Abstract
Objective:
Small cell carcinoma of the ovary, hypercalcemic type (SCCOHT) and SMARCA4-deficient undifferentiated uterine sarcoma (SMARCA4-DUS) are rare and aggressive tumors, primarily affecting pre- and perimenopausal women. Inactivating SMARCA4 mutations are thought to be the driving molecular events in the majority of these tumors. Here, we report the clinical course of a family with germline SMARCA4 mutation and compare large cohorts of these rare tumor types.
Methods:
We extracted clinico-pathological medical record data for the family with germline SMARCA4 mutation. Clinico-genomic data from SCCOHT and SMARCA4-DUS cohorts were retrospectively extracted from the archives of a large CLIA-certified reference molecular laboratory.
Results:
We identified a single family with an inherited germline SMARCA4 mutation, in which two different family members developed either SCCOHT or SMARCA4-DUS, both of whom died within one year of diagnosis, despite aggressive surgical, chemotherapy and immunotherapy treatment. Retrospective comparative analysis of large SCCOHT (n=48) and SMARCA4-DUS (n=17) cohorts revealed that SCCOHT patients were younger (median age: 28.5 vs. 49.0) and more likely to have germline SMARCA4 alterations (37.5% vs. 11.8%) than SMARCA4-DUS patients.
Conclusions:
Growing understanding of the role SMARCA4 plays in the pathogenesis of these rare cancers may inform recommended genetic testing and counseling in families with these tumor types.
1. Introduction
Small cell carcinoma of the ovary, hypercalcemic type (SCCOHT) is a rare and aggressive form of ovarian cancer, recently described to be oncogenically driven exclusively by somatic and/or germline mutations in SMARCA4 1,2. Inactivating mutations in SMARCA4 also have been observed in a rare and aggressive form of uterine sarcoma, termed SMARCA4-deficient undifferentiated uterine sarcoma (SMARCA4-DUS, also known as malignant rhabdoid tumor of the uterus) 3,4. Both of these rare cancer types affect young women. Due to their rarity and lack of clinical trials, optimal treatment strategies remain uncertain. Thus, interest has grown in further characterizing these SMARCA4 mutations to guide development of targeted therapies and genetic counseling recommendations for patients and their family members.
SMARCA4 is a member of the mammalian SWI/SNF family of chromatin regulators. Somatic mutations in the SWI/SNF complex (e.g. ARID1A, ARID2, PBRM1, SMARCA4, SMARCB1) occur in up to 20% of human malignancies 5. BRG1 (encoded by the SMARCA4 gene), a protein subunit of the SWI/SNF complex, is the most commonly mutated chromatin remodeling ATPase in cancer 5,6, occurring in lung adenocarcinomas 7, lymphomas 8, and medulloblastomas 9, which are associated with a poor prognosis 10-12. Loss of function somatic SMARCA4 mutations have also been described as secondary oncogenic events in microsatellite unstable gynecological carcinomas leading to de-differentiated carcinomas 13.
Germline mutations in SMARCA4 are best described as an inherited variant in rhabdoid tumor predisposition syndrome (RTPS) type 2 (RTPS2; OMIM 613325) 14. RTPS is a complex familial disorder with an autosomal dominant pattern of inheritance with variable penetrance predisposing to formation of tumors that develop in the brain, spine, lung, bladder, pelvis, kidney, or ovary of young children or adults 14. Genetic profiling has demonstrated these mutations in SCCOHT and SMARCA4-DUS, as well as atypical teratoid rhabdoid tumors (AT/RTs), malignant rhabdoid tumors (MRTs), and aggressive SMARCA4-deficient thoracic sarcomas (SMARCA4-DTS) 14,15,17. Unlike SCCOHT and SMARCA4-DUS, no germline mutations in SMARCA4 have been observed in SMARCA4-DTS tumors 15,16. All of these tumors represent aggressive malignant rhabdoid tumors across varying tissue types associated with SMARCA4 inactivation, though the genetic, histological, and phenotypic relationships is still an active area of research.
In this study, we describe the clinical course of a patient with SCCOHT and her mother with SMARCA4-DUS, previously noted to share a germline mutation in SMARCA4 4. In addition, we expand upon knowledge regarding known SMARCA4 variants in SCCOHT and SMARCA4-DUS with analysis of variants within large cohorts of these tumors. A somatic-germline-zygosity bioinformatics mutational algorithm is used to estimate the proportion of SMARCA4 alleles observed in a germline versus somatic context SCCOHT and SMARCA4-DUS. Understanding inherited mutations associated with SMARCA4 is critical to providing appropriate genetic counseling and treatment recommendations for SCCOHT and SMARCA4-DUS.
2. Methods
2.1. SCCOHT and SMARCA-4-DUS clinical cases
We extracted clinical and pathologic data for a mother and daughter affected by an inherited germline SMARCA4 mutation leading to SMARCA4-DUS and SCCOHT, respectively. Both patients were treated at Beth Israel Deaconess Medical Center (Boston, MA). Patient demographics, surgical management, medical management, and pathology were all extracted from medical records. The Beth Israel Deaconess Medical Center approved this study (protocol #2019000116). We obtained verbal permission from patient 2 prior to passing for release of genetic information.
2.2. Immunohistochemistry
Immunohistochemistry was performed on 4μm thick, formalin-fixed, paraffin-embedded tumor sections, using the DAKO linker 48 automated system. The following BRG1 antibody clone, dilution, vendor was used: SMARCA4/BRG1, ERP3912,1:50, Abcam. Appropriate internal positive and negative controls were evaluated.
2.3. Genomic Profiling and Germline mutation algorithm
Approval for retrospectively analyzing genomics of SCCOHT and SMARCA4-DUS cohorts at Foundation Medicine, a CLIA- and CAP-certified reference molecular pathology laboratory, including a waiver of informed consent and a HIPAA waiver of authorization, was obtained from the Western Institutional Review Board (Protocol 20152817). A retrospective database search was performed for SCCOHT and SMARCA4-DUS. All samples analyzed were processed by Foundation Medicine as part of the clinical care of these patients, prior to the initiation of this study. DNA and RNA were extracted from formalin-fixed paraffin-embedded tumors with a minimum of 20% tumor cells. Adapter-ligation and hybrid capture next-generation sequencing was performed for all coding exons in 406 cancer related genes in addition to introns from 31 highly mutated genes. The samples were fully sequenced and evaluated for all mutations, base substitutions, insertions, deletions, copy number alterations (amplifications and homozygous deletions), and select gene fusions/rearrangements. SCCOHT cases that did not have a SMARCA4 mutation were excluded. Nineteen of the forty-eight SCCOHT cases were previously described by Lin DI et al.17. Sixteen of the seventeen SMARCA4-DUS cases were also previously published by Lin DI et al.4. The SMARCA4-DUS cases were submitted to Foundation Medicine with the diagnosis of uterine sarcoma (n=17) and based on morphology and genomics, they were reclassified to SMARCA4-DUS by 2 board-certified gynecological pathologists.
The SCCOHT and SMARCA4-DUS cases had SMARCA4 inactivating mutations with no other or only few co-occurring alterations as previously described.
To assess whether a SMARCA4 alteration was potentially germline, a validated somatic germline zygosity algorithm was applied to each genomic variant as previously described18. For each sample, variants were detected using the standard FoundationOne analysis algorithm, which aligns unique sequence reads and obtains candidate mutations with associated mutant allele frequencies19. The algorithm also creates a genome-wide copy number profile based on coverage and allele frequencies (at over 3500 Single nucleotide polymorphisms (SNPs)) which are segmented and modeled to estimate the overall tumor purity, ploidy, per segment copy number, and minor allele count. To obtain a log-ratio profile of signal intensity, aligned tumor sequence reads are normalized by dividing read depth by that of a process-matched normal control, followed by a GC-content bias correction using Lowess regression. The minor allele frequency profile is obtained from the heterozygous genome-wide SNPs. These constitute the observed data for the statistical model. Given the output of the copy number model, each varian's measured allelic frequency is compared to expected at its local segment to determine whether a variant is predicted somatic, germline, or indeterminant. “Indeterminant” is used to signify mutations that were processed through the algorithm and unable to be classified as germline or somatic. “Unknown” are samples that were unable to be processed through the algorithm given incompatibility of the initial genetic sequence inputs required. These samples were either acquired prior to development of the algorithm or processed according to an alternative protocol. Statistical analysis was performed across the cohort ages using a Mann-Whitney Test using GraphPad Prism (GraphPad Software, San Diego, CA).
3. Results
3.1. SCCOHT patient
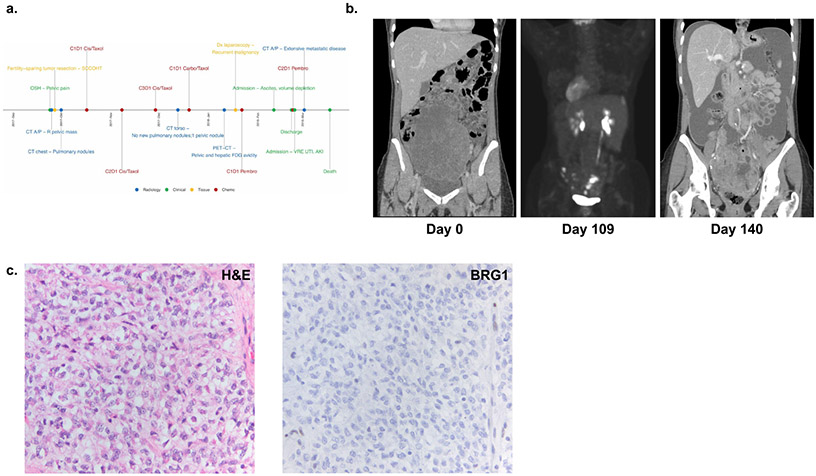
Patient 1 was a 31-year-old nulliparous woman diagnosed with stage IIIB SCCOHT (Figure 1a). On presentation, a CT of the abdomen and pelvis showed a large heterogeneous pelvic tumor and moderate ascites but no lymphadenopathy or evidence of distant metastasis (Figure 1b). The patient underwent fertility-sparing cytoreduction, including a left salpingo-oophorectomy, resection of pelvic peritoneal tumor, omentectomy, and pelvic and para-aortic lymph node dissection. There was no visible disease at the conclusion of the case.
Figure 1: Clinical Summary of Patient 1 diagnosed with SCCOHT.
(a) Timeline of major clinical events (b) Computed tomography (CT) scans at initial presentation (left panel), showing FDG-avid disease in the pelvis and liver following cycle 1 of carboplatin/ paclitaxel (middle panel), and at presentation to the hospital after cycle 2 of pembrolizumab (right panel). (c) Pathological evaluation with H&E staining (left panel) and immunohistochemistry demonstrating loss of BRG1 (right panel).
Tumor pathology showed a SCCOHT of the left ovary with metastatic disease to the peritoneum. The tumor showed classic features of SCCOHT composed of mitotically active pale spindle and epithelioid cells with irregular nuclei with scant cytoplasm (Figure 1c). The tumor architecture was diverse, characterized by arrangements in cords and islands, large cell morphology, and prominent geographic necrosis. The tumor cells demonstrated loss of BRG1 (Figure 1c). Single gene germline testing of SMARCA4 identified a deleterious mutation c.1831C>T (p.Gln611*).
Following surgery, she was treated with three cycles of cisplatin/etoposide followed by one cycle of carboplatin/paclitaxel. Interval Positron Emission Tomography/Computed Tomography (PET/CT) scan demonstrated fluorodeoxyglucose avidity in the left pericolic gutter, presacral region, and deep pelvis and prominent left external iliac lymph nodes (Figure 1b). She underwent a diagnostic laparoscopy notable for multiple peritoneal implants in the pelvis, paracolic gutters and right upper quadrant. Intra-operative biopsies confirmed progressive disease. She was initiated on second-line therapy with two cycles of pembrolizumab and continued to have disease progression. Due to advancing disease, she suffered multiple other complications precluding further treatment, including severe hydronephrosis requiring bilateral percutaneous nephrostomy tubes, pulmonary embolism, and large bowel obstruction. She ultimately died of her disease approximately six months after her initial diagnosis (Supplementary Table 1).
3.2. SMARCA4-DUS Patient
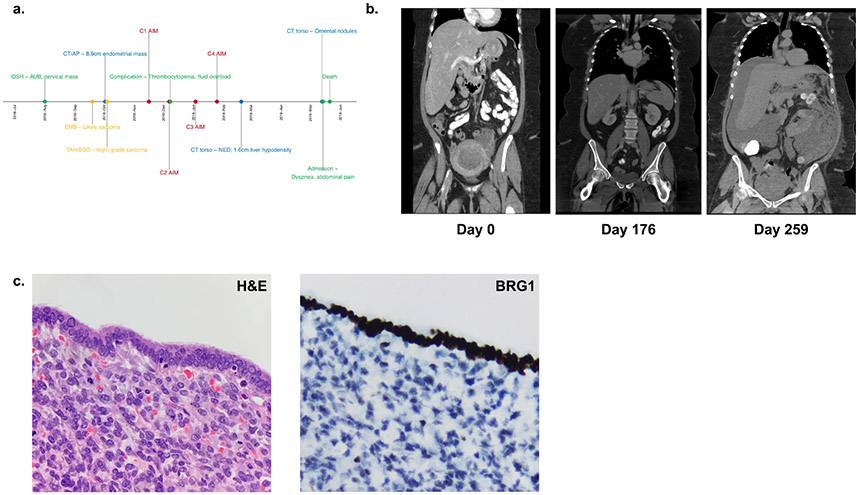
Patient 2 was the 55-year-old mother of patient 1 (Figure 2a). She presented to the emergency department approximately five months following the death of her daughter with two weeks of heavy vaginal bleeding. Exam showed a 3 cm lesion protruding from cervix. Biopsies revealed a malignant neoplasm consistent with a sarcoma.
Figure 2: Clinical Summary of Patient 2 diagnosed with SMARCA4-DUS.
(a) Timeline of major clinical events. (b) Computed tomography (CT) scans at initial presentation (left panel), showing no evidence of disease (NED) following completion of 4 cycles of AIM chemotherapy (middle panel), and after re-presentation with diffuse metastatic disease (right panel). (c) Pathological evaluation with H&E staining revealing rhabdoid morphology and sheets of highly mitotically active cells characteristic of SMARCA4-DUS (left panel), with immunohistochemistry demonstrating loss of BRG1 (right panel).
A CT scan of her chest, abdomen and pelvis showed a large endometrial mass with multifocal areas of deep myometrial invasion, as well as tumor extension to involve the bilateral adnexa (Figure 2b). There was evidence of peritoneal carcinomatosis, but no intrathoracic metastases. The patient underwent an exploratory laparotomy, total abdominal hysterectomy, bilateral salpingo-oophorectomy, omentectomy, debulking, lysis of adhesion, and cystoscopy. The tumor involved the ovaries, fallopian tubes, peritoneum, omentum, small and large bowel, diaphragm, and bladder. Lymphovascular invasion was identified. There was no gross residual disease left at the end of the procedure.
Pathology showed diffuse sheets of highly mitotically active malignant cells (Figure 2c) with significant nuclear atypia and mitotically active. There were areas of rhabdoid morphology. The immunophenotype was notable for intact expression of mismatch repair proteins, loss of BRG1 (Figure 2c), and negative PD-L1. She was diagnosed with Stage IVB high grade uterine sarcoma. Postoperative imaging revealed no evidence of residual disease. Germline genetic testing was performed for 23 genes associated with breast and gynecologic cancers including BRCA1/2, TP53, MMR genes, and SMARCA4 based on the patient’s personal and family history and identified only the same SMARCA4 mutation previously identified in the patient’s daughter (c.1831C>T (p.Gln611*)).
Patient 2 received four cycles of doxorubicin, ifosfamide, and mesna (AIM) with a 20% dose reduction between cycles 2 and 3 given development of significant thrombocytopenia requiring platelet transfusion. After completion of treatment, a repeat CT of the chest, abdomen, and pelvis was performed and showed no evidence of disease. After three months, the patient re-presented with worsening bloating, severe constipation, abdominal pain, and nausea; imaging showed disease recurrence with multiple omental nodularities, malignant ascites, and pelvic lymphadenopathy (Figure 2b). Prior to initiation of treatment, the patient was admitted to the hospital with severe dyspnea and subsequently developed septic shock with multi-organ failure and succumbed to her disease eight days following her re-presentation, and less than nine months following her initial diagnosis.
3.3. SMARCA4 Mutation
A germline SMARCA4 c.1831C>T (p.Gln611*) mutation was identified in both patients. This nonsense mutation likely results in absent or disrupted protein product. This variant was not found in the ExAC database of normal human germline exomic variants 20, supporting a pathogenic effect of this mutation. This variant has not been seen previously in patients with SCCOHT with available genetic sequencing of the SMARCA4 gene 2,21. An additional somatic frameshift variant of unknown significance in SMARCA4 (p.K1602fs*8) was identified in the mother’s tumor tissue. This mutation lies near the terminus of the 1647 amino-acid the BRG1 protein (encoded by SMARCA4 gene). The daughter’s genotype at this locus is unknown because somatic testing was not performed on Patient’s 1 tumor at the time of her diagnosis and treatment. The SMARCA4-DUS tumor was found to be microsatellite stable with low mutational burden (TMB-low, 2 mutations/megabase), consistent with other previously described SMARCA4-DUS tumors 3,4. Ten variants of unknown significance were also identified in Patient 2 (Supplementary Table 2). Though some of these missense variants occur in known cancer-related genes (e.g. JAK2), none are known to be hotspot mutations associated with tumor suppressor or oncogenic functions based on analysis of more than 40,000 cancer exomes 22,23.
Examination of a three-generation pedigree (Supplementary Figure 1) was completed to assess risk of SMARCA4-related disease in the patient’s family members. Limited phenotypic or genotypic information was available for the extended family because the mother was raised separately from her siblings and had limited contact with these individuals. Given the autosomal dominant mode of inheritance of SMARCA4-related genetic susceptibility to malignancies, the patient’s three sisters presumably each have a 50% chance of harboring increased risk for SMARCA4-related malignancy. It is also possible that the mutation occurred de novo in the mother.
3.4. Germline mutations in SCCOHT and SMARCA4-DUS cohorts
SMARCA4 inactivating genomic alterations were identified in 48 cases of SCCOHT and 17 cases of SMARCA4-DUS. In the SCCOHT cohort of 48 patients, ages ranged from 8 to 56 years, with a median of 28.5 years (Table 1). Germline mutations (Table 2) were predicted in approximately 38% of patients, while 25% were predicted to be somatic and 38% were of indeterminate origin. The median age of patients with germline mutations was 28.0 years (range: 12-45 years), while that of patients predicted to have somatic mutations was 32.0 years (range: 24-56 years; p=0.13). Patients with mutations of indeterminate origin had a median age of 26 years (range: 8-42).
Table 1:
Age demographics in SCCOHT and SMARCA4-DUS cohorts
| SCCOHT | SMARCA4-DUS | |||||
|---|---|---|---|---|---|---|
| # of Cases | Median | Range | # of Cases | Median | Range | |
| All | 48 | 28.5 | 8-56 | 17 | 49.0 | 32-70 |
| Germline | 37.5% (18/48) | 28.0 | 12-45 | 11.8% (2/17) | 47.5 | 40-55 |
| Somatic | 25% (12/48) | 32.0 | 24-56 | 23.6% (4/17) | 53.5 | 43-70 |
| Indeterminate | 37.5% (18/48) | 26.0 | 8-42 | 47.1% (8/17) | 50.0 | 33-60 |
| Unknown | n/a | n/a | n/a | 17.6% (3/17) | 48.0 | 32-51 |
Table 2:
SMARCA4 Mutations in SCCOHT
| n | Age | SMARCA4 mutation | Predicted Origin | Mutation Type | Previously Identified |
|---|---|---|---|---|---|
| 1 | 24 | p.Arg1077* c.2438+1G>A |
Somatic Somatic |
Nonsense Splice site |
*Yes *Yes |
| 2 | 24 | p.Gln987* | Indeterminate | Nonsense | No |
| 3 | 23 | p.Leu1161fs*3 | Germline | Frameshift | No |
| 4 | 31 | Deletion / loss | Somatic | Deletion / loss | No |
| 5 | 12 | p.Gln300* 3215+1G>A |
Germline | Nonsense Splice site |
No No |
| 6 | 38 | p.Gln445fs*56 p.Asp1294fs*12 |
Germline | Frameshift Frameshift |
No |
| 7 | 22 | p.Glu1300_Asn1303del | Germline | Inframe Indel | Yes |
| 8 | 8 | p.Leu1161fs*15 | Indeterminate | Frameshift | No |
| 9 | 12 | p.Leu1161fs*15 | Indeterminate | Frameshift | No |
| 10 | 45 | p.Leu361fs*50 | Germline | Frameshift | No |
| 11 | 31 | p.Gln460fs*41 | Somatic | Frameshift | No |
| 12 | 21 | c.1119-G>C homozygous loss |
Germline | Splice site Homozygous loss |
Yes |
| 13 | 42 | p.Glu798fs*33 | Germline | Frameshift | No |
| 14 | 29 | c.3215+1G>T | Germline | Splice site | No |
| 15 | 25 | c.2859+1G>C | Germline | Splice site | Yes |
| 16 | 36 | p.Ala340fs*71 c.2960_2973+2del16 |
Indeterminate | Frameshift Splice site |
No |
| 17 | 36 | p.Lys586fs*26 | Indeterminate | Frameshift | No |
| 18 | 29 | p.Phe1150fs*14 | Indeterminate | Frameshift | No |
| 19 | 27 | p.Leu1035fs*2 | Indeterminate | Frameshift | No |
| 20 | 19 | p.Lys585fs*27 p.Arg1093* |
Germline | Frameshift Nonsense |
Yes |
| 21 | 25 | p.Gln847fs*11 | Somatic | Frameshift | No |
| 22 | 40 | pLeu615fs*3 p.Gln1004* |
Indeterminate | Frameshift Nonsense |
No |
| 23 | 31 | p.Glu659* | Indeterminate | Nonsense | No |
| 24 | 40 | p.Gln1304* | Somatic | Nonsense | No |
| 25 | 45 | p.Lys1027fs*9 | Somatic | Frameshift | No |
| 26 | 17 | p.Ile542fs*71 | Indeterminate | Frameshift | No |
| 27 | 25 | p.Ala503fs*110 | Indeterminate | Frameshift | No |
| 28 | 27 | p.Arg979* | Somatic | Nonsense | Yes |
| 29 | 15 | p.Arg381* p.Ala573fs*40 |
Germline | Frameshift Frameshift |
Yes |
| 30 | 33 | 2438+1G>A | Somatic | Splice site | No |
| 31 | 33 | p.Gly334fs*78 | Germline | Frameshift | No |
| 32 | 19 | p.Arg1329fs*32 | Indeterminate | Frameshift | No |
| 33 | 21 | c.3168+1G>A | Indeterminate | Splice site | No |
| 34 | 38 | p.Glu650* | Indeterminate | Nonsense | No |
| 35 | 24 | p.Glu1056* | Indeterminate | Nonsense | No |
| 36 | 24 | p.Met1137fs*12 c.3952-2A>G |
Germline | Frameshift Splice site |
No |
| 37 | 28 | p.Leu476fs*25 | Indeterminate | Frameshift | No |
| 38 | 38 | p.Tyr1050fs*56 c.3382+2T>G |
Germline | Frameshift Splice site |
No |
| 39 | 24 | p.Cys1205fs*3 | Indeterminate | Frameshift | No |
| 40 | 33 | c.2859+1G>A | Somatic | Splice site | Yes |
| 41 | 39 | p.Arg1093* | Somatic | Nonsense | Yes |
| 42 | 31 | c.860-8_862delTCTCCCAG | Germline | Splice site | No |
| 43 | 42 | p.Arg1093* p.Ser391fs*20 |
Indeterminate | Nonsense Frameshift |
No |
| 44 | 44 | p.Phe1059fs*23 | Germline | Frameshift | No |
| 45 | 28 | p.Gln66* c.2859+1G>C |
Somatic | Nonsense Splice site |
Yes |
| 46 | 56 | Ile462fs*38 p.Gln556* |
Somatic | Frameshift Nonsense |
No |
| 47 | 27 | c.3168+2T>C | Germline | Splice site | No |
| 48 | 31 | p.Gln611* | Germline | Nonsense | No |
Overall, the 17 SMARCA4-DUS patients were older than SCCOHT patients, with a median age of 49.0 years compared with 28.5 years, respectively. This trend is independent of mutation status (germline, 28.0 vs 47.5 years, p=0.06; somatic, 32.0 vs 53.5 years, p=0.01). Fewer patients in the SMARCA4-DUS cohort were predicted to have germline SMARCA4 mutations, representing only 11.8% of the population (Table 3). Similar to the results seen in SCCOHT, the patients that were predicted to carry germline mutations versus somatic mutations were slightly younger, with a median age of 47.5 years (range: 40-55 years) compared to 53.5 years (range 43-70 year; p= 0.53). However, these results are limited by the small sample size and large fraction reported as indeterminate or unknown.
Table 3:
SMARCA4 mutations in DUS
| n | Age | SMARCA4 mutation | Predicted Origin | Mutation Type | Previously Identified |
|---|---|---|---|---|---|
| 1 | 48 | p.Ser127fs*174 p.Gln941* |
Unknown | Frameshift Nonsense |
No |
| 2 | 51 | Loss | Unknown | Loss | No |
| 3 | 63 | p.Asn175fs*105 p.Gln1053fs*53 |
Somatic | Frameshift Frameshift |
No |
| 4 | 52 | p.Arg1093* | Indeterminate | Nonsense | Yes |
| 5 | 43 | p.LysK443fs*54 p.Glu1292fs*14 |
Somatic | Frameshift Frameshift |
No |
| 6 | 51 | p.Gln1185* | Indeterminate | Nonsense | No |
| 7 | 60 | p.Arg1491* | Indeterminate | Nonsense | No |
| 8 | 49 | p.Gln201fs*102 p.Phe947fs*3 |
Indeterminate | Frameshift Frameshift |
No |
| 9 | 33 | p.Tyr862* | Indeterminate | Nonsense | No |
| 10 | 70 | c.2505+1G>A | Somatic | Splice site | No |
| 11 | 52 | p.Gly256* | Indeterminate | Nonsense | No |
| 12 | 44 | p.Lys587fs*26 | Somatic | Frameshift | No |
| 13 | 38 | p.Gly883fs*4 c.3236C>T |
Indeterminate | Frameshift Splice site |
No |
| 14 | 55 | p.Gln611* p.Lys1602fs*8 |
Germline | Nonsense Frameshift |
No |
| 15 | 45 | c.1593+1G>A p.Glu1310* |
Indeterminate | Splice site Nonsense |
No |
| 16 | 32 | p.Lys578fs*35 | Unknown | Frameshift | No |
| 17 | 40 | Loss c.3951+2_3951+20>AG |
Germline | Loss Splice site |
No |
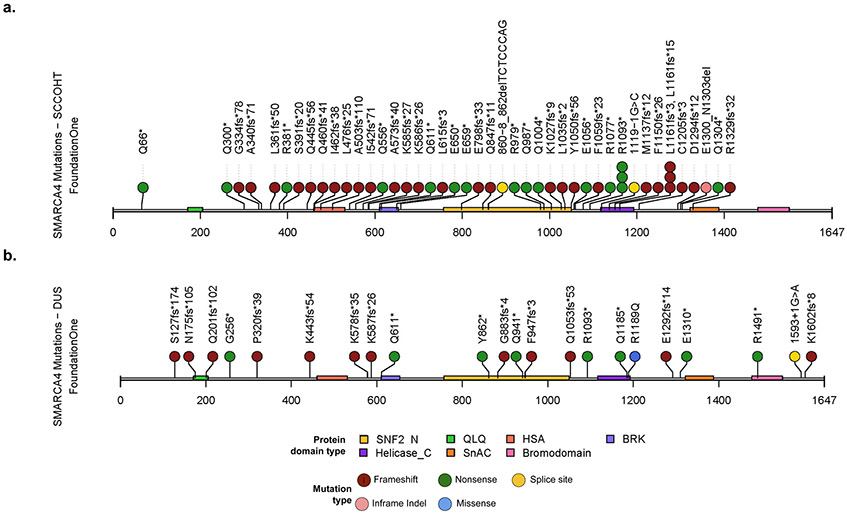
The SMARCA4 gene is composed of seven highly conserved domains (Figure 3). The majority of the mutations identified are frameshift mutations (Figure 3, Brown) followed by nonsense mutations (Figure 3, Green). A much smaller number of mutations are splice mutations (Figure 3, Yellow), missense mutations (Figure 3, Blue) and in-frame deletions (Figure 3, Pink). All of these mutations are predicted to lead to loss of function of the SMARCA4 protein.
Figure 3: Inactivating SMARCA4 mutations identified in SCCOHT and SMARCA4-DUS.
(a) Schematic of the mutations identified in the SCCOHT cohort and (b) the SMARCA-4 DUS cohorts respectively. Green represents nonsense mutations, brown represents frameshift mutations, yellow represents splice site mutations, blue represents missense mutations and pink represents in-frame deletions. QLQ; HSA, helicase/SANT-associated domain; BRK, brahma and kismet domain; SNF2_N, SNF2 family N-terminal domain; Helicase_C, helicase superfamily C-terminal domain; SnAC, Snf2-ATP coupling, chromatin remodelling complex, Bromodomain.
4. Discussion
This study details the clinical course of a mother-daughter pair with inherited SMARCA4 germline mutation leading to two aggressive and rare rhabdoid tumors - SCCOHT and SMARCA4-DUS. Despite aggressive treatment with surgery, chemotherapy, and, in the case of the daughter, immunotherapy, they both succumbed to their disease in 6 and 9 months, respectively, highlighting the aggressive nature of these tumors.
These clinical findings are consistent with previous descriptions of SCCOHT and SMARCA4-DUS. Prior studies have demonstrated that these tumors are morphologically and genetically related, 3,4 now further established through demonstration of a germline link between the diseases. Both tumors affect relatively young patients, with an average age of diagnosis for SCCOHT of 24 years, with a range described in the literature from 14 months to 71 years 21. Patients affected by SMARCA4-DUS are slightly older (median age: 49 years, range: 32-70 years), though this is younger than the average age of diagnosis for the more common uterine cancers. There is a non-significant difference in age between SCCOHT and SMARCA4-DUS patients with germline versus somatic variants in SMARCA4, suggesting that germline mutations may affect patients at slightly younger ages, consistent with previous reports 21. However, these results are limited by the small sample size and the high percentage of indeterminate or unknown results in each cohort.
Both patients harbored a nonsense mutation in SMARCA4, expected to lead to decreased production of functional BRG1 protein. Though this mutation has not been specifically described in the literature in other patients, the vast majority of mutations in the SMARCA4 gene associated with pathologically-confirmed SCCOHT are frameshift, splice-site, or nonsense mutations 2, which are expected to produce truncated, non-functional protein. There were several mutations in our cohort that overlapped with mutations previously identified in the literature 21 (Supplementary Table 3).
Prognosis is generally poor for both SCCOHT and SMARCA4-DUS, with a 5-year survival of 40% for SCCOHT 21. More recent studies using high-dose, multi-agent chemotherapy in combination with surgery, radiation, and autologous stem cell transplant have led to improved overall survival rates (up to 50% at three years) 24,25. A review of the 14 cases of SMARCA4-DUS described in the literature shows a similarly aggressive course, with most patients dying of the disease within months of diagnosis 3.
Further understanding of the shared genetic features of SCCOHT and SMARCA4-DUS and their response to targeted therapy may contribute to genomically-guided clinical decision-making for these challenging cancer types. Recent studies have suggested that SMARCA4-deficient ovarian cancers may be selectively sensitive to EZH2 inhibition 26, with possible implications for treatment of SMARCA4-DUS as well 3. Treatment of ten patients with SCCOHT with the EZH2-inhibitor tazemetostat led to clinical partial response in one patient, with duration of response of 32 weeks 27. Further study of this agent in this clinical context has been halted due to failure to pass stage 2 futility. Additionally pre-clinical data highlighted ponantinib, a tyrosine kinase receptor inhibitor, and palbociclib, a CDK4 inhibitor, as agents with selective activity against SCCOHT 28,29, though this too requires further clinical investigation.
Notably, four patients with SCCOHT have responded to anti-PD-1 monotherapy , which is unexpected for a cancer with low-mutational-burden 30. The authors additionally describe unusually high PD-L1 expression and T-cell infiltration in this cancer type. This result is especially interesting given the association between loss of PBRM1 - a member of the SWI/SNF protein family closely related to SMARCA4 - and sensitivity to anti-PD-1 immune checkpoint therapy in renal cell carcinoma, another generally low-mutational-burden cancer with a prominent immune infiltrate 31.
Reports of families affected by germline SMARCA4 mutations are rare in the literature 32,33. Growing understanding of the role of SMARCA4 in the pathogenesis of rare gynecological cancers, as well as other aggressive rhabdoid tumors, may inform recommended genetic testing and counseling for these families. Given the potential severity of the phenotype and autosomal dominant inheritance pattern, identifying germline SMARCA4 as a driver of a hereditary cancer syndrome has important implications on treatment and counseling of patients. In some of these cases, patients and their families have opted for prophylactic bilateral salpingo-oophorectomy (BSO), though the decision to proceed with this surgery is a difficult choice given incredibly young average age of onset in this population. The reliability of the medical management recommendations is further limited by insufficient information regarding the penetrance and lifetime cancer risks of carriers of these mutations.
It is critical that all individuals with suspected SCCOHT or SMARCA4-DUS receive genetic counseling. Germline testing should be recommended preferentially for the affected individual to clarify hereditary cancer risk in the family. If a germline mutation is identified, genetic testing should be offered to all at-risk family members, acknowledging the lack of published medical management guidelines. As suggested by previous authors, in the absence of a germline mutation, the tumor should be sequenced to identify possible somatic mutations 32. Male family members should also be counseled because they could be carriers, putting their female offspring at risk. Further recommendations for male carriers and association with future cancer risk is unavailable; however, the data regarding SMARCA4 in inherited cancers is rapidly expanding and future recommendations or associations may become available underlying the importance of including both male and female carriers in the genetic counseling when able.
Importantly, there is limited data at this time to make definitive recommendations regarding prophylactic surgery. All recommendations should involve shared decision making between the clinical team and the patient. In unaffected germline mutation carriers who have completed child-bearing, it is reasonable to discuss prophylactic BSO, as well hysterectomy or careful endometrial surveillance given potential risk of developing SMARCA4-DUS. Although, the optimal age to make this recommendation is unclear. This data suggests that by the third decade of life, approximately 55% of patients would have developed malignancy, heavily weighted toward SCCOHT. By the fourth decade this number increases to over 80% (Supplementary Figure 2). If a patient is an unaffected carrier over the age of 30, it may be reasonable to pursue prophylactic surgery to reduce the risk of future occurrence of SCCOHT and SMARCA4-DUS. In young women, this decision is far more complex given implications on future fertility, as well as harmful effects to mental and physical health of early menopause. Although, in highly motivated patients, long-term hormonal supplementation may be a reasonable option. In the appropriate clinical scenario, these patients may benefit from oocyte preservation prior to prophylactic surgery. As in other cancers that impact reproductive age women 34, a discussion of fertility goals should be included in the counseling of these patients. Of the two reported cases of prophylactic BSO in germline carriers, both demonstrated retained BRG1 protein expression 32,33. The interpretation of this result is unclear and highlights that more data is needed to understand the penetrance in germline carriers. However, there is currently no alternative screening modality or biomarker to offer these families. Given the complexity of the counseling and rapidly evolving treatment and diagnosis paradigm, patients with these diagnoses should be referred to high-volume centers that have experience in treatment and genetic counseling of these malignancies.
Supplementary Material
Supplementary Figure 1: Three generation family pedigree. Limited phenotypic or genotypic information was available for the family. Pathogenic mutation in SMARCA4 may have occurred de novo in patient 2.
Supplementary Figure 2: Age distribution of SMARCA4 mutations in DUS and SCCOHT. (a) Histogram of SCCOHT and SMARCA4-DUS cohorts by age (bin=10). (b) Cumulative frequency of SCCOHT and SMARCA4-DUS cohorts. By the third decade of life 70% of SCCOHT patients would have developed disease based on this cohort. The peak of SMARCA4-DUS cases is skewed by approximately 2 decades.
Supplementary Table 1: Clinical Data Summary
Supplementary Table 2: Detected variations of unknown significance
Supplementary Table 3: Previously reported mutations
8. Highlights.
SCCOHT and SMARCA4-DUS are morphological and genetically related tumors driven by loss of function mutations in SMARCA4
These tumors affect young women and generally carry a poor prognosis
Identification of germline mutations in SMARCA4 is critical to providing counseling and treatment to affected individuals
Acknowledgments:
This work was conducted with support from Harvard Catalyst ∣ The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers.
Footnotes
Conflict of Interest Statement:
DIL is a full-time employee of Foundation Medicine, Inc, which is a whole subsidiary of Roche.
6. References
- 1.Ramos P, Karnezis AN, Craig DW, et al. Small cell carcinoma of the ovary, hypercalcemic type, displays frequent inactivating germline and somatic mutations in SMARCA4. Nat Genet. 2014;46(5):427–429. doi: 10.1038/ng.2928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witkowski L, Carrot-Zhang J, Albrecht S, et al. Germline and somatic SMARCA4 mutations characterize small cell carcinoma of the ovary, hypercalcemic type. Nat Genet. 2014;46(5):438–443. doi: 10.1038/ng.2931 [DOI] [PubMed] [Google Scholar]
- 3.Kolin DL, Dong F, Baltay M, et al. SMARCA4-deficient undifferentiated uterine sarcoma (malignant rhabdoid tumor of the uterus): a clinicopathologic entity distinct from undifferentiated carcinoma. Mod Pathol. 2018;31(9):1442–1456. doi: 10.1038/s41379-018-0049-z [DOI] [PubMed] [Google Scholar]
- 4.Lin DI, Allen JM, Hecht JL, et al. SMARCA4 inactivation defines a subset of undifferentiated uterine sarcomas with rhabdoid and small cell features and germline mutation association. Mod Pathol. June 2019:1. doi: 10.1038/s41379-019-0303-z [DOI] [PubMed] [Google Scholar]
- 5.Hodges C, Kirkland JG, Crabtree GR. The Many Roles of BAF (mSWI/SNF) and PBAF Complexes in Cancer. Cold Spring Harb Perspect Med. 2016;6(8). doi: 10.1101/cshperspect.a026930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson BG, Roberts CWM. SWI/SNF nucleosome remodellers and cancer. Nat Rev Cancer. 2011;11(7):481–492. doi: 10.1038/nrc3068 [DOI] [PubMed] [Google Scholar]
- 7.Agaimy A, Fuchs F, Moskalev EA. SMARCA4-deficient pulmonary adenocarcinoma: clinicopathological, immunohistochemical, and molecular characteristics of a novel aggressive neoplasm with a consistent TTF1neg/CK7pos/HepPar-1pos immunophenotype. Virchows Arch. 2017;471. doi: 10.1007/s00428-017-2148-5 [DOI] [PubMed] [Google Scholar]
- 8.Agarwal R, Chan Y-C, Tam CS, et al. Dynamic molecular monitoring reveals that SWI–SNF mutations mediate resistance to ibrutinib plus venetoclax in mantle cell lymphoma. Nat Med. 2019;25(1):119–129. doi: 10.1038/s41591-018-0243-z [DOI] [PubMed] [Google Scholar]
- 9.Skowron P, Ramaswamy V, Taylor MD. Genetic and Molecular Alterations Across Medulloblastoma Subgroups. J Mol Med Berl Ger. 2015;93(10):1075–1084. doi: 10.1007/s00109-015-1333-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filippakopoulos P, Knapp S. Chapter 10: Bromodomains as Anticancer Targets In: Egger G, Arimondo P, eds. Drug Discovery in Cancer Epigenetics. Boston: Academic Press; 2016:239–271. doi: 10.1016/B978-0-12-802208-5.00010-2 [DOI] [Google Scholar]
- 11.Han Y, Ren J, Yu W, Terashima M, Muegge K. Chapter 7: Malignant Transformation and Epigenetics In: Lu Q, Chang CC, Richardson BC, eds. Epigenetics and Dermatology. Boston: Academic Press; 2015:113–135. doi: 10.1016/B978-0-12-800957-4.00007-2 [DOI] [Google Scholar]
- 12.Tang M, Luo H, Lu J. Chapter 12: Genetically altered cancer epigenome In: Huang S, Litt MD, Blakey CA, eds. Epigenetic Gene Expression and Regulation. Oxford: Academic Press; 2015:265–289. doi: 10.1016/B978-0-12-799958-6.00012-3 [DOI] [Google Scholar]
- 13.Köbel M, Hoang LN, Tessier-Cloutier B. Undifferentiated endometrial carcinomas show frequent loss of core switch/sucrose nonfermentable complex proteins. Am J Surg Pathol. 2017;42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solomon DA, Perry A. Chapter 22: Familial Tumor Syndromes In: Perry A, Brat DJ, eds. Practical Surgical Neuropathology: A Diagnostic Approach (Second Edition). Elsevier; 2018:505–545. doi: 10.1016/B978-0-323-44941-0.00022-9 [DOI] [Google Scholar]
- 15.Le Loarer F, Watson S, Pierron G, et al. SMARCA4 inactivation defines a group of undifferentiated thoracic malignancies transcriptionally related to BAF-deficient sarcomas. Nat Genet. 2015;47:1200. doi:10.1038/ng.339910.1038/ng.3399https://www.nature.com/articles/ng.3399#supplementary-informationhttps://www.nature.com/articles/ng.3399#supplementary-information [DOI] [PubMed] [Google Scholar]
- 16.Perret R, Chalabreysse L, Watson S. SMARCA4-deficient thoracic sarcomas. Am J Surg Pathol. 2018;43. doi: 10.1097/PAS.0000000000001188 [DOI] [PubMed] [Google Scholar]
- 17.Lin DI, Chudnovsky Y, Duggan B, et al. Comprehensive genomic profiling reveals inactivating SMARCA4 mutations and low tumor mutational burden in small cell carcinoma of the ovary, hypercalcemic-type. Gynecol Oncol. 2017;147(3):626–633. doi: 10.1016/j.ygyno.2017.09.031 [DOI] [PubMed] [Google Scholar]
- 18.Sun JX, He Y, Sanford E. A computational approach to distinguish somatic vs. germline origin of genomic alterations from deep sequencing of cancer specimens without a matched normal. PLoS Comput Biol. 2018;14. doi: 10.1371/journal.pcbi.1005965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frampton GM, Fichtenholtz A, Otto GA. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31. doi: 10.1038/nbt.2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lek M, Karczewski KJ, Minikel EV, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616):285–291. doi: 10.1038/nature19057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Witkowski L, Goudie C, Ramos P, et al. The influence of clinical and genetic factors on patient outcome in small cell carcinoma of the ovary, hypercalcemic type. Gynecol Oncol. 2016;141(3):454–460. doi: 10.1016/j.ygyno.2016.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cerami E, Gao J, Dogrusoz U. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2. doi: 10.1158/2159-8290.CD-12-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao J, Aksoy BA, Dogrusoz U. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6. doi: 10.1126/scisignal.2004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pautier P, Ribrag V, Duvillard P, et al. Results of a prospective dose-intensive regimen in 27 patients with small cell carcinoma of the ovary of the hypercalcemic type. Ann Oncol. 2007;18(12):1985–1989. doi: 10.1093/annonc/mdm376 [DOI] [PubMed] [Google Scholar]
- 25.Callegaro-Filho D, Gershenson DM, Nick AM, et al. Small cell carcinoma of the ovary-hypercalcemic type (SCCOHT): A review of 47 cases. Gynecol Oncol. 2016;140(1):53–57. doi: 10.1016/j.ygyno.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan-Penebre E, Armstrong K, Drew A. Selective killing of SMARCA2- and SMARCA4-deficient small cell carcinoma of the ovary, hypercalcemic type cells by inhibition of EZH2: In vitro and in vivo preclinical models. Mol Cancer Ther. 2017;16. doi: 10.1158/1535-7163.MCT-16-0678 [DOI] [PubMed] [Google Scholar]
- 27.Jones RL, Blay J-Y, Agulnik M, et al. 1612PDA phase II, multicenter study of the EZH2 inhibitor tazemetostat in adults (rhabdoid tumor cohort) (NCT02601950). Ann Oncol. 2018;29(suppl_8). doi: 10.1093/annonc/mdy299.011 [DOI] [Google Scholar]
- 28.Lang JD, Hendricks WPD, Orlando KA, et al. Ponatinib Shows Potent Antitumor Activity in Small Cell Carcinoma of the Ovary Hypercalcemic Type (SCCOHT) through Multikinase Inhibition. Clin Cancer Res. 2018;24(8):1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xue Y, Meehan B, Macdonald E. CDK4/6 inhibitors target SMARCA4-determined cyclin D1 deficiency in hypercalcemic small cell carcinoma of the ovary. Nat Commun. 2019;10. doi: 10.1038/s41467-018-06958-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jelinic P, Ricca J, Van Oudenhove E, et al. Immune-Active Microenvironment in Small Cell Carcinoma of the Ovary, Hypercalcemic Type: Rationale for Immune Checkpoint Blockade. J Natl Cancer Inst. 2018;110(7):787–790. doi: 10.1093/jnci/djx277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miao D, Margolis CA, Gao W, et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science. 2018;359(6377):801–806. doi: 10.1126/science.aan5951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berchuck A, Witkowski L, Hasselblatt M, Foulkes WD. Prophylactic oophorectomy for hereditary small cell carcinoma of the ovary, hypercalcemic type. Gynecol Oncol Rep. 2015;12:20–22. doi: 10.1016/j.gore.2015.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pejovic T, McCluggage WG, Krieg AJ, et al. The dilemma of early preventive oophorectomy in familial small cell carcinoma of the ovary of hypercalcemic type. Gynecol Oncol Rep. 2019;28:47–49. doi: 10.1016/j.gore.2019.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loren AW, Mangu PB, Beck LN, et al. Fertility Preservation for Patients With Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2013;31(19):2500–2510. doi: 10.1200/JCO.2013.49.2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Three generation family pedigree. Limited phenotypic or genotypic information was available for the family. Pathogenic mutation in SMARCA4 may have occurred de novo in patient 2.
Supplementary Figure 2: Age distribution of SMARCA4 mutations in DUS and SCCOHT. (a) Histogram of SCCOHT and SMARCA4-DUS cohorts by age (bin=10). (b) Cumulative frequency of SCCOHT and SMARCA4-DUS cohorts. By the third decade of life 70% of SCCOHT patients would have developed disease based on this cohort. The peak of SMARCA4-DUS cases is skewed by approximately 2 decades.
Supplementary Table 1: Clinical Data Summary
Supplementary Table 2: Detected variations of unknown significance
Supplementary Table 3: Previously reported mutations