63: 1405–1412. [DOI] [PubMed] [Google Scholar]
- 65. Choi H, Lee RH, Bazhanov N, et al. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood 2011; 118: 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liu W, Zhang S, Gu S, et al. Mesenchymal stem cells recruit macrophages to alleviate experimental colitis through TGFβ1. Cell Physiol Biochem 2015; 35: 858–865. [DOI] [PubMed] [Google Scholar]
- 67. Lo Sicco C, Reverberi D, Balbi C, et al. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: endorsement of macrophage polarization. Stem Cells Transl Med 2017; 6: 1018–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Spaggiari GM, Capobianco A, Becchetti S, et al. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 2006; 107: 1484–1490. [DOI] [PubMed] [Google Scholar]
- 69. Qu M, Cui J, Zhu J, et al. Bone marrow-derived mesenchymal stem cells suppress NK cell recruitment and activation in polyI:C-induced liver injury. Biochem Biophys Res Commun 2015; 466: 173–179. [DOI] [PubMed] [Google Scholar]
- 70. Gao WX, Sun YQ, Shi J, et al. Effects of mesenchymal stem cells from human induced pluripotent stem cells on differentiation, maturation, and function of dendritic cells. Stem Cell Res Ther 2017; 8: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jung YJ, Ju SY, Yoo ES, et al. MSC-DC interactions: MSC inhibit maturation and migration of BM-derived DC. Cytotherapy 2007; 9: 451–458. [DOI] [PubMed] [Google Scholar]
- 72. Duijvestein M, Wildenberg ME, Welling MM, et al. Pretreatment with interferon-γ enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells 2011; 29: 1549–1558. [DOI] [PubMed] [Google Scholar]
- 73. Redondo-Castro E, Cunningham C, Miller J, et al. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res Ther 2017; 8: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol 2011; 6: 425–456. [DOI] [PubMed] [Google Scholar]
- 75. Kisseleva T, Brenner DA. The phenotypic fate and functional role for bone marrow-derived stem cells in liver fibrosis. J Hepatol 2012; 56: 965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Watanabe Y, Tsuchiya A, Seino S, et al. Mesenchymal stem cells and induced bone marrow-derived macrophages synergistically improve liver fibrosis in mice. Stem Cells Transl Med 2019; 8: 271–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chai NL, Zhang XB, Chen SW, et al. Umbilical cord-derived mesenchymal stem cells alleviate liver fibrosis in rats. World J Gastroenterol 2016; 22: 6036–6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Milosavljevic N, Gazdic M, Simovic Markovic B, et al. Mesenchymal stem cells attenuate liver fibrosis by suppressing Th17 cells - an experimental study. Transpl Int 2018; 31: 102–115. [DOI] [PubMed] [Google Scholar]
- 79. Pan RL, Wang P, Xiang LX, et al. Delta-like 1 serves as a new target and contributor to liver fibrosis down-regulated by mesenchymal stem cell transplantation. J Biol Chem 2011; 286: 12340–12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Meier RP, Mahou R, Morel P, et al. Microencapsulated human mesenchymal stem cells decrease liver fibrosis in mice. J Hepatol 2015; 62: 634–641. [DOI] [PubMed] [Google Scholar]
- 81. Wang L, Bai G, Chen F. Human bone marrow mesenchymal stem cells suppress the proliferation of hepatic stellate cells by inhibiting the ubiquitination of p27. Biochem Cell Biol 2017; 95: 628–633. [DOI] [PubMed] [Google Scholar]
- 82. Jang YO, Kim MY, Cho MY, et al. Effect of bone marrow-derived mesenchymal stem cells on hepatic fibrosis in a thioacetamide-induced cirrhotic rat model. BMC Gastroenterol 2014; 14: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Li B, Shao Q, Ji D, et al. Mesenchymal stem cells mitigate cirrhosis through BMP7. Cell Physiol Biochem 2015; 35: 433–440. [DOI] [PubMed] [Google Scholar]
- 84. Zhang GZ, Sun HC, Zheng LB, et al. In vivo hepatic differentiation potential of human umbilical cord-derived mesenchymal stem cells: therapeutic effect on liver fibrosis/cirrhosis. World J Gastroenterol 2017; 23: 8152–8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Van Poll D, Parekkadan B, Cho CH, et al. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology 2008; 47: 1634–1643. [DOI] [PubMed] [Google Scholar]
- 86. Schwartz RE, Reyes M, Koodie L, et al. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest 2002; 109: 1291–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ong SY, Dai H, Leong KW. Inducing hepatic differentiation of human mesenchymal stem cells in pellet culture. Biomaterials 2006; 27: 4087–4097. [DOI] [PubMed] [Google Scholar]
- 88. Lange C, Bassler P, Lioznov MV, et al. Liver-specific gene expression in mesenchymal stem cells is induced by liver cells. World J Gastroenterol 2005; 11: 4497–4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Shu SN, Wei L, Wang JH, et al. Hepatic differentiation capability of rat bone marrow-derived mesenchymal stem cells and hematopoietic stem cells. World J Gastroenterol 2004; 10: 2818–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Choi D, Kim JH, Lim M, et al. Hepatocyte-like cells from human mesenchymal stem cells engrafted in regenerating rat liver tracked with in vivo magnetic resonance imaging. Tissue Eng Part C Methods 2008; 14: 15–23. [DOI] [PubMed] [Google Scholar]
- 91. Aurich H, Sgodda M, Kaltwasser P, et al. Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut 2009; 58: 570–581. [DOI] [PubMed] [Google Scholar]
- 92. Banas A, Teratani T, Yamamoto Y, et al. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology 2007; 46: 219–228. [DOI] [PubMed] [Google Scholar]
- 93. Yang J-F, Cao H-C, Pan Q-L, et al. Mesenchymal stem cells from the human umbilical cord ameliorate fulminant hepatic failure and increase survival in mice. Hepatobiliary Pancreat Dis Int 2015; 14: 186–193. [DOI] [PubMed] [Google Scholar]
- 94. Chamberlain J, Yamagami T, Colletti E, et al. Efficient generation of human hepatocytes by the intrahepatic delivery of clonal human mesenchymal stem cells in fetal sheep. Hepatology 2007; 46: 1935–1945. [DOI] [PubMed] [Google Scholar]
- 95. Mieli-Vergani G, Vergani D, Czaja AJ, et al. Autoimmune hepatitis. Nat Rev Dis Primers 2018; 4: 18017. [DOI] [PubMed] [Google Scholar]
- 96. Lv T, Li M, Zeng N, et al. Systematic review and meta-analysis on the incidence and prevalence of autoimmune hepatitis in Asian, European, and American population. J Gastroenterol Hepatol 2019; 34: 1676–1684. [DOI] [PubMed] [Google Scholar]
- 97. Gronbaek L, Otete H, Ban L, et al. Incidence, prevalence and mortality of autoimmune hepatitis in England 1997–2015. A population-based cohort study. Liver Int 2020; 40: 1634–1644. [DOI] [PubMed] [Google Scholar]
- 98. Pape S, Gevers TJG, Vrolijk JM, et al. High discontinuation rate of azathioprine in autoimmune hepatitis, independent of time of treatment initiation. Liver Int 2020; 40: 2164–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Van den Brand FF, Van der Veen KS, Lissenberg-Witte BI, et al. Adverse events related to low dose corticosteroids in autoimmune hepatitis. Aliment Pharmacol Ther 2019; 50: 1120–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Chen Y, Chen S, Liu LY, et al. Mesenchymal stem cells ameliorate experimental autoimmune hepatitis by activation of the programmed death 1 pathway. Immunol Lett 2014; 162: 222–228. [DOI] [PubMed] [Google Scholar]
- 101. Zhao P, Wang P, Dong S, et al. Depletion of PD-1-positive cells ameliorates autoimmune disease. Nat Biomed Eng 2019; 3: 292–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Xu M, Morishima N, Mizoguchi I, et al. Regulation of the development of acute hepatitis by IL-23 through IL-22 and IL-17 production. Eur J Immunol 2011; 41: 2828–2839. [DOI] [PubMed] [Google Scholar]
- 103. Ferreira JR, Teixeira GQ, Santos SG, et al. Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Front Immunol 2018; 9: 2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chen L, Lu F-B, Chen D-Z, et al. BMSCs-derived miR-223-containing exosomes contribute to liver protection in experimental autoimmune hepatitis. Mol Immunol 2018; 93: 38–46. [DOI] [PubMed] [Google Scholar]
- 105. Lu FB, Chen DZ, Chen L, et al. Attenuation of experimental autoimmune hepatitis in mice with bone mesenchymal stem cell-derived exosomes carrying microRNA-223-3p. Mol Cells 2019; 42: 906–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wang W, Guo H, Li H, et al. Interleukin-35 gene-modified mesenchymal stem cells protect concanavalin A-induced fulminant hepatitis by decreasing the interferon gamma level. Hum Gene Ther 2018; 29: 234–241. [DOI] [PubMed] [Google Scholar]
- 107. Castellani ML, Anogeianaki A, Felaco P, et al. IL-35, an anti-inflammatory cytokine which expands CD4+CD25+ Treg cells. J Biol Regul Homeost Agents 2010; 24: 131–135. [PubMed] [Google Scholar]
- 108. Lu M, Zhou Y, Haller IV, et al. Increasing prevalence of primary biliary cholangitis and reduced mortality with treatment. Clin Gastroenterol Hepatol 2018; 16: 1342–1350e1. [DOI] [PubMed] [Google Scholar]
- 109. Engel B, Taubert R, Jaeckel E, et al. The future of autoimmune liver diseases - understanding pathogenesis and improving morbidity and mortality. Liver Int 2020; 40(Suppl. 1): 149–153. [DOI] [PubMed] [Google Scholar]
- 110. Gong Y, Huang Z, Christensen E, et al. Ursodeoxycholic acid for patients with primary biliary cirrhosis: an updated systematic review and meta-analysis of randomized clinical trials using Bayesian approach as sensitivity analyses. Am J Gastroenterol 2007; 102: 1799–1807. [DOI] [PubMed] [Google Scholar]
- 111. Nevens F, Andreone P, Mazzella G, et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med 2016; 375: 631–643. [DOI] [PubMed] [Google Scholar]
- 112. Wang D, Zhang H, Liang J, et al. Effect of allogeneic bone marrow-derived mesenchymal stem cells transplantation in a polyI:C-induced primary biliary cirrhosis mouse model. Clin Exp Med 2011; 11: 25–32. [DOI] [PubMed] [Google Scholar]
- 113. Wang L, Li J, Liu H, et al. Pilot study of umbilical cord-derived mesenchymal stem cell transfusion in patients with primary biliary cirrhosis. J Gastroenterol Hepatol 2013; 28(Suppl. 1): 85–92. [DOI] [PubMed] [Google Scholar]
- 114. Wang L, Han Q, Chen H, et al. Allogeneic bone marrow mesenchymal stem cell transplantation in patients with UDCA-resistant primary biliary cirrhosis. Stem Cells Dev 2014; 23: 2482–2489. [DOI] [PubMed] [Google Scholar]
- 115. Boonstra K, Weersma RK, van Erpecum KJ, et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology 2013; 58: 2045–2055. [DOI] [PubMed] [Google Scholar]
- 116. Lindkvist B, Benito de, Valle M, Gullberg B, et al. Incidence and prevalence of primary sclerosing cholangitis in a defined adult population in Sweden. Hepatology 2010; 52: 571–577. [DOI] [PubMed] [Google Scholar]
- 117. Guerra I, Bujanda L, Castro J, et al. Clinical characteristics, associated malignancies and management of primary sclerosing cholangitis in inflammatory bowel disease patients: a multicentre retrospective cohort study. J Crohns Colitis 2019; 13: 1492–1500. [DOI] [PubMed] [Google Scholar]
- 118. Lindor KD, Kowdley KV, Luketic VA, et al. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology 2009; 50: 808–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Cullen SN, Chapman RW. Review article: current management of primary sclerosing cholangitis. Aliment Pharmacol Ther 2005; 21: 933–948. [DOI] [PubMed] [Google Scholar]
- 120. Broomé U, Olsson R, Lööf L, et al. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut 1996; 38: 610–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Campsen J, Zimmerman MA, Trotter JF, et al. Clinically recurrent primary sclerosing cholangitis following liver transplantation: a time course. Liver Transpl 2008; 14: 181–185. [DOI] [PubMed] [Google Scholar]
- 122. Jiang X, Karlsen TH. Genetics of primary sclerosing cholangitis and pathophysiological implications. Nat Rev Gastroenterol Hepatol 2017; 14: 279–295. [DOI] [PubMed] [Google Scholar]
- 123. Ravichandran G, Neumann K, Berkhout LK, et al. Interferon-gamma-dependent immune responses contribute to the pathogenesis of sclerosing cholangitis in mice. J Hepatol 2019; 71: 773–782. [DOI] [PubMed] [Google Scholar]
- 124. Guicciardi ME, Trussoni CE, Krishnan A, et al. Macrophages contribute to the pathogenesis of sclerosing cholangitis in mice. J Hepatol 2018; 69: 676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sugiura R, Ohnishi S, Ohara M, et al. Effects of human amnion-derived mesenchymal stem cells and conditioned medium in rats with sclerosing cholangitis. Am J Transl Res 2018; 10: 2102–2114. [PMC free article] [PubMed] [Google Scholar]
- 126. Fan J, Tang X, Wang Q, et al. Mesenchymal stem cells alleviate experimental autoimmune cholangitis through immunosuppression and cytoprotective function mediated by galectin-9. Stem Cell Res Ther 2018; 9: 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Lukomska B, Stanaszek L, Zuba-Surma E, et al. Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells Int 2019; 2019: 9628536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Lalu MM, McIntyre L, Pugliese C, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One 2012; 7: e47559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Røsland GV, Svendsen A, Torsvik A, et al. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res 2009; 69: 5331–5339. [DOI] [PubMed] [Google Scholar]
- 130. Josse C, Schoemans R, Niessen NA, et al. Systematic chromosomal aberrations found in murine bone marrow-derived mesenchymal stem cells. Stem Cells Dev 2010; 19: 1167–1173. [DOI] [PubMed] [Google Scholar]
- 131. Taléns-Visconti R, Bonora A, Jover R, et al. Hepatogenic differentiation of human mesenchymal stem cells from adipose tissue in comparison with bone marrow mesenchymal stem cells. World J Gastroenterol 2006; 12: 5834–5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Xu LJ, Wang SF, Wang DQ, et al. Adipose-derived stromal cells resemble bone marrow stromal cells in hepatocyte differentiation potential in vitro and in vivo. World J Gastroenterol 2017; 23: 6973–6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Yu YB, Song Y, Chen Y, et al. Differentiation of umbilical cord mesenchymal stem cells into hepatocytes in comparison with bone marrow mesenchymal stem cells. Mol Med Rep 2018; 18: 2009–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ma HC, Shi XL, Ren HZ, et al. Targeted migration of mesenchymal stem cells modified with CXCR4 to acute failing liver improves liver regeneration. World J Gastroenterol 2014; 20: 14884–14894. [DOI] [PMC free article] [PubMed] [Google Scholar]