Abstract
Background:
The overall prognosis of lung cancer remains unfavorable and novel prognostic biomarkers of lung cancer are needed warranted. Accumulating evidence indicate that systemic inflammation plays a vital role in lung cancer. The lymphocyte-to-monocyte ratio (LMR) is biomarker that reflects the level of systemic inflammation.
Objective:
To perform a comprehensive meta-analysis exploring the correlation of pretreatment LMR with the overall survival (OS) and progression-free survival (PFS) of lung cancer patients.
Methods:
We conducted searches of the PubMed, Embase, Cochrane Library, and Web of Science databases to May 2020 to identify relevant studies and calculated combined hazard ratios (HRs) to evaluate the association between pretreatment LMR and survival time in patients with lung cancer.
Results:
A total of 23 studies comprising 8361 lung cancer patients were included. Among the patients, 5702 (68%) were males, 4548 were current smokers and 2212 were diagnosed with squamous carcinoma. The pooled analysis revealed that decreased pretreatment LMR was significantly correlated with reduced of PFS (HR = 1.49, 95% CI: 1.34-1.67, p < 0.01) and reduced OS (HR = 1.61, 95% CI: 1.45-1.79, p < 0.01) among lung cancer patients. Furthermore, in the subgroup analyses according to histologic type, a lower level of pretreatment LMR seemed to be unrelated to the poorer OS of small cell lung cancer (SCLC) patients (HR = 1.21, 95%CI: 0.87-1.67, P = 0.25).
Conclusions:
Decreased pretreatment LMR in peripheral blood was associated with shorter OS and PFS in lung cancer patients, suggesting its potential prognostic value.
Keywords: lymphocyte-to-monocyte ratio, lung cancer, prognosis, meta-analysis, immunotherapy
Introduction
Lung cancer is the leading cause of malignant tumor-related mortality worldwide, and was responsible for an estimated 142,670 deaths in 2019.1 Non-small cell lung cancer (NSCLC) accounts for 85% of lung cancers, of which lung adenocarcinoma (LUAD) and squamous cell carcinoma (SCC) are the predominant histological subtypes. Despite the immense advances in treatment, the prognosis of lung cancer remains unsatisfactory, with a 5-year survival rate of only 15%.2 Therefore, novel prognostic biomarkers of lung cancer are needed for the development of individualized treatment strategies
Accumulating evidence indicates that systemic inflammation plays vital roles in various kinds of malignancies, including lung cancers.3-5 Derived from the routine complete blood count (CBC), LMR is defined as the ratio of the absolute lymphocyte count to the absolute monocyte count. It reflects the systemic inflammatory response and was first evaluated in hematologic malignancies.6 Recently, LMR has been increasingly investigated as a prognostic indicator in lung cancer, with lower LMR predicting worse patient prognosis.7-9 However, Cao et al observed no significant correlation between LMR and OS in lung cancer.10 The clinical significance of LMR in lung cancer remains controversial. Furthermore, the prognostic value of LMR is affected by various factors, such as the cut-off value, clinical stage, histologic type, and treatment. Considering the potential value of LMR, we performed this meta-analysis to analyze the clinical value of pretreatment LMR and the factors that may affect its prognostic ability.
Methods
Search Strategy
We systematically searched several databases such as PubMed, Embase, Cochrane Library, and Web of Science to identify potentially relevant studies published up to May 2020. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were strictly followed in the meta-analysis. The key search terms were: “Pulmonary Neoplasms,” “lymphocyte to monocyte ratio,” and “LMR.” A detailed search protocol is provided in the supplement (STable1). The reference lists of the compiled articles were manually searched for additional potentially relevant studies.
Eligibility Criteria
Studies were included if they met all the following criteria: (1) the patients had pathologically confirmed lung cancer; (2) the relationship between pretreatment LMR and prognosis was investigated; (3) the investigated survival outcomes included OS or PFS; and (4) HRs and corresponding 95% CIs were available. Articles that met any of the following criteria were eliminated: (1) the article was a conference abstract, review, or letter; and (2) the study contained duplicate data or insufficient data for analysis.
Data Extraction
Two reviewers independently extracted baseline information from each publication such as author, publication year, study period, country, sample size, gender, median age, tumor histologic type, tumor stage, smoking status, cut-off values, treatment, median follow-up time, clinical outcomes, and study design as well as data related to pretreatment LMR and prognosis. PFS was defined as the time from treatment onset to progression or death. OS was calculated from the date of inclusion in the study to the time of death from any cause.7,8 Discrepancies were resolved through discussion with the third researcher until consensus was reached.
Quality Assessment
Study quality was evaluated by 2 researchers independently based on the Newcastle Ottawa Quality Assessment Scale (NOS). The evaluation system considers 3 perspectives involving selection, comparability and exposure, with a higher score indicating higher quality. Studies with a score of at least 6 points were considered high-quality studies.
Statistical Analyses
The HRs and their associated 95% CIs in each study were utilized to investigate the relationship between LMR and prognosis in lung cancer patients, and a forest plot of the HRs from each study was constructed. According to the methods reported by Tierney et al, HRs and 95% CIs were directly extracted from the articles or estimated from the K-M curves.11 The heterogeneity within studies was evaluated using Cochran’s Q test and Higgins I2 index. A fixed-effect model or random-effect model was used according to the heterogeneity. Subgroup and sensitivity analyses were then conducted to explore the potential source of heterogeneity. The Begg’s rank correlation test was adopted to assess potential publication bias. If publication bias was detected, the trimming method was used to adjust for it. Two-sided P values were calculated, and values less than 0.05 were considered statistically significant. Statistical analyses were conducted in R (version 3.6.1).
Results
Characteristics of the Included Studies
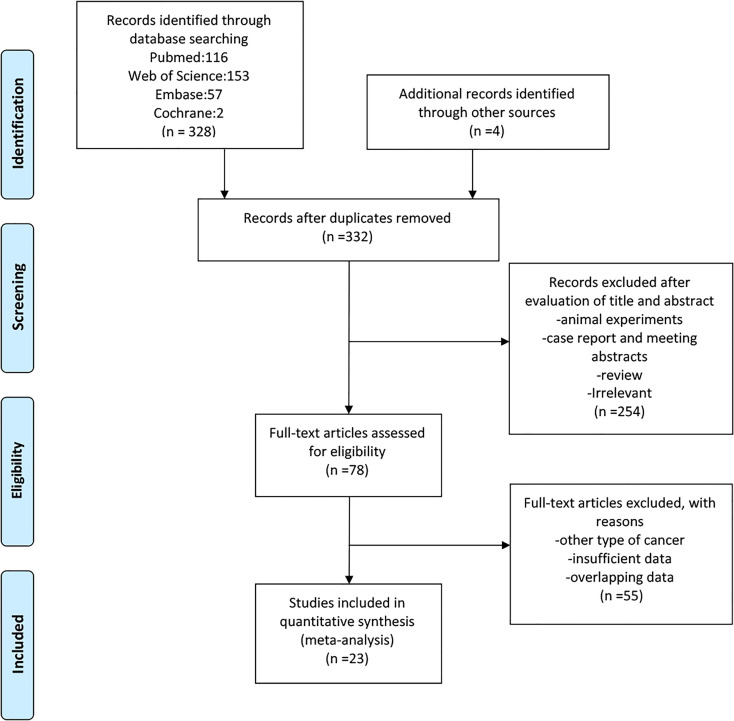
A detailed flow diagram is illustrated in Figure 1. A total of 23 studies comprising 8361 lung cancer patients were included.7-10,12-30 Among these patients, 5702 (68%) were males, 4548 were current smokers and 2212 were diagnosed with squamous carcinoma. The main features of the included studies are summarized in Table 1. Most of the studies were conducted in Asia between 2014 and 2020. The median follow-up period ranged between 25.8 and 93.8 months among all included studies. All of the studies focused on lung cancer patients, among which, 20 studies included only patients with NSCLC, 2 studies included only patients with SCLC,7,10 and one included both patients with SCLC and those with NSCLC.19 All selected studies analyzed the association between OS and pretreatment LMR. Thirteen studies reported PFS, 5 studies evaluated RR,17,20,24,26 5 studies evaluated disease-free survival(DFS),12,13,19,21,23 and 1 study analyzed recurrence-free survival (RFS).16 Chemotherapy, EGFR-TKIs, stereotactic ablative radiotherapy (SABR), chemotherapy plus TKI treatment, chemotherapy plus radiotherapy and surgery were used to treat lung cancer patients in the different studies. Various cut-off values for pretreatment LMR were utilized, ranging from 1.70 to 4.56. The quality of the included studies ranged from 6 to 8 (Table 1).
Figure 1.
Flow chart of study selection.
Table 1.
The Basic Characteristic of Enrolled Studies.
| Study | Period | Country | Histology | Sample size | MFP | Age(Median) | M/F |
|---|---|---|---|---|---|---|---|
| Huang, Q 2020 | 2012-2014 | China | NSCLC | 254 | 48 | 60.2 ± 9.5# | 186/68 |
| Katayama, Y 2020 | 2017-2018 | Japan | NSCLC | 35 | NM | 70 | 24/11 |
| Takada, K 2020 | 2016-2018 | Japan | NSCLC | 226 | 413(days) | 66 | 184/42 |
| Xue,Yuan 2020 | 2009-2011 | China | NSCLC | 538 | 54 | 60 | 343/195 |
| Guo M 2019 | 2015-2016 | China | NSCLC | 370 | NM | NM | 312/58 |
| Prelaj A 2019 | 2015-2018 | Italy | NSCLC | 154 | NM | 67 | 126/28 |
| Yan,Wang 2019 | 2014-2016 | China | NSCLC | 261 | 38 | NM | 144/117 |
| Zhang Y 2018 | 2013-2015 | China | NSCLC | 127 | 28 | 61.9¶ | 55/72 |
| Watanabe K 2018 | 2008-2017 | Japan | NSCLC | 72 | 26 | 69 | 31/41 |
| Minami S 2018 | 2007-2017 | Japan | NSCLC | 107 | NM | 70.2 ± 8.2# | 82/25 |
| Minami Seigo 2018 | 2007-2017 | Japan | NSCLC | 159 | NM | 67.2 ± 9.0# | 114/45 |
| Luo H 2018 | 2011-2015 | China | NSCLC | 63 | 30 | 73 | 49/14 |
| Lim JU 2018 | 2012-2016 | Korea | NSCLC | 217 | NM | 68 | 120 97 |
| Chen Y 2018 | 1999-2009 | China | NSCLC | 577 | 94 | 60 | 410/167 |
| Xiong Y 2017 | 2012-2015 | China | NSCLC | 78 | NM | 59 | 36/42 |
| Liu W 2017 | 2007-2011 | China | NSCLC | 1120 | 45 | 60 | 728/392 |
| Gao Y 2017 | 2007-2008 | China | NSCLC | 358 | 36 | 61 | 247/111 |
| Cao S 2017 | 2008-2010 | China | SCLC | 707 | NM | 56.24 ± 10.15# | 454/253 |
| Xia H 2016 | 2005-2010 | China | NSCLC | 439 | 57 | 62 | 287/152 |
| Song Ying-Jian 2016 | 2006-2010 | China | NSCLC | 488 | 48 | 64 | 359/129 |
| Lin Gui-Nan 2014 | 2004-2012 | China | NSCLC | 370 | NM | 64 | 213/157 |
| Pingping Hu 2014 | 2006-2011 | China | NSCLC/SCLC | 1453 | NM | 59 | 1035/418 |
| Go Se-II 2014 | 2006-2014 | Korea | SCLC | 188 | 42 | NM | 163/25 |
| Study | SCC% | Treatment | Outcome | Stage | NOS | Smoker% | Cut-off |
| Huang, Q 2020 | 44 | Chemotherapy+ Radiotherapy | OS/DFS | I-IV | 7 | 59 | 4.04 |
| Katayama, Y 2020 | 29 | Immunotherapy | OS/PFS/RR/DCR | III/IV/REC | 6 | 77 | 1.70 |
| Takada, K 2020 | 27 | Immunotherapy | OS/PFS/RR/DCR | IIIB/IV/REC | 6 | 84 | 2.12 |
| Xue,Yuan 2020 | 47 | Surgery | OS/DFS | I/II/IIIA | 7 | 64 | 3.17 |
| Guo M 2019 | 63 | Chemotherapy | OS/PFS | III | 7 | NM | 2.38 |
| Prelaj A 2019 | 42 | Immunotherapy | OS/PFS/RR/DCR | IIIB/IV | 7 | 83 | 1.80 |
| Yan,Wang 2019 | 18 | Surgery | OS/DFS | I/II/III | 6 | 47 | 4.57 |
| Zhang Y 2018 | 8 | TKI | OS/PFS | IIIB/IV | 6 | 28 | 3.37 |
| Watanabe K 2018 | NM | TKI | OS/PFS | IIIA/IIIB/IV/REC | 6 | 47 | 2.80 |
| Minami S 2018 | 100 | Chemotherapy | OS/PFS/RR | IIIB/IV/REC | 8 | NM | 2.07 |
| Minami Seigo 2018 | 0 | Chemotherapy | OS/PFS/RR | IIIB/IV | 8 | NM | 1.97 |
| Luo H 2018 | 40 | SABR | OS | I/IIA | 7 | 67 | 4.00 |
| Lim JU 2018 | 14 | Chemotherapy+TKI | OS/PFS | IV | 7 | 45 | 2.47 |
| Chen Y 2018 | 33 | Surgery | OS | IB | 8 | 59 | 3.16 |
| Xiong Y 2017 | 0 | Chemotherapy | OS/PFS | IIIB/IV | 7 | 53 | 4.30 |
| Liu W 2017 | 47 | Surgery | OS/DFS | I/II/IIIA | 6 | 66 | 3.60 |
| Gao Y 2017 | 45 | Surgery | OS | I/II/III | 7 | 68 | 3.06 |
| Cao S 2017 | 0 | Chemotherapy+ Radiotherapy | OS | LD/ED | 6 | 63 | 2.62 |
| Xia H 2016 | 41 | Surgery | OS/RFS | I | 6 | 42 | 4.00 |
| Song Ying-Jian 2016 | NM | Chemotherapy | OS/PFS | I/II | 7 | 90 | 4.50 |
| Lin Gui-Nan 2014 | 53 | Chemotherapy | OS/PFS | IIIB/IV | 6 | NM | 4.56 |
| Pingping Hu 2014 | NM | Surgery | OS/DFS | I/II/III | 8 | 54 | 3.68 |
| Go Se-II 2014 | 0 | Chemotherapy+ Radiotherapy | OS/PFS | LD/ED | 6 | 91 | 4.19 |
Abbreviation: NM: not mentioned; M/F: male/female; MFP: Median follow-up (month); SCC%: Proportion of Squamous cell carcinoma; OS: overall survival; PFS: progress free survival; RFS: recurrence-free survival; DFS: disease free survival; DCR: disease control rate; RR: response rate; REC: recurrent NSCLC who were treated with anti-PD-1 therapy; TKI: Tyrosine kinase inhibitors; SABR: Stereotactic Ablative Radiotherapy; ED: Extensive-stage; LD: Limited-stage; NOS: Ottawa quality assessment Scale; ¶: the study just provided the mean value of age; #:the study provided the mean and standard error value of age.
Relationship Between Pretreatment LMR and OS in Lung Cancer
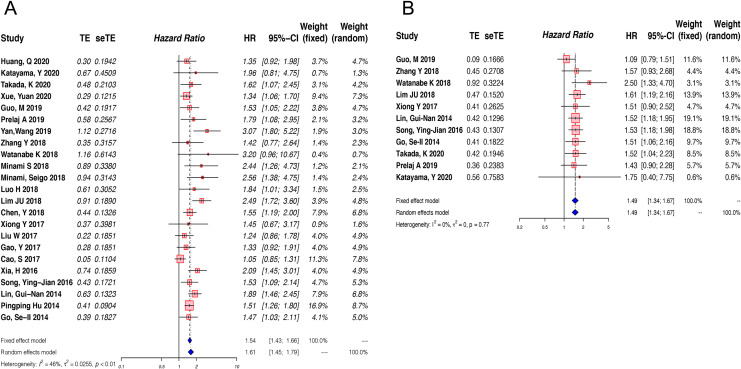
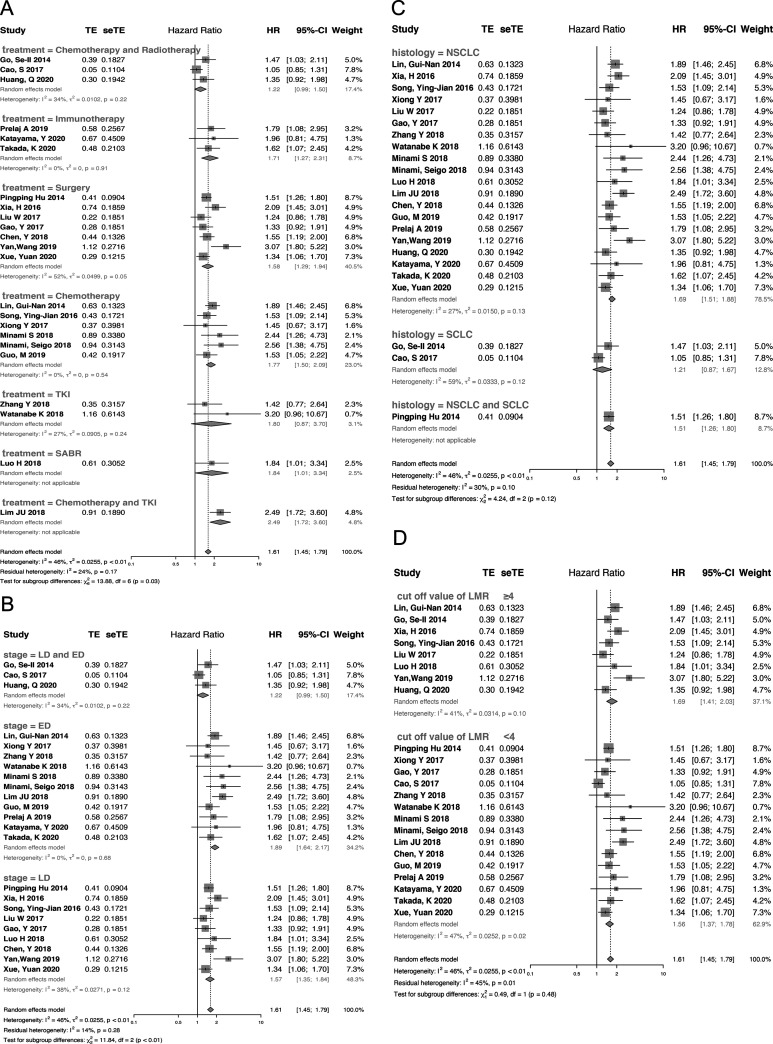
Twenty-three studies were included in the analysis of the correlation between pretreatment LMR and OS. Due to the presence of significant heterogeneity (I2 = 46%, P = 0.01), a random-effect model was used. The pooled analysis indicated that decreased pretreatment LMR was significantly correlated with reduced OS among lung cancer patients (HR = 1.61, 95% CI: 1.45-1.79, p < 0.01) (Figure 2A). To detect the potential sources of heterogeneity, subgroup analyses stratified by histologic type, tumor stage, cut-off value and treatment strategy were conducted (Figure 3). Subgroup analysis based on treatment strategy showed that pretreatment LMR was not related to OS among patients receiving TKI treatment or those receiving chemotherapy plus radiotherapy, which was in contrast to the findings among patients receiving other treatments (Figure 3A). Furthermore, stratified analysis by stage revealed that extensive-stage lung cancer was significantly associated with higher HR (extensive-stage: HR = 1.89, 95%CI: 1.64-2.17, P < 0.01; limited-stage: HR = 1.53, 95%CI: 1.39-1.69, P < 0.01, Pinteraction < 0.01) (Figure 3B, STable 2). In the subgroup analysis by histologic type, lower level of LMR seemed to be unassociated with shorter OS among SCLC patients (HR = 1.21, 95%CI: 0.87-1.67, P = 0.25) (Figure 3C). Furthermore, in the subgroup analysis by cutoff value, there were no significant differences between the high-cutoff group and the low-cutoff value group (Pinteraction = 0.48) (Figure 3D). More information is provided in STable 2 (supplement).
Figure 2.
Forest plot of the association between pretreatment LMR and OS(A) or PFS(B) in patients with lung cancer.
Figure 3.
Forest plot of the association between pretreatment LMR and OS in patients with lung cancer stratified by treatment (A), tumor stage (B), histologic type (C) and cut-off value (D).
Relationship Between Pretreatment LMR and PFS in Lung Cancer
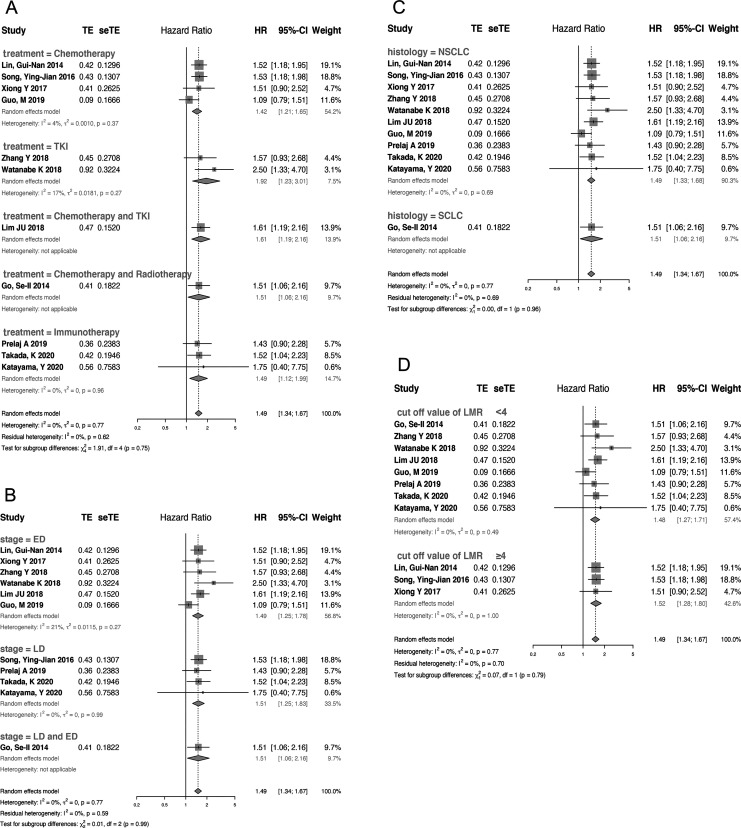
PFS was reported as a clinical outcome in 13 of the included studies, among which 2 studies did not have enough data for calculating the HR and 95% CI of PFS.17,24 Thus, 11 articles representing 2335 patients were used to estimate the association between pretreatment LMR and PFS. Owing to a lack of significant heterogeneity in PFS among the studies (I2 = 0%, P = 0.77), a fixed-effect model was used. The combined HR was 1.49 (95% CI: 1.34-1.67, P < 0.01), revealing a relationship between elevated pretreatment LMR and longer PFS in lung cancer patients (Figure 2B). The combined HRs for most subgroups were unaffected by potential influencing factors (histologic type, tumor stage, cut-off value and treatment strategy) (Figure 4). More information is provided in STable 3 (supplement)
Figure 4.
Forest plot of the association between pretreatment LMR and PFS in patients with lung cancer stratified by treatment (A), tumor stage (B), histologic type(C) and cut-off value (D).
Influence Analysis and Publication Bias
Non-significant heterogeneity was found among the studies in the analysis of the relationship between pretreatment LMR and PFS (I2 = 0%, P = 0.77). However, high heterogeneity was discovered among the studies reporting an association between pretreatment LMR and OS (I2 = 46%, P = 0.01). We evaluated the impact of each study on the pooled HRs by excluding each study individually. The sensitivity analysis revealed the robustness of the pooled HRs for OS and PFS in our meta-analysis (SFigure 1). The funnel plots of publication bias were almost symmetrical (SFigure 2) and no publication bias was detected regarding the HRs of PFS (Begg’s test, P = 0.77). However, Begg’s test suggested the presence of publication bias among the studies reporting the HRs of OS (Begg’s test, P = 0.01). Therefore, we used the trim and fill method to confirm the results of the HRs of OS after eliminating publication bias (HR = 1.46; 95% CI:1.27-1.66; P < 0.01) (SFigure 3)
Discussion
To our knowledge, the current study is the first meta-analysis to comprehensively evaluate the prognostic value of pretreatment LMR in lung cancer patients. We combined HRs and 95%CIs from 23 studies representing 8361 lung cancer patients and confirmed that decreased pretreatment LMR was significantly associated with poorer OS and poor PFS. However, the level of LMR seemed to be unrelated to OS among SCLC patients. The stratified analyses by stage, treatment and cut-off value similarly showed the potential value of LMR in predicting survival in diverse subgroup populations.
Recently, a plethora of studies have identified systemic inflammation as a crucial factor in cancer initiation, progression, treatment and survival.3,5,6 Biomarkers based on common blood tests are potential predictors of survival among patients with cancer and are easy to acquire in the clinic. Other biomarkers such as the platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR) and modified Glasgow Prognostic Score (mGPS) can reflect inflammatory status and are recognized as predictive factors in lung cancer.31-35 LMR, as one of the inflammation-associated factors implicated in tumor oncology, and based on blood tests, is potentially appealing as a prognostic indicator. Liu X et al discovered that higher LMR before treatment predicts beneficial clinical tumor response and longer PFS in patients with advanced esophageal SCC receiving definitive chemoradiotherapy (CRT).36 Consistent with the findings of Liu X, another study reported that among patients malignant pleural mesothelioma, those with LMR ≤2.6 had worse survival than those with high LMR.37 A study investigating the relationship between the change in LMR and survival time in pancreatic cancer38 indicated that a change in LMR >0.32 was significantly associated with superior OS. Go et al were the first to report the prognostic value of LMR among SCLC patients receiving chemotherapy.7 Their results demonstrated that the level of LMR at diagnosis was correlated with survival time. Many studies have investigated the predictive value of LMR in lung cancer.8,9,15,17,24,25,28,29 Mao et al reported that patients with advanced-stage epithelial cancers with higher LMR levels before treatment survived longer than those with lower levels, consistent with the findings of our study.39 Only 1343 lung cancer patients were included in the study by Mei et al and the combined HRs for lung cancer in that study were similar to ours. In another study, the cut-off value of the NLR was reported to be associated with PFS.40 We conducted a subgroup analysis according to the cut-off value of LMR. A lower cut-off value seemed to not be significantly associated with worse OS or PFS. Furthermore, from the results of the stratified analyses, we infer that the high heterogeneity for OS was mainly derived from variation in the treatment types and tumor stages of patients included in the different articles.
While high LMR is a predictor that portends better prognosis in multiple pathophysiological situations of lung cancer, the underlying molecular mechanisms are potentially complex and have not been fully elucidated. In lung cancer, the pulmonary tumor microenvironment (TME) is highly dynamic, where in a series of host-defense cells, including various inflammatory cells, play both pro- and anti-inflammatory roles in lung tumorigenesis and progression.41 Tertiary lymphoid structures (TLSs), mainly composed of T cells, B cells and mature dendritic cells (DCs), represent sites for the generation and maintenance of local and systemic adaptive antitumor responses.42,43 A relationship has consistently been found between a high frequency of TLSs and prolonged OS and PFS in lung cancer. Furthermore, monocytes have been verified to be important regulators favoring tumor invasion and metastasis,44 and their number negatively correlates with clinical outcome in lung cancer patients. Considering the interaction between lymphocytes and monocytes, LMR emerges as a more appropriate prognostic biomarker than a single event of lymphocytopenia or monocytosis.
The current study has some limitations. First, all included studies were retrospective and almost all were conducted in Asia. Hence, the conclusions of this article should be treated with caution owing to geographical limitations and the overestimation of influences of retrospective research. Second, the cut-off values vary among studies, which makes direct comparisons difficult. In our subgroup analyses, we used 4 as the boundary for cut-off value. Finally, due to the numbers of studies, no significant differences among different subgroups in LMR and PFS were observed.
Conclusion
To summarize, the current study investigated the association between pretreatment LMR and survival time in patients with lung cancer and revealed that decreased LMR is a promising predictive and prognostic biomarker for lung cancer patients. More prospective and large-scale cohort studies are warranted to validate the clinical value of LMR.
Supplemental Material
Supplemental Material, sj-doc-1-tct-10.1177_1533033820983085 for Prognostic Value of Pretreatment Lymphocyte-to-Monocyte Ratio in Lung Cancer: A Systematic Review and Meta-Analysis by Jing Jin, Lan Yang, Dan Liu and Wei Min Li in Technology in Cancer Research & Treatment
Supplemental Material, sj-docx-1-tct-10.1177_1533033820983085 for Prognostic Value of Pretreatment Lymphocyte-to-Monocyte Ratio in Lung Cancer: A Systematic Review and Meta-Analysis by Jing Jin, Lan Yang, Dan Liu and Wei Min Li in Technology in Cancer Research & Treatment
Supplemental Material, sj-pdf-1-tct-10.1177_1533033820983085 for Prognostic Value of Pretreatment Lymphocyte-to-Monocyte Ratio in Lung Cancer: A Systematic Review and Meta-Analysis by Jing Jin, Lan Yang, Dan Liu and Wei Min Li in Technology in Cancer Research & Treatment
Supplemental Material, sj-pdf-2-tct-10.1177_1533033820983085 for Prognostic Value of Pretreatment Lymphocyte-to-Monocyte Ratio in Lung Cancer: A Systematic Review and Meta-Analysis by Jing Jin, Lan Yang, Dan Liu and Wei Min Li in Technology in Cancer Research & Treatment
Supplemental Material, sj-tif-1-tct-10.1177_1533033820983085 for Prognostic Value of Pretreatment Lymphocyte-to-Monocyte Ratio in Lung Cancer: A Systematic Review and Meta-Analysis by Jing Jin, Lan Yang, Dan Liu and Wei Min Li in Technology in Cancer Research & Treatment
Supplemental Material, sj-tif-2-tct-10.1177_1533033820983085 for Prognostic Value of Pretreatment Lymphocyte-to-Monocyte Ratio in Lung Cancer: A Systematic Review and Meta-Analysis by Jing Jin, Lan Yang, Dan Liu and Wei Min Li in Technology in Cancer Research & Treatment
Supplemental Material, sj-tif-3-tct-10.1177_1533033820983085 for Prognostic Value of Pretreatment Lymphocyte-to-Monocyte Ratio in Lung Cancer: A Systematic Review and Meta-Analysis by Jing Jin, Lan Yang, Dan Liu and Wei Min Li in Technology in Cancer Research & Treatment
Abbreviations
- LMR
Lymphocyte-to-monocyte ratio
- OS
Overall survival
- PFS
Progression-free survival
- HRs
Hazard ratios
- CI
Confidence interval
- SCLC
small cell lung cancer
- NSCLC
Non-small cell lung cancer
- LUAD
lung adenocarcinoma
- SCC
squamous cell carcinoma
- CBC
Complete blood count
- PRISMA
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- NOS
The Newcastle Ottawa Quality Assessment Scale
- RFS
Recurrence-free survival
- DFS
Disease-free survival
- SABR
Stereotactic ablative radiotherapy
- TKI
Tyrosine kinase inhibitors
- PLR
Platelet-to-lymphocyte ratio
- NLR
Neutrophil-to-lymphocyte ratio
- mGPS
modified Glasgow Prognostic Score
- CRT
Chemoradiotherapy
- TME
The pulmonary tumor microenvironment
- TLSs
Tertiary lymphoid structures
Footnotes
Authors’ Contributions: (I) Conception and design: W Li, J Jin, Lan Y; II) Administrative support: D Liu, W Li; (III) Provision of study materials: J Jin; (IV) Collection and assembly of data: J Jin, Lan Y; (V) Data analysis and interpretation: D Liu, Lan Y; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Approval and Consent to Participator: All procedures performed in the studies involving human participants were by the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendment.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from Nature Science Foundation of China (81871890 and 91859203).
Supplemental Material: Supplemental material for this article is available online.
Trial Registration: The registration number of PROSPERO: CRD42019145549.
ORCID iD: Wei Min Li  https://orcid.org/0000-0003-2859-8668
https://orcid.org/0000-0003-2859-8668
References
- 1. Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2019: a review of current American Cancer Society guidelines and current issues in cancer screening. CA J Clin. 2019;69(3):184–210. [DOI] [PubMed] [Google Scholar]
- 2. Zhang XC, Wang J, Shao GG, et al. Comprehensive genomic and immunological characterization of Chinese non-small cell lung cancer patients. Nat Commun. 2019;10(1):1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–503. [DOI] [PubMed] [Google Scholar]
- 4. Munn LL. Cancer and inflammation. Wiley Interdiscip Rev Syst Biol Med. 2017;9(2):e1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wellenstein MD, de Visser KE. Cancer-cell-intrinsic mechanisms shaping the tumor immune landscape. Immunity. 2018;48(3):399–416. [DOI] [PubMed] [Google Scholar]
- 6. Porrata LF, Ristow K, Habermann TM, et al. Peripheral blood lymphocyte/monocyte ratio at diagnosis and survival in nodular lymphocyte-predominant Hodgkin lymphoma. Br J Haematol. 2012;157(3):321–330. [DOI] [PubMed] [Google Scholar]
- 7. Go SI, Kim RB, Song HN, et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with small cell lung cancer. Med Oncol. 2014;31(12):323. [DOI] [PubMed] [Google Scholar]
- 8. Guo M, Li W, Li B, et al. Prognostic value of delta inflammatory biomarker-based nomograms in patients with inoperable locally advanced NSCLC. Int Immunopharmacol. 2019;72:395–401. [DOI] [PubMed] [Google Scholar]
- 9. Watanabe K, Yasumoto A, Amano Y, et al. Mean platelet volume and lymphocyte-to-monocyte ratio are associated with shorter progression-free survival in EGFR-mutant lung adenocarcinoma treated by EGFR tyrosine kinase inhibitor. PloS One. 2018;13(9):e0203625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cao S, Jin S, Shen J, et al. Selected patients can benefit more from the management of etoposide and platinum-based chemotherapy and thoracic irradiation-a retrospective analysis of 707 small cell lung cancer patients. Oncotarget. 2017;8(5):8657–8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang Q, Diao P, Li CL, et al. Preoperative platelet-lymphocyte ratio is a superior prognostic biomarker to other systemic inflammatory response markers in non-small cell lung cancer. Medicine (Baltimore). 2020;99(4):e18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yan X, Li G. Preoperative systemic immune-inflammation index predicts prognosis and guides clinical treatment in patients with non-small cell lung cancer. Biosci Rep. 2020;40(3):BSR20200352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ying-Jian S, Li-Xin W, Yong-Qing H, et al. Lymphocyte to monocyte ratio is associated with response to first-line platinum-based chemotherapy and prognosis of early-stage non-small cell lung cancer patients. Tumor Biol. 2016;37(4):5285–5293. [DOI] [PubMed] [Google Scholar]
- 15. Luo H, Ge H, Cui Y, et al. Systemic inflammation biomarkers predict survival in patients of early stage non-small cell lung cancer treated with stereotactic ablative radiotherapy—a single center experience. J Cancer. 2018;9(1):182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xia H, Sun Z, Deng L, Zhu D, Wang D. Prognostic significance of the preoperative lymphocyte to monocyte ratio in patients with stage I non-small cell lung cancer undergoing complete resection. Cancer Invest. 2016;34(8):378–384. [DOI] [PubMed] [Google Scholar]
- 17. Minami S, Ihara S, Kim S-H, Yamamoto S, Komuta K. Lymphocyte to monocyte ratio and modified Glasgow prognostic score predict prognosis of lung adenocarcinoma without driver mutation. World J Oncol. 2018;9(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prelaj A, Rebuzzi SE, Pizzutilo P, et al. EPSILoN: a prognostic score using clinical and blood biomarkers in advanced non-small-cell lung cancer treated with immunotherapy. Preoperative systemic immune-inflammation index predicts prognosis and guides clinical treatment in patients with non-small cell lung cancer. Clin Lung Cancer. 2020;40(3):BSR20200352. [DOI] [PubMed] [Google Scholar]
- 19. Hu P, Shen H, Wang G, Zhang P, Liu Q, Du J. Prognostic significance of systemic inflammation-based lymphocyte-monocyte ratio in patients with lung cancer: based on a large cohort study. PloS One. 2014;9(9):e108062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Katayama Y, Shimamoto T, Yamada T, et al. Retrospective efficacy analysis of immune checkpoint inhibitor rechallenge in patients with non-small cell lung cancer. J Clin Med. 2019;9(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Y, Hu X, Xu W, Wang H, Huang Y, Che G. Prognostic value of a novel scoring system using inflammatory response biomarkers in non-small cell lung cancer: a retrospective study. Thorac Cancer. 2019;10(6):1402–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gui-Nan L, Jie-Wen P, Jian-jun X, Dong-Ying L, Zhong-Jun X. Prognostic impact of circulating monocytes and lymphocyte-to-monocyte ratio on previously untreated metastatic non-small cell lung cancer patients receiving platinum-based doublet. Med Oncol. 2014;31(7):70. [DOI] [PubMed] [Google Scholar]
- 23. Liu W, Ha M, Yin N. Combination of platelet count and lymphocyte to monocyte ratio is a prognostic factor in patients undergoing surgery for non-small cell lung cancer. Oncotarget. 2017;8(42):73198–73207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Minami S, Ihara S, Komuta K. Pretreatment lymphocyte to monocyte ratio as a prognostic marker for advanced pulmonary squamous cell carcinoma treated with chemotherapy. J Clin Med Res. 2018;10(8):657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen Y, Wang W, Zhang X, et al. Prognostic significance of combined preoperative platelet-to-lymphocyte ratio and lymphocyte-to-monocyte ratio in patients undergoing surgery with stage IB non-small-cell lung cancer. Cancer Manage Res. 2018;10:5411–5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Takada K, Takamori S, Yoneshima Y, et al. Serum markers associated with treatment response and survival in non-small cell lung cancer patients treated with anti-PD-1 therapy the combination of platelet count and lymphocyte to monocyte ratio is a prognostic factor in patients with resected breast cancer prognostic value of red blood cell distribution width-standard deviation (RDW-SD) in patients operated on due to non-small cell lung cancer. Lung Cancer. 2020;145(18):18–26. [DOI] [PubMed] [Google Scholar]
- 27. Gao Y, Zhang H, Li Y, Wang D, Ma Y, Chen Q. Preoperative pulmonary function correlates with systemic inflammatory response and prognosis in patients with non-small cell lung cancer: results of a single-institution retrospective study. Oncotarget. 2017;8(16):27489–27501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang Y, Feng YC, Zhu HG, et al. The peripheral blood neutrophil-to-lymphocyte ratio is a prognostic predictor for survival of EGFR-mutant nonsmall cell lung cancer patients treated with EGFR-TKIs. Medicine. 2018;97(30):e11648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lim JU, Yeo CD, Kang HS, et al. Prognostic value of platelet count and lymphocyte to monocyte ratio combination in stage IV non-small cell lung cancer with malignant pleural effusion. PloS one. 2018;13(7):e0200341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xiong Y, Zhao N, Zheng Y, Wang J, Wei F, Ren X. Prognostic value of pretreatment inflammatory biomarkers in advanced lung adenocarcinoma patients receiving first-line pemetrexed/platinum doublet. Tumour Biol. 2017;39(6):1010428317701639. [DOI] [PubMed] [Google Scholar]
- 31. Kos M, Hocazade C, Kos FT, et al. Prognostic role of pretreatment platelet/lymphocyte ratio in patients with non-small cell lung cancer. Wien Klin Wochenschr. 2016;128(17-18):635–640. [DOI] [PubMed] [Google Scholar]
- 32. Lan H, Zhou L, Chi D, et al. Preoperative platelet to lymphocyte and neutrophil to lymphocyte ratios are independent prognostic factors for patients undergoing lung cancer radical surgery: a single institutional cohort study. Oncotarget. 2016;8(21):35301–35310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sanchez-Salcedo P, de-Torres JP, Martinez-Urbistondo D, et al. The neutrophil to lymphocyte and platelet to lymphocyte ratios as biomarkers for lung cancer development. Lung Cancer. 2016;97:28–34. [DOI] [PubMed] [Google Scholar]
- 34. Wang X, Teng F, Kong L, Yu J. Pretreatment neutrophil-to-lymphocyte ratio as a survival predictor for small-cell lung cancer. Onco Targets Ther. 2016;9:5761–5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu Y, Chen Y, Yang X, Chen L, Yang Y. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were associated with disease activity in patients with systemic lupus erythematosus. Int Immunopharmacol. 2016;36:94–99. [DOI] [PubMed] [Google Scholar]
- 36. Liu X, Li M, Zhao F, et al. The lymphocyte-monocyte ratio predicts tumor response and survival in patients with locally advanced esophageal cancer who received definitive chemoradiotherapy. Oncotargets Therapy. 2017;10:871–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tanrikulu AC, Abakay A, Komek H, Abakay O. Prognostic value of the lymphocyte-to-monocyte ratio and other inflammatory markers in malignant pleural mesothelioma. Environ Health Prev Med. 2016;21(5):304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Giacomelli I, Scartoni D, Mohammadi H, Regine WF, Chuong MD. Does lymphocyte-to-monocyte ratio before, during, or after definitive chemoradiation for locally advanced pancreatic cancer predict for clinical outcomes? J Gastrointest Oncol. 2017;8(4):721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mao Y, Chen D, Duan S, et al. Prognostic impact of pretreatment lymphocyte-to-monocyte ratio in advanced epithelial cancers: a meta-analysis. Cancer Cell Int. 2018;18:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mei Z, Shi L, Wang B, et al. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: a systematic review and meta-analysis of 66 cohort studies. Cancer Treat Rev. 2017;58:1–13. [DOI] [PubMed] [Google Scholar]
- 41. Milette S, Fiset PO, Walsh LA, Spicer JD, Quail DF. The innate immune architecture of lung tumors and its implication in disease progression. J Pathol. 2019;247(5):589–605. [DOI] [PubMed] [Google Scholar]
- 42. Salmon H, Remark R, Gnjatic S, Merad M. Host tissue determinants of tumour immunity. Nat Rev Cancer. 2019;19(4):215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sautes-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019;19(6):307–325. [DOI] [PubMed] [Google Scholar]
- 44. Olingy CE, Dinh HQ, Hedrick CC. Monocyte heterogeneity and functions in cancer. J Leukocyte Biol. 2019;106(2):309–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-doc-1-tct-10.1177_1533033820983085 for Prognostic Value of Pretreatment Lymphocyte-to-Monocyte Ratio in Lung Cancer: A Systematic Review and Meta-Analysis by Jing Jin, Lan Yang, Dan Liu and Wei Min Li in Technology in Cancer Research & Treatment
Supplemental Material, sj-docx-1-tct-10.1177_1533033820983085 for Prognostic Value of Pretreatment Lymphocyte-to-Monocyte Ratio in Lung Cancer: A Systematic Review and Meta-Analysis by Jing Jin, Lan Yang, Dan Liu and Wei Min Li in Technology in Cancer Research & Treatment
Supplemental Material, sj-pdf-1-tct-10.1177_1533033820983085 for Prognostic Value of Pretreatment Lymphocyte-to-Monocyte Ratio in Lung Cancer: A Systematic Review and Meta-Analysis by Jing Jin, Lan Yang, Dan Liu and Wei Min Li in Technology in Cancer Research & Treatment
Supplemental Material, sj-pdf-2-tct-10.1177_1533033820983085 for Prognostic Value of Pretreatment Lymphocyte-to-Monocyte Ratio in Lung Cancer: A Systematic Review and Meta-Analysis by Jing Jin, Lan Yang, Dan Liu and Wei Min Li in Technology in Cancer Research & Treatment
Supplemental Material, sj-tif-1-tct-10.1177_1533033820983085 for Prognostic Value of Pretreatment Lymphocyte-to-Monocyte Ratio in Lung Cancer: A Systematic Review and Meta-Analysis by Jing Jin, Lan Yang, Dan Liu and Wei Min Li in Technology in Cancer Research & Treatment
Supplemental Material, sj-tif-2-tct-10.1177_1533033820983085 for Prognostic Value of Pretreatment Lymphocyte-to-Monocyte Ratio in Lung Cancer: A Systematic Review and Meta-Analysis by Jing Jin, Lan Yang, Dan Liu and Wei Min Li in Technology in Cancer Research & Treatment
Supplemental Material, sj-tif-3-tct-10.1177_1533033820983085 for Prognostic Value of Pretreatment Lymphocyte-to-Monocyte Ratio in Lung Cancer: A Systematic Review and Meta-Analysis by Jing Jin, Lan Yang, Dan Liu and Wei Min Li in Technology in Cancer Research & Treatment