Abstract
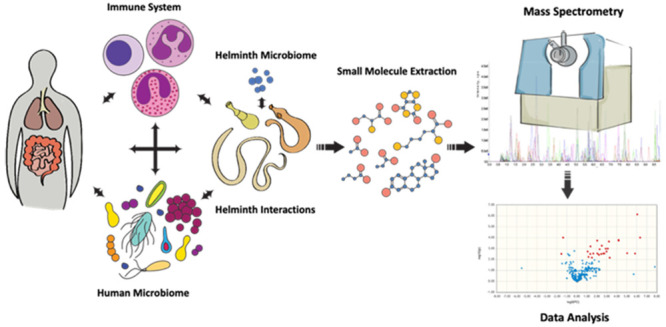
Helminths represent a diverse category of parasitic organisms that can thrive within a host for years, if not decades, in the absence of treatment. As such, they must establish mechanisms to subsist off their hosts, evade the immune system, and develop a niche among the other cohabiting microbial communities. The complex interplay of biologically small molecules (collectively known as the metabolome) derived from, utilized by, or in response to the presence of helminths within a host is an emerging field of study. In this Perspective, we briefly summarize the current existing literature, categorize key host–pathogen–microbiome interfaces that could be studied in the context of the metabolome, and provide background on mass spectrometry-based metabolomic methodology. Overall, we hope to provide a comprehensive guide for utilizing metabolomics in the context of helminthic disease.
Keywords: metabolomics, parasites, helminths, microbiome, immunology, mass spectrometry
Helminths infect over 2 billion people worldwide;1−4 that is, over 25% of the world’s population may have one or more worm infections caused by parasitic roundworms (nematodes) and/or flatworms (cestodes and trematodes). The most prevalent infections are caused by the soil-transmitted helminths (STHs), Ascaris and Trichuris, and hookworms, which are among the roughly 20 neglected tropical diseases designated by the WHO.5 These species alone infect over a billion people worldwide and account for an estimated 1.9 million disability-adjusted life years (DALYs) due to anemia, malnutrition, and cognitive deficits associated with moderate- to high-burden infections.6
To date, there are no vaccines to treat any human helminth infection, and there are only a few anthelmintic drugs that are safe and effective. With the lack of therapeutics and the rising concern over the development of drug resistance, there is a critical need to identify new therapeutic candidates. The counterparts to applying appropriate therapy are an accurate diagnosis and tests-of-cure. Microscopic exam and serology are the gold standard for diagnosing helminth infections, and few PCR-based molecular diagnostics are available;7 therefore, identification of new biomarkers and the testing of methodologies are key in exploring more sensitive, specific, and dynamic diagnostics.
Historically, the biological and molecular complexities of parasitic helminths have impeded the research on therapeutics and diagnostics, but these have been partly overcome by the wide use of systems biology “omics”. These approaches reveal information on the genome, transcriptome, and proteome of the biological agent and are revolutionizing parasitology. For example, the combination of genomics, transcriptomics, and proteomics data has provided essential molecular information about a specific tissue of interest (the nematode intestine), and advanced bioinformatic approaches provide the means to predict intestinal cell functional categories of seminal importance to parasite survival.8 Such predictions can now be experimentally tested and validated. Other examples include interfacing helminth omics with chemogenomics that have proven to identify and prioritize drugs and drug targets much more successfully compared to high-throughput screening. Moreover, such predictive models have been experimentally validated as the identified drugs have been shown to have broad potential (made possible due to the application of evolutionary genomics principles),9 and a subsequent rational drug design strategy has capitalized on parasite-specific molecular features,10 which result in improved potency and selectivity for the parasite versus the host.11
For helminth parasitologists, a growing field of investigation is metabolomics, which evaluates the downstream exogenous and endogenous small molecules in a biological system. This field has shown major growth due to major advancements in liquid chromatography combined with tandem mass spectrometry (LC-MS/MS). A multiomics approach can deepen our understanding of the parasites at a systems biology level, which is essential for making progress toward applications to prevent and treat infections by helminths.12
Metabolomics is used to profile the small molecule metabolic products of a biological specimen at a specific condition and point in time, thereby providing a snapshot of its physiological state. Profiling metabolic substrates and products and relating them to metabolic pathways can aid in defining key “chokepoints” critical to the parasite and/or host. A “chokepoint reaction” is defined as a reaction that necessitates a unique substrate or produces a unique product for a biological function.3,13 If the chokepoint enzyme (of either host or parasite origin) can be inhibited, the entire metabolic pathway can be blocked, leading to downstream parasite dysfunction. Pan-Nematoda conserved metabolic chokepoint enzymes have been identified using omics driven approaches, and a targeted repurposing approach has identified new small molecules with broad spectrum anthelmintic activity.9,11 Taylor et al.13 used an innovative approach to generate a compartmentalized metabolic and constraint-based model of the parasitic nematode Brugia malayi. This model, which divided the metabolic network into three compartments, the cytosol and mitochondria of B. malayi and the Wolbachia endosymbiont, predicted a set of 102 reactions essential to the survival of the worm, including switching between aerobic and anaerobic pathways and a novel pathway that relies on the catabolism of glutamate to aspartate to produce energy. The identification of chokepoints in conjunction with a systems biology approach can lead to a greater understanding of the metabolic pathways essential for parasite survival, as will the compartmentalized approach to study helminths and their endosymbionts that enables investigators to predict essential energy and metabolic growth pathways and identify potential therapeutic targets to treat helminthic infections.
Approaches and Limitations of Mass Spectrometry-Based Metabolomics
The direct study of biologically small molecules can be carried out by nuclear magnetic resonance (NMR) or mass spectrometry (MS). A recent publication has provided an excellent review of NMR methodology and applications to helminth metabolomics research.14 In this Perspective, we highlight recent work on the metabolomics of parasitic helminths using mass spectrometry (MS) and discuss the possibilities of what the future might hold for parasitic helminth metabolomics. Table 1 summarizes a select number of studies using MS to profile the metabolites of dogs infected with Toxocara canis, the life cycle stages of the soil-transmitted nematode (Ascaris lumbricoides), and the excretory–secretory products from Nippostrongylus brasiliensis, Trichuris muris, Ancylostoma caninum, schistosomes, and the dog/cat tapeworm to study immunomodulation and the host response. Studies to identify biomarkers in urine from individuals infected with Onchocerca volvulus and biomarkers of liver disease caused by Opisthorchis felineus are also included. Comprehensive reviews on parasite metabolomics have been published by Preidis and Hotez;12 Zheng et al.;15 Melo et al.;16 Wangchuk et al.17
Table 1. Summary of Select Investigations Highlighting the Metabolomics of Parasitic Helminths Using Mass Spectrometry.
| helminth | samples | findings | reference |
|---|---|---|---|
| Toxocara canis (dog roundworm) | infected dog serum for host metabolites | metabolic profiles of sera 24 h, 10 days, and 36 days postinfection showed alterations of bile acid, steroid hormone, and unsaturated fatty acid synthesis pathways from dog host | Zheng et al.15 |
| Ascaris lumbricoides (human roundworm) | eggs, including larval stages from stool samples from infected individuals, Brazil for biomarkers of infection | metabolites for worm eggs: hexadecenal, 21-methyl-8Z-pentatriacontene, and 3,7,11,15-tetramethyltritriacontane; for first-stage worm larvae: 2-deoxyecdysone 22-phosphate, cholesterol ester, sphingomyelin, and cardiolipin; for third-stage worm larvae: dimethylheptatriacontane | Melo et al.16 |
| Nippostrongylus brasiliensis and Trichuris muris (nematodes) | excretory–secretory products (ESPs) from worms | identified polar and nonpolar small molecules from worm ESPs with as many as 17 metabolites known to exhibit various pharmacological activities | Wangchuk et al.17 |
| Ancylostoma caninum (dog hookworm) | somatic worm extracts and excretory–secretory products from worm | low molecular weight metabolites from worm products suppressed inflammation in a murine model of colitis and reduced cytokine secretion by human peripheral blood mononuclear cells | Wangchuk et al.18 |
| Ancylostoma caninum (dog hookworm) | viable and nonviable A. caninum eggs | prostaglandin, myristic acid, and lauric acid were found in viable but not nonviable ova isolated from dog feces; metabolites may be useful in a rapid diagnostic screening test for the presence of viable hookworm ova | Gyawali et al.19 |
| Onchocerca volvulus (filarial nematode) | urine samples from onchocerciasis positive and negative individuals | N-acetyltyramine-O,β-glucuronide (NATOG) was identified as a biomarker using LC-MS on patient urine samples; NATOG-based lateral-flow immunoassay for onchocerciasis identified 85% of the 27 patient urine samples | Globisch et al.;20 Shirey et al.21 |
| Schistosoma mansoni (blood fluke) | soluble egg antigen (SEA) from schistosomes for immunomodulation | SEAs from worms bind to Dectin-1 and Dectin-2 on dendritic cells, resulting in the synthesis of eicosanoid prostaglandin E2 and the expression of OX40 ligand, enabling them to promote the Th2 response; SEA contains analytes including docosahexaenoic acid, linoleic acid, arachidonic acid, PGE2, and PGD2. | Kaisar et al.22 |
| Schistosoma mansoni (blood fluke) | S. mansoni cercariae, worms, eggs, SEA products for immunomodulation | quantified ∼350 lipid species and characterized the lipid profiles of different parasite life history stages; detected several immunomodulatory oxylipids in the different life cycle stages; prostaglandins highly enriched in egg preparations; resolvins were specifically detected in cercariae | Giera et al.23 |
| Schistosoma hematobium (blood fluke) | urine and plasma of urogenital schistosomiasis and associated bladder pathologies, Nigeria for biomarkers of pathogenesis | in infection-only and advanced cases: low levels of host sex steroids, high levels of several benzenoids, catechols, and lipids (including ganglioside, phosphatidylcholine, and phosphatidylethanolamine) | Adebayo et al.24 |
| Schistosoma hematobium (blood fluke) | urine samples of urogenital schistosomiasis and urothelial cell carcinoma, Angola for diagnostic biomarkers | estrogen-like metabolites in urogenital schistosomiasis cases but not in healthy humans; metabolites included catechol estrogen quinones (CEQ) and CEQ-DNA adducts; novel metabolites derived from 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) were found in urine from urogenital schistosomiasis cases | Gouveia et al.25 |
| Schistosoma japonicum (blood fluke) | male and female adult worms from SCID and BALB/c mice for metabolism of the host/pathogen relationship | more differential metabolites in female worms than male worms between SCID and BALB/c mice; male worms included bile acid biosynthesis, taurine and hypotaurine metabolism, sphingolipid metabolism, retinol metabolism, purine metabolism; enriched metabolite sets of differential metabolites in female worms included retinol metabolism, alpha linolenic acid and linoleic acid metabolism, purine, sphingolipid, and glutamate metabolism | Lui et al.26 |
| Opisthorchis felineus (liver fluke) | hamster sera, urine, bile; adult worms and eggs for biomarkers of liver disease | numerous oxysterols and related DNA adducts detected in the liver fluke eggs and in bile from infected hamsters | Gouveia et al.27 |
| Dipylidium caninum (dog and cat tapeworm) | excretory–secretory products (ESPs) from ex vivo worms | 49 metabolites identified from ESPs collected from worms ex vivo; 12 of which have known pharmacological properties, e.g, anti-inflammatory agents; top 10 polar metabolites were succinic acid (major component), lactic acid, myo-inositol, scyllo-inositol, tyrosine, malic acid, talose, glucose, glycerol, and phenylalanine | Wangchuk et al.28 |
Key Areas of Metabolomic Exploration in Helminth Infections
While it is easiest to think about the influence of the helminth metabolome as a direct connection between pathogen and host phenotype, the modulators are a much more complicated and interconnected system. With the explosion of microbiome and immunology-related research, major findings have begun to intertwine these interactions between many organ systems and disease states. One key facet of these interactions has been the study of metabolic products and their origins and influence on each system. While the gut microbiota has shown major metabolomic influence,29 studies including the evaluation of helminth metabolomic interactions have been lacking. Therefore, we present an outline of key areas of interaction that can be used for hypothesis generation to evaluate the metabolome’s role during helminth infections.
Helminths and the Immune System
The immune system has long been studied in the context of parasitic helminths, particularly the induction of type 2 (Th2) and regulatory T-cell (Treg) responses.30,31 Helminthic modulation of the immune system generally induces an anti-inflammatory or immune tolerance response. These changes have mainly been studied in the context of helminth excreted/secreted/cell surface proteins, leaving significant areas of investigation to explore regarding small molecule production. One central recurring finding that has been explored in the context of both helminths and the microbiome is the effect of short chain fatty acids (SCFAs) on the induction of Treg immune suppression responses.32,33
Giera et al.23 used lipidomics to characterize the lipid profiles of different life history stages of Schistosoma mansoni and found several immunomodulatory oxylipids; specifically, prostaglandins were highly enriched in egg preparations and resolvins were specifically detected in cercariae. Lipidomic analyses of soluble egg antigens (SEAs) of S. mansoni also revealed that SEAs bind to the pattern-recognition receptors Dectin-1 and Dectin-2 on dendritic cells, resulting in the synthesis of eicosanoid prostaglandin E2 and the expression of the OX40 ligand, enabling them to promote a Th2 response.22 Though these studies may not be broadly applicable to all helminth interactions with the immune system, they demonstrate distinct immunomodulatory components of some helminth-derived small molecules, which suggest further evaluation is needed at this host–pathogen interface.
Helminths and the Host Microbiome
The dysbiosis of the gut microbiome is generally thought of in the context of infections related to pathogenic bacteria or viruses; however, helminths can also inhabit this community as pathogens. When discussing this interface, one must explore the interactions between both helminths and microbial communities as well as the microbial communities and host health. A number of studies in animal models and humans have shown various chronic helminth infections can change microbiome populations and relate to disease states.34−36 For example, human studies have shown an increased diversity of the gut microbiome and identified conserved bacterial taxa positively and negatively associated with soil-transmitted helminths (STHs) in different endemic countries, suggesting changes associated with infection regardless of baseline variation of the microbiomes in different countries.37 Furthermore, the gut microbiota responds to anthelmintic treatment; however, after deworming, the microbiome assembles in a state that does not resemble an uninfected state.38 These cross-kingdom interactions in the human gut ecosystem in individuals infected with STH species have been studied at the taxonomic, genetic, and functional levels, opening the door for undertaking key mechanistic studies in the future. There is a need for a better understanding of the microbiome changes following albendazole treatment since a study with samples from Kenya had shown that albendazole treatment alters the microbiome structure, especially in individuals infected with Necator americanus,39 but a study in Indonesia showed that albendazole does not affect the microbiome composition.43 Of importance is to note that changes in the microbiome are likely dependent on the methodologies used (e.g., depth of coverage generated by next generation sequencing platforms), burden of infection (e.g., low vs moderate to heavy worm infections), and the type/tropism of infection.
These population changes have been studied in the context of direct or indirect (i.e., helminth-produced antibacterial molecules) changes in the mechanical barriers of the GI epithelium and immune regulation. In terms of the direct influence of the metabolome, the microbiome has been extensively studied in the context of small molecule regulators with significant findings in relation to host pathologies and are beyond the scope of this Perspective.29 In a recent study of human volunteers infected with Strongyloides stercoralis, the authors report that, in addition to the observed increased alpha diversity and decreased beta diversity of the fecal profiles of infected vs noninfected individuals, there was an increased abundance of specific metabolites in the infected individuals (specific amino acids) and SCFAs in the noninfected individuals.40 Suffice it to say, there is a wealth of emerging evidence on the sources and effects of the metabolite interplay between host, microbes, and macrobes that could be due to shifts in metabolite consumption/production or altered gastrointestinal absorption.
Microbiomes of Helminths
The complex interactions of microbiota with host organisms also applies to helminths themselves. This is well-known in the filarial nematodes (Onchocerca volvulus, Wuchereria bancrofti, and Brugia malayi) and their endosymbiotic bacterium Wolbachia.41 This bacterium is necessary for the reproduction and survival of these organisms, largely due to the production of essential nutrients for the helminths including heme, riboflavin, and flavin adenine dinucleotide (FAD).42
Other helminth endosymbionts (e.g., Neorickettsia) and models of microbiome acquisitions are emerging with great potential to discover necessary or novel metabolites as a source of novel biology in this system.43,44 As with any endosymbiotic relationship, challenges remain separating the symbiont from its host but new technologies such as single cell analyses can be applied to omic investigations with bacteria and their helminth hosts.
Helminth/Host Metabolites as Biomarkers of Infection
As one may imagine, the diversity of parasitic helminths and their life cycles greatly complicates the ability to study and discuss metabolism in a cohesive manner. Classical biochemical pathways have been established for a number of helminths, though the subject has not been revisited in recent literature, to our knowledge.45,46 An emerging area of study in microbial communities within animals is the production of xenometabolites or small molecule products either synthesized de novo or derived from host metabolites. Though this is not a new subject, given the knowledge of the gut microbiome’s role in bilirubin derivation/excretion and vitamin K production, advances in small molecule mass spectrometry have opened up the ability to discover and characterize novel molecules.47
One example that may be applied to this category is the discovery of numerous estrogen-like metabolites from urogenital schistosomiasis cases but not in healthy humans that were observed in urine samples and included catechol estrogen quinones (CEQ) and CEQ-DNA adducts. Novel metabolites derived from 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) were also identified in urine from all 40 urogenital schistosomiasis cases.24 Similar experiments in broader surveys of helminth infections will be of great importance for characterizing biologically relevant compounds and biomarkers of pathogenesis.
An interesting example of a biomarker that is both a parasite- and host-derived metabolite is N-acetyltyramine-O,β-glucuronide (NATOG).20 NATOG is a neurotransmitter derived from a combination of metabolic steps occurring in both the nematode and host; tyramine, a neurotransmitter from Onchocerca volvulus, is acetylated by the worm to form N-acetylturamine, which is glucuronidated by the host. This biomarker is being used to develop a lateral-flow immunoassay for the diagnosis of onchocerciasis or river blindness,21 a chronic disease that affects roughly 18 million people in mostly low/middle income countries in sub-Saharan Africa.
Other potential biomarkers were identified in serum and urine samples from humanized mice infected with O. volvulus using LC-MS/MS.48 The study focused on worm-derived proteins and found that, of all the proteins that were identified, 155 were detected from infected mice and not the from the control mice. This study also noted that the proteins that were most likely to be useful as biomarkers were among those with an unknown function, and due to the low number and abundance of O. volvulus-specific proteins, samples had to be pooled from each group of mice for analysis. The study points to the challenges helminthologists face when attempting to identify many of the novel parasite metabolites. It is essential that newly generated helminth-related metabolomic data (including metabolite structures and their reference spectra) are submitted in public databases (e.g., MetaboLights49) along with information on the metabolomics experiment and derived information, so that novel metabolites that have not been previously reported can be documented and, in the future, be confirmed in independent experiments in the same or different helminth species.
Overview of Mass Spectrometry-Based Metabolomics
With a number of interesting metabolomic interfaces to explore, understanding the methodology is key to designing and controlling for meaningful results. Below, we have provided a comprehensive overview of small molecule mass spectrometry techniques and how they may be applied to the study of parasitic helminths.
Targeted versus Untargeted Mass Spectrometry
Mass spectrometry techniques for metabolomics can be broadly separated into three categories: targeted, untargeted, and flux studies (Figure 1).
Figure 1.
Mass spectrometry methods used in metabolomics. (A) Targeted metabolomics analyze individual compounds defined by the user. Individual methods must be developed for each analyte. (B) A specialized form of targeted metabolomics called flux analysis or fluxomics is used to identify and quantify metabolites as they move through the metabolic pathways, achieved by the introduction of heavy isotope labeled (e.g., 13C, 15N, 2H) substrates. (C) Untargeted metabolomics generally uses a different type of mass spectrometer to detect and measure analytes without a priori knowledge of what is being analyzed. The heterogeneity in sensitivity and quantification is the major limitation in comparison to targeted methods.
Targeted mass spectrometry utilizes triple quadrupole mass analyzers, which isolate each analyte of interest for the detection and quantification with an individually optimized method. Compound-related stable isotope internal standards are used to normalize for preanalytical and analytical variation of the assay, greatly improving accuracy and precision. Untargeted metabolomics utilizes a newer generation of quadrupole high-resolution mass spectrometers (QHRMS), which combine front-end tandem mass spectrometry and back-end high-resolution time-of-flight or orbitrap technologies to obtain mass resolutions below 1 × 10–4 daltons. This allows for data acquisition techniques that can parse analytes using the quadrupole mass filters and then trigger fragmentation into product ions and collect high-resolution spectra. The fingerprint of an analyte’s product ion spectra can give specific structural information about the compound and greatly increase confidence in the metabolite identification. While QHRMS methods greatly increase the number of analytes that can potentially be surveyed, the results may be skewed by the fact that not all analytes will be equally detectable nor quantifiable by one general mass spectrometry method. When one both designs a study and compares evidence, understanding the utility and limitations of both targeted and untargeted metabolomics approaches is essential.
Metabolomic flux (also referred to as “fluxomics”) can measure and trace analytes through metabolic pathways.50,51 To do this, stable isotope (2H, 13C, 15N) labeled primary metabolites (e.g., glucose or amino acids) are added to a model system (e.g., parasite culture or infected animals) and identified throughout downstream pathways due to the measurable mass difference and quantifiable ratio of heavy isotope incorporation in metabolized products. This is particularly attractive for the study of parasitic infections considering labeled metabolites can hypothetically be traced between both host and parasite to elucidate essential metabolic relationships.52
When Should Investigators Use Targeted versus Untargeted Mass Spectrometry?
In general, these experiments can be broken into hypothesis testing and hypothesis generating. For targeted metabolomics, one would want to evaluate the key intermediates of a metabolic pathway(s) that are known to be dysregulated in an experimental system, such as SCFA production from an in vivo infection model. Fluxomics, as a subset of targeted metabolomics, is useful when dynamic information about metabolites within a pathway is needed. An example of this could be exploration on the utilization of a class of metabolites or metabolic pathways (e.g., glycolysis/gluconeogenesis, fatty acid oxidation, purine metabolism, etc.) under various infection or treatment conditions. Untargeted metabolomics is useful when a researcher can modulate a system and wants to broadly explore the metabolic changes, e.g., exploring changes in profiles following anthelmintic therapy.
Harmonizing Helminth Metabolomics Methodologies
The field of metabolomics is currently adapting to the constant evolution of instrumentation and informatics. As such, the harmonization of methods and materials to ensure accurate and reproducible findings has been a moving target for the entirety of the field.53 Therefore, it is incumbent upon the investigators to clearly publish detailed methods for their metabolomics experiments and promote protocol sharing and communication whenever possible. Below, we will detail areas of variance in metabolomics studies to propose a framework for key areas of methodologic harmonization going forward.
Preanalytical variables can be a major point of discordance between metabolomics studies.54 Because metabolites do not represent one distinct class of biological macromolecules (such as nucleic acids or polypeptides), there can be many sources of variability and interference. These can include the type of specimen collection devices, metabolite extraction protocols, and lipid depletion strategies to name a few. Overall, how the sample is handled and prepared may lead to a differential representation of individual metabolites or metabolite classes entering the mass spectrometer for analysis.
Understanding natural variabilities of specimen types is also important for appropriate metabolomics study design. For example, the metabolomic variability of stool specimens is expected to be much higher than that of peripheral blood considering the gut contents will include all variations in diet, where the serum reflects only a physiologically balanced net absorption/excretion of gut products. Delineations must even be accounted for between seemingly equivalent specimens, such as serum and plasma, considering the higher content of protein from clotting factors can affect extraction efficiency.
Analytically, one must take into consideration both the chromatography and modality of mass spectrometry when analyzing study design. Both gas chromatography (GC) and liquid chromatography (LC) are established for the upfront separation of analytes and as a parameter of data analysis. Advances in ultra high-performance liquid chromatography (UHPLC) and column chemistry have brought LC-MS to the forefront of metabolomic methods. However, GC is still predominantly used for the analysis of volatiles (alcohols, esters, ketones, etc.) and SCFAs. Even within LC, it is not uncommon that two UHPLC methods, such as reverse phase and hydrophilic interaction (HILIC), will be run on each sample to achieve appropriate separation for polar, semipolar, and nonpolar metabolites. Even then, the exact choice of column chemistry can affect confidence in compound detection.55
When confronting the variability of the mass spectrometry component, the most important factor in study design is the source of library spectra for metabolite identification. Differences in mass spectrometry source and compound-dependent parameters of a method can create different product ion spectra, which can confound identification and quantification. Ideally, one’s metabolomics experiments should be analyzed with the same method parameters in the same mass spectrometry facility when at all possible.
Postanalytical Variables
Postanalytical variables in metabolomics mostly relate to data analysis, which encompasses confidence in metabolite identification (for untargeted data) and statistical analysis approaches for large metabolite data sets. Each metabolite identification by LC-MS has three major components: (1) LC retention time (RT), (2) mass accuracy to the theoretical chemical formula, and (3) product ion spectra library match. Metabolomics standards initiatives (MSIs) have sought to grade confidence in metabolite identification by assigning confidence levels utilizing these components.56,57 The decision to align with MSI confidence level criteria or to define a different system is largely up to the researcher but should be clearly outlined within the methodology of a subsequent publication. The ability to get to high confidence identifications relies greatly on the quality and source of product ion spectral libraries, which are produced by curating method-specific product ion spectra of authentic standards or in silico prediction. Online spectral libraries58 provide an attractive resource for searching the largest number of metabolites possible; however, the quality of the spectral data can vary greatly depending on the analyte standard used to generate the library entry and the MS method parameters (as noted above). When possible, using an in-house spectral library, where all standards were run on the same instrument parameters as the specimens, will provide the highest confidence in accurate metabolite identification.
The statistical approach of data analysis becomes a second source of postanalytical variance; however, this is not specific to metabolomic studies and therefore beyond the scope of this article. It will suffice to say that the most common forms of metabolomic data analysis include volcano plots, hierarchical clustering, and multivariate component analyses, of which the specifics of the data set and experimental questions are key for choosing the appropriate statistical approach. While many of these approaches can be performed using general statistical software, resources such as MetaboAnalyst provide robust data analysis tools appropriate for metabolomics data with curated tutorials and guidance.59
Harmonization of Metabolomic Methodology and Future Directions for Studying Parasitic Helminths
Given the diversity of parasitic helminths, it is essential for the field to harmonize metabolomic experimentation to increase confidence in the reproducibility of the findings and in undertaking comparative metabolomic studies. One key bottom-up approach would be the utilization of helminth-specific protocols for sample collection, preparation, and metabolite extraction. Metabolomics data repositories, such as Metabolomics Workbench60 (https://www.metabolomicsworkbench.org), provide the capacity for standardized formatting and open access for these protocols. As the field evolves, a more centralized parasitic helminth database for combining metabolomic, proteomic, and transcriptomic/genomic data would be an ideal source for the community.
Another direction for increasing harmonization would be specialized metabolomics centers for parasitic diseases. Metabolomics-driven science is often broadly generalized to any type of sample sets, yet aspects of study design and biological interpretation may be key to understanding the unique and divergent biology of helminths under the dynamic conditions of host–parasite interactions. The development of a center combining primary metabolomics methodologists and infectious diseases experts will be essential for producing high quality studies that innovate the field.
Conclusions
Metabolomics can be studied in the context of helminth infections in a number of ways including in vitro parasite culture, in vivo animal models, or clinical studies in humans. The results and conclusions derived from this research must encompass the interconnected systems of helminth infections, human immunology, and microbiome dysbiosis (of both the worm and host). Major areas of focus for studying metabolomics in helminth infections include understanding and characterizing helminth-derived small molecule and metabolic interactions in the context of pathogenesis, drug design, and biomarker discovery. Lastly, for this research to provide meaningful and reproducible metabolomic findings, researchers must take it upon themselves to be versed in the strengths and limitations of the methodology and advocate best practices of this mass spectrometry-driven science.
Acknowledgments
The participation of J.D.W. was supported by a grant from the National Heart, Lung, and Blood Institute at the National Institutes of Health under award number K38HL154203. J.A.S. was supported by the Bill and Melinda Gates Foundation under award number OPP1017584. M.M. was supported by the National Institute of General Medical Sciences Grant R01GM097435. The funding sources had no role in the study design, collection, analysis and interpretation of the data, preparation of the manuscript, or the decision to submit for publication. This publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of their sponsors.
The authors declare no competing financial interest.
References
- Hotez P. J. (2018) Human Parasitology and Parasitic Diseases: Heading Towards 2050. Adv. Parasitol. 100, 29–38. 10.1016/bs.apar.2018.03.002. [DOI] [PubMed] [Google Scholar]
- King C. H. (2019) Chapter Two: Helminthiasis Epidemiology and Control: Scoring Successes and Meeting the Remaining Challenges. In Advances in Parasitology (Keiser J., Ed.) Vol. 103, pp 11–30, Academic Press, 10.1016/bs.apar.2018.08.001. [DOI] [PubMed] [Google Scholar]
- (2019) Comparative genomics of the major parasitic worms. Nat. Genet. 51 (1), 163–174. 10.1038/s41588-018-0262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392 (10159), 1789–1858. 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (accessed 2020-03-21) Neglected tropical diseases: Summary, https://www.who.int/neglected_diseases/diseases/en/.
- Freeman M. C.; Akogun O.; Belizario V. Jr.; Brooker S. J.; Gyorkos T. W.; Imtiaz R.; Krolewiecki A.; Lee S.; Matendechero S. H.; Pullan R. L.; Utzinger J. (2019) Challenges and opportunities for control and elimination of soil-transmitted helminth infection beyond 2020. PLoS Neglected Trop. Dis. 13, e0007201. 10.1371/journal.pntd.0007201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant J. R.; Pilotte N.; Williams S. A. (2019) A Case for Using Genomics and a Bioinformatics Pipeline to Develop Sensitive and Species-Specific PCR-Based Diagnostics for Soil-Transmitted Helminths. Front. Genet. 10, 883. 10.3389/fgene.2019.00883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmer D. P.; Rosa B. A.; Tyagi R.; Mitreva M. (2019) Omics Driven Understanding of the Intestines of Parasitic Nematodes. Front. Genet. 10, 652. 10.3389/fgene.2019.00652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. M.; Martin J.; Rao R. U.; Powell K.; Abubucker S.; Mitreva M. (2013) Using existing drugs as leads for broad spectrum anthelmintics targeting protein kinases. PLoS Pathog. 9 (2), e1003149. 10.1371/journal.ppat.1003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.; Heizer E.; Rosa B. A.; Wildman S. A.; Janetka J. W.; Mitreva M. (2016) Characterization of parasite-specific indels and their proposed relevance for selective anthelminthic drug targeting. Infect., Genet. Evol. 39, 201–211. 10.1016/j.meegid.2016.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi R.; Maddirala A. R.; Elfawal M.; Fischer C.; Bulman C. A.; Rosa B. A.; Gao X.; Chugani R.; Zhou M.; Helander J.; Brindley P. J.; Tseng C. C.; Greig I. R.; Sakanari J.; Wildman S. A.; Aroian R.; Janetka J. W.; Mitreva M. (2018) Small Molecule Inhibitors of Metabolic Enzymes Repurposed as a New Class of Anthelmintics. ACS Infect. Dis. 4 (7), 1130–1145. 10.1021/acsinfecdis.8b00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preidis G. A.; Hotez P. J. (2015) The newest ″omics″--metagenomics and metabolomics--enter the battle against the neglected tropical diseases. PLoS Neglected Trop. Dis. 9 (2), e0003382. 10.1371/journal.pntd.0003382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. M.; Wang Q.; Rosa B. A.; Huang S. C.; Powell K.; Schedl T.; Pearce E. J.; Abubucker S.; Mitreva M. (2013) Discovery of anthelmintic drug targets and drugs using chokepoints in nematode metabolic pathways. PLoS Pathog. 9 (8), e1003505. 10.1371/journal.ppat.1003505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokova D.; Mayboroda O. A. (2019) Twenty Years on: Metabolomics in Helminth Research. Trends Parasitol. 35 (4), 282–288. 10.1016/j.pt.2019.01.012. [DOI] [PubMed] [Google Scholar]
- Zheng W. B.; Zou Y.; Elsheikha H. M.; Liu G. H.; Hu M. H.; Wang S. L.; Zhu X. Q. (2019) Serum metabolomic alterations in Beagle dogs experimentally infected with Toxocara canis. Parasites Vectors 12 (1), 447. 10.1186/s13071-019-3703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo C. F.; Esteves C. Z.; de Oliveira R. N.; Guerreiro T. M.; de Oliveira D. N.; Lima E. O.; Mine J. C.; Allegretti S. M.; Catharino R. R. (2016) Early developmental stages of Ascaris lumbricoides featured by high-resolution mass spectrometry. Parasitol. Res. 115 (11), 4107–4114. 10.1007/s00436-016-5183-2. [DOI] [PubMed] [Google Scholar]
- Wangchuk P.; Kouremenos K.; Eichenberger R. M.; Pearson M.; Susianto A.; Wishart D. S.; McConville M. J.; Loukas A. (2019) Metabolomic profiling of the excretory-secretory products of hookworm and whipworm. Metabolomics 15 (7), 101. 10.1007/s11306-019-1561-y. [DOI] [PubMed] [Google Scholar]
- Wangchuk P.; Shepherd C.; Constantinoiu C.; Ryan R. Y. M.; Kouremenos K. A.; Becker L.; Jones L.; Buitrago G.; Giacomin P.; Wilson D.; Daly N.; McConville M. J.; Miles J. J.; Loukas A. (2019) Hookworm-Derived Metabolites Suppress Pathology in a Mouse Model of Colitis and Inhibit Secretion of Key Inflammatory Cytokines in Primary Human Leukocytes. Infect. Immun. 87 (4), e00851-18. 10.1128/IAI.00851-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyawali P.; Beale D. J.; Ahmed W.; Karpe A. V.; Magalhaes R. J.; Morrison P. D.; Palombo E. A. (2016) Determination of Ancylostoma caninum ova viability using metabolic profiling. Parasitol. Res. 115 (9), 3485–92. 10.1007/s00436-016-5112-4. [DOI] [PubMed] [Google Scholar]
- Globisch D.; Moreno A. Y.; Hixon M. S.; Nunes A. A.; Denery J. R.; Specht S.; Hoerauf A.; Janda K. D. (2013) Onchocerca volvulus-neurotransmitter tyramine is a biomarker for river blindness. Proc. Natl. Acad. Sci. U. S. A. 110 (11), 4218–23. 10.1073/pnas.1221969110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirey R. J.; Globisch D.; Eubanks L. M.; Hixon M. S.; Janda K. D. (2018) Noninvasive Urine Biomarker Lateral Flow Immunoassay for Monitoring Active Onchocerciasis. ACS Infect. Dis. 4 (10), 1423–1431. 10.1021/acsinfecdis.8b00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaisar M. M. M.; Ritter M.; Del Fresno C.; Jonasdottir H. S.; van der Ham A. J.; Pelgrom L. R.; Schramm G.; Layland L. E.; Sancho D.; Prazeres da Costa C.; Giera M.; Yazdanbakhsh M.; Everts B. (2018) Dectin-1/2-induced autocrine PGE2 signaling licenses dendritic cells to prime Th2 responses. PLoS Biol. 16 (4), e2005504. 10.1371/journal.pbio.2005504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giera M.; Kaisar M. M. M.; Derks R. J. E.; Steenvoorden E.; Kruize Y. C. M.; Hokke C. H.; Yazdanbakhsh M.; Everts B. (2018) The Schistosoma mansoni lipidome: Leads for immunomodulation. Anal. Chim. Acta 1037, 107–118. 10.1016/j.aca.2017.11.058. [DOI] [PubMed] [Google Scholar]
- Adebayo A. S.; Mundhe S. D.; Awobode H. O.; Onile O. S.; Agunloye A. M.; Isokpehi R. D.; Shouche Y. S.; Santhakumari B.; Anumudu C. I. (2018) Metabolite profiling for biomarkers in Schistosoma haematobium infection and associated bladder pathologies. PLoS Neglected Trop. Dis. 12 (4), e0006452. 10.1371/journal.pntd.0006452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia M. J.; Santos J.; Brindley P. J.; Rinaldi G.; Lopes C.; Santos L. L.; da Costa J. M.; Vale N. (2015) Estrogen-like metabolites and DNA-adducts in urogenital schistosomiasis-associated bladder cancer. Cancer Lett. 359 (2), 226–32. 10.1016/j.canlet.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Liu R.; Cheng W. J.; Tang H. B.; Zhong Q. P.; Ming Z. P.; Dong H. F. (2019) Comparative Metabonomic Investigations of Schistosoma japonicum From SCID Mice and BALB/c Mice: Clues to Developmental Abnormality of Schistosome in the Immunodeficient Host. Front. Microbiol. 10, 440. 10.3389/fmicb.2019.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia M. J.; Pakharukova M. Y.; Laha T.; Sripa B.; Maksimova G. A.; Rinaldi G.; Brindley P. J.; Mordvinov V. A.; Amaro T.; Santos L. L.; Costa J.; Vale N. (2017) Infection with Opisthorchis felineus induces intraepithelial neoplasia of the biliary tract in a rodent model. Carcinogenesis 38 (9), 929–937. 10.1093/carcin/bgx042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangchuk P.; Constantinoiu C.; Eichenberger R. M.; Field M.; Loukas A. (2019) Characterization of Tapeworm Metabolites and Their Reported Biological Activities. Molecules 24 (8), 1480. 10.3390/molecules24081480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon G.; Garg N.; Debelius J.; Knight R.; Dorrestein P. C.; Mazmanian S. K. (2014) Specialized metabolites from the microbiome in health and disease. Cell Metab. 20 (5), 719–730. 10.1016/j.cmet.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizels R. M.; McSorley H. J. (2016) Regulation of the host immune system by helminth parasites. J. Allergy Clin. Immunol. 138 (3), 666–675. 10.1016/j.jaci.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizels R. M.; Smits H. H.; McSorley H. J. (2018) Modulation of Host Immunity by Helminths: The Expanding Repertoire of Parasite Effector Molecules. Immunity 49 (5), 801–818. 10.1016/j.immuni.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiss M. M.; Rapin A.; Lebon L.; Dubey L. K.; Mosconi I.; Sarter K.; Piersigilli A.; Menin L.; Walker A. W.; Rougemont J.; Paerewijck O.; Geldhof P.; McCoy K. D.; Macpherson A. J.; Croese J.; Giacomin P. R.; Loukas A.; Junt T.; Marsland B. J.; Harris N. L. (2015) The Intestinal Microbiota Contributes to the Ability of Helminths to Modulate Allergic Inflammation. Immunity 43 (5), 998–1010. 10.1016/j.immuni.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tielens A. G.; van Grinsven K. W.; Henze K.; van Hellemond J. J.; Martin W. (2010) Acetate formation in the energy metabolism of parasitic helminths and protists. Int. J. Parasitol. 40 (4), 387–97. 10.1016/j.ijpara.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Reynolds L. A.; Finlay B. B.; Maizels R. M. (2015) Cohabitation in the Intestine: Interactions among Helminth Parasites, Bacterial Microbiota, and Host Immunity. J. Immunol. 195 (9), 4059–66. 10.4049/jimmunol.1501432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gause W. C.; Maizels R. M. (2016) Macrobiota - helminths as active participants and partners of the microbiota in host intestinal homeostasis. Curr. Opin. Microbiol. 32, 14–18. 10.1016/j.mib.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds L. A.; Redpath S. A.; Yurist-Doutsch S.; Gill N.; Brown E. M.; van der Heijden J.; Brosschot T. P.; Han J.; Marshall N. C.; Woodward S. E.; Valdez Y.; Borchers C. H.; Perona-Wright G.; Finlay B. B. (2017) Enteric Helminths Promote Salmonella Coinfection by Altering the Intestinal Metabolome. J. Infect. Dis. 215 (8), 1245–1254. 10.1093/infdis/jix141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C.; Tang M. S.; Lim Y. A.; Choy S. H.; Kurtz Z. D.; Cox L. M.; Gundra U. M.; Cho I.; Bonneau R.; Blaser M. J.; Chua K. H.; Loke P. (2014) Helminth colonization is associated with increased diversity of the gut microbiota. PLoS Neglected Trop. Dis. 8 (5), e2880. 10.1371/journal.pntd.0002880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa B. A.; Supali T.; Gankpala L.; Djuardi Y.; Sartono E.; Zhou Y.; Fischer K.; Martin J.; Tyagi R.; Bolay F. K.; Fischer P. U.; Yazdanbakhsh M.; Mitreva M. (2018) Differential human gut microbiome assemblages during soil-transmitted helminth infections in Indonesia and Liberia. Microbiome 6 (1), 33. 10.1186/s40168-018-0416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton A. V.; Quinones M.; Vujkovic-Cvijin I.; Oliveira R. G.; Kepha S.; Odiere M. R.; Anderson R. M.; Belkaid Y.; Nutman T. B. (2019) The Impact of Anthelmintic Treatment on Human Gut Microbiota Based on Cross-Sectional and Pre- and Postdeworming Comparisons in Western Kenya. mBio 10 (2), e00519-19. 10.1128/mBio.00519-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins T. P.; Formenti F.; Castro C.; Piubelli C.; Perandin F.; Buonfrate D.; Otranto D.; Griffin J. L.; Krause L.; Bisoffi Z.; Cantacessi C. (2018) A comprehensive analysis of the faecal microbiome and metabolome of Strongyloides stercoralis infected volunteers from a non-endemic area. Sci. Rep. 8 (1), 15651. 10.1038/s41598-018-33937-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. J.; Voronin D.; Johnston K. L.; Ford L. (2013) Wolbachia filarial interactions. Cell. Microbiol. 15 (4), 520–6. 10.1111/cmi.12084. [DOI] [PubMed] [Google Scholar]
- Slatko B. E.; Taylor M. J.; Foster J. M. (2010) The Wolbachia endosymbiont as an anti-filarial nematode target. Symbiosis 51 (1), 55–65. 10.1007/s13199-010-0067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins T. P.; Brindley P. J.; Gasser R. B.; Cantacessi C. (2019) Helminth Microbiomes - A Hidden Treasure Trove?. Trends Parasitol. 35 (1), 13–22. 10.1016/j.pt.2018.10.007. [DOI] [PubMed] [Google Scholar]
- McNulty S. N.; Tort J. F.; Rinaldi G.; Fischer K.; Rosa B. A.; Smircich P.; Fontenla S.; Choi Y. J.; Tyagi R.; Hallsworth-Pepin K.; Mann V. H.; Kammili L.; Latham P. S.; Dell’Oca N.; Dominguez F.; Carmona C.; Fischer P. U.; Brindley P. J.; Mitreva M. (2017) Genomes of Fasciola hepatica from the Americas Reveal Colonization with Neorickettsia Endobacteria Related to the Agents of Potomac Horse and Human Sennetsu Fevers. PLoS Genet. 13 (1), e1006537. 10.1371/journal.pgen.1006537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saz H. J. (1981) Energy metabolisms of parasitic helminths: adaptations to parasitism. Annu. Rev. Physiol. 43, 323–41. 10.1146/annurev.ph.43.030181.001543. [DOI] [PubMed] [Google Scholar]
- Barrett J. (1991) Amino acid metabolism in helminths. Adv. Parasitol. 30, 39–105. 10.1016/S0065-308X(08)60306-1. [DOI] [PubMed] [Google Scholar]
- Postler T. S.; Ghosh S. (2017) Understanding the Holobiont: How Microbial Metabolites Affect Human Health and Shape the Immune System. Cell Metab. 26 (1), 110–130. 10.1016/j.cmet.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J. B.; Bennuru S.; Eberhard M. L.; Hess J. A.; Torigian A.; Lustigman S.; Nutman T. B.; Abraham D. (2018) Development of Onchocerca volvulus in humanized NSG mice and detection of parasite biomarkers in urine and serum. PLoS Neglected Trop. Dis. 12 (12), e0006977. 10.1371/journal.pntd.0006977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug K.; Cochrane K.; Nainala V. C.; Williams M.; Chang J.; Jayaseelan K. V.; O’Donovan C. (2019) MetaboLights: a resource evolving in response to the needs of its scientific community. Nucleic Acids Res. 48 (D1), D440–D444. 10.1093/nar/gkz1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A.; Kowalski G. M.; Callahan D. L.; Meikle P. J.; Creek D. J. (2016) Strategies for Extending Metabolomics Studies with Stable Isotope Labelling and Fluxomics. Metabolites 6 (4), 32. 10.3390/metabo6040032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni N.; Saghatelian A.; Patti G. J. (2015) Defining the metabolome: size, flux, and regulation. Mol. Cell 58 (4), 699–706. 10.1016/j.molcel.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafsack B. F.; Llinas M. (2010) Eating at the table of another: metabolomics of host-parasite interactions. Cell Host Microbe 7 (2), 90–9. 10.1016/j.chom.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker E. S.; Patti G. J. (2019) Perspectives on Data Analysis in Metabolomics: Points of Agreement and Disagreement from the 2018 ASMS Fall Workshop. J. Am. Soc. Mass Spectrom. 30 (10), 2031–2036. 10.1007/s13361-019-02295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin P.; Lehmann R.; Xu G. (2015) Effects of pre-analytical processes on blood samples used in metabolomics studies. Anal. Bioanal. Chem. 407 (17), 4879–92. 10.1007/s00216-015-8565-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naser F. J.; Mahieu N. G.; Wang L.; Spalding J. L.; Johnson S. L.; Patti G. J. (2018) Two complementary reversed-phase separations for comprehensive coverage of the semipolar and nonpolar metabolome. Anal. Bioanal. Chem. 410 (4), 1287–1297. 10.1007/s00216-017-0768-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creek D. J.; Dunn W. B.; Fiehn O.; Griffin J. L.; Hall R. D.; Lei Z.; Mistrik R.; Neumann S.; Schymanski E. L.; Sumner L. W.; Trengove R.; Wolfender J.-L. (2014) Metabolite identification: are you sure? And how do your peers gauge your confidence?. Metabolomics 10 (3), 350–353. 10.1007/s11306-014-0656-8. [DOI] [Google Scholar]
- Sumner L. W.; Amberg A.; Barrett D.; Beale M. H.; Beger R.; Daykin C. A.; Fan T. W.; Fiehn O.; Goodacre R.; Griffin J. L.; Hankemeier T.; Hardy N.; Harnly J.; Higashi R.; Kopka J.; Lane A. N.; Lindon J. C.; Marriott P.; Nicholls A. W.; Reily M. D.; Thaden J. J.; Viant M. R. (2007) Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 3 (3), 211–221. 10.1007/s11306-007-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metabolomics Society (accessed 2020-12-05) Databases, http://metabolomicssociety.org/resources/metabolomics-databases.
- Chong J.; Soufan O.; Li C.; Caraus I.; Li S.; Bourque G.; Wishart D. S.; Xia J. (2018) MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 46 (W1), W486–W494. 10.1093/nar/gky310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sud M.; Fahy E.; Cotter D.; Azam K.; Vadivelu I.; Burant C.; Edison A.; Fiehn O.; Higashi R.; Nair K. S.; Sumner S.; Subramaniam S. (2016) Metabolomics Workbench: An international repository for metabolomics data and metadata, metabolite standards, protocols, tutorials and training, and analysis tools. Nucleic Acids Res. 44 (D1), D463–70. 10.1093/nar/gkv1042. [DOI] [PMC free article] [PubMed] [Google Scholar]



