Abstract
BACKGROUND & AIMS:
Single measurements of liver stiffness (LS) by magnetic resonance elastography (MRE) have been associated with outcomes of patients with primary sclerosing cholangitis (PSC), but the significance of changes in LS over time are unclear. We investigated associations between changes in LS measurement and progression of PSC.
METHODS:
We performed a retrospective review of 204 patients with patients who underwent 2 MREs at a single center between January 1, 2007 and December 31, 2018. We collected laboratory data and information on revised Mayo PSC risk and model for end-stage liver disease scores, the PSC risk estimate tool, and levels of aspartate transferase at the time of each MRE. The ΔLS/time was determined by the change in LS between the second MRE compared to the first MRE divided by the time between examinations. The primary endpoint was development of hepatic decompensation (ascites, variceal hemorrhage or hepatic encephalopathy).
RESULTS:
The median LS measurement was 2.72 kPa (interquartile range, 2.32–3.44 kPa) and the overall change in LS was 0.05 kPa/y. However, ΔLS/y was 10-fold higher in patients anticipated to have cirrhosis (0.31 kPa/y) compared to patients with no fibrosis (0.03 kPa/y). The median LS increased over time in patients who ultimately developed hepatic decompensation (0.60 kPa/y; interquartile range, 0.21–1.26 kPa/y) vs but remained static in patients who did not (reduction of 0.04/y; interquartile range, reductions of 0.26 to 0.17 kPa/y) (P < .001). The ΔLS/y value associated with the highest risk of hepatic decompensation was Δ0.34 kPa/y (hazard ratio [HR], 13.29; 95% Cl, 0.23–33.78). After we adjusted for baseline LS and other risk factors, including serum level of alkaline phosphatase and the Mayo PSC risk score, ΔLS/y continued to be associated with hepatic decompensation. The optimal single LS cut-off associated with the hepatic decompensation was 4.32 kPa (HR, 60.41; 95% Cl, 17.85–204.47). A combination of both cut-off values was associated with risk of hepatic decompensation (concordance score, 93; 95% Cl, 0.88–0.98)
CONCLUSIONS:
A single LS measurement and changes in LS over time are independently associated with hepatic decompensation in patients with PSC. However, changes in LS occur slowly in patients without advanced fibrosis or hepatic decompensation.
Keywords: Biomarker, Prognostic Factor, Development, Severity
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disorder characterized by inflammation and fibrosis of the intra and or extrahepatic bile ducts.1 Over time, PSC can lead to cirrhosis and complications associated with portal hypertension. There is no effective medical therapy for PSC. In part, this is related to the phenotypic heterogeneity of the disorder and outcomes such as death or hepatic decompensation may take years to develop. Consequently, the limited array of biomarkers that can both accurately predict clinical endpoints and change in parallel with the disease course makes clinical trials and drug development challenging.2
Liver stiffness (LS), a surrogate marker for hepatic fibrosis that can be quantified using elastography, has been proposed as a surrogate endpoint candidate.2–4 Magnetic resonance elastography (MRE) and transient elastography (TE) are the 2 most common techniques used to measure LS.5,6 A single LS measured by either MRE or TE has been found to correlate with fibrosis stage and predict clinical outcomes among those with PSC.7,8 As previously described, MRE has several advantages over TE among those patients with PSC.8,9 Several of these advantages include an ability to sample a larger volume of the liver and an ability to perform MRE with magnetic resonance cholangiography, which can in turn detect disease related complications and flow-limiting strictures that can increase the LS beyond what would be expected for fibrosis alone.10–14
Our understanding of the significance of changes in LS over time (ΔLS/time) in PSC is limited. In a cohort of 142 PSC patients who underwent repeated LS assessments using TE, the ΔLS/time was associated with a heterogeneous composite endpoint which included both portal hypertension and non-portal hypertension-related outcomes.7 However, the ΔLS/time had a similar performance to the ΔMayo PSC risk score and it is unclear if ΔLS/time remained an independent predictor of outcomes after adjusting for the baseline LS value, which may correlate with the rapidity of LS increases. Moreover, when serial LS assessments were obtained using TE in a prospective PSC clinical trial, ΔLS/time was not associated with the primary outcome after adjusting for the baseline LS value.15 Therefore, it is unknown if ΔLS/time obtained using real-world LS measurements offers an independent prognostic metric beyond a single LS value. Furthermore, ΔLS/time as measured by MRE has not been studied in PSC to date. Consequently, using MRE, our aims were to describe the natural history of ΔLS/time and determine if ΔLS/time are associated with hepatic decompensation regardless of the baseline LS value and other prognostic factors.
Materials And Methods
Patients
This study was approved by the institutional review board at Mayo Clinic (Rochester, MN) and conforms to the ethical guidelines of the 1975 Declaration of Helsinki. A retrospective review was conducted between January 1, 2007, and December 31, 2018. Patients were included if they had diagnostic features of PSC as previously defined and had 2 or more MREs at our institution.16 Patients were excluded if they developed hepatic decompensation (ascites, variceal hemorrhage, or hepatic encephalopathy) or underwent a liver transplant before the second MRE. MRE exams were performed as previously described and LS values were expressed in kilopascals.5,8Among the subjects included, 146 were previously included in our original MRE article because they subsequently had a second MRE and additional follow-up data.
Data Collection and Key Definitions
Laboratory data, including the revised Mayo PSC risk and Model for End-Stage Liver Disease (MELD) scores, PSC risk estimate tool (PREsTo), and aspartate aminotransferase-to-platelet ratio, were collected at the time of the MRE. Given different reference ranges for serum alkaline phosphatase (SAP) utilized at our institution, the SAP was divided by the respective upper limit of normal. Thrombocytopenia was defined as a platelet value <150 × 109/L. Features of portal hypertension among those without decompensation included thrombocytopenia, splenomegaly, or nonbleeding varices. A dominant stricture is generally defined as a stricture with a diameter 1 mm or less in the hepatic duct or 1.5 mm or less in the common bile duct.16 An untreated dominant stricture was defined as the presence of a dominant stricture and a bilirubin 2.0 mg/dL or greater at the time of the MRE.
Statistical Analysis
Statistical analysis was performed with JMP and SAS software (SAS Institute, Cary, NC). All tests were 2-sided, Q3 with a level of significance of P < .05. Categorical data were compared using the Pearson chi-square test and continuous variables were compared using the nonparametric Wilcoxon test Categorical data are presented as numbers and percentage, whereas continuous variables are expressed as median and interquartile range, unless otherwise stated.
Anticipated changes in LS overtime was expressed as a slope and determined using random-effects linear regression model, the timing of the MRE treated as a random effect. All MRE’s performed were considered. This analysis was performed in 4 groups, categorizing each patient based on their initial LS value and prior published data for the prediction of fibrosis stage.8 The correlation between continuous variables and change in LS was assessed using a Pearson correlation coefficient.
The prognostic significance of a single LS measurement and ΔLS/time were assessed. Unless otherwise stated, the second MRE was considered baseline time point. The single LS measurement was obtained from the second MRE. The ΔLS/time was determined by the change in LS between the second MRE compared with the first MRE divided by the time between examinations. The optimal cutoff for both a single LS measurement and ΔLS/time to predict hepatic decompensation was determined as suggested by Contal and O’Quigley.17
The primary endpoint was the development of hepatic decompensation (ascites, variceal hemorrhage, or hepatic encephalopathy), which may have occurred at any time point provided it was not diagnosed before the second MRE. Patients were censored at the time of liver transplantation, death, or date of last follow-up (whichever one occurred earlier). Cox proportional hazards regression analysis was utilized in both univariable and multivariable analyses to examine associations between covariates and the primary endpoint, and the results were expressed as hazard ratio (HR) and 95% confidence interval (Cl). The LS covariate plus 1 other prognostic variable that was significant in the univariate analysis were included in a series of multivariate models. The discriminative ability of LS to categorize individuals at various risks for developing hepatic decompensation was assessed with the concordance score from the Cox model.
The ability of either a single LS or ΔLS/time to predict decompensation among individuals within 4 risk categories (low, mild, moderate, and high) was described. The LS cutoffs for these 4 categories were determined based on the LS quartiles from either the LS at MRE 2 or ΔLS/y for the entire cohort. The Hosmer-Lemeshow goodness-of-fit test compared the predicted probability of hepatic decompensation in these 4 risk groups with the actual number of observed hepatic decompensation events.
Results
Patients
Two-hundred and four patients with PSC were included in this study and followed for a median of 4.00 (IQR, 3.10 to 4.80) years. The median time between the first and second MRE was 1.10 (IQR, 1.00 to 2.00) years. The majority (n = 194) of subjects were part of a cohort in which MRE was done a priori among all individuals undergoing annual cholangiocarcinoma (CCA) screening. The clinical features at the time of the second MRE are shown in Table 1. Subjects with features of portal hypertension at baseline had a higher LS compared with those who did not: 3.85 (IQR, 2.94 to 4.88) kPa vs 2.39 (IQR, 2.11 to 2.90) kPa (P < .01).
Table 1.
Baseline Characteristics of Cohort
| Age, y | 47.00 (34.00 to 61.00) |
| Female | 33.80 (69/204) |
| BMI, kg/m2) | 25.60 (22.90 to 28.90) |
| IBD presenta | 81.90 (167/204) |
| PSC duration, y | 8.50 (2.90 to 14.20) |
| PSC-AIH overlap | 5.90 (12/204) |
| UDCA use | 34.30 (70/204) |
| Platelets (×109/L) | 242.80 (194.00 to 285.00) |
| Platelets <150 × 109/L | 10.8 (21/195) |
| Features of portal hypertension | 31.90 (65/204) |
| APRI | 0.50 (0.30 to 0.90) |
| SAP/ULN | 1.40 (0.90 to 2.60) |
| Total bilirubin, mg/dL | 0.80 (0.50 to 1.30) |
| MELD score | 6.50 (6.40 to 7.00) |
| Mayo PSC risk score | −0.20 (−0.80 to 0.50) |
| PREsTo | 4.30 (3.30 to 8.30) |
| Baseline LSb | 2.70 (2.30 to 3.50) |
Values are median (interquartile range) or % (n/n).
AIH, autoimmune hepatitis; APRI, aspartate aminotransferase-to-platelet ratio index; BMI, body mass index; IBD, inflammatory bowel disease; LS, liver stiffness; MELD, Model for End-Stage Liver Disease; PREsTo, primary sclerosing cholangitis risk estimate tool; PSC, primary sclerosing cholangitis; SAP, serum alkaline phosphatase; UDCA, ursodeoxycholic acid; ULN, upper limit of normal.
Among those with inflammatory bowel disease: n = 135: ulcerative colitis; n = 29; Crohn’s disease; n = 3 (indeterminate colitis).
Baseline was assessed at second magnetic resonance enterography.
Changes in LS Over Time
We estimated the rates of LS progression from the first MRE to last LS assessment. The median LS at the first MRE for the entire cohort was normal, at 2.72 (IQR, 2.32 to 3.44) kPa, and the overall change in LS was minimal (slope = 0.05 kPa/y; P = .002). LS progression was slow among those with lower baseline LS values and faster among those with higher baseline LS values (Figure 1). For example, those with a baseline LS consistent with stage 0 fibrosis had a slope of 0.03 kPa/y (P = .06), while those estimated to have stage 4 fibrosis had a slope of 0.31 kPa/y (P = .04).
Figure 1.

Stratified changes in liver stiffness over time.
We examined clinical and laboratory variables obtained at the first MRE to assess their correlation with subsequent ΔLS/y between the first and second MRE (Supplementary Tables 1 and 2). Both LS (R2 = .05, P = .001) and the total bilirubin (R2 = .03, P = .02) at the first MRE had a small association with a subsequent LS increase. Increases in prognostic scores between the first and second MRE correlated with a rise in LS (Δtotal bilirubin: R2 = .41, P < .0001; ΔMELD: R2 = .16, P < .0001; ΔMayo PSC risk score: R2 = .17, P < .0001; ΔPREsTo: R2 = .35, P < .0001) (Supplementary Table 3).
There were 9 individuals with an untreated dominant stricture at the time of the first MRE. Individuals with an untreated dominant stricture had a higher median LS compared with those who did not: 3.82 (IQR, 3.41 to 4.45) kPa vs 2.69 (IQR, 2.28 to 3.35) kPa (P < .01). However, those with an untreated dominant stricture detected at the first MRE had a similar ΔLS/y compared with those without a dominant stricture (Supplementary Table 2). Of those 9 subjects, 8 underwent an endoscopic retrograde cholangiopancreatography with dilation plus or minus stenting. However, endoscopic therapy did not result in a significant reduction in LS when the second MRE was performed: 0.38 (IQR, −0.03 to 1.29) kPa/y. This may suggest that the LS was elevated regardless of the degree of biliary obstruction.
Prognostic Significance of LS and LS Progression
Hepatic decompensation developed in 23 subjects. Ascites occurred in all individuals with decompensation, while 3 also had variceal hemorrhage, and 4 had hepatic encephalopathy. Malignant hepatobiliary complications occurred in 10 individuals (CCA n = 9; HCC, n = 1). Seven participants underwent liver transplantation (indications: hepatic decompensation, n = 4; CCA, n = 2; other, n = 1), while 7 died (CCA, n = 2; liver failure, n = 2; other, n = 3). Table 2 illustrates the covariates examined in the univariate analysis to predict hepatic decompensation.
Table 2.
Covariates Associated With Hepatic Decompensation (Unadjusted)
| Variable | HR (95% Cl) | P Value |
|---|---|---|
| Age | 1.04 (0.81–1.34) | .75 |
| Female | 1.20 (0.52–2.78) | .68 |
| BMI | 1.20 (0.84–1.65) | .34 |
| IBD present | 2.96 (0.55–16.05) | .21 |
| PSC duration | 1.24 (0.81–1.90) | .31 |
| PSC-AIH overlap | 2.29 (0.68–7.71) | .18 |
| Untreated dominant stricture | 1.16 (0.07–20.54) | .92 |
| Recent ascending cholangitisa | 4.64 (0.62–34.89) | .14 |
| UDCA use | 1.90 (0.83–4.32) | .13 |
| Features of portal hypertensionb | 10.68 (3.630–31.45) | <.0001 |
| APRI | 1.51 (1.29–1.76) | <.0001 |
| SAP/ULN | 1.49 (1.24–1.63) | .67 |
| Total bilirubin | 1.28 (1.17–1.39) | <.0001 |
| MELD score | 1.11 (0.67–1.85) | .68 |
| Mayo PSC risk score | 3.91 (2.47–6.18) | <C.0001 |
| PREsTo | 1.06 (1.04–1.07) | <.0001 |
| Baseline LS | 1.80 (1.57–2.07) | <C.0001 |
| LS >4.32 kPa at baseline | 60.41 (17.85–204.47) | <.0001 |
| ΔLS/y | 2.24 (1.73–2.89) | <.0001 |
| LS >Δ0.34 kPa/y | 13.29 (5.23–33.78) | <.0001 |
AIH, autoimmune hepatitis; APRI, aspartate aminotransferase-to-platelet ratio index; BMI, body mass index; IBD, inflammatory bowel disease; LS, liver stiffness; MELD, Model for End-Stage Liver Disease; PREsTo, primary sclerosing cholangitis risk estimate tool; PSC, primary sclerosing cholangitis; SAP, serum alkaline phosphatase; UDCA, ursodeoxycholic acid; ULN, upper limit of normal.
Episode of ascending cholangitis within 2 weeks of elastography.
Defined by presence of any of the following: splenomegaly, varices, or other collaterals or thrombocytopenia (platelets <150 × 109/L).
A single LS measurement (obtained at the second MRE) was associated with hepatic decompensation (HR, 1.80; 95% Cl, 1.57–2.07; P < .0001). It was well calibrated to predict events in 4 respective risk categories (low, mild, moderate, and high), as indicated by the similar number of predicted (vs observed) events: 0.1 (vs 0), 0.4 (vs 0), 1.2 (vs 1), 21.2 (vs 22) (P = .90). The optimal cutoff for a single LS measurement to predict decompensation was 4.32 kPa (HR, 60.41; 95% Cl, 17.85–204.47; P < .0001) (Figure 2A). A single LS of >4.32 kPa remained associated with hepatic decompensation after adjusting for the ΔLS/y: (HR, 44.17; 95% Cl, 12.57–155.16; P < .0001). Indeed, >4.32 kPa was highly discriminative in its ability to categorize individuals at high risk of hepatic decompensation (concordance score, 0.90; 95% Cl, 0.83–0.96).
Figure 2.
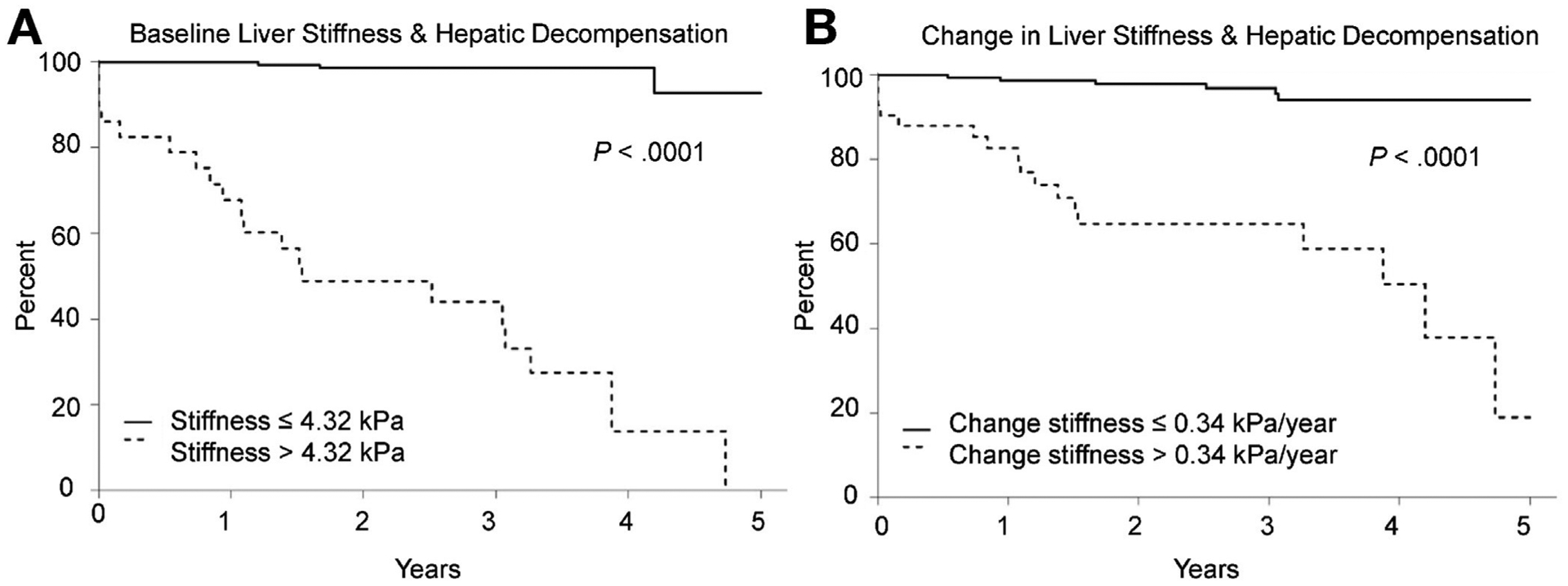
Optimal cutoffs for (A) baseline liver stiffness and (B) change in liver stiffness to predict hepatic decompensation.
LS increased prior to the development of hepatic decompensation compared with those who did not develop the endpoint (Figure 3A and B). Indeed, the median LS increased over time among those who developed hepatic decompensation, but remained static in those who did not reach the endpoint: 0.60 (IQR, 0.21 to 1.26) kPa/y vs −0.04 (IQR, −0.26 to 0.17) kPa/y (P < .001). ΔLS/y was associated with hepatic decompensation after adjusting for the baseline LS (HR, 1.51; 95% Cl, 1.11–2.07; P = .01). We also examined the ΔLS/y as a continuous variable to determine if it retained its prognostic value after adjusting for changes in other prognostic variables. In contrast to Δtotal bilirubin, ΔSAP, ΔMELD, and ΔMayo PSC risk score, the ΔLS/y, ΔPREsTo, and change in aspartate aminotransferase-to-platelet ratio all continued to be associated with hepatic decompensation (Supplementary Table 4). ΔLS/y was well calibrated to predict events in 4 respective risk categories (low, mild, moderate, and high) as indicated by the similar number of predicted (vs observed) events: 0.7 (vs 0), 2.3 (vs 2), 3.8 (vs 4), 16.1 (vs 17) (P = .73).The optimal ΔLS/y cutoff to predict hepatic decompensation was 0.34 kPa/y (HR, 13.29; 95% Cl, 5.23–33.78; P < .0001) (Figure 2B). Clinically significant increases in LS tended to occur among those who already had elevated LS. For example, the initial LS was higher among individuals with a ΔLS >0.34 kPa compared with those who did not have a significant change in their LS: 3.22 (IQR, 2.38 to 3.85) kPa vs 2.67 (IQR, 2.28 to 3.31) kPa (P = .02).
Figure 3.
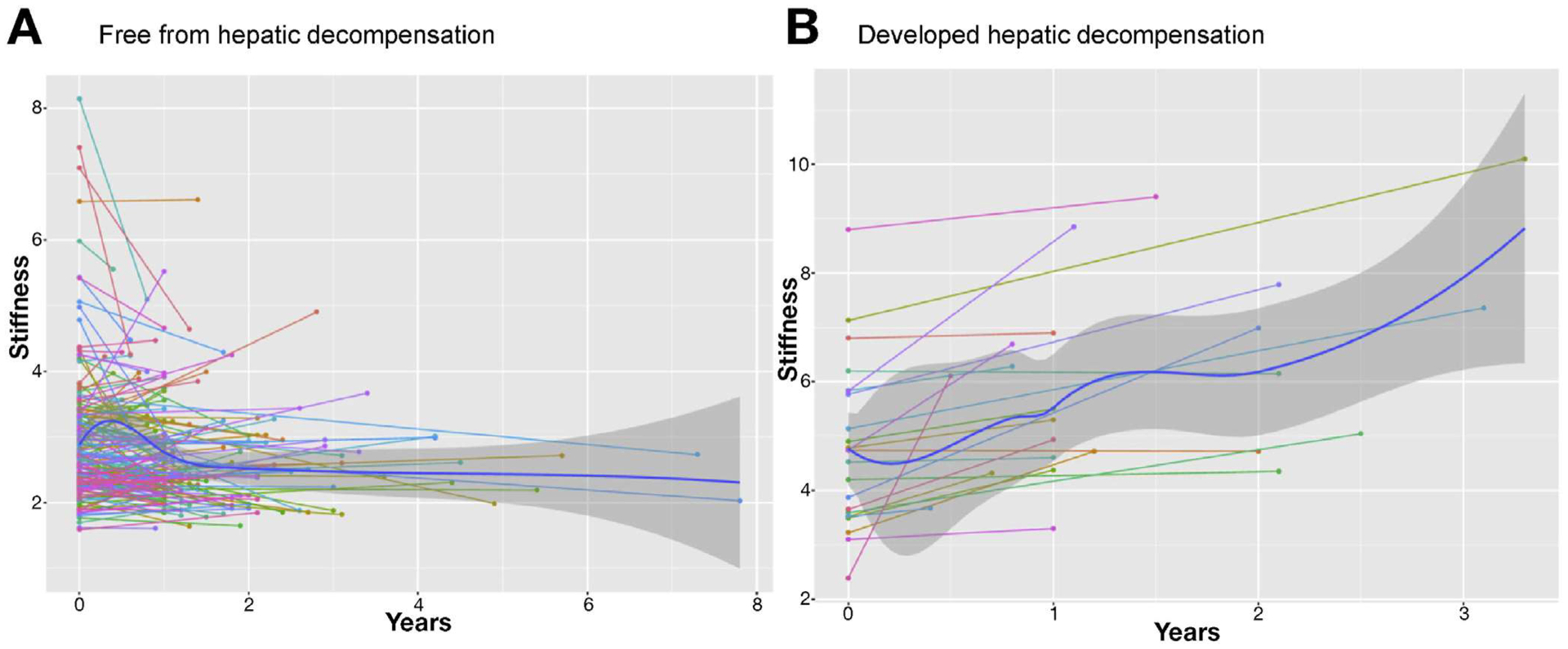
Liver stiffness changes among those who (A) were free from and (B) developed hepatic decompensation.
ΔLS >0.34 kPa remained an independent predictor of hepatic decompensation after adjusting for baseline LS values and other prognostic covariates (Table 3). Notably, it continued to be predictive of hepatic decompensation even when manifestations of portal hypertension were already present (Table 3). While less robust than a single LS value of >4.39 kPa, an increase LS of >0.34 kPa/y was also able to discriminate individuals at a high risk of hepatic decompensation (concordance score, 0.79; 95% Cl, 0.70–0.88). There was no difference in the time interval between the first and second MRE among those who had a baseline LS more than 4.32 kPa or an increase by more than 0.34 kPa/y, compared with those who did not (data not shown).
Table 3.
Ability of Change in LS to Predict Hepatic Decompensation (Adjusted)
| Variable | HR (95% Cl) | P Value |
|---|---|---|
| Model 1 | ||
| LS at baseline | 1.54 (1.41–1.94) | <.0001 |
| LS >Δ0.34 kPa/y | 4.32 (1.38–13.56) | .01 |
| Model 2 | ||
| LS >4.32 kPa at baseline | 34.32 (9.37–125.77) | <.0001 |
| LS >Δ0.34 kPa/y | 3.43 (1.24–9.46) | .02 |
| Model 3 | ||
| Features of portal hypertensiona | 9.33 (3.13–27.79) | <.0001 |
| LS >Δ0.34 kPa/y | 14.76 (5.34–40.80) | <.0001 |
| Model 4 | ||
| APRI | 1.48 (1.24–1.77) | <.0001 |
| LS >Δ0.34 kPa/y | 12.36 (4.79–31.93) | <.0001 |
| Model 5 | ||
| Total bilirubin | 1.21 (1.11–1.32) | <.0001 |
| LS >Δ0.34 kPa/y | 11.23 (4.32–29.21) | <.0001 |
| Model 6 | ||
| Mayo PSC risk score | 2.80 (1.78–4.40) | <.0001 |
| LS >Δ0.34 kPa/y | 4.56 (1.50–13.85) | <.001 |
| Model 7 | ||
| PREsTo | 1.04 (1.02–1.06) | <.0001 |
| LS >Δ0.34 kPa/y | 8.49 (3.12–23.09) | <.0001 |
APRI, aspartate aminotransferase-to-platelet ratio index; Cl, confidence interval; HR, hazard ratio; LS, liver stiffness; PREsTo, primary sclerosing cholangitis risk estimate tool; PSC, primary sclerosing cholangitis.
Defined by presence of any of the following: splenomegaly, varices or other collaterals or thrombocytopenia (platelets <150 × 109/L).
Both the current LS value and the magnitude of change over time are important. Combining both of the cutoffs (1 time stiffness assessment of >4.32 kPa and change in LS of >0.34 kPa/y) improved the ability of the individual LS covariates to predict hepatic decompensation (concordance score, 0.93; 95% Cl, 0.88–0.98). However, it is important to note that the short term prognostic implications of an increased rate of LS progression are attenuated when the current LS value is low. For example, the median LS among those who had an increase in LS >0.34 kPa/y was significantly higher among those who developed hepatic decompensation vs those who did not: 6.20 (IQR, 4.96 to 7.68) kPa vs 3.24 (IQR, 2.69 to 3.98) kPa (P < .001).
Discussion
To date, this is the largest series that describes changes in LS and its association with outcomes among those with PSC. Our results highlight several key findings. First, changes in LS generally occur slowly. However, subgroups anticipated to have a more rapid LS increase include those with more advanced fibrosis (or higher baseline LS) and those who are at a higher risk for complications stemming from portal hypertension. Second, the changes in LS correlate with changes in other known prognostic scores. Third, changes in LS over time independently predict hepatic decompensation after adjusting for baseline LS values and other prognostic factors. Last, our findings reaffirm the prognostic value of a single LS measurement.
In general, LS increases slowly among those with PSC when advanced fibrosis is absent. This is observation is consistent with the lack of change in LS as measured by TE after nearly 2 years in the simtuzumab PSC clinical trial, as well as in other studies that examined serial LS changes in other chronic liver diseases.15,18,19 In contrast, utilizing TE, Corpechot et al7 described progression rates that were 4- to 5-fold higher than those reported here for stage 0 and stage 4 fibrosis (after using a conversion factor of 3 to more directly compare the shear-based MRE measurement with the Young’s modulus TE measurement), despite having a similar duration of follow-up. Variations in elastography techniques and patient characteristics may explanation these differences. For example, MRE samples a larger area of the liver compared with TE and PSC can be patchy with segmental atrophy and hypertrophy. Consequently, TE could measure either the atrophic or hypertrophic segments and fail to provide a global LS average. TE may also be more susceptible to changes of transient segmental biliary obstruction, which can also increase LS. However, our results confirmed the observation by Corpechot et al7 that progression rates depend on the baseline LS value, and individuals expected to have stage 4 fibrosis will have a more rapid increase in LS over time when compared with those without fibrosis (Figure 1). It is possible that a more exponential increase in LS among those who had a low baseline LS may occur after a longer period of observation.
Describing the dynamic changes in LS and its prognostic relevance is important. Indeed, both the change in LS overtime and a single LS measurement are associated with hepatic decompensation. Unlike changes in SAP, the most widely used surrogate marker in both clinical practice and clinical trials, the change in LS remained associated with the endpoint (Supplementary Table 4). While it is important to note that the change in this biomarker is meaningful, the change in LS does not supersede the prognostic relevance of a single contemporary LS measurement. For example, among those who had a LS increase >0.34 kPa/y, the median baseline LS was 2-fold higher among those who did (compared with those who did not) develop hepatic decompensation. This illustrates that the short-term risk of decompensation would be low if a patient’s LS increased from 2.00 kPa to 2.50 kPa over the course of 1 year. Indeed, our findings reinforce the observation that the development of hepatic decompensation is rare when the baseline LS is less than 4.32 kPa.
These findings raise new questions and have implications for both clinical trials and patient care. First, a single LS measurement is well suited to stratify patients into risk groups when entering a clinical trial. However, the slow change in LS over time is a limitation for its widespread adaptation as a primary surrogate endpoint in most early -phase trials. For example, using our cohort’s median LS of 2.72 kPa (comparable to the median LS in the simtuzumab trial), we would anticipate a change in LS of 0.05 kPa/y.15 However, changes in LS may serve as a niche primary endpoint for selective drugs (eg, antifibrotic agents) in early-phase trials in which the study population can be enriched with participants with advanced liver fibrosis, who are more likely to have rapid changes in LS over a shorter period of time. Similar to prior studies, our results highlight that there are other prognostic biomarkers that have an enhanced performance when compared with SAP changes.15,20 This illustrates the importance of weaning our 20th century addiction of using SAP alone as a surrogate endpoint and moving toward a more sophisticated 21st century strategy of selecting from a pool of biomarkers based on their performance to predict the outcome of interest and the mechanism of action of the study drug. Second, it would be advantageous to incorporate both TE and MRE in future clinical trials to enable a direct comparison between their diagnostic and prognostic performances. Third, patients with a low LS can be reassured that their short-term risk of hepatic decompensation is low. Indeed, measuring LS adds prognostic value to patient care beyond routine laboratory tests and prognostic scores.
Our study has several limitations. First, it was a single-center retrospective study, and validating our LS cutoff values will be important to ensure external validity and to replicate the performance of LS cutoffs to predict outcomes, as the concordance scores presented herein could overestimate their actual discriminatory ability. However, our patient baseline characteristics are typical for adults with large-duct PSC and the MELD score, Mayo PSC risk score, and median LS in this cohort were comparable to the multicenter simtuzumab study.15 Second, we were unable to compare the changes in LS to changes in histology or other novel biomarkers such as the enhanced liver fibrosis score. Third, the LS cutoff to separate stage 2 and 3 fibrosis using MRE is unknown. The inability to separate these groups and our sample size may explain why we did not see a significant increase in LS among those expected to have stage 2–3 fibrosis. Last, our study was not designed or powered to assess if endoscopic therapy via balloon dilation or stenting can affect LS. While a flow-limiting dominant stricture may influence the LS, excluding the small number of patients with an untreated dominant stricture did not impact our findings (data not shown).10,12
In conclusion, both baseline LS and changes in LS over time are independently and robustly associated with hepatic decompensation. While LS progression accelerates when the baseline LS is high, the overall rate of LS change is slow. Therefore, if LS is used as a primary surrogate endpoint in early-phase clinical trials, it may be advantageous to exclude individuals with a low baseline LS.
Supplementary Material
What You Need to Know.
Background
Single measurements of liver stiffness (LS) by magnetic resonance elastography have been associated with outcomes of patients with primary sclerosing cholangitis (PSC). We investigated the significance of changes in LS over time.
Findings
A single LS measurement and changes in LS over time are independently associated with hepatic decompensation in patients with PSC. However, changes occur slowly in patients without advanced fibrosis or hepatic decompensation.
Implications for patient care
LS measurements can be tracked over time to monitor progression of liver disease in patients with PSC.
Acknowledgments
The authors would like to thank Carlos Fund in Primary Sclerosing Cholangitis for supporting this project.
Abbreviations used in this paper.
- CCA
cholangiocarcinoma
- Cl
confidence interval
- HR
hazard ratio
- LS
liver stiffness
- MRE
magnetic resonance elastography
- MELD
Model for End-Stage Liver Disease
- PSC
primary sclerosing cholangitis
- PREsTo
primary sclerosing cholangitis risk estimate tool
- SAP
serum alkaline phosphatase
- TE
transient elastography
Footnotes
Conflicts of Interest
The Mayo Clinic has intellectual property rights and a financial interest related to magnetic resonance elastography, a technology used in this study. The authors disclose no conflicts.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.Org/10.1016/j.cgh.2019.10.041.
References
- 1.Eaton JE, Talwalkar JA, Lazaridis KN, et al. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology 2013;145:521–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ponsioen CY, Chapman RW, Chazouilleres O, et al. Surrogate endpoints for clinical trials in primary sclerosing cholangitis: Review and results from an International PSC Study Group consensus process. Hepatology 2016;63:1357–1367. [DOI] [PubMed] [Google Scholar]
- 3.Castera L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005;128:343–350. [DOI] [PubMed] [Google Scholar]
- 4.Singh S, Venkatesh SK, Wang Z, et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol 2015;13:440–451 .e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging 2013;37:544–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shire NJ, Yin M, Chen J, et al. Test-retest repeatability of MR elastography for noninvasive liver fibrosis assessment in hepatitis C. J Magn Reson Imaging 2011. ;34:947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corpechot C, Gaouar F, El Naggar A, et al. Baseline values and changes in liver stiffness measured by transient elastography are associated with severity of fibrosis and outcomes of patients with primary sclerosing cholangitis. Gastroenterology 2014; 146:970–979; quiz e15-e16. [DOI] [PubMed] [Google Scholar]
- 8.Eaton JE, Dzyubak B, Venkatesh SK, et al. Performance of magnetic resonance elastography in primary sclerosing cholangitis. J Gastroenterol Hepatol 2016;31:1184–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schramm C, Eaton J, Ringe Kl, et al. Recommendations on the use of magnetic resonance imaging in PSC-A position statement from the International PSC Study Group. Hepatology 2017;66:1675–1688. [DOI] [PubMed] [Google Scholar]
- 10.Bookwalter CA, Venkatesh SK, Eaton JE, et al. MR elastography in primary sclerosing cholangitis: correlating liver stiffness with bile duct strictures and parenchymal changes. Abdom Radiol (NY) 2018;43:3260–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Millonig G, Reimann FM, Friedrich S, et al. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology 2008;48:1718–1723. [DOI] [PubMed] [Google Scholar]
- 12.Ehlken H, Lohse AW, Schramm C. Transient elastography in primary sclerosing cholangitis-the value as a prognostic factor and limitations. Gastroenterology 2014;147:542–543. [DOI] [PubMed] [Google Scholar]
- 13.Attia D, Pischke S, Negm AA, et al. Changes in liver stiffness using acoustic radiation force impulse imaging in patients with obstructive cholestasis and cholangitis. Dig Liver Dis 2014; 46:625–631. [DOI] [PubMed] [Google Scholar]
- 14.Ichikawa S, Motosugi U, Morisaka H, et al. Comparison of the diagnostic accuracies of magnetic resonance elastography and transient elastography for hepatic fibrosis. Magn Reson Imaging 2015;33:26–30. [DOI] [PubMed] [Google Scholar]
- 15.Muir AJ, Levy C, Janssen HLA, et al. Simtuzumab for primary sclerosing cholangitis: phase 2 study results with insights on the natural history of the disease. Hepatology 2019;69:684–698. [DOI] [PubMed] [Google Scholar]
- 16.Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010; 51:660–678. [DOI] [PubMed] [Google Scholar]
- 17.Contal C, O’Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Computati Stat Data Anal 1999;30:253–270. [Google Scholar]
- 18.Arima Y, Kawabe N, Hashimoto S, et al. Reduction of liver stiffness by interferon treatment in the patients with chronic hepatitis C. Hepatol Res 2010;40:383–392. [DOI] [PubMed] [Google Scholar]
- 19.Egbe A, Miranda WR, Connolly HM, et al. Temporal changes in liver stiffness after Fontan operation: Results of serial magnetic resonance elastography. Int J Cardiol 2018;258: 299–304. [DOI] [PubMed] [Google Scholar]
- 20.Eaton JE, Vesterhus M, McCauley BM, et al. Primary sclerosing cholangitis risk estimate tool (PREsTo) predicts outcomes in PSC: a derivation and validation study using machine learning. Hepatology 2020;71:214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


