Abstract
Objective:
We aim to characterize the incidence and relative risk of rheumatic and systemic immune-related adverse effects (irAEs) among immune checkpoint inhibitor (ICI) therapy compared with those after placebo treatment.
Methods:
Randomized clinical trial studies with placebo control with the following keywords were searched from Embase, PubMed, Cochrane databases: immune checkpoint inhibitors, neoplasms, randomized controlled trials, and adverse effects.
Results:
Among the 5444 published and 316 registration records, nine placebo-controlled randomized clinical trials met our selection criteria, and included data from 5560 patients. Compared with placebo use, using ICIs increases the risk of overall-rheumatic irAEs. The incidence and relative risk of all-grade rheumatic irAEs were 18.40% [95% confidence interval (CI) 12.16–25.59%, p < 0.01] and 2.30 (95% CI 1.32–4.02), respectively, while musculoskeletal irAEs were 11.30% (95% CI 9.76–12.85%) and 1.01 (95% CI 0.84–1.22). The incidence and relative risk of severe rheumatic irAEs were 5.72% (95% CI 3.92–7.82%), and 8.29 (95% CI 3.75–18.35), respectively. Arthralgia was the most common rheumatic irAE (incidence 11.00%, 95% CI 9.55–12.64%; relative risk 0.99, 95% CI 0.82–1.19), although usually not severe. Colitis (incidence 3.23%, 95% CI 1.27–7.98%; relative risk 6.53, 95% CI 2.66–16.04) and pneumonitis (incidence 3.11%, 95% CI 1.56–6.21; relative risk 4.04, 95% CI 1.65–9.89) were commonly observed and tended to be severe. Hepatitis, hypophysitis, thyroiditis, and myositis were rare and less recorded, yet can be severe and life threatening. Other extremely rare severe rheumatic irAEs included sarcoidosis (n = 11), autoimmune arthritis (n = 8), autoimmune uveitis (n = 3), autoimmune pericarditis, bursitis, osteochondrosis, psoriasis, polymyalgia rheumatica, systemic inflammatory response syndrome, and Sjögren syndrome (n = 1, each).
Conclusion:
ICI therapy increased the incidence and relative risk of all-grade and severe rheumatic irAEs. Arthralgia was the most commonly observed non-severe irAE, while colitis and pneumonitis were commonly observed severe irAEs. Rare rheumatic irAEs like hepatitis, hypophysitis, thyroiditis, and myositis warrant special attention.
Keywords: adverse effects, immune checkpoint inhibitors, meta-analysis, neoplasm, placebo, rheumatic
Introduction
Immune checkpoint inhibitors (ICIs) are a class of immunotherapy agents that block inhibitory immune checkpoint pathways, mainly including anti-programmed death-1 (PD-1) antibody, anti-programmed death-ligand 1 (PD-L1), and anti-cytotoxic T-lymphocyte associated protein 4 (CTLA-4) antibodies. In recent years, ICIs have gained great attention due to their promising ability to reactivate immune responses against cancer. However, inappropriate immune activation by ICIs during immunotherapy can lead to idiosyncratic adverse effects, termed immune-related adverse events (irAEs). The incidence of irAEs is 17.1–72%, varying from different types and dosages of ICI, malignant type, etc.1–3 They can affect almost any organ system and range widely in severity, including rash, pruritus, colitis, hypothyroidism, hyperthyroidism, and pneumonitis.
Meanwhile, these immune checkpoints are also relevant in rheumatic disease pathogenesis.4,5 Since ICIs block negative T-cell co-stimulation, immunotherapy would lead to an enhanced immune response, including autoimmune responses. So several rheumatic-like manifestations and the relapse of pre-existing rheumatic diseases were reported during and after ICI therapy, such as lupus-like, arthritis, sicca symptom, vasculitis.6 Thus, the rheumatic irAEs during the immunotherapy of malignancy have garnered attention. However, limited data have been documented, especially the incidence.
In this meta-analysis, we aimed to compare the incidence and relative risk of rheumatic irAEs (both musculoskeletal and systemic) related to ICI therapy compared with those related to placebo treatment in oncologic patients in clinical trial studies.
Methods
Search strategy
This systematic review and meta-analysis were performed according to the PRISMA guidelines. We systematically reviewed the articles published up to 31 December 2019 in MEDLINE, Web of Science, EMBASE, and Cochrane databases, without any language restriction. Keywords included “PD-1,” “PD-L1,” “CTLA-4,” “nivolumab (anti-PD-1 antibody),” “pembrolizumab (anti-PD-1 antibody),” “pidilizumab (anti-PD-1 antibody),” “atezolizumab (anti-PD-L1 antibody),” “durvalumab (anti-PD-L1 antibody),” “ipilimumab (anti-CTLA-4 antibody),” “tremelimumab (anti-CTLA-4 antibody)” (with their chemical names and brand names), “cancer,” “tumor,” “carcinoma,” “neoplasm,” “malignancy,” “randomized controlled trial,” and “adverse effect,” “safety,” “security,” “harms,” “complications,” and “toxicity.” The detailed search strategy used is shown in Table 1 in Supplemental material. We also screened the references of the included studies, relevant reviews, and conference abstracts to find potential studies. This study is registered with PROSPERO, number CRD42020170511.
Table 1.
Characteristics of the included studies.
| Study | NCT number | Cancer | Size | Intervention | Recording of adverse effect | Follow-up* (months) | Monitoring** (days) |
|---|---|---|---|---|---|---|---|
| Antonia et al.9 | NCT02125461 | NSCLC | 709 | PD-L1 durvalumab 10 mg/kg every 2 weeks | CTCAE4.0; MedDRA 19.1 | 14.5 | 90 |
| Beer et al.11 | NCT01057810 | CRPC | 598 | CTLA-4 ipilimumab 10 mg/kg every 3 weeks × 4 doses → 10 mg/kg every 3 months | N/A; MedDRA 17.1 |
37.3# | 90 |
| CHECKMATE451-single | NCT02538666 | SCLC | 830 | PD-1 nivolumab 240 mg Q2W IV infusion <24 months | CTCAE4.0; MedDRA 21.1 |
10.4 | 30 |
| CHECKMATE451-double | NCT02538666 | SCLC | 830 | PD-1+CTLA-4 nivolumab 1 mg/kg IV + ipilimumab 3 mg/kg IV every 3 weeks × 4 doses → nivolumab 240 mg every 2 weeks | CTCAE4.0; MedDRA 21.1 |
9.2 | 30 |
| Eggermont et al.12 | NCT00636168 | Melanoma | 939 | CTLA-4 ipilimumab 10 mg/kg every 3 weeks × 4 doses → 10 mg/kg every 3 months <3 years | CTCAE3.0; MedDRA 22.0 |
32.9 | 70 |
| Eggermont et al.17 | NCT02362594 | Melanoma | 1011 | PD-1 pembrolizumab 200 mg every 3 weeks (⩽18 doses) | CTCAE4.0; MedDRA 20.1 |
14.7 | 30; 90 for serious AE |
| Finn et al.13 | NCT02702401 | HCC | 413 | PD-1 pembrolizumab 200 mg every 3 weeks (⩽35 doses) | CTCAE4.0; MedDRA 21.1 | 13.8 | 30 |
| Maio et al.15 | NCT01843374 | relapsed malignant mesothelioma | 569 | CTLA-4 tremelimumab 10 mg/kg every 4 weeks × 7 doses → 10 mg/kg every 12 weeks | CTCAE; MedDRA 18.0 | 1.9 | 90 |
| Kang et al.10 | NCT02267343 | advanced gastric or GEJ cancer | 491 | PD-1 nivolumab 3 mg/kg every 2 weeks | CTCAE4.0; N/A | 8.9 | 28 |
Follow-up is the reported median follow-up time for the treatment arm.
Monitoring duration is the period after the end of treatment for reporting adverse effects.
Follow-up time of the study Beer et al. was calculated from the published data.
AE, adverse effect; CRPC, castration-resistant prostate cancer; CTCAE, Common Terminology Criteria for Adverse Events; GEJ cancer, gastro-esophageal junction cancer; HCC, hepatocellular carcinoma; MedDRA, Medical Dictionary for Regulatory Activities; NCT number, clinical trial registry numbers; NCT, ClinicalTrials.gov Identifier; NSCLC, Non-Small Cell Lung Cancer; SCLC, Small Cell Lung Cancer.
Selection criteria
The studies meeting the following criteria were included: (1) randomized, placebo-controlled clinical trials comparing the ICI (anti-PD-1, anti-PD-L1, and anti-CTLA-4) with placebo; (2) adverse effects were clearly described; and (3) patients diagnosed with malignancies and treated with anti-PD-1/PD-L1/CTLA-4 agents. Other oncologic therapies prior to ICI treatment were acceptable.
The exclusion criteria were as follows: (1) non-oncologic patients; (2) oncologic patients treated with ICI agents combined with other treatments simultaneously (for example, chemotherapy, radiotherapy); (3) retrospective studies, reviews, systematic reviews and meta-analyses, case reports, basic research, and expert opinions; and (4) duplicate publications or unpublished studies.
Data extraction
Two investigators independently reviewed the titles and abstracts. They then assessed the full texts and protocols of all potential studies and extracted relevant information from the included studies using a predefined data collection form. For each trial, the patient and control group characteristics, treatment modalities (inhibitor type, dosage, and duration), characteristics of irAEs [type and grade, from 1 to 5 according to Version 3 or 4 of the Common Terminology Criteria for Adverse Events (CTCAE) of the National Cancer Institute], follow-up duration, and therapeutic response were collected. Any disagreement between them over the eligibility of the study was resolved through discussion with a third reviewer.
Outcomes
The primary endpoints in our study were the irAE incidence and risk ratio (RR). IrAEs are adverse effects (AEs) potentially caused by immunological effects. Here we focused on rheumatic irAEs in a generalized manner, defining them as a subset of irAEs related to but not limited to the musculoskeletal systems, as non-musculoskeletal irAEs may occur along with the musculoskeletal symptoms.7 The rheumatic irAEs of interest included arthralgia, arthritis, synovitis, xerostomia, xerophthalmia, Sjögren syndrome, systemic lupus erythematosus, myositis, vasculitis, colitis, thyroiditis, hypophysitis, pneumonitis, vitiligo, and hepatitis, according to a prospective cohort study.7 Rheumatic irAEs with abundant records were collected for meta-analysis, and the data of rheumatic irAEs were extracted from the original publications and information reported on clinicaltrial.gov.
Quality assessment and risk of bias
Two investigators independently assessed the methodological quality of the included studies. The quality of evidence and risk of bias for the included studies were evaluated according to Cochrane collaboration’s tool for assessing risk of bias. Assessing categories included sequence generation, allocation concealment, blinding, completeness of outcome data, incomplete outcome data, and other sources of bias.8 Disagreements were resolved first by discussion and then by consulting a third investigator for arbitration.
Statistical analysis
Statistical analysis was performed using RevMan 5.3 (The Cochrane Collaboration; Oxford, UK) and R statistical software (packages metafor, meta, robvis Version 3.6.2, R Foundation). Event risks, relative risk values, and 95% confidence intervals (CIs) were calculated using both random-effects and fixed-effect models and presented by forest plots. Publication bias was assessed using funnel plots. Statistical heterogeneity between studies in our meta-analyses was assessed by the I2 statistic (<30%, no heterogeneity; 30–60%, moderate heterogeneity; >60%, strong heterogeneity). Strong heterogeneity was further explored by sensitivity analysis. We performed subgroup analysis according to single and combined ICI use to investigate the potential additional effect of concomitant. A two-tailed p-value < 0.05 was considered significant.
Results
Characteristics of the included studies
The search strategy originally generated 5444 published records and 316 registration records from the databases. After screening and eligibility assessment, 35 records were retrieved and assessed for eligibility. After removing publications of follow-ups and medico-economic analysis, we finally included seven published studies, (designated as Antonia 2017, Beer 2017, Eggermont 2015, Eggermont 2018, Finn 2017, Maio 2017, Kang 2017)9–15 and one unpublished study from the registration database (CHECKMATE 451),16 which include data from 5560 patients. The detailed search and study selection process is shown in Figure 1.
Figure 1.
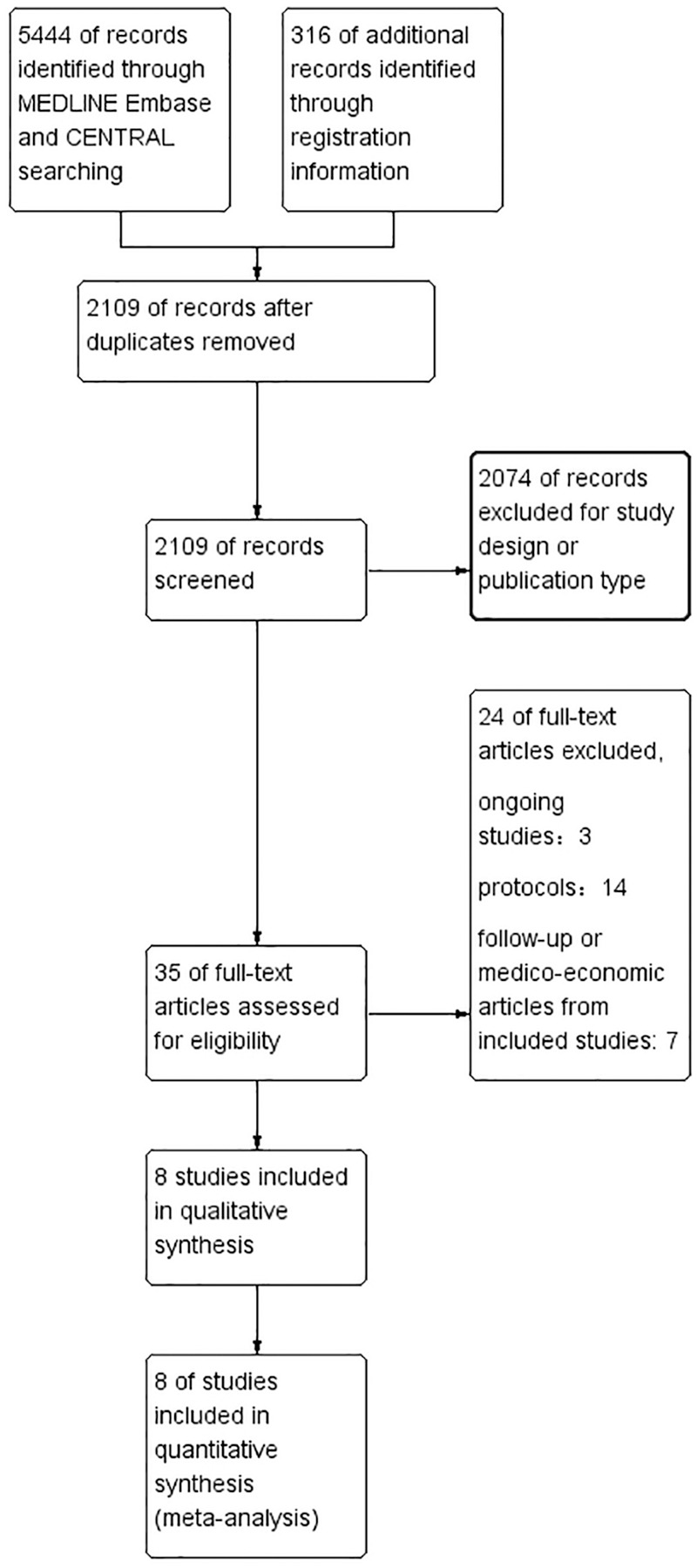
Flow diagram of search and study selection process.
All studies were placebo-controlled, multicenter, randomized trials with available protocols. Among the included studies, seven were phase III clinical trials and one was phase IIb. Four trials used PD-1/PD-L1 inhibitors, three used CTLA-4 inhibitors, and one used both PD-1 and CTLA-4 inhibitors. All the studies recorded AEs according to the most updated CTCAE criteria available. The detailed study characteristics are presented in Table 1.9–16
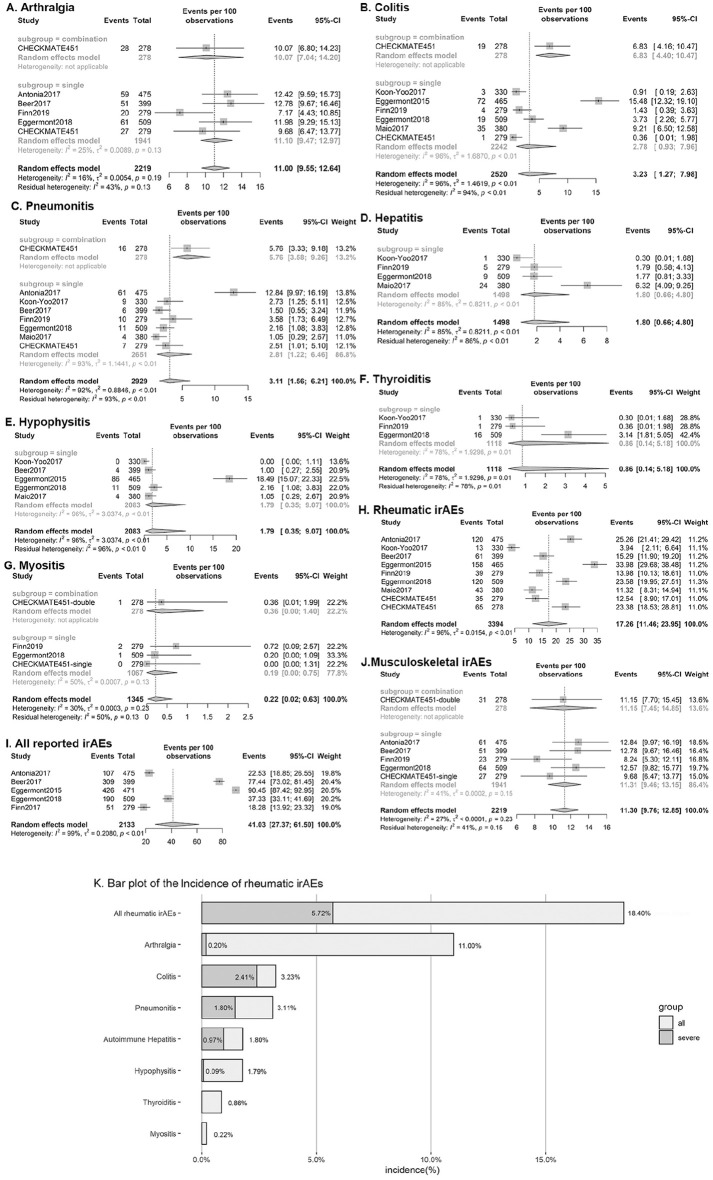
Incidence and relative risk of all irAEs
We first analyzed all-grade irAEs in five original publications that defined and presented irAEs. All-grade irAEs occurred in 41.03% participants in the treatment arms (95% CI 27.37–61.50%, p < 0.01, I2 = 99%), with 2.79 relative risk (95% CI 2.21–3.53 p < 0.01, I2 = 71%) (Figure 2I, Supplemental Figure 3J). The considerable heterogeneity may arise from the definition of irAEs. Three trials prespecified a list of irAEs recorded,10,12,14 while one trial defined irAEs as events with an immune-mediated mechanism requiring specific treatment.11
Figure 2.
Forest plots of incidence of all-grade immune-related adverse effects.
Incidence is presented by events per 100 observations: (A) Arthralgia; (B) Colitis; (C) Pneumonitis; (D) Autoimmune hepatitis; (E) Hypophysistis; (F) Thyroiditis; (G) Myositis ;(H) All rheumatic irAEs; (I) All irAEs reported by the included studies; (J) Musculoskeletal irAEs; and (K) Bar plot of the incidence of overall and interested rheumatic irAEs. One severe thyroiditis of 1118 and three severe myositis of 1345 were observed in treatment group, while 0 reported in control group.
CI, confidence interval.
The incidence and relative risk of severe irAEs (defined as CTCAE grade 3–5) was 12.61% (95% CI 6.02–26.43%, p < 0.01, I2 = 98%) and 12.09 (95% CI 8.17–17.89, p < 0.01, I2 = 84%), respectively (Supplemental Figures 1H, 2H). The relative risk of developing severe irAEs is strongly promoted by ICI therapy, although studies displayed considerable heterogeneity, which may arise from the different types of cancers and patients.
Incidence and relative risk of frequently observed rheumatic irAEs
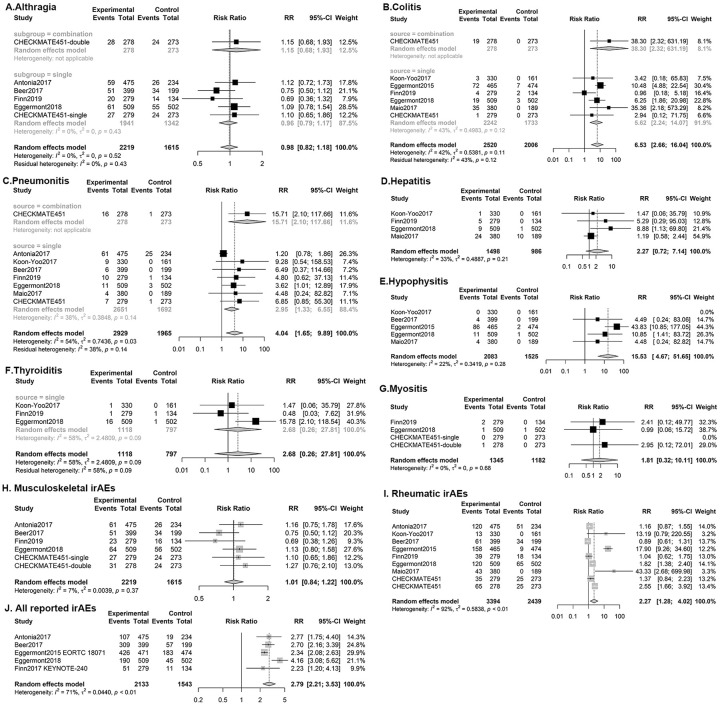
The overall incidence and relative risk values of all-grade rheumatic irAEs were 18.40% (95% CI 12.16–25.59%, p < 0.01, I2 = 96%) and 2.30 (95% CI 1.32–4.02, p < 0.01, I2 = 92%) (Figures 2, 3), respectively, while those of severe rheumatic irAEs were 5.72% (95% CI 3.92–7.82%, p < 0.01, I2 = 84%) and 8.29 (95% CI 3.75–18.35, p = 0.04, I2 = 50%), respectively. Musculoskeletal irAEs, including arthralgia, myositis, and rare conditions like polymyalgia rheumatica and autoimmune arthritis occurred in 11.30% (95% CI 9.76–12.85%, p < 0.01, I2 = 27%) of the study population, among whom 0.36% (95% CI 0.10–0.80%, p < 0.01, I2 = 66%) were severe (Figures 2, 3). The relative risk of all musculoskeletal irAEs was 1.01 (95% CI 0.84–1.22, I2 = 7%), and severe musculoskeletal irAEs was 1.68 (95% CI 0.58–4.89, I2 = 12%) (Supplemental Figure 1, 2).
Figure 3.
Forest plots of relative risk of all-grade immune-related adverse effects.
Relative risk of all-grade adverse events: (A) Arthralgia; (B) Colitis; (C) Pneumonitis; (D) Autoimmune hepatitis; (E) Hypophysitis; (F) Thyroiditis; (G) Myositis; (H) Musculoskeletal irAEs (I) all rheumatic irAEs; and (J) All irAEs reported by the included studies.
CI, confidence interval.
Seven rheumatic irAEs that were reported by more than three trials and enough events were considered for the final meta-analysis, while others were summarized by description. The most frequently observed rheumatic irAE was arthralgia, followed by pneumonitis and colitis, whereas hepatitis, hypophysitis, thyroiditis, and myositis were rare and less recorded.
The incidence and relative risk of all-grade arthralgia (n = 2219) were 11.00% (95% CI 9.55–12.64%, p = 0.19, I2 = 16%) and 0.99 (95% CI 0.82–1.19, p = 0.52, I2 = 0%), respectively, while grade 3–5 arthralgia occurred in 0.20% (95% CI 0.06–0.69%, p = 1.0, I2 = 15%) of treatment arm participants with 1.13 relative risk (95% CI 0.22–4.47, p = 5.90, I2 = 16%).
Pneumonitis, including interstitial lung diseases, was recorded in all studies. The incidence of all-grade pneumonitis in the treatment arms was 3.11% (95% CI 1.56–6.21, p < 0.01, I2 = 92%), while the relative risk compared with that in the control was 4.04 (95% CI 1.65–9.89, p = 0.03, I2 = 54%). The incidence and relative risk of severe pneumonitis were 1.47% (95% CI 0.70–3.09%, p < 0.01, I2 = 79%) and 3.48 (95% CI 1.61–7.55, p = 0.34, I2 = 11%), respectively.
The incidence and relative risk of all-grade colitis (n = 2919) were 3.23% (95% CI 1.27–7.98%, p < 0.01, I2 = 96%) and 6.53 (95% CI 2.66–16.04, p = 0.11, I2 = 42%), respectively, while those of grade 3–5 colitis were 2.41% (95% CI 0.99–5.77%, p = 0.31, I2 = 95%) and 9.59 (95% CI 4.54–20.27, p = 0.64, I2 = 0%), respectively.
The incidences of all-grade autoimmune hepatitis, hypophysitis, thyroiditis, myositis, severe hepatitis, and severe hypophysitis were 1.80%, 1.79%, 0.86%, 0.22%, 0.97% and 0.09%, respectively, and two patients died of hepatitis. In the treatment arm, only one patient receiving PD-1 inhibitor developed severe thyroiditis among 1118 patients13 and three had severe myositis among 1345 patients (CHECKMATE 451)10,12 leading to one death, while none were reported in the control arm10 (Table 2).
Table 2.
Incidence and relative risks of frequently observed rheumatic irAEs.
| All | Severe | |||
|---|---|---|---|---|
| Incidence in treatment arm, % (95% CI%) | Relative risk (95% CI) | Incidence in treatment arm, % (95% CI%) | Relative risk (95% CI) | |
| Arthralgia | 11.00 (9.55–12.64) | 0.99 (0.82–1.19) | 0.20 (0.06–0.69) | 1.13 (0.22–5.90) |
| Colitis | 3.23 (1.27–7.98) | 6.53 (2.66–16.04) | 2.41 (0.99–5.77) | 9.59 (4.54–20.27) |
| Pneumonitis* | 3.11 (1.56–6.21) | 4.04 (1.65–9.89) | 1.47 (0.70–3.09) | 3.48 (1.61–7.55) |
| Autoimmune hepatitis | 1.80 (0.66–4.80) | 2.27 (0.72–7.14) | 0.97 (0.41–1.71) | 5.03 (1.96–12.88) |
| Hypophysitis | 1.79 (0.35–9.07) | 15.53 (4.67–51.65) | 0.09 (0.07–2.94) | 8.98 (2.10–38.38) |
| Thyroiditis# | 0.86 (0.14–5.18) | 2.68 (0.26–27.81) | / | / |
| Myositis$ | 0.22 (0.02–0.63) | 1.88 (0.35–10.10) | / | / |
| Musculoskeletal irAEs | 11.30 (9.76–12.85) | 1.01 (0.84–1.22) | 0.36 (0.10–0.80) | 1.68 (0.58–4.89) |
| All rheumatic irAEs | 18.40 (12.16–25.59) | 2.30 (1.32–4.02) | 5.72 (3.92–7.82) | 8.29 (3.75–18.35) |
| All irAEs | 41.03 (27.37–61.50) | 2.79 (2.21–3.53) | 12.61 (6.02–26.43) | 12.09 (8.17–17.89) |
“pneumonitis” includes immune-related pneumonitis and interstitial lung diseases.
One patient with severe thyroiditis was detected among 1118 patients in the treatment arm, while none were reported in the control arm.13
Three patients with severe myositis were detected among 1345 patients in the treatment arm, leading to one death, while none were reported in the control arm.
All data were analyzed using a random-effects model.
CI, confidence interval.
Results of the subgroup analysis
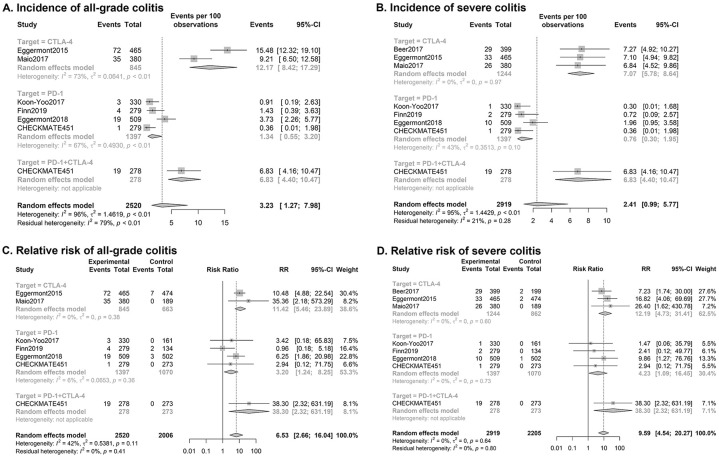
The incidence and relative risk of pneumonitis (Figure 3C, Supplemental Figures 1C, 2C, and 3C) and colitis displayed considerable overall heterogeneity in the included studies. Thus, we performed subgroup analysis to further examine the heterogeneity.
Subgroup analysis of all-grade pneumonitis showed heterogeneity from an increased incidence with therapy combining CTLA-4 and PD-1 inhibitors. The relative risk of pneumonitis with single ICI use was 2.95 [95% CI (1.33, 6.55), p = 0.14, I2 = 38%], whereas it increased to 15.71 [95% CI (2.10, 117.66)] with combined use of ICIs, as reported in CHECKMATE 451.
Subgroup analysis according to drug target showed an increased risk of colitis related to CTLA-4 therapy, particularly severe colitis. The incidence of grade 3–5 colitis was 7.07% (95% CI 5.78–8.64%, p = 0.97, I2 = 0%), combining results of three trials applying only CTLA-4 inhibitors, while it was 0.76% (95% CI 0.30–1.95%, p = 0.10, I2 = 43%) of PD-1 inhibitor therapy. Similarly, the relative risk values of severe colitis with CTLA-4 treatment, PD-1 treatment, and combined therapy were 12.19 (95% CI 4.73–31.41, p = 0.60, I2 = 0%), 4.23 (95% CI 1.09–16.45, p = 0.73, I2 = 0%), and 38.30 (95% CI, 2.32–631.19), respectively. Colitis of all severity displayed similar but less distinct results, as displayed in Figure 4.
Figure 4.
Forest plots of subgroup analysis of ICI-related colitis.
Subgroup analysis by drug of: (A) Incidence of all-grade colitis; (B) Incidence of severe colitis; (C) Relative risk of all-grade colitis; (D) Relative risk of severe colitis.
Less recorded rheumatic irAEs
The included studies also reported several severe but rarely observed rheumatic irAEs. Sarcoidosis occurred in 11 of 1449 patients in the treatment arm,9,12,14 of whom four were grade 3–4, compared with only one case of severe sarcoidosis in 474 patients in the placebo arm.12 Autoimmune arthritis occurred in five of 1911 patients in the treatment arm, among whom one patient was diagnosed with rheumatoid arthritis14 compared with 2 of 1438 patients in the control arm12,14–16 (CHECKMATE451). Other severe rheumatic irAEs occurred only in the treatment arm, including autoimmune uveitis (n = 3), autoimmune pericarditis, bursitis, osteochondrosis, psoriasis, polymyalgia rheumatica, systemic inflammatory response syndrome, and Sjögren syndrome (n = 1 each).
Non-severe rheumatic irAEs have been much less reported. One study of standard pembrolizumab maintenance therapy in completely resected melanoma patients recorded 30 patients with CTCAE grade 1–3 dry mouth in the treatment arm versus 10 in the control arm, and 24 patients with vitiligo in the treatment arm and none recorded in the control arm.14
Quality assessment and publication bias
The risk of bias plots was used to evaluate the methodological qualities of the included studies. The overall risk of bias was evaluated as low. Therefore, the quality of the studies was satisfactory (Supplemental Figure 3). The funnel plot for publication bias based on the RR of all-grade treatment-related AEs presented symmetric distribution on either side of the funnel, indicating that no significant publication bias existed in this meta-analysis (Figure 5). Findings from Egger’s test (p = 0.9644) were consistent with the funnel plot.
Figure 5.
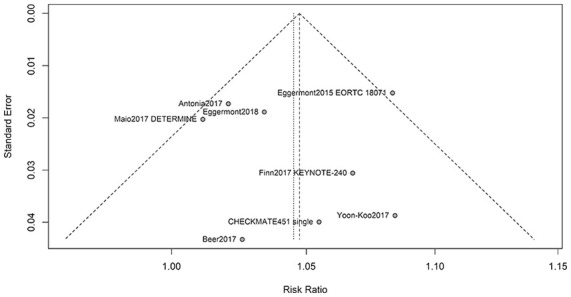
Funnel plot of publication bias.
Discussion
As agents resetting the checks and balances of T-cell cytotoxicity against tumors, ICIs have recently become a promising approach for treating many types of cancer. However, ICI therapy is accompanied by increased challenges in irAE management. Despite its low incidence, rheumatic irAEs exhibit considerable complexity and uniqueness among all irAEs. An in-depth study providing high-grade evidence is crucial for obtaining good understanding of rheumatic irAEs. Although a large number of case reports and some systematic reviews of rheumatic irAEs have been reported, no meta-analysis focusing on the incidence and RR of rheumatic irAEs has been published. To the best of our knowledge, this is the first systematic review and meta-analysis investigating ICI-related irAEs with placebo as the control group.
Since there are some shared pathways and pathogenesis during the process of rheumatic diseases and ICI treatment, there is a theoretical basis for rheumatic irAE development. The mechanisms leading to irAEs involve T-cell and B-cell responses.18 ICI might increase T-cell repertoire diversification and mobilize large numbers of T cells, leading to irAE.17,19 Another study20 found that circulating B cells decreased after combination ICI treatment, correlated with increased rates of grade 3 or higher irAEs 6 months after therapy initiation.21 Furthermore, ICIs can affect B-cell responses and induce autoantibody production, and 19.2% of patients developed new autoantibodies under ICI treatment.21
Our results indicated that ICI therapy increased incidences and relative risks of all-grade irAEs and rheumatic irAEs compared with placebo. However, although musculoskeletal irAEs were not rare in the included studies, the overall risk of musculoskeletal irAEs was not significantly increased by ICI therapy. The calculated relative risk of each severe irAE exceeded that of all-grade irAEs, except for hypophysitis and pneumonitis, which implied that generalized rheumatic irAEs were severe in a large proportion of patients. However, significant heterogeneity existed in the included studies reporting overall irAEs, which may be justified by the difference in drug targets, histological tumor types, and disease phases among the included studies.
Arthralgia, colitis, and pneumonitis are common and well-recorded generalized rheumatic irAEs, whereas hepatitis, hypophysitis, thyroiditis, and myositis occurred in less than 2% of the patients. However, recognition and recording of some irAEs were not adequate in many included studies as some rare irAEs such as hypophysitis, thyroiditis, and myositis were not reported in many trials. Our knowledge of irAEs is broadening with increasing clinical experience of ICIs, which may partly explain the inadequate reporting and observed heterogeneity.
Arthralgia was the most frequent rheumatic irAE. As reported in an earlier systematic review, arthralgia rates ranged widely from 1% to 43% in a randomized controlled study.6 Our results also showed that the all-grade arthralgia incidence was 10%, yet the relative risk showed insignificant risk increase by ICI therapy. Severe arthralgia and arthritis were reported in seven of 2219 patients in the treatment arm. Thus, although arthralgia was common, it may not be severe or directly relevant to ICI therapy in most cases, which should be considered when managing arthralgia.
Hypophysitis had the largest relative risk among the all-grade irAEs. One study of ipilimumab maintenance treatment in patients with melanoma contributed the most to the observed hypophysitis events.12 Another meta-analysis of endocrine AEs also discovered that 76 of 85 observed hypophysitis events occurred in the patients with melanoma, and patients receiving CTLA-4 inhibitors were more likely to experience hypophysitis than patients receiving PD-1 inhibitors.22 The exact mechanism is unknown, yet a former study on autopsy of pituitary glands discovered that pituitary cells express CTLA-4, and the patients with hypophysitis and high pituitary CTLA-4 expression experienced extensive anti-CTLA-4-mediated adenohypophyseal architecture destruction.23 Thus, these studies provide some insight into the observed heterogeneity in hypophysitis incidence.
Colitis, the most common severe rheumatic irAE, was the second most frequent all-grade rheumatic irAE. Upon subgroup analysis, we found that CTLA-4 inhibitor-induced colitis may be more common and severe than PD-1 inhibitor-induced colitis, and the combined use of PD-1 and CTLA-4 inhibitors was even worse, which was in agreement with findings from a previous meta-analysis.24 The exact mechanism is unclear, but anti-CTLA-4-induced colitis may be mechanistically different from anti-PD-1-induced colitis. CD4+ T-cell infiltration and high mucosal TNF-α concentration were found in colonic biopsies of the patients with anti-CTLA-4-induced colitis, whereas Tregs were dominant in anti-PD-1-induced colitis.25 Gut microflora may also be involved in anti-CTLA-4-induced colitis, as findings from human gut microflora showed that baseline colonization of Firmicutes is associated with colitis, while increased representation of baseline Bacteroidetes protects against colitis.26,27 Further studies in high-risk groups and cautious use of anti-CTLA-4 inhibitors are warranted.
Myositis was a rare but severe rheumatic irAE, which led to one death in the ICI group and only one patient with non-severe myositis was observed in the control group.14 Current knowledge of ICI-induced myositis mainly comes from retrospective studies and case reports. A Japanese pharmacovigilance study explored the Japanese Adverse Drug Event Report database and found that 127 of 7604 (1.67%) inflammatory myositis occurred with ICI use.28 The inconsistency between our results and those of the retrospective study may be due to the inadequate identification and report of myositis and myocarditis in clinical trials. However, ICI-associated myositis can be severe, with mortality as high as 21.2% in patients treated with ICI monotherapy or combination, of whom 87.7% were oncologic patients and 13.3% had unknown indication.29 As reported in another retrospective study, among 10 European oncologic patients with ICI-induced myositis nine developed CTCAE grade 3–4 myositis, and received corticosteroid therapy.30 Thus, myositis should be identified and treated properly.
Both all-grade and severe autoimmune hepatitis occurred less frequently than colitis and pneumonitis. A previous meta-analysis of irAEs in non-placebo-controlled ICI trials observed that hepatitis incidence ranged from 0.6% to 1.8%,31,32 which was lower than our results and was partly explained by the hepatotoxicity caused by therapy in the control arm. We observed that half of the ICI-associated hepatitis incidences were severe. According to a meta-analysis focusing on fatal irAEs, hepatitis was among the main causes of death. Hepatitis may be asymptomatic, and increased transaminase is the most common initial presentation of hepatitis.24 Therefore, special attention should be paid to liver function tests of patients receiving ICIs.
The combined use of PD-1 and CTLA-4 inhibitors resulted in an increased risk and incidence of all irAEs, especially severe irAEs, and this phenomenon was the most prominent in pneumonitis. Previous meta-analyses of ICI-induced AEs by Su et al. and Gu et al. also supported increased risks of AEs in combination with ICI use,33,34 but the exact relative risks of combining ICI therapy-induced pneumonitis was lower than our results (3.25 and 2.25 versus 4.04 and 3.48 in all-grade and grade 3–5 pneumonitis, respectively). The underestimation of relative risks may be explained by the inclusion of non-placebo-controlled randomized trials.
These findings have practical implications. In clinical practice, it is difficult to differentiate rheumatic irAEs from generalized symptoms from underlying cancer, infection, pre-existing rheumatic diseases, or side effects of other medications. Thus, ICI-related irAEs with placebo as the control group provide important insights into the incidence and RRs of the rheumatic irAEs. Rheumatic irAEs contribute to almost one-third of all irAEs and the systemic involvement can be severe and lethal; thus, attention should be paid to these specific situations in ICI therapy. Future research should focus on diagnostic and severity biomarkers, mechanisms, and treatment for rheumatic irAEs.
Our analysis was limited by several factors, which should be taken into account when interpreting the findings in the real world. First, heterogeneity due to clinical and methodological diversity was inevitable, including malignancy types, treatment types, and therapies before the current study. This may affect the reliability of the analysis results. Second, the studies included were insufficient to carry out more subgroup analysis, for example, by malignancy type and ICI mechanisms in some rare AEs. Third, this is a meta-analysis of literature and data extraction was based on collecting and reporting of AEs by the investigators, so publication bias due to under-diagnosis and under-reporting might exist. Fourth, monitoring duration (1.9 to over 37 months) was inconsistent between studies and especially restricted in one trial in mesothelioma with poor prognosis, which possibly influenced the performance of this meta-analysis to describe the ICI-induced rheumatic irAEs. However, as reported in observational studies, most rheumatic irAEs occur shortly after treatment initiation. Fifth, although there was no uniform definition of rheumatic irAE across the studies, the report of AEs in all the included studies is uniform, according to the most recent versions of CTCAE and MedDRA. Moreover, rheumatic irAEs can only be well explained by ICI administration in the included trials, so they are unlikely to be influenced by ascertainment biases or different thresholds. Finally, there is a lack of reports about the details of events of interest in most studies, which made it difficult to observe treatment and previous history of rheumatic diseases.
Conclusion
The overall incidence and relative risk of all-grade rheumatic irAEs were 18.40% and 2.30, respectively, while for overall musculoskeletal irAEs were 11.30% and 1.01. Arthralgia was the most common non-severe irAE; colitis and pneumonitis were the most common severe irAEs. Special attention is needed to identify rare irAEs such as hypophysitis, thyroiditis, and myositis. It was also seen that combining anti-PD-1 and anti-CTLA-4 inhibitors increased rheumatic irAEs risk. Thus, the patients undergoing ICI therapy, especially combined ICI, should be closely monitored for rheumatic irAEs.
Supplemental Material
Supplemental material, sj-docx-1-taj-10.1177_2040622320976996 for Rheumatic immune-related adverse events associated with immune checkpoint inhibitors compared with placebo in oncologic patients: a systemic review and meta-analysis by Shuo Zhang, Ziyue Zhou, Li Wang, Mengtao Li, Fengchun Zhang and Xiaofeng Zeng in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-2-taj-10.1177_2040622320976996 for Rheumatic immune-related adverse events associated with immune checkpoint inhibitors compared with placebo in oncologic patients: a systemic review and meta-analysis by Shuo Zhang, Ziyue Zhou, Li Wang, Mengtao Li, Fengchun Zhang and Xiaofeng Zeng in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-3-taj-10.1177_2040622320976996 for Rheumatic immune-related adverse events associated with immune checkpoint inhibitors compared with placebo in oncologic patients: a systemic review and meta-analysis by Shuo Zhang, Ziyue Zhou, Li Wang, Mengtao Li, Fengchun Zhang and Xiaofeng Zeng in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-4-taj-10.1177_2040622320976996 for Rheumatic immune-related adverse events associated with immune checkpoint inhibitors compared with placebo in oncologic patients: a systemic review and meta-analysis by Shuo Zhang, Ziyue Zhou, Li Wang, Mengtao Li, Fengchun Zhang and Xiaofeng Zeng in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-5-taj-10.1177_2040622320976996 for Rheumatic immune-related adverse events associated with immune checkpoint inhibitors compared with placebo in oncologic patients: a systemic review and meta-analysis by Shuo Zhang, Ziyue Zhou, Li Wang, Mengtao Li, Fengchun Zhang and Xiaofeng Zeng in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-6-taj-10.1177_2040622320976996 for Rheumatic immune-related adverse events associated with immune checkpoint inhibitors compared with placebo in oncologic patients: a systemic review and meta-analysis by Shuo Zhang, Ziyue Zhou, Li Wang, Mengtao Li, Fengchun Zhang and Xiaofeng Zeng in Therapeutic Advances in Chronic Disease
Supplemental material, sj-pdf-7-taj-10.1177_2040622320976996 for Rheumatic immune-related adverse events associated with immune checkpoint inhibitors compared with placebo in oncologic patients: a systemic review and meta-analysis by Shuo Zhang, Ziyue Zhou, Li Wang, Mengtao Li, Fengchun Zhang and Xiaofeng Zeng in Therapeutic Advances in Chronic Disease
Footnotes
Author contributions: Wang L had full access to all the data in the study and takes responsibility for data integrity and data analysis accuracy. Zhang S and Zhou Z collected, analyzed, and interpreted the data. All authors prepared and revised the manuscript.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Nonprofit Central Research Institute Fund of the Chinese Academy of Medical Science (2019XK320022), National Key Research and Development Program (2016YFA0101003, 2016YFC0903901), National Natural Science Fund (81771764, 81571594).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Ziyue Zhou  https://orcid.org/0000-0002-6580-4419
https://orcid.org/0000-0002-6580-4419
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Shuo Zhang, Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China; National Clinical Research Center for Dermatologic and Immunologic Diseases, Ministry of Science & Technology, Beijing, China; Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, Beijing, China.
Ziyue Zhou, Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China; National Clinical Research Center for Dermatologic and Immunologic Diseases, Ministry of Science & Technology, Beijing, China; Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, Beijing, China.
Li Wang, Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, 1, Shuaifuyuan, Beijing, 100730, China; National Clinical Research Center for Dermatologic and Immunologic Diseases, Ministry of Science & Technology, Beijing, China; Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, Beijing, China.
Mengtao Li, Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China; National Clinical Research Center for Dermatologic and Immunologic Diseases, Ministry of Science & Technology, Beijing, China; Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, Beijing, China.
Fengchun Zhang, Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China; National Clinical Research Center for Dermatologic and Immunologic Diseases, Ministry of Science & Technology, Beijing, China; Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, Beijing, China.
Xiaofeng Zeng, Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China; National Clinical Research Center for Dermatologic and Immunologic Diseases, Ministry of Science & Technology, Beijing, China; Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, Beijing, China.
References
- 1. Bertrand A, Kostine M, Barnetche T, et al. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med 2015; 13: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. El Osta B, Hu F, Sadek R, et al. Not all immune-checkpoint-inhibitors are created equal: meta-analysis and systematic review of immune-related adverse events in cancer trials. Crit Rev Oncol Hematol 2017; 119: 1–12. [DOI] [PubMed] [Google Scholar]
- 3. Wang PF, Chen Y, Song SY, et al. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: a meta-analysis. Front Pharmacol 2017; 8: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pedoeem A, Azoulay-Alfaguter I, Strazza M, et al. Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol 2014; 153: 145–152. [DOI] [PubMed] [Google Scholar]
- 5. Dai S, Jia R, Zhang X, et al. The PD-1/PD-L1s pathway and autoimmune diseases. Cell Immunol 2014; 290: 72–79. [DOI] [PubMed] [Google Scholar]
- 6. Cappelli LC, Gutierrez AK, Bingham CO, et al. Rheumatic and musculoskeletal immune-related adverse events due to immune checkpoint inhibitors: a systematic review of the literature. Arthritis Care Res 2017; 69: 1751–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kostine M, Rouxel L, Barnetche T, et al. Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer-clinical aspects and relationship with tumour response: a single-centre prospective cohort study. Ann Rheum Dis 2018; 77: 393–398. [DOI] [PubMed] [Google Scholar]
- 8. Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med 2017; 377: 1919–1929. [DOI] [PubMed] [Google Scholar]
- 10. Kang Y-K, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 390: 2461–2471. [DOI] [PubMed] [Google Scholar]
- 11. Beer TM, Kwon ED, Drake CG, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol 2017; 35: 40–47. [DOI] [PubMed] [Google Scholar]
- 12. Eggermont AMM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 2015; 16: 522–530. [DOI] [PubMed] [Google Scholar]
- 13. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-Blind, phase III trial. J Clin Oncol 2020; 38: 193–202. [DOI] [PubMed] [Google Scholar]
- 14. Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med 2018; 378: 1789–1801. [DOI] [PubMed] [Google Scholar]
- 15. Maio M, Scherpereel A, Calabrò L, et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol 2017; 18: 1261–1273. [DOI] [PubMed] [Google Scholar]
- 16. Ready N, Owonikoko TK, Postmus PE, et al. CheckMate 451: a randomized, double-blind, phase III trial of nivolumab (nivo), nivo plus ipilimumab (ipi), or placebo as maintenance therapy in patients (pts) with extensive-stage disease small cell lung cancer (ED-SCLC) after first-line platinum-based d. J Clin Oncol 2016; 34: TPS8579. [Google Scholar]
- 17. Calabrese C, Kirchner E, Kontzias K, et al. Rheumatic immune-related adverse events of checkpoint therapy for cancer: case series of a new nosological entity. RMD Open 2017; 3: e000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weinmann SC, Pisetsky DS. Mechanisms of immune-related adverse events during the treatment of cancer with immune checkpoint inhibitors. Rheumatology (Oxford) 2019; 58: vii59–vii67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Robert L, Tsoi J, Wang X, et al. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clin Cancer Res 2014; 20: 2424–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Das R, Bar N, Ferreira M, et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J Clin Invest 2018; 128: 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Moel EC, Rozeman EA, Kapiteijn EH, et al. Autoantibody development under treatment with immune-checkpoint inhibitors. Cancer Immunol Res 2019; 7: 6–11. [DOI] [PubMed] [Google Scholar]
- 22. Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens a systematic review and meta-analysis. JAMA Oncol 2018; 4: 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Caturegli P, Di Dalmazi G, Lombardi M, et al. Hypophysitis secondary to cytotoxic T-lymphocyte–associated protein 4 blockade: insights into pathogenesis from an autopsy series. Am J Pathol 2016; 186: 3225–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 2018; 4: 1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coutzac C, Adam J, Soularue E, et al. Colon immune-related adverse events: anti-CTLA-4 and anti-PD-1 blockade induce distinct immunopathological entities. J Crohn’s Colitis 2017; 11: 1238–1246. [DOI] [PubMed] [Google Scholar]
- 26. Dubin K, Callahan MK, Ren B, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun 2016; 7: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chaput N, Lepage P, Coutzac C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol 2017; 28: 1368–1379. [DOI] [PubMed] [Google Scholar]
- 28. Sato K, Mano T, Iwata A, et al. Neurological and related adverse events in immune checkpoint inhibitors: a pharmacovigilance study from the Japanese adverse drug event report database. J Neurooncol 2019; 145: 1–9. [DOI] [PubMed] [Google Scholar]
- 29. Anquetil C, Salem JE, Lebrun-Vignes B, et al. Immune checkpoint inhibitor–associated myositis: expanding the spectrum of cardiac complications of the immunotherapy revolution. Circulation 2018; 138: 743–745. [DOI] [PubMed] [Google Scholar]
- 30. Touat M, Maisonobe T, Knauss S, et al. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology 2018; 91: e985–e994. [DOI] [PubMed] [Google Scholar]
- 31. Chen C, Zhang F, Zhou N, et al. Efficacy and safety of immune checkpoint inhibitors in advanced gastric or gastroesophageal junction cancer: a systematic review and meta-analysis. Oncoimmunology. Epub ahead of print 5 March 2019. DOI: 10.1080/2162402X.2019.1581547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reynolds K, Thomas M, Dougan M. Diagnosis and management of hepatitis in patients on checkpoint blockade. Oncologist 2018; 23: 991–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Su Q, Zhu EC, Wu JB, et al. Risk of pneumonitis and pneumonia associated with immune checkpoint inhibitors for solid tumors: a systematic review and meta-analysis. Front Immunol. Epub ahead of print 4 February 2019. DOI: 10.3389/fimmu.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gu L, Khadaroo PA, Su H, et al. The relative risk and incidence of immune checkpoint inhibitors related pneumonitis in patients with advanced cancer: a meta-analysis. BMC Cancer 2018; 9: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taj-10.1177_2040622320976996 for Rheumatic immune-related adverse events associated with immune checkpoint inhibitors compared with placebo in oncologic patients: a systemic review and meta-analysis by Shuo Zhang, Ziyue Zhou, Li Wang, Mengtao Li, Fengchun Zhang and Xiaofeng Zeng in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-2-taj-10.1177_2040622320976996 for Rheumatic immune-related adverse events associated with immune checkpoint inhibitors compared with placebo in oncologic patients: a systemic review and meta-analysis by Shuo Zhang, Ziyue Zhou, Li Wang, Mengtao Li, Fengchun Zhang and Xiaofeng Zeng in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-3-taj-10.1177_2040622320976996 for Rheumatic immune-related adverse events associated with immune checkpoint inhibitors compared with placebo in oncologic patients: a systemic review and meta-analysis by Shuo Zhang, Ziyue Zhou, Li Wang, Mengtao Li, Fengchun Zhang and Xiaofeng Zeng in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-4-taj-10.1177_2040622320976996 for Rheumatic immune-related adverse events associated with immune checkpoint inhibitors compared with placebo in oncologic patients: a systemic review and meta-analysis by Shuo Zhang, Ziyue Zhou, Li Wang, Mengtao Li, Fengchun Zhang and Xiaofeng Zeng in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-5-taj-10.1177_2040622320976996 for Rheumatic immune-related adverse events associated with immune checkpoint inhibitors compared with placebo in oncologic patients: a systemic review and meta-analysis by Shuo Zhang, Ziyue Zhou, Li Wang, Mengtao Li, Fengchun Zhang and Xiaofeng Zeng in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-6-taj-10.1177_2040622320976996 for Rheumatic immune-related adverse events associated with immune checkpoint inhibitors compared with placebo in oncologic patients: a systemic review and meta-analysis by Shuo Zhang, Ziyue Zhou, Li Wang, Mengtao Li, Fengchun Zhang and Xiaofeng Zeng in Therapeutic Advances in Chronic Disease
Supplemental material, sj-pdf-7-taj-10.1177_2040622320976996 for Rheumatic immune-related adverse events associated with immune checkpoint inhibitors compared with placebo in oncologic patients: a systemic review and meta-analysis by Shuo Zhang, Ziyue Zhou, Li Wang, Mengtao Li, Fengchun Zhang and Xiaofeng Zeng in Therapeutic Advances in Chronic Disease