Abstract
Objectives
This study provides the first comparison of trends in dementia prevalence in the U.S. population using 3 different dementia ascertainments/data sources: neuropsychological assessment, cognitive tests, and diagnosis codes from Medicare claims.
Methods
We used data from the nationally representative Health and Retirement Study and Aging, Demographics, and Memory Study, and a 20% random sample of Medicare beneficiaries. We compared dementia prevalence across the 3 sources by race, gender, and age. We estimated trends in dementia prevalence from 2006 to 2013 based on cognitive tests and diagnosis codes utilizing logistic regression.
Results
Dementia prevalence among older adults aged 70 and older in 2004 was 16.6% (neuropsychological assessment), 15.8% (cognitive tests), and 12.2% (diagnosis codes). The difference between dementia prevalence based on cognitive tests and diagnosis codes diminished in 2012 (12.4% and 12.9%, respectively), driven by decreasing rates of cognitive test-based and increasing diagnosis codes-based dementia prevalence. This difference in dementia prevalence between the 2 sources by sex and for age groups 75–79 and 90 and older vanished over time. However, there remained substantial differences across measures in dementia prevalence among blacks and Hispanics (10.9 and 9.8 percentage points, respectively) in 2012.
Discussion
Our results imply that ascertainment of dementia through diagnosis may be improving over time, but gaps across measures among racial/ethnic minorities highlight the need for improved measurement of dementia prevalence in these populations.
Keywords: Cognitive tests, Diagnosis codes, Neuropsychological assessment, Racial/ethnic minorities, Trends
Rising life expectancy has led to an increasing prevalence of diseases more common at old age, such as Alzheimer’s disease (AD) and related dementias. The number of Americans aged 65 and older with AD and other dementias was about 7 million in 2012 and is projected to increase to almost 12 million by 2040 (Zissimopoulos et al., 2018).
The annual per case cost of AD is estimated to increase from $71,303 to $140,000 from 2010 to 2050, with total population annual costs increasing from $307 billion, including $181 billion in medical costs paid out-of-pocket and by Medicare and Medicaid, and $126 billion in the value of unpaid caregiving by family, to $1.5 trillion (Zissimopoulos et al., 2014). Accurate estimates and forecasts of dementia prevalence in the United States will aid families, policy makers, and health care providers in planning for the social, economic, and health burden of this costly and formidable disease. However, estimates of dementia prevalence for the U.S. population vary widely, driven in large part by differences in dementia ascertainment and study populations (Brookmeyer et al., 2011; Prince et al., 2016).
Neuropsychological assessment of dementia may provide high accuracy in ascertaining dementia prevalence, but is typically performed only in small and nonrepresentative samples, thus limiting its usefulness for quantifying population levels and changes over time (Brookmeyer et al., 2011; Demirovic et al., 2003; Gurland et al., 1999; Hebert et al., 2013; Rocca et al., 2011). One study, the Aging, Demographics, and Memory Study (ADAMS), identified dementia using neuropsychological assessment conducted by experts including neuropsychologists and neurologists in a sample that, when weighted, is nationally representative of older adults in the United States. Sample sizes, however, are small and the study has not been repeated over time (Crimmins et al., 2011; Plassman et al., 2007).
Cognitive tests of respondents in large-scale surveys such as the Health and Retirement Study (HRS) are another source for quantifying dementia prevalence and trends in the population (Chen & Zissimopoulos, 2018; Crimmins et al., 2011, 2018; Langa et al., 2017). The HRS is well-suited for studying trends in dementia prevalence for two reasons. First, the longitudinal, panel data provide multiple assessments of the same individual’s cognition over time. Second, HRS allows for estimating population trends in dementia by observing nationally representative samples over time (Langa et al., 2017; Rocca et al., 2011). However, dementia ascertainment from cognitive tests may overestimate rates for some individuals, such as those with low education or nonnative English speakers (Crum et al., 1993; Ganguli et al., 2010; Gianattasio et al., 2019; Spering et al., 2012).
Medicare claims data are a potentially rich source for estimating dementia prevalence and trends because of Medicare’s broad coverage of Americans aged 65 and older. Beneficiaries’ health care claims, from both outpatient services and inpatient facilities, are recorded over long periods, usually from age 65 until the beneficiary’s death. Studies, however, have found that these records have measurement errors (Amjad et al., 2018; Bradford et al., 2009; Chodosh et al., 2004; Taylor et al., 2009).
Taylor et al. (2009) compared dementia diagnosis in Medicare claims from 1993 to 2005 with neuropsychological assessment in the ADAMS 2001–2003 and reported that 14.5% of individuals classified as having dementia based on neuropsychological assessment were without a diagnosis code for dementia in their Medicare claims records. Amjad et al. (2018) compared cognitive test-based dementia and dementia diagnosis using the 2011 wave of the National Health and Aging Trends Study (NHATS) and Medicare claims and found that 39.5% of those with dementia based on cognitive testing were undiagnosed in Medicare. However, this number may overestimate the measurement error as ascertainment of dementia based on cognitive test performance at a single interview was found in other studies to overestimate dementia (Freedman et al., 2018; Zissimopoulos et al., 2018). Chen et al. (2019) compared cognitive decline and diagnosis using HRS data linked with respondents’ Medicare claims and found that 85% of respondents with incident dementia measured by cognitive decline received a diagnosis or died within 4 years, with lower odds of diagnosis among blacks and Hispanics compared to whites. In this study, we used data from three samples, broadly representative of the U.S. population, to estimate the level and trends over time of dementia prevalence. We quantified prevalence differences from three measurement approaches and data sources: neuropsychological assessment from the ADAMS, cognitive tests from the HRS, and diagnosis codes from Medicare claims records. We improved upon prior comparisons of survey-based and claims-based prevalence measures with methods that require an individual to have more than one dementia ascertainment over time to reduce measurement error in dementia estimates. Additionally, we analyzed levels in 2004 and time trends in prevalence rates previously measured, from 2006 to 2013, and separately for whites, blacks, and Hispanics, men and women, and persons of different ages. Quantifying the differences in estimated prevalence across measures and sources over time improved our understanding of strengths and weaknesses in using Medicare claims and survey-based cognitive tests for tracking dementia trends over time and in different racial and ethnic populations.
Method
Data and Study Population
We used data from the HRS, the ADAMS, and a 20% random sample of Medicare beneficiaries and their Medicare Parts A and B health care claims. We compared the prevalence of dementia at a point in time that is common across all three data sources, 2004, and for respondents aged 70 and older. We then analyzed trends in dementia prevalence for the subsequent years, 2006–2013 for the two longitudinal data sources (HRS and Medicare claims) for respondents aged 67 and older. Internal review board approval was granted by the University of Southern California.
Health and Retirement Study
The HRS is a biennial, nationally representative longitudinal study of adults aged 51 years and older. Since the first study year, 1992, it has included oversamples of African Americans and Hispanics. HRS collects data on a wide range of topics including cognition, health, family, employment, income, and wealth. We selected respondents from the 2004 wave of the study, aged 70 years and older (7,768 persons). Also, we selected respondents aged 67 and older from survey waves 2006, 2008, 2010, and 2012 (13,922 persons and 40,234 person-waves). For analyses, respondents were both community-dwelling and in nursing homes. Participants were compensated about $80 for their participation, and verbal informed consent was obtained from all respondents. Ethics approval was from the Health Sciences and Behavioral Sciences institutional review board at the University of Michigan. The average participation rate across waves among nondeceased eligible individuals in the HRS 1992–2014 sample is 86% at the population level and 87%, 87%, and 85% among white, black, and Hispanic nondeceased eligible persons (unweighted; numbers are calculated by authors), respectively.
Aging, Demographics, and Memory Study
A random subsample of 1,770 individuals, aged 70 and older, stratified based on cognitive performance, age, and sex, were selected from HRS 2000 and 2002 interview waves for participation in ADAMS. Assessments were completed for 856 individuals between August 2001 and December 2003 with follow-up through March 2005 (Heeringa et al., 2009). We used information from the initial wave and first follow-up wave (approximately 1.5 years later) that included reassessments of the initial diagnosis that were ambiguous or for persons with cognitive impairment without dementia (Plassman et al., 2011). When weighted, the sample is representative of the aged 70 and older population over the 2-year data collection window. Consent was obtained from respondents and approved by the University of Michigan institutional review board. The participation rate in ADAMS wave A (2001–2003) among nondeceased and eligible persons at the population level is 56% (Heeringa et al., 2009). The participation rate among nondeceased and eligible whites is lower than that among nondeceased and eligible African Americans and Hispanics (54%, 61%, and 60% among whites, African Americans, and Hispanics, respectively; unweighted). We selected all respondents with completed neuropsychological assessments (856 persons).
Medicare claims
We used Medicare Parts A (hospital stays) and B (outpatient) claims data from a 20% random sample of Medicare beneficiaries enrolled in fee-for-service (FFS) in the years 2004–2013. Board of the University of Southern California granted a waiver of participant consent. We selected beneficiaries aged 70 and older in 2004 (3,649,190 persons) and beneficiaries aged 67 and older in years 2006–2013 (6,603,477 persons and 32,886,153 person-years). All selected Medicare beneficiaries were continuously enrolled in Medicare FFS for at least 3 years. The 3-year continuous enrollment requirement excludes 15.6% (1,362,927 persons) of all Medicare beneficiaries enrolled in FFS for at least 12 months from 2004 to 2013 at age 65 and older.
Measurement of Dementia in ADAMS, HRS, and Medicare Claims Data Sources
Dementia measured using cognitive tests (HRS)
HRS assessed cognitive functions through an adapted version of the Telephone Interview for Cognitive Status (TICS). TICS was modeled after the Mini-Mental State Examination (MMSE) which has been extensively used in neuropsychological assessment of cognition (Brandt et al., 1988; Folstein et al., 1975). Spanish versions were developed for each questionnaire and were administered by bilingual interviewers to Spanish-speaking respondents (http://hrsonline.isr.umich.edu/sitedocs/surveydesign.pdf). Imputation for item nonresponse was performed and described in the study of Fisher et al., 2017. We followed prior studies on the classification of dementia, which is based on the concordance of HRS cognitive functioning scores and consensus diagnosis of dementia in a subset of HRS respondents who had an extensive neuropsychological assessment in ADAMS (Crimmins et al., 2011; Langa et al., 2017; Zissimopoulos et al., 2014). Scores on several questions that measured cognition determined dementia ascertainment. Specifically, we assigned cognitive state based on scores from three cognitive assessments for self-respondents—immediate and delayed word recall (scale 0–10 for each test), counting down from 100 by 7’s (scale 0–5), and counting back from 20 (scale 0–2). Those with a total cognitive score (scale 0–27) were categorized into three groups based on their cognitive status: dementia (score 0–6), cognitively impaired no dementia (CIND; score 7–11), and cognitively normal (score 12–27). Cognitive status for respondents who had a proxy respondent was determined by summing the following: number (0–5) of limitations with instrumental activities of daily living; interviewer’s rating of the respondent’s difficulty finishing the interview due to cognitive limitations (0 = no cognitive limitations, 1 = some limitations, and 2 = cognitive limitations); and proxy informant’s rating of the respondent’s memory (from 0 = excellent to 4 = poor). Individuals with proxy scores were also classified into three groups: dementia (score 6–11), CIND (score 3–5), and cognitively normal (score 0–2). Some respondents in HRS changed dementia status over time, that is, transitioned into or out of dementia. For dementia ascertainment in HRS, we required an individual to be classified as having dementia in one wave and classified as having dementia or CIND in the subsequent wave. Those with dementia at one wave who died before the next wave were assumed to have dementia. Once an individual was classified as having dementia, we assumed they had dementia thereafter. Zissimopoulos et al. (2018) reported that the difference between dementia prevalence based on a single assessment is 5 percentage points higher than based on this two-wave assessment (21% compared to 16%) at age 85. A similar two-wave assessment was used and validated in another recent study using NHATS data (Freedman et al., 2018). Although the measurement of dementia status in HRS is based on the concordance with neuropsychological assessment in ADAMS, dementia prevalence may differ across samples due to differences in sample characteristics and ascertainment of dementia in the two studies.
Dementia measured using neuropsychological assessment (ADAMS)
Diagnosis of dementia in ADAMS was based on neuropsychological assessments structured in a 3- to 4-h in-home interview, which was conducted by a neuropsychology technician and a nurse. The final diagnosis was established by consensus conferences consisting of neuropsychologists, a cognitive neuroscientist, neurologists, neuropsychiatrists, and internists. Several cognitive tests were conducted including the MMSE, Boston naming test, digit span, Symbol Digit Modality Test, animal fluency, word list three trial learning, construction praxis copying, Trail Making Test, Wechsler Memory Scale, Fuld Object Memory Test, Shipley vocabulary test, and the WRAT 3 blue reading test. Proxy reports were based on the Blessed Dementia Ratings, including, for example, questions about the ability to accomplish household tasks, manage small amounts of money, and remembering a short list (Blessed et al., 1968; Crimmins et al., 2011; Langa et al., 2005).
Dementia measured using diagnosis codes (Medicare Claims)
Providers that bill Medicare use codes for patient diagnoses, and more than one diagnosis code is allowed. In Medicare claims, we ascertained dementia based on the Chronic Conditions Data Warehouse (CCW) algorithm for AD or related disorders or senile dementia using the following International Classification of Disease, ninth revision diagnosis codes: 331.0, 331.11, 331.19, 331.2, 331.7, 290.0, 290.10, 290.11, 290.12, 290.13, 290.20, 290.21, 290.3, 290.40, 290.41, 290.42, 290.43, 294.0, 294.10, 294.11, 294.20, 294.21, 294.8, and 797. We added additional diagnostic codes for dementia with Lewy bodies, cerebral degeneration, senile psychosis, and dementia classified elsewhere: 331.82, 331.89, 331.9, 290.8, 290.9, and 294.9. The CCW algorithm requires at least one inpatient, facility, home health, or outpatient billed claim with one of the dementia diagnosis codes during 3 years. For example, a dementia diagnosis code in 2004 uses claims data from 2002, 2003, and 2004. We additionally required a second diagnosis claim over the study period to reduce measurement error from false positives (verified dementia). Beneficiaries who died within 2 years after the first diagnosis were assumed to have dementia. We compared dementia prevalence based on this verification to a different method requiring no second claim for dementia diagnosis (unverified dementia). Dementia prevalence was 14.1% based on verified ascertainment and 15.3% based on unverified in 2013.
Demographic Variables
We grouped respondents into six age groups (67–69, 70–74, 75–79, 80–84, 85–89, and 90 and older) and four racial/ethnic groups (non-Hispanic white, non-Hispanic black, Hispanic, and other races). We included the distribution of education in HRS/ADAMS, and we divided the sample into three education levels (less than high school, high school, and some college and above). Race was self-reported in HRS/ADAMS and claims data. Race/ethnicity was determined with the beneficiary race code in the Center for Medicare & Medicaid Services (CMS) enrollment data using the application of a name-based identification algorithm from the Research Triangle Institute to improve accuracy (Eicheldinger & Bonito, 2008).
Analysis
We compared dementia prevalence based on neuropsychological assessment, cognitive tests, and diagnosis codes from Medicare claims records by race, gender, and age in 2004—a point in time that was comparable across all three data sources. We estimated trends over time in dementia prevalence using logistic regression adjusting for age, sex, and race and separately for HRS and Medicare claims data (Equation 1). We chose the years 2006 to 2013 to study trends in dementia prevalence.
Equation 1:
| (1) |
The same model is estimated using data from the HRS with the exception that year indicators are 2008, 2010, and 2012, reflecting the biennial nature of the HRS. We computed the predicted values of dementia prevalence by age, sex, race, and year. For example, we estimated predicted values of dementia prevalence rates among whites in 2012 with age and sex measured at their mean values among whites in 2012. We additionally used direct standardization to standardize the 2012 sample based on the age-, sex-, and race distribution of the 2006 sample. We compared standardized and nonstandardized prevalence rates in 2012. We tested for differences in time trends across data sources and measures by pooling the HRS and Medicare claims data, re-estimating the model including an indicator variable for HRS as the data source and interacting this indicator with the race, sex, age, and year covariates.
Results
Dementia Prevalence in 2004 Based on Neuropsychological Assessment, Cognitive Tests, and Diagnosis Codes
Table 1 displays the demographic characteristics (race, sex, age group, and education) of persons aged 70 and older in the three data sources: ADAMS, HRS, and Medicare claims in 2004. The racial/ethnic population distribution in ADAMS was blacks 7.6%, Hispanics 5.3%, and whites 87.1%; in HRS: blacks 8.0%, Hispanics 5.4%, whites 84.8%, and other race 1.8%; and in Medicare claims: blacks 7.0%, Hispanics 4.3%, whites 86.3%, and other race 2.3%. Individuals in the youngest age group, 70 to 74, and in the oldest, 90 and older, respectively, were 26.9% and 6.7% of ADAMS respondents, 33.6% and 5.3% of HRS respondents, and 32.6% and 5.4% of the sample of Medicare beneficiaries.
Table 1.
Sample Characteristics From ADAMS, HRS, and Medicare Claims Data Sources, Aged 70 and Older, 2004
| ADAMS (2001–2005) | HRS 2004 | Claims 2004 | |
|---|---|---|---|
| Race | |||
| White | 87.1% (746) | 84.8% (6,589) | 86.3% (31,499,03) |
| Black | 7.6% (65) | 8.0% (618) | 7.0% (256,446) |
| Hispanic | 5.3% (45) | 5.4% (421) | 4.3% (158,082) |
| Other race | N/A | 1.8% (139) | 2.3% (84,759) |
| Gender | |||
| Male | 39.3% (336) | 40.2% (3,125) | 39.1% (1,427,157) |
| Female | 60.7% (520) | 59.8% (4,643) | 60.9% (2,222,033) |
| Age group (years) | |||
| 70–74 | 26.9% (230) | 33.6% (2,608) | 32.6% (1,188,062) |
| 75–79 | 31.5% (269) | 28.6% (2,220) | 29.4% (1,072,628) |
| 80–84 | 22.5% (193) | 21.1% (1,635) | 21.3% (776,827) |
| 85–89 | 12.4% (106) | 11.5% (896) | 11.3% (413,713) |
| 90 and older | 6.7% (58) | 5.3% (408) | 5.4% (197,960) |
| Education | |||
| Less than high school | 34.8% (299) | 32.3% (2,491) | N/A |
| High school | 28.1% (240) | 33.1% (2,574) | N/A |
| College | 37.1% (317) | 34.7% (2,704) | N/A |
| Total | 856 | 7,768 | 3,649,190 |
Notes: ADAMS = the Aging, Demographics, and Memory Study; HRS = Health and Retirement Study; Claims = Medicare claims. Values in ADAMS are weighted by the ADAMS sampling weights. Values in HRS are weighted by the HRS sampling weights.
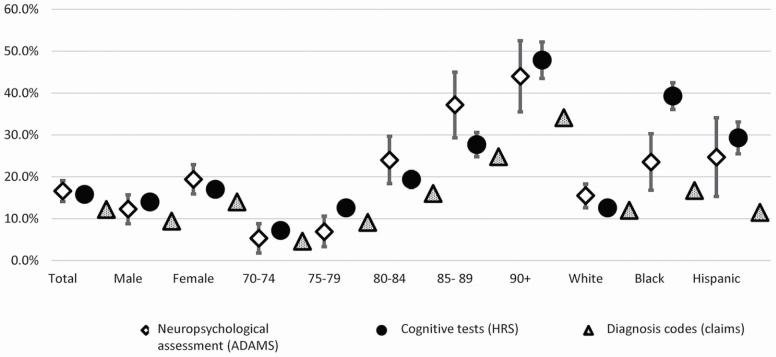
Figure 1 (and Supplementary Table 1) shows dementia prevalence based on neuropsychological assessment, cognitive tests, and diagnostic codes on claims by sex, age, and race. Dementia prevalence in 2004 was 16.6% (neuropsychological assessment), 15.8% (cognitive tests), and 12.2% (diagnosis codes). Dementia prevalence for both men and women was higher based on neuropsychological assessment (men, 12.3%; women, 19.4%) and cognitive tests (men, 14.0%; women,17.0%) than based on diagnosis codes (men, 9.4%; women, 14.0%). Percentage point differences between neuropsychological assessment and diagnosis codes were higher among women (5.4 percentage point difference) than among men (2.9 percentage point difference).
Figure 1.
Dementia prevalence for the U.S. population and by race, gender, and age in neuropsychological assessment (ADAMS), cognitive tests (HRS), and diagnosis codes (Medicare claims), aged 70 and older, 2004 with 95% confidence intervals. ADAMS = the Aging, Demographics, and Memory Study; HRS = Health and Retirement Study; Claims = Medicare claims. Values in ADAMS are weighted by the ADAMS sampling weights. Values in HRS are weighted by the HRS sampling weights.
Differences across ascertainment methods and data sources were particularly pronounced for the oldest age group, 90 and older, with rates of 44.0% based on neuropsychological assessment, 47.9% based on cognitive tests, and 34.1% based on diagnosis codes (Figure 1; Supplementary Table 1). There were substantial differences across measures in dementia prevalence among ethnic minorities. Dementia prevalence for blacks based on neuropsychological assessment, cognitive test, and diagnosis codes was 23.5% (95% confidence interval [CI], 16.8%–30.1%), 39.3% (95% CI, 36.1%–42.5%), and 16.7% (95% CI, 16.6%–16.8%), respectively. Dementia prevalence among Hispanics was 24.7% (95% CI, 15.3%–34.1%), 29.3% (95% CI, 25.5%–33.1%), and 11.5% (95% CI, 11.4%–11.7%), respectively.
Dementia Prevalence Based on Cognitive Tests and Diagnosis Codes Over Time
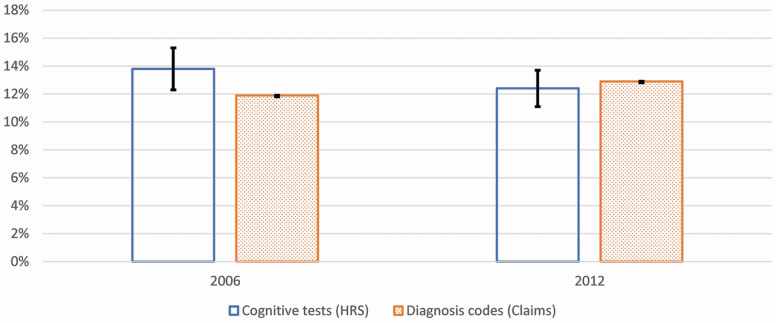
Figure 2 shows the predicted values of dementia prevalence at the population level in 2006 and 2012 from cognitive tests and diagnosis codes. We used estimates obtained from logistic regressions adjusting for age, race, and sex to compute predicted values of dementia prevalence (Table 2 reports the odds ratio estimates). In 2006, the prevalence was higher based on cognitive tests than that based on diagnosis codes. In 2012, however, there was no such difference in prevalence rates. Prevalence based on cognitive tests was 13.8% (95% CI, 12.3%–15.5%) in 2006 and, statistically significantly lower, 12.4% (95% CI, 11.0%–14.0%) in 2012 (Table 2). Dementia prevalence in 2008 and 2010 was not statistically significantly different from that in 2006 (Table 2). Dementia prevalence based on diagnosis codes increased from 2006 to 2012 (diagnosis codes in 2006, 11.9%; 95% CI, 11.9%–12.0% and in 2012, 12.9%; 95% CI, 12.9%–13.0%). Time trends across data sources were statistically different (Supplementary Table 5, Model 5). Standardizing the composition of the population in 2012 based on the age, sex, and race/ethnicity of the population in 2006 did not result in substantively different rates of dementia prevalence in 2012 (12.3% and 12.7% based on cognitive test and diagnosis codes in claims, respectively; Supplementary Table 4).
Figure 2.
Predicted values of dementia prevalence based on cognitive tests (HRS) and diagnosis codes (claims) from logistic models adjusting for race, sex, age group, and wave in HRS and claims, aged 67 and older, 2006 and 2012. HRS = Health and Retirement Study; Claims = Medicare claims; predicted values of dementia prevalence in HRS are weighted by the HRS sampling weights; 95% confidence intervals (CIs) are included in the figure and 95% CI adjusted by Bonferroni correction.
Table 2.
Odds Ratios for Presence of Dementia Based on Cognitive Tests (HRS) and Diagnosis Codes (Medicare Claims), Aged 67 and Older, 2006–2013
| (1) | (2) | |||
|---|---|---|---|---|
| Presence of dementia | Cognitive tests (HRS) | Diagnosis codes (Claims) | ||
| Odds ratio | 95% CI | Odds ratio | 95% CI | |
| Male | 1 | 1–1 | 1 | 1–1 |
| Female | 1.05 | 0.95–1.13 | 1.28* | 1.28–1.28 |
| White | 1 | 1–1 | 1 | 1–1 |
| Black | 4.91* | 4.41–5.42 | 1.68* | 1.67–1.69 |
| Hispanic | 4.22* | 3.69–4.79 | 1.46* | 1.45–1.47 |
| Other races | 1.89* | 1.52–2.33 | 0.98* | 0.97–0.99 |
| 67–69 | 1 | 1–1 | 1 | 1–1 |
| 70–74 | 1.44* | 1.22–1.71 | 1.88* | 1.87–1.9 |
| 75–79 | 2.43* | 2.04–2.85 | 3.91* | 3.89–3.94 |
| 80–84 | 4.36* | 3.66–5.14 | 7.51* | 7.46–7.57 |
| 85–89 | 8.86* | 7.42–10.51 | 13.09* | 12.99–13.19 |
| 90 and older | 17.22* | 14.19–20.71 | 21.91* | 21.74–22.08 |
| 2006 | 1 | 1–1 | 1 | 1–1 |
| 2007 | 1.05* | 1.04–1.05 | ||
| 2008 | 0.95 | 0.84–1.06 | 1.08* | 1.08–1.09 |
| 2009 | 1.11* | 1.11–1.12 | ||
| 2010 | 0.97 | 0.86–1.1 | 1.12* | 1.12–1.12 |
| 2011 | 1.10* | 1.1–1.11 | ||
| 2012 | 0.85* | 0.75–0.96 | 1.083* | 1.08–1.09 |
| 2013 | 1.02* | 1.01–1.02 | ||
| Constant | 0.04* | 0.03–0.04 | 0.0232* | 0.02–0.02 |
| Observations | 40,224 | 32,855,743 | ||
| Pseudo R2 | 0.146 | 0.1194 | ||
Notes: CI = confidence interval; HRS = Health and Retirement Study; Claims = Medicare claims; samples restricted to age 67 and older. 95% CI adjusted by Bonferroni correction.
*p < .001.
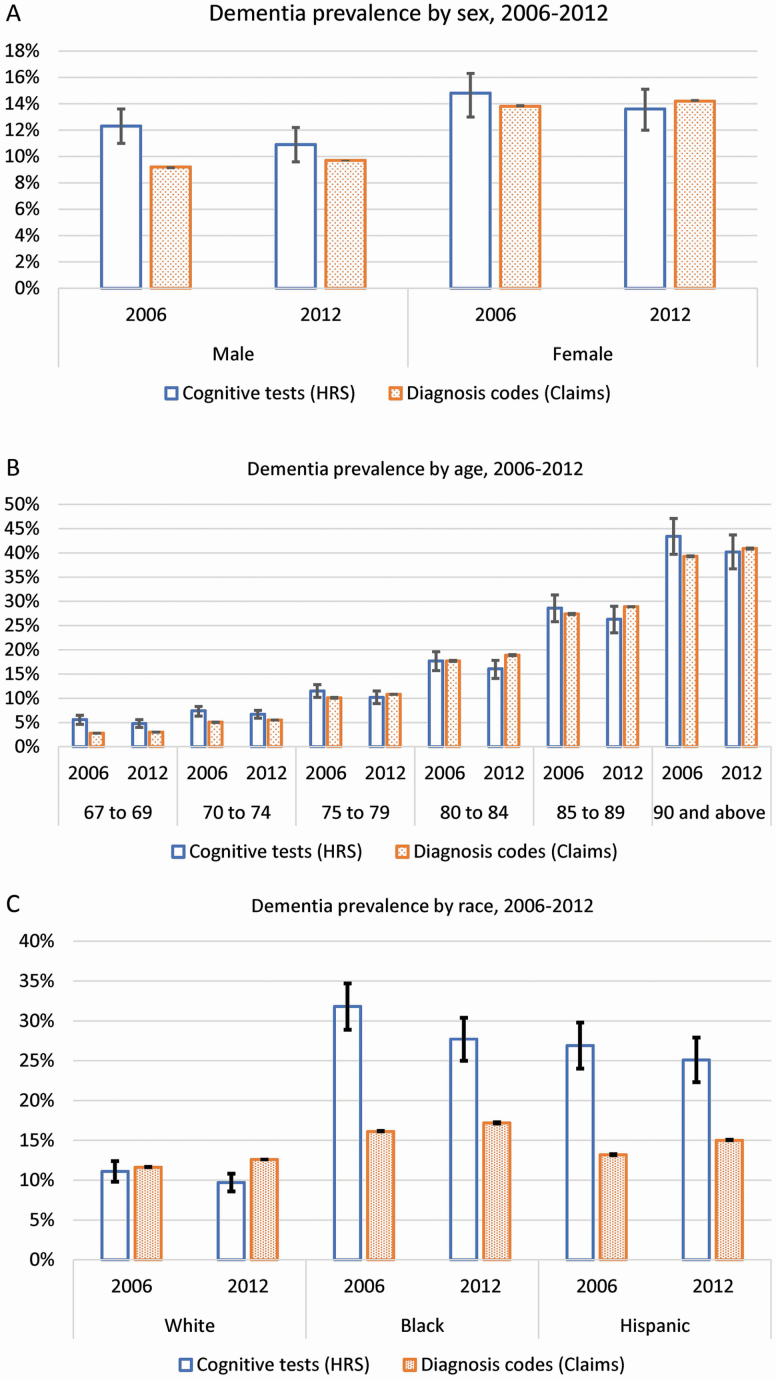
Figure 3 shows estimates of dementia prevalence based on cognitive tests and diagnosis codes separately by sex (Figure 3A), age (Figure 3B), and race/ethnicity (Figure 3C) in 2006 and 2012 from logistic regressions (estimates for all years in Supplementary Table 2). There was a 3.1 percentage point difference in dementia prevalence based on cognitive tests and diagnosis codes among men in 2006 that reduced to 1.2 percentage points by 2012 (cognitive tests, 10.9%; 95% CI, 9.6%–12.2% and diagnosis codes, 9.7%; 95% CI, 9.7%–9.7%). Among women, the difference across measures in 2006 was 1 percentage point and 0.6 in 2012 (cognitive tests, 13.6%; 95% CI, 12.1%–15.2% and diagnosis codes,14.2%; 95% CI, 14.1%–14.2%; Figure 3A and Supplementary Table 2 report predicted values of dementia prevalence by sex).
Figure 3.
Dementia prevalence by sex (A), age (B), and race (C) based on cognitive tests (HRS) and diagnosis codes (claims) from logistic models adjusting for race, sex, age group, and wave in HRS and claims, aged 67 and older, 2006 and 2012. (A) Dementia prevalence by sex, 2006 and 2012; (B) dementia prevalence by age, 2006 and 2012; and (C) dementia prevalence by race, 2006 and 2012. HRS = Health and Retirement Study; Claims = Medicare claims; predicted values of dementia prevalence in HRS are weighted by the HRS sampling weights; 95% confidence intervals (CIs) are included in the figure and 95% CI adjusted by Bonferroni correction.
The percentage difference in dementia prevalence across measures was most pronounced at age 67–74 compared to age 75 and older in 2006 and 2012. In 2012, dementia prevalence based on cognitive tests was statistically significantly higher than that based on diagnosis codes among persons aged 67–69 (cognitive tests, 4.8%; 95% CI, 4.0%–5.6% and diagnosis codes, 3.0%; 95% CI, 3.0%–3.0%) and aged 70–74 (cognitive tests, 6.7%; 95% CI, 5.9%–7.5% and diagnosis codes, 5.5%; 95% CI, 5.5%–5.5%). At the oldest age, persons 90 and older, the 4.1 percentage point difference between these two measures in 2006 (cognitive tests, 43.4%; 95% CI, 39.7%–47.1% and diagnosis codes, 39.3%; 95% CI, 39.2%–39.4%) reduced to 0.7 percentage points in 2012 (cognitive tests, 40.2%; 95% CI, 36.7%–43.7% and diagnosis codes, 40.9%; 95% CI, 40.8%–41.0%) and was not statistically different (Figure 3B; Supplementary Table 2).
There was a large difference in dementia prevalence from the two sources based on cognitive tests and based on diagnosis codes among blacks and Hispanics, which decreased over time. In 2006 the difference between the two measures was 15.1 percentage points among blacks (cognitive tests, 31.2%; 95% CI, 28.3%–34.1% and diagnosis codes, 16.1%; 95% CI, 16.0%–16.1%) and 14.0 percentage points among Hispanics (cognitive tests, 27.2%; 95% CI, 24.3%–30.5% and diagnosis codes, 13.2%; 95% CI, 13.1%–13.3%). The gap across measures was smaller in 2012: 10.9 percentage points among blacks (cognitive tests, 28.1%; 95% CI, 25.4%–31.1% and diagnosis codes, 17.2%; 95% CI, 17.1%–17.3%) and 9.8 percentage points among Hispanics (cognitive tests, 24.8%; 95% CI, 22%–27.6% and diagnosis codes, 15.0%; 95% CI, 14.9%–15%; Figure 3C and Supplementary Table 2).
Discussion
We analyzed the prevalence of dementia in samples broadly representative of the U.S. population and by sex, age, and race/ethnicity for years from 2004 through 2013 using three different measurement approaches and data sources: neuropsychological assessment (ADAMS), cognitive tests (HRS), and diagnosis codes (Medicare claims). We found dementia prevalence for age 70 and older in 2004 was highest based on neuropsychological assessment and lowest based on diagnosis codes from claims (16.6% [neuropsychological assessment], 15.8% [cognitive tests], and 12.2% [diagnosis codes]). Dementia prevalence based on cognitive tests was lower in 2012 (12.4%) compared to 2006 (13.8%), reflecting a decline from 2010 to 2012 and no difference in prevalence in years 2006, 2008, and 2010. This decline was also consistent with that reported in the studies of Langa et al. (2017) and Freedman et al. (2018). In contrast, dementia prevalence based on diagnosis codes rose over time (2006, 11.9%; 2012, 12.9%). Changes in the age, sex, and racial/ethnic composition of the population did not explain the time trends.
Subgroup analysis of the three measures in 2004 and for cognitive tests and diagnosis codes in 2006–2012 revealed several consistencies across all measures: dementia prevalence was higher for women than men, increased with age, and was higher for blacks and Hispanics than whites.
Over time, dementia prevalence for all age groups, for men and women, and for whites, blacks, and Hispanics declined based on cognitive tests and increased based on diagnosis codes from claims, thereby reducing the difference across measures for these subpopulations over time. The notable difference in dementia prevalence based on cognitive tests and diagnosis codes for both men and women in 2006 was not statistically different for either sex by 2012. Similarly, the differences between these measures of prevalence among those aged 90 and older vanished over time. Notably, dementia prevalence based on both cognitive tests and diagnosis codes among people aged 90 and older in 2012 was 40.2% and 40.9%, respectively. This estimate combined with an increase in the proportion of older Americans aged 90 and older from 5.2% of those aged 65 and older to 9.9% by 2050 (Vincent & Velkoff, 2010) will drive the growth in number of Americans with dementia and in burden of dementia in the future. Across racial/ethnic populations, there were substantial differences in dementia prevalence across measures and data sources and these differences reduced, but did not vanish over time. Cognitive test-based prevalence was the highest and diagnosis code-based prevalence was the lowest among ethnic minorities. Our results highlight the strengths and weaknesses of the different data sources and different measures for quantifying dementia prevalence for the U.S. population and for different subpopulations. Medicare claims data are available for a large population of older Americans and as such have large samples of ethnic minorities. There are no other data sources with such significant numbers of minorities from across the regions of the United States. Doctors may take into account many factors such as cardiovascular health, education level, and including but not limited to cognition in determining a dementia diagnosis. Diagnosing dementia in racial and ethnic populations may be improving over time. Although we do not analyze what is driving these changes, factors may include changing social and cultural norms about the stigma associated with dementia and/or improvement in awareness of dementia by both physicians and patients (Amjad et al., 2018; Chodosh et al., 2004). However, there are two primary limitations. The sample only includes older Americans enrolled in Medicare FFS and not those enrolled in Medicare Advantage (MA). The portion of beneficiaries enrolled in MA has been increasing over time and these populations may have different rates of dementia. Second, despite potential improvements in identifying dementia in racial/ethnic minority populations, dementia may still be underdiagnosed (Chen et al., 2019; Gianattasio et al., 2019). Increases in diagnosis code measures of dementia prevalence over time among racial/ethnic minorities that are explained by changing norms and awareness may obscure information about changes in dementia risk.
The HRS sample includes older Americans enrolled in either FFS or MA and when weighted, is nationally representative. The cognitive test-based measures of dementia in HRS were validated by neuropsychological exams and are correlated with well-known risk factors of dementia including low education and cardiovascular risk factors (Chen & Zissimopoulos, 2018; Langa et al., 2017). Thus, the declining trend in dementia over time may provide the signal of declines in dementia risk in the U.S. population. However, these data and measures also have limitations for measuring the prevalence of dementia in the population and among racial/ethnic subpopulations. Several studies have found that a cognitive test-based measurement approach had lower specificity among low-educated persons and nonnative English speakers (Crum et al., 1993; Ganguli et al., 2010; Gianattasio et al., 2019; Spering et al., 2012) and algorithmic approaches that do not adjust for educational attainment will overestimate dementia rates among racial/ethnic minorities (Gianattasio et al., 2019). Indeed, dementia prevalence among blacks in 2012 from NHATS, and based on criteria that included but were not limited to cognitive tests, founds rates (15%) more similar to those based on diagnosis in Medicare claims than cognitive tests from HRS data (Freedman et al., 2018; Kasper et al., 2013).
Our study has limitations. We require 3 years of continuous enrollment in FFS, standard practice for measuring disease conditions based on the Chronic Conditions Warehouse algorithm. This may underestimate diagnosis codes-based prevalence by excluding individuals who die within 3 years. This requirement will affect older beneficiaries more than younger ones and, in particular, may narrow differences in prevalence rates across measures among persons aged 80–84 (Supplementary Table 2). Medicare claims data exclude beneficiaries enrolled in MA plans. Consistent with other studies (St. Clair et al., 2017), respondents enrolled in MA plans are younger, more likely to be ethnic minorities, male, and less educated than those in FFS plans (Supplementary Table 3). We used data from the HRS that included both MA and FFS enrollees and found only small differences in dementia prevalence in 2012 among MA beneficiaries (9.7%) and FFS beneficiaries (10.5%).
Attributing differences (or concordance) in dementia prevalence to how dementia is measured in analyses of cognitive tests and neuropsychological assessment in 2004 was complicated by potential differences in sample composition and also by small sample sizes in subpopulations that led to imprecise estimates. ADAMS is a stratified (by age, sex, and cognition) random sample of HRS self- and proxy respondents aged 70 and older who contributed data to wave 2000 or 2002. Thus, cohorts are not totally independent, yet there were compositional differences in sample characteristics (Table 1). In supplementary analyses (results not shown), we used linked HRS and ADAMS data to exclude variation in sample composition and found gaps in dementia prevalence in racial/ethnic subpopulations and among persons aged 70–89 were due to dementia measurement. In analyses of concordance between cognitive tests and diagnosis code measures, we distinguished between sample composition and dementia measurement as drivers of differences in prevalence rates from 2006 to 2012. We found that differential changes in the age, sex, and race composition of the HRS and Medicare sample populations did not explain the reduction in differences in dementia prevalence rates between the two measures (Supplementary Table 4). The improvement in diagnostic practices and/or awareness is a likely driver of the increase in prevalence based on diagnosis codes and the convergence in dementia prevalence across data sources. Although this convergence across measures is a positive sign for measuring population dementia prevalence, limitations remain for measuring dementia prevalence among racial/ethnic populations. In these subpopulations, dementia is likely underestimated based on diagnosis codes and likely overestimated based on cognitive tests. The level of under- and overestimation is unknown.
More recent longitudinal data sources, such as NHATS, with broadly population-representative samples and several different measures for ascertaining dementia may add to our understanding of levels and time trends in dementia prevalence (Freedman et al., 2018; Kasper et al., 2013). New data collection efforts may improve the measurement of dementia in the U.S. population and in subpopulations at a point in time and over time. The HRS Harmonized Cognitive Assessment Protocol collected data on a sample of HRS respondents in 2016 and is proposed for follow-up in 2020 (Weir et al., 2016). The study included an in-home 1-h battery of cognitive tests and an informant interview. There are new opportunities for clinicians to better detect and diagnose dementia. The Annual Wellness Visit, introduced in 2011, requires cognitive screening among Medicare FFS beneficiaries and may improve the detection of dementia among all Medicare beneficiaries (Chodosh et al., 2018; Ganguli et al., 2017). Another opportunity is the collaboration of community health workers, consisting of paraprofessionals who work in communities, share health care information and resources among the minorities, and advocate dementia detection (Chodosh et al., 2018). Considering this potential increasing detection of dementia among blacks and Hispanics, continued tracking of these trends in diverse populations, and using different methods of ascertainment will aid in understanding how risk may be changing over time as well as the extent to which high-risk populations are receiving diagnoses. Studies analyzing individual-level agreement in dementia ascertainment based on cognitive tests and diagnosis codes will provide additional insight into changes in risk of dementia over time for different racial/ethnic groups, aid in targeting resources to populations who are underdiagnosed, and assist health care providers and policy makers prepare for future dementia burden in the United States.
Funding
This work was supported by the National Institute on Aging (R01AG055401, R03AG054120, P30AG024968, P30 AG 017265, and P30 AG043073).
Supplementary Material
Acknowledgments
We thank Dr. Geoffrey Joyce, Bryan Tysinger, Johanna Thunell, and Patricia Ferido for reviewing and providing comments on data analysis.
Author Contributions
J. Zissimopoulos conceptualized the study, planned the study, supervised data analysis, organized funding for the research, and contributed to writing and revising the manuscript. Y. Zhu helped plan the study, conducted data analysis, and wrote and revised the manuscript. Y. Chen helped plan the study, conducted data analysis, and contributed to writing and revising the manuscript. E. Crimmins contributed to writing and revising the manuscript.
Conflict of Interest
None declared.
References
- Amjad, H., Roth, D. L., Sheehan, O. C., Lyketsos, C. G., Wolff, J. L., & Samus, Q. M (2018). Underdiagnosis of dementia: An observational study of patterns in diagnosis and awareness in US older adults. Journal of General Internal Medicine, 33(7), 1131–1138. doi: 10.1007/s11606-018-4377-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessed, G., Tomlinson, B. E., & Roth, M (1968). The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. The British Journal of Psychiatry, 114(512), 797–811. doi: 10.1192/bjp.114.512.797 [DOI] [PubMed] [Google Scholar]
- Bradford, A., Kunik, M. E., Schulz, P., Williams, S. P., & Singh, H (2009). Missed and delayed diagnosis of dementia in primary care: Prevalence and contributing factors. Alzheimer Disease and Associated Disorders, 23(4), 306–314. doi: 10.1097/WAD.0b013e3181a6bebc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt, J., Spencer, M., & Folstein, M (1988). The telephone interview for cognitive status. Neuropsychiatry, Neuropsychology and Behavioral Neurology, 1(2), 111–117. https://journals.lww.com/cogbehavneurol/Abstract/1988/00120/The_Telephone_Interview_for_Cognitive_Status.4.aspx [Google Scholar]
- Brookmeyer, R., Evans, D. A., Hebert, L., Langa, K. M., Heeringa, S. G., Plassman, B. L., & Kukull, W. A (2011). National estimates of the prevalence of Alzheimer’s disease in the United States. Alzheimer’s & Dementia, 7(1), 61–73. doi: 10.1016/j.jalz.2010.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y., Tysinger, B., Crimmins, E., & Zissimopoulos, J. M (2019). Analysis of dementia in the US population using Medicare claims: Insights from linked survey and administrative claims data. Alzheimer’s & Dementia (New York, N. Y.), 5, 197–207. doi: 10.1016/j.trci.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C., & Zissimopoulos, J. M (2018). Racial and ethnic differences in trends in dementia prevalence and risk factors in the United States. Alzheimer’s & Dementia (New York, N. Y.), 4, 510–520. doi: 10.1016/j.trci.2018.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodosh, J., Petitti, D. B., Elliott, M., Hays, R. D., Crooks, V. C., Reuben, D. B., Buckwalter, J. G., & Wenger, N (2004). Physician recognition of cognitive impairment: Evaluating the need for improvement. Journal of the American Geriatrics Society, 52(7), 1051–1059. doi: 10.1111/j.1532-5415.2004.52301.x [DOI] [PubMed] [Google Scholar]
- Chodosh, J., Thorpe, L. E., & Trinh-Shevrin, C (2018). Changing faces of cognitive impairment in the U.S.: Detection strategies for underserved communities. American Journal of Preventive Medicine, 54(6), 842–844. doi: 10.1016/j.amepre.2018.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins, E. M., Kim, J. K., Langa, K. M., & Weir, D. R (2011). Assessment of cognition using surveys and neuropsychological assessment: The Health and Retirement Study and the Aging, Demographics, and Memory Study. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 66(Suppl. 1), 162–171. doi: 10.1093/geronb/gbr048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins, E. M., Saito, Y., Kim, J. K., Zhang, Y. S., Sasson, I., & Hayward, M. D (2018). Educational differences in the prevalence of dementia and life expectancy with dementia: Changes from 2000 to 2010. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 73(Suppl. 1), 20–28. doi: 10.1093/geronb/gbx135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum, R. M., Anthony, J. C., Bassett, S. S., & Folstein, M. F (1993). Population-based norms for the Mini-Mental State Examination by age and educational level. Journal of the American Medical Association, 269(18), 2386–2391. doi: 10.1001/jama.1993.03500180078038 [DOI] [PubMed] [Google Scholar]
- Demirovic, J., Prineas, R., Loewenstein, D., Bean, J., Duara, R., Sevush, S., & Szapocznik, J (2003). Prevalence of dementia in three ethnic groups: The South Florida program on aging and health. Annals of Epidemiology, 13(6), 472–478. doi: 10.1016/s1047-2797(02)00437-4 [DOI] [PubMed] [Google Scholar]
- Eicheldinger, C., & Bonito, A (2008). More accurate racial and ethnic codes for Medicare administrative data. Health Care Financing Review, 29(3), 27–42. https://www.cms.gov/Research-Statistics-Data-and-Systems/Research/HealthCareFinancingReview/Downloads/08Springpg27.pdf [PMC free article] [PubMed] [Google Scholar]
- Fisher, G. G., Hassan, H., Faul, J. D., Rodgers, W. L., & Weir, D. R (2017). Health and Retirement Study imputation of cognitive functioning measures: 1992–2014 (final release version) http://hrsonline.isr.umich.edu/modules/meta/xyear/cogimp/desc/COGIMPdd.pdf
- Folstein, M. F., Folstein, S. E., & McHugh, P. R (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Freedman, V. A., Kasper, J. D., Spillman, B. C., & Plassman, B. L (2018). Short-term changes in the prevalence of probable dementia: An analysis of the 2011–2015 National Health and Aging Trends Study. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 73, 48–56. doi: 10.1093/geronb/gbx144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli, M., Snitz, B. E., Lee, C. W., Vanderbilt, J., Saxton, J. A., & Chang, C. C (2010). Age and education effects and norms on a cognitive test battery from a population-based cohort: The Monongahela-Youghiogheny Healthy Aging Team. Aging & Mental Health, 14(1), 100–107. doi: 10.1080/13607860903071014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli, I., Souza, J., McWilliams, J. M., & Mehrotra, A (2017). Trends in use of the US Medicare annual wellness visit, 2011–2014. JAMA, 317(21), 2233–2235. doi: 10.1001/jama.2017.4342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianattasio, Z. K., Wu, M. Q., Glymour, C. M., & Power, C. M (2019). Comparison of methods for algorithmic classification of dementia status in the Health and Retirement Study. Epidemiology, 30(2), 291–302. doi: 10.1097/EDE.0000000000000945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurland, B. J., Wilder, D. E., Lantigua, R., Stern, Y., Chen, J., Killeffer, E. H., & Mayeux, R (1999). Rates of dementia in three ethnoracial groups. International Journal of Geriatric Psychiatry, 14(6), 481–493. doi: [DOI] [PubMed] [Google Scholar]
- Hebert, L. E., Weuve, J., Scherr, P. A., & Evans, D. A (2013). Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology, 80(19), 1778–1783. doi: 10.1212/WNL.0b013e31828726f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeringa, S. G., Fisher, G. G., Hurd, M., Langa, K. M., Ofstedal, M. B., Plassman, B. L., Rodgers, W. L., & Weir, D. R (2009). Aging, Demographics and Memory Study (ADAMS). Sample design, weighting, and analysis for ADAMS Retrieved from http://hrsonline.isr.umich.edu/sitedocs/userg/ADAMSSampleWeights_Jun2009.pdf
- Kasper, J. D., Freedman, V. A., & Spillman, B. C (2013). Classification of persons by dementia status in the National Health and Aging Trends Study Retrieved from www.NHATS.org
- Langa, K. M., Larson, E. B., Crimmins, E. M., Faul, J. D., Levine, D. A., Kabeto, M. U., & Weir, D. R (2017). A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Internal Medicine, 177(1), 51–58. doi: 10.1001/jamainternmed.2016.6807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa, K. M., Plassman, B. L., Wallace, R. B., Herzog, A. R., Heeringa, S. G., Ofstedal, M. B., Burke, J. R., Fisher, G. G., Fultz, N. H., Hurd, M. D., Potter, G. G., Rodgers, W. L., Steffens, D. C., Weir, D. R., & Willis, R. J (2005). The Aging, Demographics, and Memory Study: Study design and methods. Neuroepidemiology, 25(4), 181–191. doi: 10.1159/000087448 [DOI] [PubMed] [Google Scholar]
- Plassman, B. L., Langa, K. M., Fisher, G. G., Heeringa, S. G., Weir, D. R., Ofstedal, M. B., Burke, J. R., Hurd, M. D., Potter, G. G., Rodgers, W. L., Steffens, D. C., Willis, R. J., & Wallace, R. B (2007). Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology, 29(1–2), 125–132. doi: 10.1159/000109998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman, B. L., Langa, K. M., McCammon, R. J., Fisher, G. G., Potter, G. G., Burke, J. R., … Wallace, R. B (2011). Incidence of dementia and cognitive impairment, not dementia in the United States. Annals of Neurology, 70(3), 418–426. doi: 10.1002/ana.22362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince, M., Ali, G. C., Guerchet, M., Prina, A. M., Albanese, E., & Wu, Y. T (2016). Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimer’s Research & Therapy, 8(1), 23. doi: 10.1186/s13195-016-0188-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca, W. A., Petersen, R. C., Knopman, D. S., Hebert, L. E., Evans, D. A., Hall, K. S., Gao, S., Unverzagt, F. W., Langa, K. M., Larson, E. B., & White, L. R (2011). Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimer’s & Dementia, 7(1), 80–93. doi: 10.1016/j.jalz.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spering, C. C., Hobson, V., Lucas, J. A., Menon, C. V., Hall, J. R., & O’Bryant, S. E (2012). Diagnostic accuracy of the MMSE in detecting probable and possible Alzheimerʼs disease in ethnically diverse highly educated individuals: An analysis of the NACC database. Journals of Gerontology, Series A: Biomedical Sciences and Medical Sciences, 67(8), 890–896. doi: 10.1093/gerona/gls006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Clair, P. P., Gaudette, P. É., Zhao, P. H., Tysinger, P. B., Seyedin, P. R., & Goldman, P. D (2017). Using self-reports or claims to assess disease prevalence: It’s complicated. Medical Care, 55(8), 782–788. doi: 10.1097/MLR.0000000000000753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, D. H.Jr, Østbye, T., Langa, K. M., Weir, D., & Plassman, B. L (2009). The accuracy of Medicare claims as an epidemiological tool: The case of dementia revisited. Journal of Alzheimer’s Disease, 17(4), 807–815. doi: 10.3233/JAD-2009-1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent, G. K., & Velkoff, V. A.(2010). The next four decades: The older population in the United States: 2010 to 2050 Retrieved from https://www.census.gov/content/dam/Census/library/publications/2010/demo/p25-1138.pdf
- Weir, D., Langa, K., & Ryan, L (2016). Harmonized Cognitive Assessment Protocol (HCAP): Study protocol summary Retrieved from http://hrsonline.isr.umich.edu/index.php?p=shoavail&iyear=ZU
- Zissimopoulos, J. M., Crimmins, E. M., & St Clair, P. A (2014). The value of delaying Alzheimer’s disease onset. Forum for Health Economics & Policy, 18(1), 25. doi: 10.1515/fhep-2014-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zissimopoulos, J. M., Tysinger, B. C., St Clair, P. A., & Crimmins, E. M (2018). The impact of changes in population health and mortality on future prevalence of alzheimer’s disease and other dementias in the United States. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 73(suppl_1), 38–47. doi: 10.1093/geronb/gbx147 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.