Key Points
Beneficial effects of Ts-serpin regulation on T. spiralis survival were identified.
A Ts-serpin–triggered mechanism was found in macrophages independent of IL-4Rα.
The anti-inflammatory effect of Ts-serpin in a TNBS-induced IBD model was verified.
Abstract
Trichinella spiralis is recognized for its ability to regulate host immune responses via excretory/secretory (ES) products. Serine protease inhibitors (serpins) play an important role in ES product-mediated immunoregulatory effects during T. spiralis infection. In this study, the immunoregulatory properties of a serpin derived from T. spiralis (Ts-serpin) were explored in BALB/c mice. The results showed that naturally occurring Ts-serpin was detected in the stichosomes of muscle larvae and adult worms. Moreover, enhancing (by injection of a soluble-expressed recombinant Ts-serpin [rTs-serpin]) or blocking (by passive immunization with anti–rTs-serpin serum) the effects of Ts-serpin changed the levels of cytokines related to inflammation induced by T. spiralis infection in the serum, mesenteric lymph nodes, and peritoneal cavity, which then led to a change in the adult worm burden in early T. spiralis infection. Moreover, the phenotypic changes in peritoneal macrophages were found to be related to Ts-serpin–mediated immunoregulation. Furthermore, a STAT6 activation mechanism independent of IL-4Rα has been found to regulate protein-mediated alternative activation of bone marrow–derived macrophages and mimic the immunoregulatory role of Ts-serpin in T. spiralis infection. Finally, the anti-inflammatory properties of rTs-serpin and bone marrow–derived macrophage alternative activation by rTs-serpin were demonstrated using a trinitrobenzene sulfonic acid–induced inflammatory bowel disease model. In summary, a protein-triggered anti-inflammatory mechanism was found to favor the survival of T. spiralis in the early stage of infection and help to elucidate the immunoregulatory effects of T. spiralis on the host immune response.
Introduction
Helminths are considered to have the ability to regulate the host immune system (1, 2). In recent years, the immunoregulation mechanism involved in helminth infection has been preliminarily elucidated (3, 4). It has been recognized that excretory/secretory (ES) products are responsible for regulating the immune response through their anti-inflammatory effects (5). However, because of life cycle diversity and various parasite histotropism in different helminths, the immunoregulatory mechanisms vary greatly. Moreover, because of the absence of analyses of the definitive effective proteins and the immune cells with which they interact, research on the immunoregulatory properties of helminths has encountered a bottleneck for a long time. Generally, the immunoregulatory effects of helminths on the host immune system include stimulation of the Th2-type immune response and immunosuppression (6, 7). Trichinella spiralis), as one of most epidemic species within the genus of Trichinella, alternates generations in the same host, accompanied by changes of histotropism (8, 9). Its specific immune response, including the two different regulatory effects produced above in different developmental stages (10, 11), makes it a good model organism to study the immunoregulatory mechanism of worms.
The type of immune response in the host varies in different stage of infection during T. spiralis invasion (12). The immunosuppressive immune response, which is considered to be very important for the survival of adult worms and the parasitism of muscle larvae (ML), is mainly detected in the intestinal period of infection (13, 14). Recently, to identify the immunological characteristics of the immune response to T. spiralis infection, both innate and adaptive immunity have been studied with regard to the target immune cells and the stage of immune response in which the immunoregulatory proteins participate (10, 15). Generally, innate cells are thought to be indispensable in the early stage of infection to initiate the immune response, including immunosuppressive effects (16). Therefore, screening and exploring the interaction effect between a single component derived from the ES products of adult worms and innate immune cells are helpful for elucidating the mechanism of immunosuppression. Moreover, clarifying the trigger mechanism of the interaction effect is the basis and the key to elucidating the role of single-component protein-mediated immune regulation in the early stage of T. spiralis infection. In previous studies, serine protease inhibitors (serpins) from T. spiralis (high-abundance proteins expressed at different developmental stages that contribute to the protection of T. spiralis from serine protease hydrolysis and contribute to the escape of the parasite from immune attack by the host) (17) showed regulatory effects involved in the anti-inflammatory immune response (18). These data suggest the potential of serpins to regulate the immune system and suggest that they may play an important role in T. spiralis infection.
To explore the initiation mechanism of the anti-inflammatory immune response in the early stage of T. spiralis infection and to identify the major single-component protein that is responsible for the early immunoregulatory effect from the ES products, a serpin derived from T. spiralis (Ts-serpin) was selected to investigate its effects on the survival of worm burden and the immune response in T. spiralis infection by different intervention methods. Moreover, regulatory effect-dependent protein-interacting cells were detected, including macrophages and T cells in the peritoneal cavity. Related mechanisms were also explored in bone marrow–derived macrophages (BMDMs). Finally, the anti-inflammatory properties of Ts-serpin were evaluated in a trinitrobenzene sulfonic acid (TNBS)–induced inflammatory bowel disease (IBD) model.
Materials and Methods
Animals and parasites
Eight-week-old female BALB/c mice were purchased from Shanghai Laboratory Animal Care (Shanghai, China). T. spiralis (ISS534) was raised and preserved in our laboratory. ML from 35-d postinfection (dpi) and adult worms from 3, 6, 9 and 12 dpi were obtained as previously described (19, 20).
Animal experiments were performed strictly in accordance with the guidelines for the care and use of laboratory animals (21). All animal experiments in this study were approved by the Ethical Committee of Jilin University affiliated with the Provincial Animal Health Committee (Changchun, China) (ethical clearance number IZ-2009-08).
Isolation and culture of cells
To isolate and culture cells from different organs and tissues, BALB/c mice were anesthetized and immediately killed by isoflurane and CO2 inhalation. Peritoneal cells were collected by irrigating the peritoneal cavity with PBS, and mesenteric lymph node (mLN) cells were disaggregated, filtered, and collected by being passed through 70-μm nylon mesh (22, 23). BMDMs were cultured as previously described (24). The bone marrow cells were flushed from the long bones of eight-week-old female BALB/c mice, seeded and cultured in DMEM with 10% FBS and 25 ng/ml recombinant mouse (rm) M-CSF at 37°C with 5% CO2 for 48 h. Subsequently, all adherent cells were cultured in DMEM with 10% FBS and 25 ng/ml rmM-CSF for 72 h.
Preparation and identification of recombinant Ts-serpin
The sequence of Ts-serpin (GenBank accession number DQ864973) excluding the N-terminal signal peptide was subcloned into the pCold I vector. The soluble recombinant protein was expressed in Escherichia coli BL21 (DE3) and purified by nickel affinity chromatography according to the manufacturer’s instructions (GE Healthcare). Endotoxin was removed from the purified protein sample by an Endotoxin Removal Kit (Abbkine) and was detected by an Amplite Fluorimetric Endotoxin Detection Kit (AAT Bioquest). The immunogenicity of recombinant Ts-serpin (rTs-serpin) was analyzed by Western blot and agar gel double-immunodiffusion tests. The serine protease inhibitory activity of the recombinant protein was characterized by single-stage kinetic assays.
Production of anti–rTs-serpin serum and T. spiralis–infected serum
BALB/c mice were first injected i.m. with ∼60 μg of purified rTs-serpin mixed 1:1 (v/v) with CFA. Additional injections of 60 μg of protein with IFA were administered to mice twice at a 2-wk interval. Preimmune serum samples were collected as control serum. One week after the last injection, serum was collected for the analysis of passive immune protection and immunofluorescence staining or purified by protein G affinity chromatography according to the manufacturer’s instructions (GE Healthcare) for the analysis of the interaction between rTs-serpin and BMDMs.
To prepare T. spiralis–infected serum, BALB/c mice were orally infected with 350 T. spiralis ML, and the serum was collected at 21 dpi and stored at −20°C. The non-T. spiralis–infected serum was used as negative serum.
Immunofluorescence staining
T. spiralis ML and adult worms were washed with PBS and fixed in 4% (w/v) paraformaldehyde for 2 h at room temperature (RT). All samples were subsequently permeabilized with 1% Triton X-100 in PBS overnight at 4°C, blocked with 5% (v/v) natural goat serum in PBS for 1 h at RT, and incubated with anti–rTs-serpin serum (1:200) or control serum overnight at 4°C. After washing three times with PBS, the samples were incubated with Alexa Fluor 594–conjugated anti-mouse IgG for 1 h at 37°C (1:400; Life Technologies). The ML and adult worms were incubated with Hoechst 33342 for 5 min at RT for nuclear staining. The expression of Ts-serpin in T. spiralis was visualized with a laser scanning confocal microscope (FluoView FV 1000; Olympus).
Passive immune protection of anti–rTs-serpin serum and immunoregulatory effects of rTs-serpin in the T. spiralis infection model
To establish the infection model, BALB/c mice were orally infected with 350 T. spiralis ML. The anti–rTs-serpin serum was passively immunized i.v. 12 h before infection and 2, 4, and 6 dpi (200 μl per mouse at each time point). As a control, control serum of an equal volume was injected i.v. in parallel.
The operation process of the immunoregulatory test of rTs-serpin was similar to that of the immune protection of anti–rTs-serpin serum. Briefly, the recombinant protein was injected i.p. into the T. spiralis infection model 12 h before infection and 3 and 6 dpi (20 μg of rTs-serpin in 200 μl of PBS per mouse at each time point). As a control, infection model mice were injected with an equal volume of PBS.
Worm burden in all infection models was assessed by counting the number of ML (35 dpi) and adult worms (3, 6, 9, 12 dpi). Serum samples (3, 6, 35 dpi) were collected for cytokine detection.
Detection of the immunoregulatory effects of rTs-serpin in the early immune response
Recombinant protein (50 μg per mouse) was injected i.p. into BALB/c mice (i.v. injection with control serum 12 h before and 2 d after rTs-serpin injection). Three days after injection, immune cells derived from the peritoneal cavity and mLNs were isolated for the detection of cell phenotypes and/or cytokine secretion. Specifically, changes in T cell subsets and macrophage phenotypes among peritoneal cells were immediately detected by flow cytometry analysis and the cytokine levels in the supernatants of the cells derived from the peritoneal cavity and mLNs were detected after the cells were stimulated with PMA (0.05 μg/ml; MedChemExpress) and ionomycin (1 μg/ml; MedChemExpress) for 24 h. As a control, the detection of peritoneal and mLN cells was also performed in BALB/c mice (i.p. injected with PBS) that were infected with 350 T. spiralis ML (detected at 3 dpi) plus i.v. injection of anti–rTs-serpin serum/control serum as previously described and in uninfected BALB/c mice injected with PBS (i.p.) and control serum (i.v.).
Furthermore, the rat anti-mouse IL-4Rα neutralizing Ab (IL-4Rα blocking mAb, mIL4R-M1, BD Biosciences) was used to block the rTs-serpin–mediated immunoregulatory effects. The IL-4Rα blocking mAb (diluted to 50 μg/ml in PBS, 200 μl per mouse) or equivalent isotype control Ab (isotype control mAb, R35-95; BD Biosciences) was injected i.p. into the BALB/c mice 2 h before i.p. injection of rTs-serpin (50 μg per mouse) or rmIL-4 (50 ng/ml, 10 ng per mouse; PeproTech). Only phenotypic changes were analyzed in the peritoneal macrophage population by flow cytometry 24 h after injection of rTs-serpin or rmIL-4.
Detection of the interaction between rTs-serpin and BMDMs
All BMDMs were preincubated with rat anti-mouse CD16/32 blocking Ab (1 μg in 100 μl of PBS per 1 × 106 cells) for 30 min at 37°C. Additionally, rTs-serpin (20 μg/ml) was incubated with an equal volume of T. spiralis–infected serum or negative serum for 2 h at 37°C. After the pretreated BMDMs were washed with PBS, the cells were resuspended and incubated in the premix of rTs-serpin and T. spiralis–infected serum/negative serum for 3 h. As a control, CD16/32 blocking Ab–pretreated BMDMs were incubated in an equal volume of the premix of PBS and negative serum.
To investigate the interaction between the recombinant protein and IL-4Rα, BMDMs pretreated with CD16/32 blocking Ab were incubated with IL-4Rα blocking mAb (10 μg/ml) or an equal volume of isotype control mAb for 2 h at 37°C. Subsequently, rTs-serpin (10 μg/ml) interacted with BMDMs for 3 h at 37°C. As a control, isotype control mAb-treated cells (pretreated with CD16/32 blocking Ab) without rTs-serpin interaction (treated with PBS) were also carried out.
All BMDM samples were washed with PBS and incubated with purified mouse anti–rTs-serpin polyclonal Ab (2 μg in 1 × 106 cells per 100 μl of PBS for 30 min) followed by rat anti-mouse IgG1 mAb (0.5 μg in 1 × 106 cells per 100 μl of PBS for 30 min; A85-1, FITC conjugated; BD Biosciences). The samples were analyzed using a BD FACSCalibur flow cytometer, and the mean fluorescence intensity (MFI) was analyzed using FlowJo software (Tree Star).
Detection of the immunoregulatory effects of rTs-serpin on BMDMs
To analyze the regulatory effects of rTs-serpin on macrophages, BMDMs were incubated with rTs-serpin alone (10 μg/ml) or with rTs-serpin plus negative serum (5%, v/v) for 48 h. BMDMs stimulated with rTs-serpin plus T. spiralis–infected serum (5%, v/v) and untreated BMDMs were used as inhibitory and control administration, respectively.
To elucidate the regulatory mechanism of rTs-serpin on BMDMs, the IL-4Rα blocking mAb (10 μg/ml) and STAT6 inhibitor AS1517499 (50 nM; MedChemExpress) were added to the rTs-serpin–incubated BMDMs. The isotype control mAb (10 μg/ml) and solvent of AS1517499 (100× stock solvent: 10% DMSO, 40% PEG300, 5% Tween 80, 45% saline) were added to the rTs-serpin–incubated BMDMs as controls. The same blocking operation was performed on rmIL-4 (10 ng/ml)–treated BMDMs.
To explore the role of the enzyme inhibitory activity in rTs-serpin–mediated macrophage polarization, 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (AEBSF; 90 nM; Sigma-Aldrich) was added to replace rTs-serpin in incubating BMDMs. Untreated BMDMs that were incubated with the solvent of AEBSF (0.1% PBS, v/v) were used as a control.
The incubated BMDMs were collected for flow cytometry analysis directly or collected for Western blot analysis after incubation with RIPA lysis buffer mixed with 1 mM PMSF for 10 min. The BMDM culture supernatants were collected for the detection of cytokines.
To verify the immunoregulatory effects of treated BMDMs, all BMDMs were adoptively transferred i.p. to the T. spiralis infection model (12 h before infection and 3 dpi, 11 × 106 cells per mouse at each time point). Only the adult worm (3, 6, 9, 12 dpi) burden was assessed.
Detection of the anti-inflammatory effects of rTs-serpin or protein-treated BMDMs in the TNBS-induced IBD model
The IBD model was established in BALB/c mice, as previously described (25). BALB/c mice (20–21 g) were anesthetized by isoflurane inhalation and injected rectally with a single dose of TNBS (5%, 0.5 mg; Sigma-Aldrich) in an equal volume of ethanol. As a negative control, normal mice received an equal volume of 50% ethanol–PBS solution. For analysis of the anti-inflammatory effects, PBS (50 μl per mouse at each time point), rTs-serpin (1 mg/ml, 50 μg per mouse at each time point), and BMDMs (1 × 106 cells per mouse at each time point) incubated with rTs-serpin plus T. spiralis–infected serum/negative serum were injected i.p. into the TNBS-induced IBD model mice (2 d before and 3 d after TNBS administration). Additionally, the mice in the negative control received an equal volume of PBS i.p. To evaluate the course of disease and the level of inflammation, the disease activity index (DAI), including weight loss, stool consistency, and hematochezia, was evaluated daily after the IBD model was established, and colonic tissue histology was performed as previously described (26, 27) on the seventh day after TNBS administration. Inflammation-related cytokines in the serum were also detected on the fourth day.
Agar gel double-immunodiffusion test
An agar gel double-immunodiffusion test was carried out on a 1% agar gel prepared with 0.9% NaCl solution according to a routine protocol. Forty microliters of purified recombinant protein (1 mg/ml) was added to the central pore, and equal volumes of different dilutions of T. spiralis–infected serum (21 dpi, dilutions: 2, 4, 8, 16 times), negative serum, and 0.9% NaCl solution were added to the peripheral pores. The reaction was incubated at 37°C for 24 h, then the precipitation of the antigen–antibody complex was observed.
Enzyme inhibitory activity assay
Single-stage kinetic assays were used to characterize the inhibitory activity of rTs-serpin against the human neutrophil elastase (elastase H) and the mouse mast cell protease-1 (mMCP-1) (28, 29). Increasing concentrations of purified recombinant protein (1, 2, 5, 10, 15 μg/ml) were preincubated with each of the enzymes for 10 min at 25°C followed by the addition of the appropriate chromogenic substrate in a total volume of 200 μl in individual wells of a 96-well microtiter plate. The concentrations of enzyme/substrate are shown in Table I. Absorbance changes at 405 nm were monitored over 5 min using a kinetic microplate reader to analyze the hydrolysis of the proteases to evaluate the inhibitory activity of recombinant proteins. The reaction system without recombinant protein or with AEBSF (0.1 mM) was employed as negative and positive controls, respectively. The inhibition rates were calculated as follows: (ODnegative−ODrTs-serpin)/(ODnegative−ODAEBSF).
Table I. Enzyme/substrate used for rTs-serpin inhibitory activity assay.
| Enzyme | Substrate |
|---|---|
| Elastase H (17 nM; Sigma-Aldrich) | N-succinyl-Ala-Ala-Pro-Leu-p-nitroanilide (100 mM; Sigma-Aldrich) |
| mMCP-1 (3 nM; Sigma-Aldrich) | N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide (100 mM; Sigma-Aldrich) |
Western blot analysis
Equal amounts of purified rTs-serpin or lysates of incubated BMDMs (20 μg per well) were electrophoresed on a 12% SDS-PAGE gel and electrotransferred to a PVDF membrane (Millipore). The recombinant protein samples were incubated with negative serum or T. spiralis–infected serum (1:2000) and then incubated with HRP-conjugated goat anti-mouse IgG for 1 h at 37°C (1:5000). The cell lysate samples were treated with a variety of primary Abs (rabbit anti–phospho-Y705 STAT3, mouse anti-STAT3, rabbit anti–phospho-Y641 STAT6, rabbit anti-STAT6, rabbit anti–arginase 1 (Arg 1), rabbit anti–chitinase-like molecule, and mouse anti–β-actin; Abcam) and HRP-conjugated secondary Abs (goat anti-mouse IgG and rabbit anti-mouse IgG; Abcam). The Western blots were developed using the ECL Plus Western Blotting Detection System (GE Healthcare). The results of the signaling pathways and effector molecules in the cell lysates were quantified with ImageJ software version 1.49 for Windows.
Flow cytometry staining and gating strategy
BMDMs and peritoneal cells were washed with PBS, and the number was adjusted to 1 × 106 cells per 100 μl of PBS containing 1% FBS. The cells were first incubated with fluorescein-labeled Abs for surface marker staining. The surface markers included CD3 (145-2C11, allophycocyanin conjugated), CD4 (GK1.5, FITC conjugated), CD8 (53-6.7, PE conjugated), CD44 (IM7, allophycocyanin conjugated), CD62L (MEL-14, PE conjugated), and CD25 (PC61, allophycocyanin conjugated) for T cells (BD Biosciences) and F4/80 (BM8, allophycocyanin conjugated) and CD16/32 (93, FITC conjugated) for polarized macrophages (BioLegend). Each cell suspension was incubated with the appropriate Ab for 30 min at 4°C and washed three times with PBS. To detect intracellular markers, the samples were subsequently fixed in 4% (w/v) paraformaldehyde for 30 min, permeabilized with 1% Triton X-100 in PBS for 40 min at 4°C, then stained with the Foxp3 Ab (R16-715, PE conjugated; BD Biosciences) for regulatory T cells (Tregs) or the CD206 Ab (MR5D3, PE conjugated; BioLegend) for alternatively activated macrophages. After a final washing with PBS, the samples were analyzed using a BD FACSCalibur flow cytometer, and the results were analyzed using FlowJo software (Tree Star). Positive subsets were classified by comparison with isotype control Ab-incubated samples.
To define the groups of macrophages in BMDMs and peritoneal cells, a single nonlymphocytic population (peritoneal cells) or all single cells (BMDMs) were selected individually to be defined and counted by the F4/80 marker. The polarization of macrophages was analyzed in F4/80+ cells.
Multicytokine immunoassay
The levels of inflammation-related cytokines in serum samples or cell culture supernatants were detected by a sandwich multicytokine immunoassay (Meso Scale Diagnostics).
Statistical analysis
The data are expressed as the mean ± SD. Statistical analysis was performed using GraphPad Prism 5 software for Windows. One-way and two-way ANOVA and independent exponent t test were used to compare the means and determine statistically significant differences between different conditions.
Results
Identification of recombinant and naturally occurring Ts-serpin
The endotoxin residue in the purified recombinant protein was <0.06 U/ml after removal of endotoxin four times (Supplemental Fig. 1). The Western blot analysis results showed that recombinant protein with a relative molecular mass of 38 kDa was specifically recognized by T. spiralis–infected serum and was not detected by incubation with negative serum. In the double-immunodiffusion test, a precipitation line was formed at the reaction site between the low dilution of T. spiralis–infected serum and the recombinant protein, and the titer of specific Ab against the purified protein was determined to be 1:4 (Fig. 1B).
FIGURE 1.
Identification of recombinant and naturally occurring Ts-serpin. (A) Western blot analysis. M is the prestained marker. Samples are purified rTs-serpin incubated with negative serum (line 1) and T. spiralis–infected serum (21 dpi; line 2). (B) Agar gel double-immunodiffusion test of purified rTs-serpin (original magnification ×4). Samples in the peripheral pores: 1–4 are different dilutions of T. spiralis–infected serum (21 dpi; dilutions in pores 1–4: 2, 4, 8, 16 times, respectively); pore 5 is 0.9% NaCl solution; pore 6 is negative serum. T. spiralis ML incubated with (C) control serum or (D) anti–rTs-serpin serum (original magnification ×200). At (E) 3 dpi and (F) 6 dpi, the adult worms were probed with anti–rTs-serpin serum (original magnification ×50). Positive staining (red fluorescence signals) located the naturally occurring Ts-serpin at stichocytes (red arrows). High nuclear density (blue fluorescence signals) indicated the gonads of T. spiralis (blue arrows).
In the immunofluorescence detection and the density and morphology of the cells indicated the anatomical and tissue structure of T. spiralis at different developmental stages. As shown in Fig. 1C–F, the gonads of T. spiralis, which exhibit a higher nuclear density, were distributed in the posterior part of the body (indicated by the blue arrows), and stichocytes, which are composed of stichosomes with secretory function, were mainly located around the column in the forebody (indicated by the red arrows) (30). The specific positive signal of naturally occurring Ts-serpin in ML was mainly concentrated in stichosomes, compared with negative serum (Fig. 1D). In adult worms, the distribution of natural Ts-serpin at 3 and 6 dpi was similar to that in ML and extended to the epidermis (Fig. 1E, 1F).
Adoptive anti–rTs-serpin serum or rTs-serpin changed the elimination of T. spiralis and the levels of inflammatory cytokines in the early stage of infection
Passive immune protection and immunoregulatory experiments were performed as shown in Figs. 2A, 3A. In the process of T. spiralis infection, the adult worm burden in the intestine remained stable at 3 and 6 dpi, decreased rapidly from 6 to 12 dpi, and remained low at 12 dpi. The passive immunoprotection of anti–rTs-serpin serum caused a significant decrease in adult worm burden from 3 dpi, and the elimination of adult worms was advanced and almost complete at 12 dpi, compared with the control serum (Fig. 2B). Similarly, the ML burden at 35 dpi was also significantly decreased (Fig. 2C). In contrast, i.p. injection of rTs-serpin increased the intestinal survival number of adult worms at 9 and 12 dpi but had no effect at 3 and 6 dpi (Fig. 3B). The ML burden at 35 dpi was also upregulated by injection of recombinant protein (Fig. 3C). The levels of different cytokines in the serum varied at different stages of T. spiralis infection. In general, the levels of inflammatory-related cytokines (except TGF-β1) were higher in the early stage of infection than in ML (Fig. 2D). Both immunoprotection and injection of rTs-serpin had no effect on the levels of any cytokines at 35 dpi (Figs. 2D, 3D).
FIGURE 2.
Changes in worm burden and serum cytokines in passive immunity of anti–rTs-serpin serum in the T. spiralis infection model. (A) Anti–rTs-serpin serum-based passive immunization protocol in T. spiralis infection model. Statistics of (B) adult worm burden in small intestine and (C) larvae burden in skeletal muscle in T. spiralis infection after injection of control serum or anti–rTs-serpin serum. (D) Levels of inflammatory-related cytokines in the serum at 3, 6, and 35 dpi. The values are the mean ± SD of at least eight independent experiments (n = 8–11). Significant differences were analyzed by t test. **p < 0.01.
FIGURE 3.
Changes in worm burden and serum cytokines in the i.p. injection of rTs-serpin in T. spiralis infection model. (A) Immunoregulatory experiment protocol of rTs-serpin in T. spiralis infection model. Statistics of (B) adult worm burden in small intestine and (C) larvae burden in skeletal muscle in T. spiralis infection under injection of PBS or rTs-serpin. (D) Levels of inflammatory-related cytokines in the serum at 3, 6, and 35 dpi. The values are the mean ± SD of at least eight independent experiments (n = 8–12). Significant differences were analyzed by t test. **p < 0.01.
Specifically, as shown in Fig. 2D, the high levels of IFN-γ and IL-17 detected at 3 dpi in the normal process of infection were enhanced and extended at 6 dpi by inducing anti–rTs-serpin serum. However, the level of IL-4 peaked at 6 dpi and was then unchanged. In the detection of innate immunity-related proinflammatory cytokines, passive immunization with anti–rTs-serpin serum upregulated the IL-1β and TNF-α levels only at 3 dpi but had no effect at 6 dpi. Continuous upregulation of IL-12 was induced by passive immunization at 3 and 6 dpi. The changes in anti-inflammatory cytokines exhibited different trends than the changes in proinflammatory cytokines. Neither T. spiralis infection nor passive immunoprotection affected the level of TGF-β1 at 3 and 6 dpi. The high level in IL-10 induced by T. spiralis infection was decreased by anti–rTs-serpin serum injection.
In the analysis of serum cytokines after injection of rTs-serpin (Fig. 3D), the changes in cytokine levels were generally opposite to those of passive immunoprotection. Briefly, high levels of IFN-γ (3 dpi), IL-4, IL-12 (6 dpi), and IL-1β (both 3 and 6 dpi) were suppressed by rTs-serpin, but there were no differences in the levels of IL-17, TNF-α, and TGF-β1 at 3 and 6 dpi, compared with the normal infection model. Furthermore, the high level of IL-10 induced by T. spiralis infection was enhanced at both 3 and 6 dpi.
Injection of rTs-serpin in uninfected BALB/c mice mimicked the formation of an immunosuppressive cytokine environment and phenotypic changes of peritoneal macrophages but not T cell activation in the early immune response to T. spiralis infection
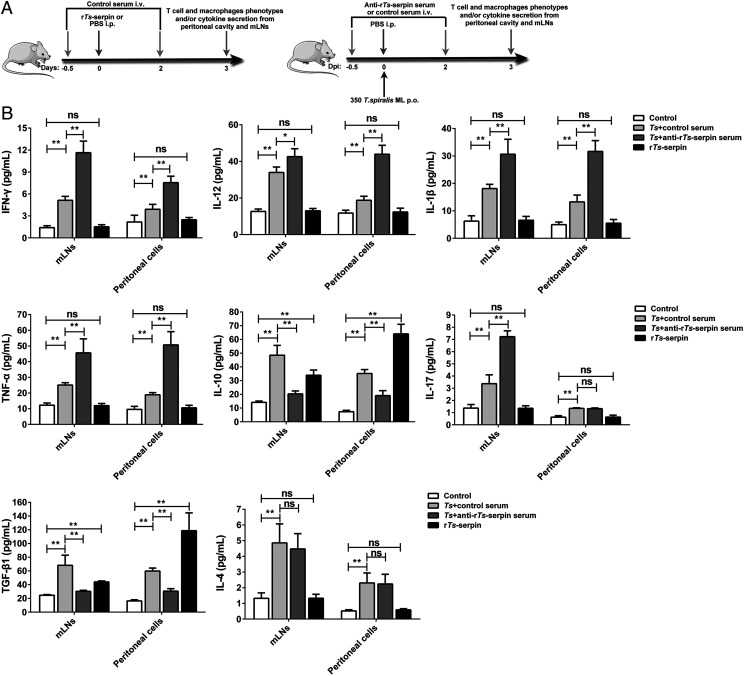
The experimental design of the early immune regulation effect of Ts-serpin is shown in Fig. 4A. The effects of T. spiralis infection or passive immunity of anti–rTs-serpin serum on cytokines derived from mLNs was more obvious than those in the peritoneal cavity, and the changes in the main cytokines were similar to those observed previously in serum (Fig. 4B). Generally, all inflammatory-related cytokines in mLNs, including proinflammatory and anti-inflammatory cytokines, were upregulated by T. spiralis infection, and passive immunization with anti–rTs-serpin serum enhanced the upregulation of proinflammatory cytokines (except IL-4) but weakened the upregulation of anti-inflammatory cytokines. In contrast, the IL-17 level was not enhanced by the anti–rTs-serpin serum as detected in immune cells from the peritoneal cavity. Injection of rTs-serpin only increased the levels of IL-10 and TGF-β1 but had no effect on other cytokines in mLNs and the peritoneal cavity. In addition, upregulation induced by the recombinant protein was more significant in peritoneal cells than in mLNs.
FIGURE 4.
Early immune regulation effect of Ts-serpin on inflammatory-related cytokines in the supernatants of mLNs and peritoneal cells. (A) The experimental scheme of early immune regulation effect of recombinant or naturally occurring Ts-serpin in T. spiralis–infected or normal mice. (B) Control serum-pretreated normal mice were injected i.p. with rTs-serpin or PBS (control group), and PBS-treated, T. spiralis–infected mice were injected with different sera (control serum, T. spiralis plus control serum group; anti–rTs-serpin serum, T. spiralis plus anti–rTs-serpin serum group). The values are the mean ± SD of five independent experiments. Significant differences were analyzed by one-way ANOVA (compared with the control group) or by t test (compared with the T. spiralis plus control serum group). *p < 0.05, **p < 0.01. ns, not significant.
From flow cytometry analysis, infection with T. spiralis induced a change in the proportion of T cell subsets and phenotypic changes in macrophages in the peritoneal cavity, but the injection of rTs-serpin only affected the phenotype of macrophages (Fig. 5A, 5B). More concretely, the proportion of CD4+ T cells increased in the T. spiralis infection model along with the proportion of CD3+ T cells. Meanwhile, the ratio of resident CD4+ T cells decreased (CD44+, CD62L−), and cells participating in lymphocyte recirculation (CD44+, CD62L+) showed an enhanced ratio. In the Treg (CD4+, CD25+, Foxp3+) subset, the proportion also increased. When anti–rTs-serpin serum was administered, the increasing ratio of CD3+ and CD4+ cells was more intense; the decreased proportion of resident CD4+ T cells was restored, and the increasing ratio in Tregs was slightly inhibited. In contrast, there were no significant changes in each T cell subset in rTs-serpin–treated mice (Fig. 5A). In the analysis of macrophages, an increase in F4/80+ cells was found both in T. spiralis infection and after rTs-serpin injection. Along with the increase in F4/80+ cells, a new subset of macrophages was detected with a high expression level of F4/80 as well as high expression levels of CD206 and CD16/32 in F4/80+ cells in both the T. spiralis infection and rTs-serpin injection groups. Similar to the previous results of cytokine changes, the addition of anti–rTs-serpin serum eliminated the effects of T. spiralis infection on macrophages (Fig. 5B).
FIGURE 5.
Regulation of rTs-serpin and naturally occurring Ts-serpin on peritoneal cells in the early stage of the immune response. Flow cytometry analysis of (A) T cells and (B) macrophages in peritoneal cells treated by i.p. injection of rTs-serpin or PBS (control group) and PBS-treated, T. spiralis–infected mice that were injected with different sera (control serum, T. spiralis plus control serum group; anti–rTs-serpin serum, T. spiralis plus anti–rTs-serpin serum group). The values are the mean ± SD of five independent experiments. Significant differences were analyzed by one-way ANOVA (compared with the control group) or by t test (compared with the T. spiralis plus control serum group). *p < 0.05, **p < 0.01. ns, not significant.
rTs-serpin induced the alternative activation of BMDMs, which contributed to the parasitism of T. spiralis
The purity of BMDMs before protein treatment (F4/80+ cell ratio) was more than 99% (Supplemental Fig. 2). In the incubation experiment, rTs-serpin induced the upregulation of CD206 expression in BMDMs. Slightly different from the previous results obtained in peritoneal macrophages, the phenotypic change in BMDMs induced by rTs-serpin did not include upregulation of CD16/32 expression (Fig. 6A). Moreover, the expression and phosphorylation levels of STAT6 and other effector molecules (Arg 1; chitinase-like molecule, Ym1) related to alternative activation of macrophages were also upregulated by rTs-serpin (Fig. 6B). However, the expression and phosphorylation of STAT3 were not related to the rTs-serpin–mediated activation of BMDMs. In contrast, the addition of T. spiralis–infected serum abolished the rTs-serpin–mediated upregulation of CD206 expression and other effector molecules related to the alternative activation of macrophages. Moreover, the expression and phosphorylated STAT6 levels were also inhibited by the addition of T. spiralis–infected serum. The effects of rTs-serpin and T. spiralis–infected serum on cytokine levels were similar to those in peritoneal cells in the previously reported results. Generally, rTs-serpin mediated the upregulation of IL-10 and TGF-β1 in BMDMs but had no significant effect on other cytokines. Adding T. spiralis–infected serum to rTs-serpin–treated BMDMs increased the expression of IL-12 and IL-1β and blocked the upregulation of IL-10 and TGF-β1 (Fig. 6C). In the analysis of the interaction between rTs-serpin and BMDMs, the specific fluorescent signal of the recombinant protein was found on the surface of the treated BMDMs by comparison with the cells without incubation with rTs-serpin. Moreover, the MFI of rTs-serpin on BMDMs was downregulated by T. spiralis–infected serum (Fig. 6D).
FIGURE 6.
The phenotypic and functional changes in BMDMs induced by rTs-serpin and reversed by inhibition with T. spiralis–infected serum. (A) Flow cytometry analysis, (B) Western blot analysis, and (C) levels of inflammatory-related cytokines in the supernatant of BMDMs treated with PBS (control group), rTs-serpin, rTs-serpin combined with negative serum (rTs-serpin plus negative serum group), rTs-serpin combined with T. spiralis–infected serum (rTs-serpin plus T. spiralis–infected serum group). (D) Analysis of the positive fluorescence signal of rTs-serpin on the surface of BMDMs treated with negative serum, rTs-serpin combined with negative serum (rTs-serpin plus negative serum group) and rTs-serpin combined with T. spiralis–infected serum (rTs-serpin plus T. spiralis–infected serum group). (E) Adoptive transfer protocol of nonstimulated BMDMs or rTs-serpin–treated BMDMs with or without different sera in T. spiralis infection model. (F) Statistics of adult worm burden after the adoptive transfer of BMDMs stimulated with different conditions. The values are the mean ± SD of eight (adult worm burden detection) or five (flow cytometry analysis, Western blot analysis, and cytokines detection) independent experiments. Significant differences were analyzed by t test. **p < 0.01, rTs-serpin group compared with control group; ##p < 0.01, rTs-serpin plus T. spiralis–infected serum group compared with rTs-serpin plus negative serum group; §§p < 0.01 in (D).
Finally, when nonstimulated BMDMs or treated BMDMs were transferred to the T. spiralis infection model (Fig. 6E), a similar decrease in adult worm burden to passive immunity of anti–rTs-serpin serum was found when the injected BMDMs were pretreated with rTs-serpin plus T. spiralis–infected serum. In contrast, adoptive transfer of BMDMs pretreated with rTs-serpin showed no significant change in adult worm burden at 3 or 6 dpi but increased the number of adult worms in the intestine at 9 and 12 dpi (Fig. 6F).
Ts-serpin–induced alternative activation of macrophages depended on the activation of STAT6 and was independent of IL-4Rα
Enzyme activity inhibition analysis showed that rTs-serpin significantly inhibited the hydrolysis activity of elastase H and mMCP-1. The inhibitory activity was dose-dependent and reached a stable peak at 10 μg/ml (Supplemental Fig. 3A). In the detection of BMDMs incubated with protease inhibitor, there was no significant upregulation in the percentage of CD206+ cells in all levels of secretory cytokines when BMDMs were treated with AEBSF (Supplemental Fig. 3B, 3C). High expression levels of CD206, Arg 1, and Ym1 were found in BMDMs treated with rmIL-4 plus different control reagents (isotype control mAb/solvents of AS1517499), accompanied by increased phosphorylation and expression of STAT6 (Supplemental Fig. 4A, 4B). When rmIL-4–treated BMDMs were coincubated with IL-4Rα blocking mAb or AS1517499, all high-level markers were suppressed. Moreover, the levels of IL-10 and TGF-β1 in the supernatant were significantly downregulated (Supplemental Fig. 4C). In contrast, the expression levels of markers and anti-inflammatory cytokines in rTs-serpin–treated BMDMs did not change with the addition of the IL-4Rα blocking mAb but were downregulated by AS1517499 (Fig. 7A–C). In the detection of proinflammatory cytokines, significant changes were not found either in IL-4Rα blocking mAb-blocked or in AS1517499-inhibited rTs-serpin– or rmIL-4–treated BMDMs (Fig. 7C, Supplemental Fig. 4C). Similarly, the IL-4Rα blocking mAb could only counteract the upregulation effects of rmIL-4 on single CD206+ subsets and the ratio of F4/80+ cells in peritoneal cells but had no effect on the phenotype and subsets of macrophages mediated by rTs-serpin in vivo (Fig. 8). In the interaction experiment, the addition of the isotype control mAb did not affect the detection of the fluorescence signal of rTs-serpin on the surface of BMDMs, and the IL-4Rα blocking mAb did not change the MFI displayed on the cell surface (Fig. 7D).
FIGURE 7.
Activation of BMDMs induced by rTs-serpin depends on STAT6 activation without the involvement of IL-4Rα. (A) Flow cytometry analysis, (B) Western blot analysis, and (C) levels of inflammatory-related cytokines in the supernatant of rTs-serpin–treated BMDMs coincubated with the isotype control mAb, IL-4Rα blocking mAb, STAT6 inhibitor (AS1517499), and solvents of AS1517499 (solvent control group). (D) Analysis of the positive fluorescence signal of rTs-serpin on the surface of BMDMs treated with isotype control mAb, rTs-serpin combined with isotype control mAb (rTs-serpin plus isotype control mAb group), and rTs-serpin combined with IL-4Rα blocking mAb (rTs-serpin plus IL-4Rα blocking mAb group). (E) Adoptive transfer protocol of rTs-serpin–treated BMDMs (inhibited with different blocking reagent) in T. spiralis infection model. (F) Statistics of the adult worm burden after adoptive transfer of BMDMs stimulated with different conditions. The values are the mean ± SD of eight (adult worm burden) or five (flow cytometry analysis, Western blot analysis, and cytokine detection) independent experiments. Significant differences were analyzed by t test. ##p < 0.01, AS1517499 group compared with solvent control group; §§p < 0.01 in (D). ns, not significant.
FIGURE 8.
The phenotypic effect of IL-4Rα blocking mAb on rmIL-4–treated or rTs-serpin–treated peritoneal macrophages in vivo. (A) Blocking effect design of IL-4Rα blocking mAb in rTs-serpin–treated or rmIL-4–treated peritoneal macrophages in vivo. Flow cytometry analysis of the F4/80+ ratio in nonlymphocyte subsets (B) and polarization in F4/80+ subsets (C) in rmIL-4–treated or rTs-serpin–treated peritoneal cells coincubated with isotype control mAb or IL-4Rα blocking mAb. The values are the mean ± SD of eight independent experiments. Significant differences were analyzed by t test. **p < 0.01.
In the adoptive transfer experiment, rmIL-4–treated BMDMs had no significant effect on the intestinal survival of T. spiralis, regardless of whether the activation of macrophages was counteracted by the IL-4Rα blocking mAb or by AS1517499 (Supplemental Fig. 4D). Conversely, inhibition of rTs-serpin–mediated activation of BMDMs by AS1517499 reduced the intestinal survival of T. spiralis at 9 and 12 dpi (Fig. 7E, 7F).
Injection of rTs-serpin or rTs-serpin–treated BMDMs in the peritoneal cavity alleviated the course of disease and the inflammatory response in the TNBS-induced IBD model
The anti-inflammatory effects were analyzed as shown in Fig. 9A. In the TNBS-induced IBD model, weight loss and DAI scores significantly changed when TNBS was administered to the colon (Fig. 9B, 9C). Briefly, weight loss occurred rapidly in the first 4 d after model establishment and then the weight recovered slightly. Similarly, high DAI scores were observed and maintained for 1–4 d after TNBS treatment. In the detection of serum cytokines, the implementation of TNBS induced increases in the levels of all detected cytokines except TGF-β1 (Fig. 9D). Histology and macrodissection revealed obvious pathological changes related to acute inflammation, including colonic atrophy, splenomegaly, and inflammatory infiltration of colonic tissue (Fig. 9E–G). When rTs-serpin or rTs-serpin–treated BMDMs were injected, the acute pathological process was significantly relieved. In short, severe weight loss was relieved, and the high DAI scores decreased. In addition, there were no significant pathological changes in the colon or spleen, and the colon maintained its normal structure at the histological level. Similarly, the upregulation of proinflammatory cytokines induced by TNBS was relieved by both rTs-serpin and adoptive transfer therapy in rTs-serpin–treated BMDMs (Fig. 9D). Moreover, rTs-serpin–treated BMDMs had a weaker anti-inflammatory effect than treatment with rTs-serpin alone. Conversely, in stark contrast to the success of rTs-serpin and rTs-serpin–treated BMDMs in treating colitis, inhibition of the alternative activation of BMDMs by T. spiralis–infected serum abolished the anti-inflammatory effects.
FIGURE 9.
Anti-inflammatory effects of rTs-serpin or rTs-serpin–induced BMDMs in a TNBS-induced IBD model. (A) Experimental scheme of rTs-serpin–mediated or treated BMDMs-mediated inflammation intervention in TNBS-induced IBD model. (B) Weight and (C) DAI score during the pathological process in TNBS-induced IBD model mice (TNBS) compared with mice treated with rTs-serpin, rTs-serpin–treated BMDMs, or rTs-serpin–treated BMDMs inhibited by T. spiralis–infected serum (rTs-serpin plus T. spiralis–infected serum-treated BMDMs group) and normal BALB/c mice (negative control group). (D) Inflammatory cytokine levels in the serum on the fourth day after IBD model establishment. Evaluation of (E) colonic atrophy and (F) splenomegaly in the negative control (A), rTs-serpin (B), rTs-serpin, rTs-serpin–treated BMDMs (C), rTs-serpin plus T. spiralis–infected serum-treated BMDMs (D), and TNBS (E) groups on the seventh day after IBD model establishment. (G) Representative H&E staining of the colon (original magnification ×100). The values are the mean ± SD of eight independent experiments. Significant differences were analyzed by one-way (D–G) or two-way (B and C) ANOVA (compared with the TNBS group). *p < 0.05, **p < 0.01, negative control group compared with TNBS group; #p < 0.05, ##p < 0.01, rTs-serpin group compared with TNBS group; §p < 0.05, §§p < 0.01, rTs-serpin-treated BMDMs group compared with TNBS group; &p < 0.05, &&p < 0.01, rTs-serpin-treated BMDMs group compared with TNBS group.
Discussion
T. spiralis belongs to the Trichinella spp. with a unique life cycle of “dead end” in the host. Unlike other helminths, infectious T. spiralis is not released into the environment, and it requires a greater ability to reduce the immune response in the process of infection to ensure its long-term coexistence with the host and to complete its life cycle (8). Unless there is an infection with a large number of ML, the host will not produce a serious immune response, either Th1 or Th2 type, to resist T. spiralis in the stage of intestinal infection (31, 32). The secretory protein components in ES products of T. spiralis were confirmed to be responsible for the ability to regulate the host immune response (33). In this study, immunofluorescence analysis of Ts-serpin determined that the protein was located on stichocytes, a specialized protein-secreting cell of T. spiralis. Considering that the localization of Ts-serpin extended to the epidermis in the adult worm stage, it was determined that the protein was secreted mainly in the adult worm stage and might play an important role in the early stage of the immune response to T. spiralis infection. In addition, as the specific Ab against ES products of T. spiralis can only be detected 3–4 wk postinfection (34, 35), the secretory properties of the protein in the early stage of infection were also preliminarily implied by the reaction between rTs-serpin and T. spiralis–infected serum at 21 dpi.
To elucidate the regulatory effects of Ts-serpin, it was necessary to replicate the natural structure of the protein on which the immunoregulatory properties depend and separate the immunoregulatory properties from the others. Concretely, the complex molecular structure of Ags is considered to be indispensable for immune regulation (36–38), and the potential spatial conformation was confirmed to be involved in the purified soluble rTs-serpin by the positive reaction in the agar gel double-immunodiffusion test because the formation of the precipitation line required at least two spatial epitopes on the surface of the Ag to form a continuous antigen–antibody complex (39). In contrast, like two sides of a coin, the antigenicity and immunoregulatory properties of proteins, including Ts-serpin as a heterologous agent, are inseparable and interact with each other during the process of recognition and elimination by the host immune system (40, 41). In fact, active immunization against a protein through vaccination often causes confusion of the two roles, which leads to instability of the effects, and the use of adjuvants can also interfere with the evaluation of the immunoregulatory properties of proteins. Specifically, our previous research on Ts-serpin indicated the disadvantages of active immunity (42). In our research, passive immunization with anti–rTs-serpin serum or injection of the recombinant protein without adjuvants was used to block or enhance the immunoregulatory characteristics in the T. spiralis infection model without changing its antigenicity. In comparison, a stable correlation in the survival burden of both adult worms and ML was found to be related to treatment with anti–rTs-serpin serum or rTs-serpin, and it was speculated to be mainly attributed to the change of immunoregulatory properties. In addition, in view of the short-term effectiveness of passive immunity on the immune response, the reduction in ML burden, which was consistent with the effects of the treatment strategies for T. spiralis infection reported previously, was considered to be caused by a decline in adult worms (43). Furthermore, because the upregulated IL-4 level was inhibited at 6 dpi, the immunoregulatory effect of rTs-serpin in the process of T. spiralis infection was supposed to be derived from immunosuppression rather than from the Th2-type immune response. All these results led to the investigation of the regulatory properties of Ts-serpin associated with early immunosuppression during T. spiralis infection.
In the early stage of T. spiralis infection, infective ML are released and undergo four molts to reach sexual maturity in intestinal columnar epithelial cells (9, 44, 45). The immune response is first concentrated in the intestine and the associated lymphoid tissue for ∼6 d (46, 47). During the early stage of infection, the intensity of inflammation in the bowel is less intense and sluggish, and it is due to immunosuppression of the host immune system by T. spiralis (48). In our study, an immunosuppressive effect was found in the early stage of T. spiralis infection mediated by Ts-serpin. In short, natural Ts-serpin induced the upregulation of anti-inflammatory cytokines and inhibited the level of proinflammatory cytokines in mLNs and peritoneal cells. According to classical immune theory, increasing levels of anti-inflammatory cytokines are thought to directly induce immunosuppression and are the main strategy for identifying target immune cells with which proteins interact (49, 50). The secretion of anti-inflammatory cytokines is mainly derived from alternatively activated macrophages or Tregs (4, 51, 52). From flow cytometry analysis, although T. spiralis induced a change in both the proportion of subsets in T cells (CD3+, CD4+, Treg) in the early stage of infection compared with the uninfected group, the data were only slightly changed after the induction of passive immunity, which implied that the regulatory effects induced by T. spiralis on T cells were independent of Ts-serpin. The effects of i.p. injection of rTs-serpin also supported our speculation, and it ruled out the possibility of secretion by Tregs as a Ts-serpin–mediated source of the high expression of IL-10 in the early infection stage. Similarly, changes in CD44 and CD62L, as markers of T cell migration and residence, were also not affected by Ts-serpin–induced immune regulation.
In previous studies on innate immune cells with immunosuppressive functions, the immune microenvironment of the tissue in which the immune cells are located is believed to play a decisive role (53). Furthermore, parasites are generally considered to use the immune response in local tissues to exert their immunoregulatory effects (54, 55). In the intestine and the peritoneal cavity, the immune microenvironments are considered to have positive defensive and anti-inflammatory functions and are often used by parasites to resist immune attacks to survive in the host (56). Moreover, macrophages residing in abdominal organs are considered to be mainly responsible for the anti-inflammatory response (57). In this study, Ts-serpin was found to regulate the phenotypes of peritoneal macrophages. Briefly, CD206 was upregulated in macrophages induced by rTs-serpin or T. spiralis infection. Interestingly, flow cytometry analysis revealed a new subset of macrophages with high expression of F4/80 that was associated with macrophage activation. This was mainly due to the different populations of macrophages in the peritoneal cavity. According to previous research, large peritoneal macrophages, which reside in the peritoneal cavity and express high levels of F4/80, are thought to mainly constitute the alternatively activated subset of Ts-serpin–mediated macrophages (58). Moreover, the F4/80 highly expressed macrophages showed the characteristics of the double-positive markers CD206 and CD16/32, which was different from the result that we found after induction by rmIL-4 in vivo, suggesting that the mechanism of rTs-serpin–mediated macrophage activation may be different from that of rmIL-4.
In response to different stimuli, macrophages are polarized into a classical or an alternative phenotype (59, 60). Traditionally, alternatively activated macrophages are induced by IL-4 after the Th2-type immune response is activated, leading to anti-inflammatory and tissue reparative effects (61). Recently, even early immune activation in T. spiralis infection was thought to be related to calcium responses evoking the mediated rapid release of Th2-type immune cytokines (62). In other words, unlike classically activated macrophages that can be directly activated by pathogen-associated molecular patterns (such as LPS) independent of autologous cytokines (such as IFN-γ), there has been no evidence for direct interaction of definitive exogenous substance that can trigger the alternative activation of macrophages independent of IL-4 or other Th2-type immune cytokines (63). Surprisingly, the inhibition of IL-4 by rTs-serpin found in the immunoregulatory experiments provides the potential for direct activation of macrophages by a single component from T. spiralis. In addition, the in vitro incubation of BMDMs with rTs-serpin preliminarily confirmed the direct interaction and regulatory effect on macrophages. Moreover, the neutralization of T. spiralis–infected serum against rTs-serpin–mediated macrophages suggested that the regulatory mechanism may be based on the structural information or biological function of the protein. As a homologous protein derived from Trichinella pseudospiralis in our previous research (64), similar enzyme inhibitory activity of rTs-serpin was confirmed in this study and was presumed to play an important role in T. spiralis infection. However, because no significant alternative activation was found in BMDMs when the recombinant protein was replaced by AEBSF with equal inhibitory units, the enzyme inhibitory activity was excluded from the regulatory mechanism of macrophages mediated by rTs-serpin. Furthermore, the interaction results showed that rTs-serpin could be displayed on the surface of BMDMs with a neutralizing serum (T. spiralis–infected serum)-mediated reversible inhibition relationship at the early stage of coincubation, which suggested the possibility of receptor-dependent activation in macrophages.
In the process of alternative activation of macrophages, activation of the STAT3 and STAT6 signaling pathways is considered to be crucial (63, 65). In this study, the upregulatory expression/phosphorylation of STAT6 was found to be consistent with protein-mediated macrophage activation, suggesting that it was involved in the internal signal transmission of protein–cell interactions. Moreover, in view of the fact that various alternative activation markers and effector molecules can be counteracted with the inhibition of STAT6, STAT6 was suggested to be a key molecule and checkpoint in rTs-serpin–mediated macrophage activation. However, as a major agonist receptor of STAT6 signaling pathway activation, IL-4Rα was not necessary for protein-mediated STAT6 activation in macrophages, and it did not participate in the interaction between rTs-serpin and BMDMs. The same results have also been demonstrated in peritoneal macrophages. This result suggested that rTs-serpin and rTs-serpin–mediated macrophages may be a key initial trigger of immunosuppression independent of Th2 cytokines in the early stage of T. spiralis infection. In addition, similar adult worm survival changes in the adoptive transfer of treated BMDMs also confirmed the importance of the regulatory properties and mechanisms of macrophages based on rTs-serpin to mediate the survival of adult worms and suppression of the host immune system in the early stage of T. spiralis infection.
Finally, to verify the anti-inflammatory properties to reverse the inflammation-mediated pathological changes, rTs-serpin and rTs-serpin–treated BMDMs were used in the TNBS-induced IBD model. In previous studies, the reversal effect on the pathological process in colitis model has been confirmed through injection of helminth-derived secretory proteins or adoptive transfer of helminth-induced macrophages (66–68). As a rapid and highly reproducible animal model, the TNBS-induced IBD model is characterized by acute colitis and exhibits a condition similar to Crohn disease (69). In this model, alleviation of pathological inflammatory processes appeared after treatment with rTs-serpin or rTs-serpin–treated BMDMs and was associated with a significant upregulation of anti-inflammatory cytokines, which supported the anti-inflammatory and regulatory effects of rTs-serpin identified previously. Moreover, based on the results of nonsymptom relief in the treatment of T. spiralis–infected serum-mediated failed-activated BMDMs and downregulation of IL-4 levels after treatment with rTs-serpin, it was demonstrated that the anti-inflammatory properties of rTs-serpin were derived from protein conformation-induced macrophage activation with high levels of anti-inflammatory cytokines independent of IL-4. In addition, reversing the experiments in the IBD model indicated the potential for the use of rTs-serpin and protein-treated functional macrophages for the intervention in acute inflammatory diseases.
In conclusion, the secretory protein Ts-serpin was found to play a key role in activating initial immunosuppression by directly activating macrophages with an alternatively activated phenotype in early T. spiralis infection and maintaining the survival of adult worms during intestinal infection. Furthermore, the anti-inflammatory properties of Ts-serpin–induced alternative activation of macrophages were demonstrated to be mediated by STAT6 activation independent of IL-4Rα. In addition, the anti-inflammatory properties of the protein were indicated to reverse the pathological changes in an IBD model. In summary, this study provides important clues for the study of immune regulation mechanisms in early T. spiralis infection and directions for the applications of T. spiralis–related proteins for acute inflammatory disease intervention.
Supplementary Material
Acknowledgments
We thank Xinrui Wang for help with evaluation of pathological processes and changes.
This work was supported by the National Natural Science Foundation of China (31520103916, 31872467), the National Key Research and Development Program of China (2017YFD0501302, 2017YFC1601206), the Guangdong Innovative and Entrepreneurial Research Team Program (2014ZT05S123), the Jilin Provincial Science and Technology Development Project (20180520042JH), and the Program for JLU Science and Technology Innovative Research Team.
The online version of this article contains supplemental material.
- AEBSF
- 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride
- BMDM
- bone marrow–derived macrophage
- DAI
- disease activity index
- dpi
- day postinfection
- elastase H
- human neutrophil elastase
- ES
- excretory/secretory
- IBD
- inflammatory bowel disease
- MFI
- mean fluorescence intensity
- ML
- muscle larvae
- mLN
- mesenteric lymph node
- mMCP-1
- mouse mast cell protease-1
- rm
- recombinant mouse
- RT
- room temperature
- rTs-serpin
- recombinant Ts-serpin
- serpin
- serine protease inhibitor
- TNBS
- trinitrobenzene sulfonic acid
- Treg
- regulatory T cell
- Ts-serpin
- serpin derived from T. spiralis Treg.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Ramanan, D., Bowcutt R., Lee S. C., Tang M. S., Kurtz Z. D., Ding Y., Honda K., Gause W. C., Blaser M. J., Bonneau R. A., et al. 2016. Helminth infection promotes colonization resistance via type 2 immunity. Science 352: 608–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reese, T. A., Wakeman B. S., Choi H. S., Hufford M. M., Huang S. C., Zhang X., Buck M. D., Jezewski A., Kambal A., Liu C. Y., et al. 2014. Helminth infection reactivates latent γ-herpesvirus via cytokine competition at a viral promoter. Science 345: 573–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gazi, U., Taylan-Ozkan A., Mumcuoglu K. Y. 2019. Immune mechanisms in human and canine demodicosis: a review. Parasite Immunol. 41: e12673. [DOI] [PubMed] [Google Scholar]
- 4.Maizels, R. M., McSorley H. J. 2016. Regulation of the host immune system by helminth parasites. J. Allergy Clin. Immunol. 138: 666–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Los Reyes Jiménez, M., Lechner A., Alessandrini F., Bohnacker S., Schindela S., Trompette A., Haimer P., Thomas D., Henke F., Mourão A., et al. 2020. An anti-inflammatory eicosanoid switch mediates the suppression of type-2 inflammation by helminth larval products. Sci. Transl. Med. 12: eaay0605. [DOI] [PubMed] [Google Scholar]
- 6.Doetze, A., Satoguina J., Burchard G., Rau T., Löliger C., Fleischer B., Hoerauf A. 2000. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by T(h)3/T(r)1-type cytokines IL-10 and transforming growth factor-beta but not by a T(h)1 to T(h)2 shift. Int. Immunol. 12: 623–630. [DOI] [PubMed] [Google Scholar]
- 7.Grogan, J. L., Kremsner P. G., Deelder A. M., Yazdanbakhsh M. 1996. Elevated proliferation and interleukin-4 release from CD4+ cells after chemotherapy in human Schistosoma haematobium infection. Eur. J. Immunol. 26: 1365–1370. [DOI] [PubMed] [Google Scholar]
- 8.Despommier, D. D. 1993. Trichinella spiralis and the concept of niche. J. Parasitol. 79: 472–482. [PubMed] [Google Scholar]
- 9.Stewart, G. L., Despommier D. D., Burnham J., Raines K. M. 1987. Trichinella spiralis: behavior, structure, and biochemistry of larvae following exposure to components of the host enteric environment. Exp. Parasitol. 63: 195–204. [DOI] [PubMed] [Google Scholar]
- 10.Scales, H. E., Ierna M. X., Lawrence C. E. 2007. The role of IL-4, IL-13 and IL-4Ralpha in the development of protective and pathological responses to Trichinella spiralis. Parasite Immunol. 29: 81–91. [DOI] [PubMed] [Google Scholar]
- 11.Faubert, G. M. 1977. Trichinella spiralis: immunosuppression in challenge infections of Swiss mice. Exp. Parasitol. 43: 336–341. [DOI] [PubMed] [Google Scholar]
- 12.Yu, Y. R., Deng M. J., Lu W. W., Jia M. Z., Wu W., Qi Y. F. 2013. Systemic cytokine profiles and splenic toll-like receptor expression during Trichinella spiralis infection. Exp. Parasitol. 134: 92–101. [DOI] [PubMed] [Google Scholar]
- 13.Ding, J., Bai X., Wang X., Shi H., Cai X., Luo X., Liu M., Liu X. 2017. Immune cell responses and cytokine profile in intestines of mice infected with Trichinella spiralis. Front. Microbiol. 8: 2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helmby, H., Grencis R. K. 2002. IL-18 regulates intestinal mastocytosis and Th2 cytokine production independently of IFN-γ during Trichinella spiralis infection. J. Immunol. 169: 2553–2560. [DOI] [PubMed] [Google Scholar]
- 15.Herwald, H., Egesten A. 2013. Macrophages: past, present and future. J. Innate Immun. 5: 657–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith, K. A., Löser S., Varyani F., Harcus Y., McSorley H. J., McKenzie A. N., Maizels R. M. 2018. Concerted IL-25R and IL-4Rα signaling drive innate type 2 effector immunity for optimal helminth expulsion. eLife 7: e38269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molehin, A. J., Gobert G. N., McManus D. P. 2012. Serine protease inhibitors of parasitic helminths. Parasitology 139: 681–695. [DOI] [PubMed] [Google Scholar]
- 18.Song, Y., Xu J., Wang X., Yang Y., Bai X., Pang J., Wang X., Yu M., Liu M., Liu X., Sun S. 2019. Regulation of host immune cells and cytokine production induced by Trichinella spiralis infection. Parasite 26: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang, L., Wang Z. Q., Hu D. D., Cui J. 2013. Proteomic analysis of Trichinella spiralis muscle larval excretory-secretory proteins recognized by early infection sera. BioMed Res. Int. 2013: 139745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson, M. W., Greig R., Beattie K. A., Lamont D. J., Connolly B. 2007. Comparative analysis of the excretory-secretory proteome of the muscle larva of Trichinella pseudospiralis and Trichinella spiralis. Int. J. Parasitol. 37: 139–148. [DOI] [PubMed] [Google Scholar]
- 21.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals 2011. Guide for the Care and Use of Laboratory Animals. National Academies Press (US), Washington, DC. [PubMed] [Google Scholar]
- 22.Lee, M. C., Lacey D. C., Fleetwood A. J., Achuthan A., Hamilton J. A., Cook A. D. 2019. GM-CSF- and IRF4-dependent signaling can regulate myeloid cell numbers and the macrophage phenotype during inflammation. J. Immunol. 202: 3033–3040. [DOI] [PubMed] [Google Scholar]
- 23.Steel, N., Faniyi A. A., Rahman S., Swietlik S., Czajkowska B. I., Chan B. T., Hardgrave A., Steel A., Sparwasser T. D., Assas M. B., et al. 2019. TGFβ-activation by dendritic cells drives Th17 induction and intestinal contractility and augments the expulsion of the parasite Trichinella spiralis in mice. PLoS Pathog. 15: e1007657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei, P., Lu Q., Cui G., Guan Z., Yang L., Sun C., Sun W., Peng Q. 2015. The role of TREM-2 in internalization and intracellular survival of Brucella abortus in murine macrophages. Vet. Immunol. Immunopathol. 163: 194–201. [DOI] [PubMed] [Google Scholar]
- 25.Wu, L.-H., Xu Z.-L., Dong D., He S.-A., Yu H. 2011. Protective effect of anthocyanins extract from blueberry on TNBS-induced IBD model of mice. Evid. Based Complement. Alternat. Med. 2011: 525462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo, J., Yang L. J., Sun M., Xu L. F. 2019. Inhibiting microRNA-7 expression exhibited a protective effect on intestinal mucosal injury in TNBS-induced inflammatory bowel disease animal model. Inflammation 42: 2267–2277. [DOI] [PubMed] [Google Scholar]
- 27.Chen, L., Wang J., You Q., He S., Meng Q., Gao J., Wu X., Shen Y., Sun Y., Wu X., Xu Q. 2018. Activating AMPK to restore tight junction assembly in intestinal epithelium and to attenuate experimental colitis by metformin. Front. Pharmacol. 9: 761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milstone, A. M., Harrison L. M., Bungiro R. D., Kuzmic P., Cappello M. 2000. A broad spectrum Kunitz type serine protease inhibitor secreted by the hookworm Ancylostoma ceylanicum. J. Biol. Chem. 275: 29391–29399. [DOI] [PubMed] [Google Scholar]
- 29.Rhoads, M. L., Fetterer R. H., Hill D. E., Urban J. F. Jr 2000. Trichuris suis: a secretory chymotrypsin/elastase inhibitor with potential as an immunomodulator. Exp. Parasitol. 95: 36–44. [DOI] [PubMed] [Google Scholar]
- 30.Despommier, D. D., Müller M. 1976. The stichosome and its secretion granules in the mature muscle larva of Trichinella spiralis. J. Parasitol. 62: 775–785. [PubMed] [Google Scholar]
- 31.Khan, W. I. 2008. Physiological changes in the gastrointestinal tract and host protective immunity: learning from the mouse-Trichinella spiralis model. Parasitology 135: 671–682. [DOI] [PubMed] [Google Scholar]
- 32.Bell, R. G. 1998. The generation and expression of immunity to Trichinella spiralis in laboratory rodents. Adv. Parasitol. 41: 149–217. [DOI] [PubMed] [Google Scholar]
- 33.Gerencer, M., Marinculić A., Rapić D., Franković M., Valpotić I. 1992. Immunosuppression of in vivo and in vitro lymphocyte responses in swine induced by Trichinella spiralis or excretory-secretory antigens of the parasite. Vet. Parasitol. 44: 263–273. [DOI] [PubMed] [Google Scholar]
- 34.Møller, L. N., Petersen E., Gamble H. R., Kapel C. M. 2005. Comparison of two antigens for demonstration of Trichinella spp. antibodies in blood and muscle fluid of foxes, pigs and wild boars. Vet. Parasitol. 132: 81–84. [DOI] [PubMed] [Google Scholar]
- 35.Kapel, C. M., Gamble H. R. 2000. Infectivity, persistence, and antibody response to domestic and sylvatic Trichinella spp. in experimentally infected pigs. Int. J. Parasitol. 30: 215–221. [DOI] [PubMed] [Google Scholar]
- 36.Kaufmann, S. H. E., Dorhoi A. 2016. Molecular determinants in phagocyte-bacteria interactions. Immunity 44: 476–491. [DOI] [PubMed] [Google Scholar]
- 37.Yin, Q., Fu T. M., Li J., Wu H. 2015. Structural biology of innate immunity. Annu. Rev. Immunol. 33: 393–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor, P. R., Martinez-Pomares L., Stacey M., Lin H. H., Brown G. D., Gordon S. 2005. Macrophage receptors and immune recognition. Annu. Rev. Immunol. 23: 901–944. [DOI] [PubMed] [Google Scholar]
- 39.Shale, D. J., Faux J. A. 1985. The report of Kaufman et al. on the relationship between agar gel double-diffusion and ELISA results for Aspergillus fumigatus IgG antibody. J. Allergy Clin. Immunol. 76: 128. [DOI] [PubMed] [Google Scholar]
- 40.Dorhoi, A., Reece S. T., Kaufmann S. H. 2011. For better or for worse: the immune response against Mycobacterium tuberculosis balances pathology and protection. Immunol. Rev. 240: 235–251. [DOI] [PubMed] [Google Scholar]
- 41.O’Shea, J. J., Paul W. E. 2010. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science 327: 1098–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu, J., Bai X., Wang L. B., Shi H. N., van der Giessen J. W. B., Boireau P., Liu M. Y., Liu X. L. 2017. Influence of adjuvant formulation on inducing immune response in mice immunized with a recombinant serpin from Trichinella spiralis. Parasite Immunol. 39: e12437. [DOI] [PubMed] [Google Scholar]
- 43.Singh, A., Frazee B., Talan D. A., Ng V., Perez B. 2018. A tricky diagnosis. N. Engl. J. Med. 379: 1364–1369. [DOI] [PubMed] [Google Scholar]
- 44.Guiliano, D. B., Oksov Y., Lustigman S., Gounaris K., Selkirk M. E. 2009. Characterisation of novel protein families secreted by muscle stage larvae of Trichinella spiralis. Int. J. Parasitol. 39: 515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Capo, V., Despommier D. D., Silberstein D. S. 1984. The site of ecdysis of the L1 larva of Trichinella spiralis. J. Parasitol. 70: 992–994. [PubMed] [Google Scholar]
- 46.Vos, J. G., Ruitenberg E. J., Van Basten N., Buys J., Elgersma A., Kruizinga W. 1983. The athymic nude rat. IV. Immunocytochemical study to detect T-cells, and immunological and histopathological reactions against Trichinella spiralis. Parasite Immunol. 5: 195–215. [DOI] [PubMed] [Google Scholar]
- 47.Ruitenberg, E. J., Elgersma A. 1976. Absence of intestinal mast cell response in congenitally athymic mice during Trichinella spiralis infection. Nature 264: 258–260. [DOI] [PubMed] [Google Scholar]
- 48.Ilic, N., Gruden-Movsesijan A., Sofronic-Milosavljevic L. 2012. Trichinella spiralis: shaping the immune response. Immunol. Res. 52: 111–119. [DOI] [PubMed] [Google Scholar]
- 49.Li, Y., Guan X., Liu W., Chen H. L., Truscott J., Beyatli S., Metwali A., Weiner G. J., Zavazava N., Blumberg R. S., et al. 2018. Helminth-induced production of TGF-β and suppression of graft-versus-host disease is dependent on IL-4 production by host cells. J. Immunol. 201: 2910–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marple, A., Wu W., Shah S., Zhao Y., Du P., Gause W. C., Yap G. S. 2017. Cutting edge: helminth coinfection blocks effector differentiation of CD8 T cells through alternate host Th2- and IL-10-mediated responses. [Published erratum appears in 2017 J. Immunol. 199: 3005.] J. Immunol. 198: 634–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith, P. L., Lombardi G., Foster G. R. 2005. Type I interferons and the innate immune response--more than just antiviral cytokines. Mol. Immunol. 42: 869–877. [DOI] [PubMed] [Google Scholar]
- 52.Stenger, S., Röllinghoff M. 2001. Role of cytokines in the innate immune response to intracellular pathogens. Ann. Rheum. Dis. 60(Suppl 3): iii43–iii46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hepworth, M. R., Fung T. C., Masur S. H., Kelsen J. R., McConnell F. M., Dubrot J., Withers D. R., Hugues S., Farrar M. A., Reith W., et al. 2015. Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4+ T cells. Science 348: 1031–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meli, A. P., Fontés G., Leung Soo C., King I. L. 2017. T follicular helper cell-derived IL-4 is required for IgE production during intestinal helminth infection. J. Immunol. 199: 244–252. [DOI] [PubMed] [Google Scholar]
- 55.Reynolds, L. A., Finlay B. B., Maizels R. M. 2015. Cohabitation in the intestine: interactions among helminth parasites, bacterial microbiota, and host immunity. J. Immunol. 195: 4059–4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kennedy, J. C., Treadwell P. E., Lennox E. S. 1970. Antigen-specific synergism in the immune response of irradiated mice given marrow cells and peritoneal cavity cells or extracts. J. Exp. Med. 132: 353–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jenkins, S. J., Ruckerl D., Cook P. C., Jones L. H., Finkelman F. D., van Rooijen N., MacDonald A. S., Allen J. E. 2011. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332: 1284–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghosn, E. E., Cassado A. A., Govoni G. R., Fukuhara T., Yang Y., Monack D. M., Bortoluci K. R., Almeida S. R., Herzenberg L. A., Herzenberg L. A. 2010. Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc. Natl. Acad. Sci. USA 107: 2568–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Biswas, S. K., Mantovani A. 2010. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat. Immunol. 11: 889–896. [DOI] [PubMed] [Google Scholar]
- 60.Varin, A., Mukhopadhyay S., Herbein G., Gordon S. 2010. Alternative activation of macrophages by IL-4 impairs phagocytosis of pathogens but potentiates microbial-induced signalling and cytokine secretion. Blood 115: 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bosurgi, L., Cao Y. G., Cabeza-Cabrerizo M., Tucci A., Hughes L. D., Kong Y., Weinstein J. S., Licona-Limon P., Schmid E. T., Pelorosso F., et al. 2017. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science 356: 1072–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luo, X. C., Chen Z. H., Xue J. B., Zhao D. X., Lu C., Li Y. H., Li S. M., Du Y. W., Liu Q., Wang P., et al. 2019. Infection by the parasitic helminth Trichinella spiralis activates a Tas2r-mediated signaling pathway in intestinal tuft cells. Proc. Natl. Acad. Sci. USA 116: 5564–5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gordon, S., Martinez F. O. 2010. Alternative activation of macrophages: mechanism and functions. Immunity 32: 593–604. [DOI] [PubMed] [Google Scholar]
- 64.Xu, N., Liu X., Tang B., Wang L., Shi H. N., Boireau P., Liu M., Bai X. 2017. Recombinant Trichinella pseudospiralis serine protease inhibitors alter macrophage polarization in vitro. Front. Microbiol. 8: 1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chávez-Galán, L., Olleros M. L., Vesin D., Garcia I. 2015. Much more than M1 and M2 macrophages, there are also CD169(+) and TCR(+) macrophages. Front. Immunol. 6: 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang, S. A., Park M. K., Park S. K., Choi J. H., Lee D. I., Song S. M., Yu H. S. 2019. Adoptive transfer of Trichinella spiralis-activated macrophages can ameliorate both Th1- and Th2-activated inflammation in murine models. Sci. Rep. 9: 6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang, S., Xie Y., Yang X., Wang X., Yan K., Zhong Z., Wang X., Xu Y., Zhang Y., Liu F., Shen J. 2016. Therapeutic potential of recombinant cystatin from Schistosoma japonicum in TNBS-induced experimental colitis of mice. Parasit. Vectors 9: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ziegler, T., Rausch S., Steinfelder S., Klotz C., Hepworth M. R., Kühl A. A., Burda P. C., Lucius R., Hartmann S. 2015. A novel regulatory macrophage induced by a helminth molecule instructs IL-10 in CD4+ T cells and protects against mucosal inflammation. J. Immunol. 194: 1555–1564. [DOI] [PubMed] [Google Scholar]
- 69.Silva, I., Pinto R., Mateus V. 2019. Preclinical study in vivo for new pharmacological approaches in inflammatory bowel disease: a systematic review of chronic model of TNBS-induced colitis. J. Clin. Med. 8: 1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.