Abstract
Increasing concern over rising levels of antibiotic resistance among pathogenic bacteria has prompted significant research into developing efficacious alternatives to antibiotic treatment. Previously, we have reported on the therapeutic activity of a dinuclear ruthenium(II) complex against pathogenic, multi-drug-resistant bacterial pathogens. Herein, we report that the solubility properties of this lead are comparable to those exhibited by orally available therapeutics that in comparison to clinically relevant antibiotics it induces very slow evolution of resistance in the uropathogenic, therapeutically resistant, E. coli strain EC958, and this resistance was lost when exposure to the compound was temporarily removed. With the aim of further investigating the mechanism of action of this compound, the regulation of nine target genes relating to the membrane, DNA damage, and other stress responses provoked by exposure to the compound was also studied. This analysis confirmed that the compound causes a significant transcriptional downregulation of genes involved in membrane transport and the tricarboxylic acid cycle. By contrast, expression of the chaperone protein-coding gene, spy, was significantly increased suggesting a requirement for repair of damaged proteins in the region of the outer membrane. The complex was also found to display activity comparable to that in E. coli in a range of other therapeutically relevant Gram-negative pathogens.
Keywords: antimicrobials, pathogens, resistance, ruthenium, transcriptomics
Over recent years, there has been a significant increase in bacterial infections that display multi-drug resistance, leading to a concomitant increase in mortality rates.1−3 As last-line treatments such as carbapenems increasingly fail,3−6 antimicrobial resistance is rapidly becoming a global threat to public health and the economy.7−9 It has been estimated that by 2050 a total of 10 million lives per year and a cumulative $100 trillion of economic output will be lost due to the rise of drug-resistant infections.10
Escherichia coli strains are a significant cause of infection within clinical settings and are linked to high morbidity and mortality globally causing a wide range of infections including meningitis, pneumonia, and bacteraemia.11E. coli is highly prevalent in urinary tract infections and accounts for 80% of all community-acquired urinary tract infections.12,13 High antimicrobial resistance within uropathogenic E. coli (UPEC) strains is common; a study of antibiotic resistance in cases of urinary tract infections in Nigeria found that from a total of 137 E. coli isolates 36% were resistant to 10 out of 11 urine line antibiotics.14
In this context, UPEC sequence type ST131 is an emerging pathogen of particular concern. Apart from being commonly resistant to fluoroquinolones, this strain produces the CTX-M extended spectrum β-lactamase that confers resistance to oxyimino-cephalosporins and monobactams.15−18 Furthermore, CTX-M-encoding genes are found on plasmids which frequently carry additional resistance genes. As a consequence, geographical variants commonly possess quinolone-modifying enzymes that provide fluoroquinolone resistance, as well as the enzymes carbapenemases and cephamycinases.19
As global concern increased, the United States surveillance programs SENTRY and MYSTIC estimated through extrapolation that ST131 accounted for approximately 17% of all E. coli isolates, 44% of all antimicrobial resistant isolates, and around 68% of fluoroquinolone-resistant isolates.16,20 This is problematic as within the United States fluoroquinolones are prescribed as a first-line treatment against urinary tract infections. Given these facts, it is unsurprising that even when treated with standard antibiotic regimes urinary tract infections caused by ST131 can dangerously progress into pyelonephritis and sepsis.21,22
With one-third of women having a course of antibiotics to treat a urinary tract infection by the age of 26,23 multi-drug-resistant uropathogenic bacteria are clearly a significant threat, with ST131 being at the forefront of concern. It is therefore apparent that urine line antibiotics are a pivotal tool in healthcare, and as they are quickly becoming less effective, it is crucial that novel treatment options are identified.24
Metal complexes are a class of compounds that demonstrated significant early promise as therapeutic leads but are underdeveloped. As early as the 1950s, the Dwyer group reported that RuII polypyridyl complexes had potential as antimicrobials.25,26 Their work demonstrated that increasing the lipophilicity of the parent [Ru(phen)3]2+ cation resulted in enhanced antimicrobial action, leading to a derivative that displayed promising activity against Gram-positive bacteria. However, due to the wide range of effective conventional antibiotics clinically available at that time, no further development of these distinctive leads occurred for decades.
In the past decade or so, due to the growing antibiotic resistance crisis, the use of metals as antimicrobials has been revisited,27−32 with particular focus on the RuII systems,28,33 although most of these newly reported systems still exhibit higher activities against Gram-positive bacteria. As part of a program to develop novel metal-complex-based imaging probes,34−36 therapeutics,37−40 and phototherapeutics,41−45 we recently identified a series of dinuclear ruthenium(II) complexes that exhibit higher activity against Gram-negative species.46 Subsequent detailed studies involving several strains of Staphylococcus aureus indicated that the lowered activity against Gram-positive bacteria is due to the complexes binding to cell wall teichoic acids residues, leading to reduced internalization.47
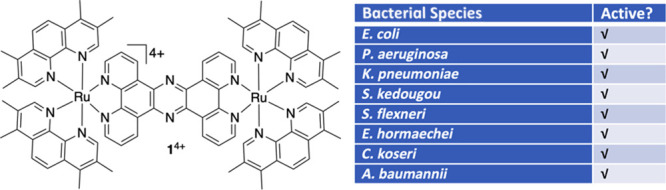
Although these reports provided preliminary insights into the mechanism of action of these new potential therapeutics, in this study a deeper and more focused understanding of the antimicrobial activity of the main lead complex, 14+ (Figure 1) is developed. Although the complex was synthesized as a hexafluorophosphate salt, for biological studies anion metathesis was used to convert the complex into the Cl− salt form. Using the multi-drug-resistant uropathogenic strain of E. coli as a model Gram-negative pathogen, quantitative PCR (qPCR) was used to monitor the regulation of key genes thought to be responsible for sensing and reacting to the presence of 14+, providing further insights into its mechanism of action. As we also find that the complex displays solubility comparable with established orally available therapeutics, that it is active against a range of Gram-negative pathogens, and that resistance toward it emerges only very slowly, these data further underline its therapeutic potential.
Figure 1.
Structure of the ruthenium(II) complex relevant to this report.
Results
Kinetic Turbidimetric Solubility
Over the last two decades, largely as a consequence of high-through-put screening and demand for structurally complex drug leads, poor aqueous solubility has increasingly become a limiting factor in drug development; compounds with poor solubility present high attrition risks and increased drug development costs.48−50 As complex 14+ displays activity against Gram-negative pathogens and it is known that solubility is a key criterion for oral availability of any drug lead,51 the complex’s solubility was assessed through kinetic turbidimetric stability assays (Figure 2).
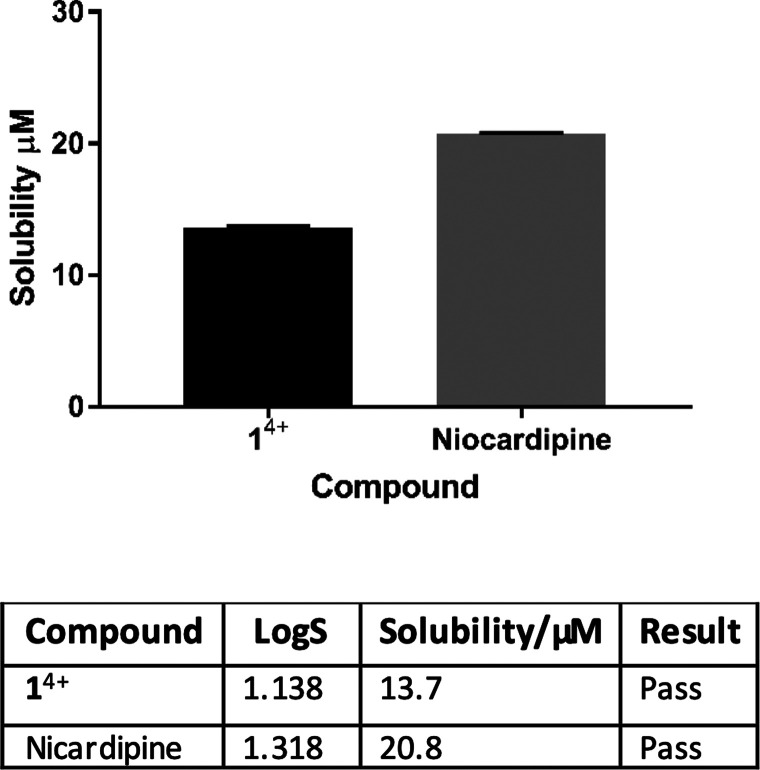
Figure 2.
Kinetic turbidimetric solubility. The kinetic solubility of 14+ measured through turbidimetry and compared with a positive control, nicardipine. Turbidimetry was measured at seven controls (0.2–100 μM) in DMSO (1%). Samples were incubated for 5 min at 25 °C. Absorbance was determined at 620 nm. N = 4 ± SD. Any complex with a solubility of <1 μM is considered insoluble and therefore fails the solubility assay.
Nicardipine, a drug used to treat high blood pressure and angina that is frequently employed in drug metabolism and pharmacokinetic studies, was used as a positive control. It was found that 14+ exhibits solubility in the range of this control; see the Supporting Information for details. Under the Biopharmaceutics Classification System provided by the FDA, which is used to predict intestinal drug absorption, [1]Cl4 is seen to be freely soluble.52 As aqueous solubility is a major factor in the bioavailability of antimicrobial compounds, the high solubility of 14+ indicates that it is a good orally delivered drug candidate.
Resistance of Escherichia coli EC958 to Clinical Antibiotics
The uropathogenic E. coli strain EC958 used in this study is a sequence type ST131 isolate. Due to the presence of the extended-spectrum β-lactamase gene blaCTX-M-15 on its pEC958 virulence plasmid it has been designated as a Priority 1 Critical Pathogen by the World Health Organization that urgently requires new treatments.18 To confirm the categorization and antibiotic resistance profile of this EC958 clinical isolate, phenotypic testing of its sensitivity to several β-lactam antibiotics was carried out. These studies (which included a monobactam and different generations of cephalosporins, as well as various other major groups of antibiotics) were accomplished through a standardized EUCAST disc diffusion assay (Table 1).53
Table 1. EUCAST Disc Diffusion Antibiotic Sensitivity Testing against E. coli Strain EC958.
| antibiotic | disk content (μg) | mean zone diameter (mm) ± SEM | sensitivitya |
|---|---|---|---|
| Cephalosporins | |||
| cefotaxime | 3 | 10 ± 0 | resistant |
| ceftazidime | 30 | 9.5 ± 0.47 | resistant |
| cefuroxime | 30 | 0 ± 0 | resistant |
| Tetracyclines | |||
| tigecycline | 15 | 23 ± 0 | sensitive |
| Monobactams | |||
| aztreonam | 30 | 19.5 ± 0.47 | resistant |
| Carbapenems | |||
| doripenem | 10 | 29 ± 0 | sensitiveb |
| meropenem | 10 | 31.5 ± 0.47 | sensitive |
| ertapenem | 10 | 26 ± 0.47 | sensitive |
| imipenem | 10 | 29.7 ± 0.47 | sensitive |
| Aminoglycosides | |||
| gentamicin | 10 | 15 ± 0 | resistant |
| Fluoroquinolones | |||
| ciprofloxacin | 5 | 0 ± 0 | resistant |
| levofloxacin | 5 | 0 ± 0 | resistant |
| Miscellaneous | |||
| rifampicin | 2 | 0 ± 0 | resistant |
| fosfomycin | 50 | 28.5 ± 0.82 | sensitive |
| nitrofurantoin | 100 | 22 ± 0 | sensitive |
The tests revealed that the strain shows resistance to all tested cephalosporins and the monobactam aztreonam, thus confirming its Priority 1 categorization; however, it is still sensitive to carbapenems, tigecycline, fosfomycin, and nitrofurantoin.
Growth and Viability of Growing E. coli Strain EC958 Cultures Is Diminished upon Exposure to 14+
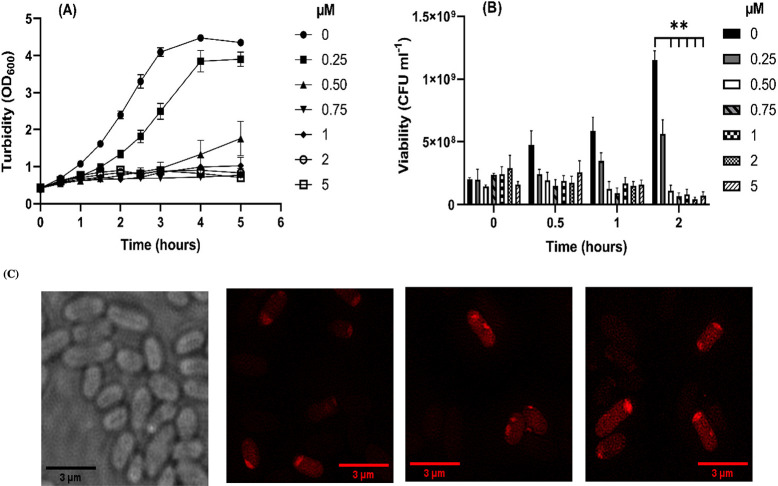
Having established the multi-drug-resistance properties of the model EC958 strain, we investigated its sensitivity to complex, 14+. Previously, we determined that the minimal inhibitory concentration (MIC) of 14+ was 2.8 μM;46 however, the MIC is measured using very low turbidity cultures recently diluted from the stationary phase. As treatment of most infections occurs when the bacterial load is already high and actively growing, we investigated what effects exposure to the compound had on actively growing cultures. Growth assays in which cultures in the early exponential growth phase were injected with a range of concentrations of 14+ below and above the MIC were performed. As Figure 3 illustrates, very little effect on growth is observed within the first 30 min of exposure to the compound. Subsequently, growth in the presence of 14+ becomes inhibited for at least 2.5 h, and the extent of growth inhibition correlates with increasing concentration of the compound. After this period, there is some recovery at concentrations up to 1 μM, but above this concentration, little recovery in growth is seen. To understand whether 14+ was affecting viability or causing bacteriostasis at these concentrations, viability assays were performed (Figure 3B). From 2 h postinjection, a significant difference in viability is observed between untreated cultures and those exposed to 0.5–5 μM of 14+. In agreement with this data, fluorescence microscopy shows accumulation of the RuII complex between 20 and 120 min and a significant increase in fluorescence at the 60–120 min time points, indicating further accumulation of the compound (Figure 3C). An increase in fluorescence intensity was measured using the integrated density measurement function on ImageJ. At 60 min, the average corrected total cell fluorescence (CTCF) for the cells was 9308.9; at 120 min, the average CTCF for the cells was 29956.9. This is in agreement with previous findings indicating that ruthenium accumulates within the cell during this time.46
Figure 3.
Effect of 14+ concentration on the growth and viability of E. coli strain EC958 in glucose defined minimal medium. (A) Cultures were grown to early exponential phase at 37 °C and 150 rpm shaking in 250 mL flasks with defined minimal medium containing glucose as the sole carbon source. Upon reaching an OD600 ∼ 0.4 differing concentrations of 14+ (0–5 μM) were added to the cultures and growth was monitored at regular intervals. N ≥ 3 ± SEM. (B) Samples of the cultures described in (A) were removed and viable counts performed to determine the bactericidal effect of 14+ at differing concentrations between 0–5 μM. N ≥ 3 ± SEM. Statistical significance (**) was determined with two-way ANOVA and Tukey’s multiple comparisons (p < 0.05). (C) Localization of 14+ in E. coli EC958 cells was visualized through structured illumination microscopy at 0, 20, 60, and 120 min. Cells were imaged using the emission of 14+ on excitation at 450 nm using A568 filter. After treatment with 0.8 μM 14+, cells were washed with nitric acid before fixing with paraformaldehyde (4%). Images were taken using a 1516× oil objective and SlowFade Gold Antifade Mountant.
Evolution of Resistance of E. coli Strain EC958 to 14+
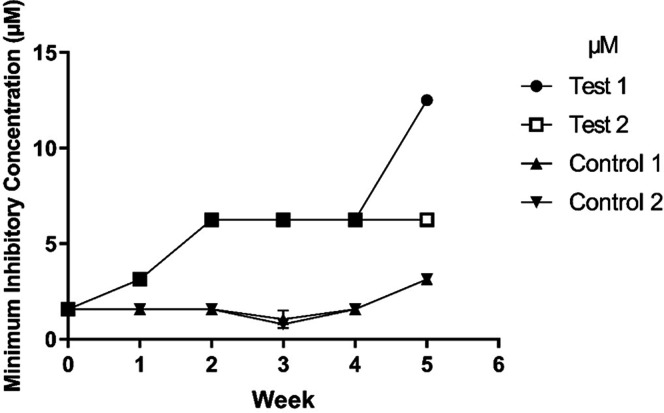
The potential of 14+ as a putative antimicrobial lead would be enhanced if therapeutic resistance either does not develop rapidly or does not develop to a high degree. To investigate this, we serially passaged EC958 cultures containing concentrations of 14+ at half the MIC for 5 weeks. The wild-type (WT) MIC in minimal media (1.5 μM) was used for initiation of the experiment. New MIC assays were undertaken each week, and changes to 14+ concentrations were made if an MIC increase was observed to maintain the 0.5 × MIC concentration in the growing cultures (Figure 4).
Figure 4.
Evolution of 14+ resistance of E. coli strain EC958 in glucose defined minimal media (GDMM). E. coli cultures were grown in liquid GDMM containing 0.5 × MIC of 14+, cultures were passaged every 24 h and weekly MIC’s were determined. Experiments were performed as biological duplicates and results displayed are weekly mean MIC results (N ≥ 3 ± SD for each biological repeat). Control samples (upward and downward facing triangles) were treated the same as test samples (●, □), except no compound was added. Two-way ANOVA with Tukey’s multiple comparison test showed significant differences between the original WT cultures and both test cultures by week five and between the test and control cultures in week five (p < 0.0001).
Both independent cultures that were passaged in the presence of 14+ showed a small increase in resistance over the first two weeks of exposure, with the MIC increasing from 1.5 to 6.1 μM. A further increase in resistance was observed in one of the test cultures after 5 weeks of exposure (MIC: 12.5 μM), whereas resistance in the other test culture remained constant between weeks 2 and 5. Significant differences were found between both test cultures after 5 weeks of exposure to 14+, compared with the initial MIC of the WT strain (p < 0.001). We observed a 4- to 8-fold increase in MIC over the course of 5 weeks of constant exposure to 14+. Additionally, tests were performed to determine whether resistance to 14+ would evolve due to serial passage in the absence of the ruthenium complex. Figure 4 shows a minor increase in resistance to the compound in week 5 where 14+ was omitted; however, this is a small increase, suggesting exposure to the ruthenium complex is required to cause significant resistance to 14+.
Samples from all cultures were streaked weekly to check for morphological changes or contaminants. Cultures exposed to the RuII complex consistently showed different colony size and morphology when subsequently streaked onto rich media in the absence of 14+ (Figure S-1). This effect was transient, as re-streaking of the differently sized colonies subsequently produced normal growth (data not shown).
All cultures in the evolution experiment depicted in Figure 4 were cryo-preserved weekly. When cultures that had demonstrated a 4- to 8-fold increase in MIC were revived from these frozen stocks and 14+ susceptibility was retested, the MIC was reduced to WT levels. This indicates that the strains may not have become resistant to 14+ via mutational change; instead, it suggests that the altered expression of resistance genes such as those coding for efflux pumps and modulated membrane permeability may be the cause of the small increase in resistance after prolonged exposure to 14+. Upon re-streaking from resistant cryo-preserved stocks, these genes appear to revert to their pre-exposure expression levels, resulting in the reduction in MIC to the original value.
Transcriptomic Analysis Reveals That Membrane Repair Plays a Significant Role in the Response to 14+ Exposure
To further study the effects of 14+ on E. coli strain EC958, its mechanism of action was probed through qPCR. From the study on bacterial growth after exposure to a range of concentrations of 14+ illustrated in Figure 3, we determined that a final RuII complex concentration of 1.5 μM would allow gene expression to be accurately assessed. Significant cell death occurs within 60 min of treatment when a lethal dose of the compound is administered. We therefore monitored changes in gene expression at time points ranging from 20 to 120 min to determine whether early changes in the response to the compound are altered after continued exposure.
Cultures in GDMM were grown in triplicate to the early exponential phase, after which samples of the culture were removed to act as the pre-exposure control. Subsequently, at each time point, postexposure samples were removed from the culture, and RNA was extracted. The expression of selected genes was then compared to a reference.
Prompted by our initial experiments indicating a dual mechanism of action of 14+,46 the nine target genes selected for study have functional roles in membrane permeability/stability and DNA repair, and qPCR analysis was performed at all time points to assess expression of these genes after exposure to 14+. The reference gene hcaT was selected for this study as it is a well-defined constitutively expressed gene under many conditions and showed no significant change in expression in whole-transcriptome analyses of E. coli exposed to other ruthenium-based compounds.56 In these experiments, it was found that three of the nine genes tested showed significant changes in expression upon exposure to 14+ (Figure 5).
Figure 5.
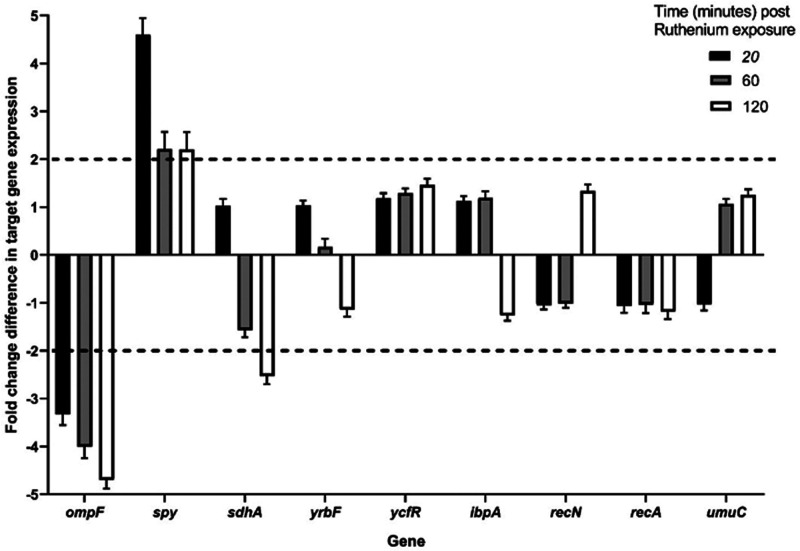
Relative E. coli gene expression levels of nine target genes after exposure to 14+ show effects on membrane permeability and protein repair. The qPCR expression profiles of nine genes after exposure to 1.5 μM 14+ are shown over a time course of 20 (black bars), 60 (gray bars), and 120 min (white bars). N ≥ 3 ± SEM. Expression levels were normalized against the reference gene hcaT. The dashed horizontal lines represent significant expression changes (≥2-fold change).
A steady decrease in expression of the ompF gene was observed across the time-course. This gene encodes a nonspecific porin found within E. coli,57 and its primary function is to facilitate passive diffusion of small hydrophilic molecules, including tetracycline and fluoroquinolones, across the cell membrane.57,58 Therefore, downregulation of this gene upon exposure to 14+ suggests an attempt by the cell to prevent uptake of the compound by reducing membrane permeability. However, exposure to 14+ of a WT E. coli strain and several porin knockout mutants, including ompF and ompF-ompC deletions,59 showed no significant change in the minimal inhibitory or bactericidal concentrations (Figure S-2).
Raised expression of the chaperone gene spy was observed over the time-course, with initial rapid growth in expression slowly plateauing at a constant, increased level. These observations suggest that the Spheroplast protein Y (Spy) protein encoded by this gene is rapidly required after exposure to 14+. In Gram-negative bacteria, changes in external environment can potentially affect the periplasmic space, resulting in unfavorable conditions that cause proteins to aggregate and/or unfold, and under these circumstances chaperones, like Spy are vital in maintaining protein folding homeostasis.60,61
A significant decrease in expression of the sdhA gene, encoding succinate dehydrogenase flavoprotein subunit A (SdhA), was observed after 120 min of exposure to 14+. SdhA is a key protein involved in the tricarboxylic acid cycle, the generation of precursor metabolites, and aerobic respiration. Genes coding for key components of aerobic respiration have previously shown to be highly downregulated in E. coli when treated with another ruthenium-containing compound.62
To determine the wider effects of exposure to 14+, three other genes were tested for changes in expression after exposure: the yrbF gene that encodes for a component of an ATP-binding cassette transporter system that maintains lipid asymmetry in the outer membrane which can be disrupted by chemicals or assembly defects,63ibpA that encodes for a small heat shock protein that protects various proteins from thermal and oxidative stress,64 and ycfR that encodes for a protein considered as both a biofilm regulator and multistress response protein whose expression increases as a result of multiple environmental changes.65 However, the expression levels of none of these genes altered significantly (by 2-fold or greater) upon exposure to 14+.
14+ Shows Antimicrobial Activity of against Different Strains of Pathogenic Bacteria
Given that antimicrobial resistance is a serious problem across many species of pathogenic bacteria, and it is not always possible to know the identity of the infecting pathogen prior to initiating treatment, it is important to understand the spectrum of activity of 14+. As our previous work has indicated that this complex appears to be more active in Gram-negative bacteria,47 we explored its activity against a wider spectrum of multi- and pan-drug-resistant E. coli strains as well as other Gram-negative pathogenic species (Table 2). The E. coli strains include a KPC-producing (Klebsiella pneumonia carbapenase) E. coli,2 an avian pathogenic E. coli, the E. coli EC958 strain (for comparison) and an antimicrobial testing control strain, NCTC 12923. The other Gram-negative pathogens included in this panel were clinical isolates of Pseudomonas aeruginosa, Salmonella kedougou, Shigella flexneri, Enterobacter hormaechei, Citrobacter koseri, and Acinetobacter baumannii. Interestingly, the potent activity of 14+ against the control strain is retained in all the tested drug-resistant strains, including carbapenem-resistant pathogens, indicating its broad spectrum of activity.
Table 2. MIC for 14+ Demonstrates Significant Antimicrobial Activity against a Variety of Bacterial Pathogens.
| bacterial species/strain | type | MIC (μM) |
|---|---|---|
| E. coli EC958 | multi-drug-resistant, clinical isolate | 1.7 |
| E. coli NCTC12923 | antimicrobial control strain | 2.4 |
| E. coli EC_160_KPC2 | carbapenem resistant | 2.4 |
| Avian pathogenic E. coli | multi-drug-resistant | 1.6 |
| P. aeruginosa | clinical isolate | 1.9 |
| K. pneumoniae | clinical isolate | 1.6 |
| S. kedougou | clinical isolate | 1.4 |
| S. flexneri | clinical isolate | 4.2 |
| E. hormaechei | clinical isolate | 1.7 |
| C. koseri | clinical isolate | 1.1 |
| A. baumannii | clinical isolate | 1.6 |
Discussion
Examination of the antibiotic susceptibility profile of this uropathogenic E. coli strain demonstrates its significant multi-drug resistance. Current treatment recommendations for uncomplicated urinary tract infections where antibiotic therapy is indicated include the administration of nitrofurantoin, trimethoprim/sulfamethoxazole, fluoroquinolones, fosfomycin, or oral β-lactam agents. Clinicians are guided in their choice of treatment by local susceptibility patterns of E. coli and other uropathogens, as strain-specific antimicrobial susceptibility profiles are usually not determined.66 The EC958 strain tested here showed resistance to several β-lactam antibiotics and both fluoroquinolones tested; however, both nitrofurantoin and fosfomycin were shown to be effective (Table 2). Much of the antibiotic-resistance profile demonstrated herein is expected when interrogating the whole-genome sequence of this organism.18 Totsika et al. report the presence of the pEC958 plasmid in this strain that contains multiple antibiotic-resistance genes. The presence of tetA and tetR found on the pEC958 plasmid provide resistance to early members of the tetracyclines; however, they do not confer resistance to the third-generation tigecycline tested here.67,68 Resistance to the fluoroquinolones and aminoglycosides can be explained by the presence of aac(6′)-Ib-cr, encoding an aminoglycoside acetyltransferase capable of causing resistance to aminoglycosides and fluoroquinolones via modification of the drug.69−71 However, interestingly no obvious genomic explanation for rifampicin resistance could be found. Resistance to rifampicin commonly occurs via mutation of the rpoB gene, and resistance is easily evolved.72 Therefore, our strain of EC958 may be a variant of the one described previously18,73 that has acquired this mutation. With no apparent carbapenem-resistance genes in the genome sequence and EC958 displaying sensitivity to all four types tested, this drug class would provide a good treatment option in the case of disease progression from EC958 causing a urinary tract infection to pyelonephritis and bloodstream infection.
Given the extensive multi-drug resistance of this strain and many other bacterial pathogens, new antimicrobial leads that do not readily succumb to evolving resistance are urgently needed. The rate at which resistance to an antimicrobial arises is dependent on its mechanism of action and whether single or multiple changes are required for significant resistance to arise. We compared the resistance changes of EC958 when exposed to 14+ with literature reports of resistance evolution in E. coli exposed to commonly used antibiotics. One study showed that continuous exposure to levofloxacin caused E. coli to increase resistance to this antibiotic by 16-fold within the first 24 h of exposure and 64-fold after 14 days. The mechanism of resistance was identified as mutation of targets of the fluoroquinolone and changes in membrane permeability.74 Resistance to fluoroquinolones such as levofloxacin only requires a single point mutation in DNA gyrase to emerge, so the rapid increase observed in this study is not surprising. A separate study exposed E. coli to three antibiotics in different evolution experiments: trimethoprim, chloramphenicol, and doxycycline. Trimethoprim, an antibiotic commonly used to treat urinary tract infections, showed a consistent increase in resistance of around 1680-fold after 20 days of exposure. Increases of 870- and 10-fold were observed against chloramphenicol and doxycycline, respectively.75 One further study found that clinical resistance could be evolved in previously susceptible uropathogenic E. coli strains to ciprofloxacin, amoxacillin, and aminoglycosides by as little as 1, 2, and 3–5 day(s) of passage, respectively.76 Thus, the evolution of resistance to 14+ is slow in comparison to that of many clinically available antibiotics. The slow and low-level resistance gains of EC958 against 14+ may be due to its multimechanism mode of action which targets both the cell membrane and intracellular targets.46,47 Therefore, it seems likely that resistant isolates would take significantly longer to arise, further adding promise to 14+ as a therapeutic tool.
In addition to gaining further insight into the potential for resistance to the RuII complex, it is important to fully understand how 14+ causes bacterial growth inhibition and cell death. Figure 2 demonstrates that very little impact on growth is observed within the first 30 min postinjection of the compound. This is in agreement with previous stimulated emission–depletion imaging that identified a 20 min period when compound accumulation at cell membranes occurs before localization at cell poles.46 Alexa Fluor NHS-ester-405 counterstaining experiments also demonstrated that membrane damage is not present within at least the first 5 min of exposure to 14+ with subsequent bacterial inner and outer membrane damage identified 60 min postexposure to 14+.
To gain insight into how EC958 senses and responds to the presence of 14+ at a transcriptomic level, we selected nine genes associated with the proposed targets of the complex, the bacterial membranes and DNA, to better understand the bacterial response to membrane damage, and to determine whether DNA is a secondary target.
We observed a steady decrease in expression of ompF upon exposure to 14+, OmpF is a nonspecific porin found within E. coli.57 This porin allows passive diffusion of small hydrophilic charged molecules and antibiotics including tetracyclines and fluoroquinolones across the cell membrane.57 As downregulation of ompF causes resistance to multiple antibiotics, this change in gene expression may be a cause of the transient resistance gained during prolonged exposure to 14+ (Figure 2).77 However, with upper limits for molecular weight, the cutoff for porins of E. coli is around 600 Da.78 As the molecular weight of 14+ is considerably larger than this, OmpF-mediated transport is unlikely to be a mechanism of passage into the bacterial cell.46 Aside from the generalized porins, there are solute-specific facilitated diffusion channels through the outer membrane into the periplasm that allow solutes to bypass the porin-specific size and charge requirements; 14+ may utilize one of these routes to gain entry to the cell.78
An increase in expression of the spy gene was observed upon exposure to 14+. Spy is a non-ATP-dependent periplasmic chaperone, vital in maintaining the homeostasis of protein folding under cellular stress.60,61 In Gram-negative bacteria, such as EC958, changes in external environment impact the periplasmic space, resulting in unfavorable conditions for proteins leading to aggregation and unfolding. Under these conditions, spy expression is upregulated to help prevent unfolding and aggregation and to refold substrates without ATP. Stresses that have been found to cause induction in the spy gene include ethanol, indole, tannins, and metals such as zinc, copper, and other ruthenium-based complexes with differing mechanistic actions.62,79,80 In this case, the upregulation of spy upon treatment with 14+ is likely due to the compound damaging the bacterial membrane and altering the conditions of the periplasmic space. Therefore, this increase in expression may be an attempt by the cell to regain the balance of protein aggregation and unfolding in this region. The expression of spy is controlled by the two-component systems CpxAR and BaeSR; both are responsible for regulation of the envelope stress response. Accumulation of 14+ at the poles of the cell is demonstrated herein (Figure 3C) and supports previous findings.46 This transcriptomic data provides further evidence that the membrane is a major site of activity for this compound, which appears to selectively target and damage bacterial membranes, but neither damages mammalian cells nor demonstrates significant toxicity in animal models at similar concentrations.46 This is an important finding in the selectivity and therapeutic potential of 14+.
After 2 h of exposure to 14+, a significant decrease in the expression of sdhA was observed. SdhA is part of the succinate dehydrogenase enzyme complex, bound to the inner surface of the cytoplasmic membrane; its primary functions are to catalyze the oxidation of succinate to fumarate in the citric acid cycle and to participate in the aerobic electron transport pathway to generate energy for the cell by oxidative phosphorylation.81 At 2 h, 14+ has penetrated the membranes of E. coli and entered the cell where it could potentially cause significant intracellular damage; therefore, it is likely that treated bacteria downregulate various metabolic pathways in order to conserve energy and prevent the production of further potentially harmful species, causing the reduction in sdhA expression.
We previously showed that 14+ binds intracellular targets at 60 min.46,47 To decipher whether this binding could cause damage to bacterial DNA, we monitored the regulation of three genes that would be upregulated as a response to DNA damage and the SOS response: recA, recN, and umuC. No significant change was observed in any of these genes, suggesting that the bacteria did not produce an SOS response. Therefore, at the low concentrations used, DNA damage is unlikely to be a target for 14+ in E. coli. The gene expression levels of three further proteins were examined upon exposure to 14+. The protein expressed by ycfR is a biofilm regulator. It is a multi-stress-response protein, expression of which increases as a result of environmental changes.65 The gene yrbF encodes for a component of an ATP-binding cassette transporter system, which is one mechanism that the cell may use to maintain lipid asymmetry in the outer membrane when chemically disrupted.63 Finally, the heat shock protein gene, ibpA protects various substrates and proteins when the cell undergoes thermal and oxidative stress.64 Interestingly, no significant change in gene expression was observed for these genes after exposure to 14+, indicating that exposure to the complex results in a relatively specific disruption to cellular function.
This gene expression data provides further evidence to support the hypothesis that the bacterial cell membrane is a significant target of 14+ with disruption to the stability of periplasmic proteins being a major stressor for the cell. The pathogen screen for sensitivity to this ruthenium complex showed significant activity against several Gram-negative species; each of these species also contain homologues of the spy and sdhA genes. Thus, it is likely that these pathogens will elicit similar responses upon exposure (Table 2). However, the outer membrane proteins expressed by the Gram-negative pathogens differ significantly between the species tested, with P. aeruginosa containing a porin of unusually low permeability (OprF) and A. baumannii containing an E. coli OmpA homologue (OmpAAB). These porins are thought to contribute to the intrinsic antibiotic resistance of these species, but as shown in Table 2, they do not appear to contribute to resistance against 14+. Recently, Smitten et al. examined the differing activity of 14+ against Gram-positive bacterial pathogens47 demonstrating that the level and type of teichoic acids present within the Gram-positive cell wall has a significant impact on the antimicrobial activity of the compound. Testing antimicrobial activity of 14+ against several Gram-negative bacterial species has confirmed the broad-spectrum activity of this compound against Gram-negative pathogens, including clinical isolates and multi-drug-resistant strains (Table 2), further demonstrating the potential of this compound for future use as a therapeutic in the fight against antimicrobial resistant infections.
Conclusions
As with previous studies with 14+ and the EC958 pathogenic strain of E. coli, it was found the compound is highly potent against several pathogenic E. coli strains and other Gram-negative pathogens. Although a small rise in resistance was observed over 5 weeks of exposure, the slow rate and relatively low level of evolution in comparison to that of clinically available organic antibiotics makes 14+ a strong potential candidate for antimicrobial therapeutics. In addition, the good kinetic solubility indicates the compound will have good bioavailability and could be used as an oral antimicrobial. Transcriptomic analysis suggests that 14+ does cause damage both to the inner and outer membrane, resulting in unfavorable, stressful conditions in the periplasmic space. This work adds to the growing volume of research supporting the hypothesis that this class of RuII complexes can be developed into effective antimicrobial drugs.
Future research will further consider the potential emergence of resistance to this lead with a particular focus on mechanisms of cellular entry and efflux and any proteins capable of binding to the complex making it unavailable to bind other more vulnerable targets within the cell. Such studies will facilitate the circumvention of these mechanism if they prove to be potential routes of resistance.
Materials and Methods
Bacterial Strains and Culture Conditions
Strains used in this study are listed in Table 1; the primary strain used was a CTX-M-15-type extended-spectrum β-lactamase (EBSL)-producing clinical isolate E. coli EC958 (ST131).73 Bacteria were routinely grown aerobically at 37 °C in either Mueller–Hinton broth (Sigma-Aldrich, UK) or GDMM glucose as the sole carbon source as described previously.46 Prior to experiments, bacterial starter cultures were prepared by inoculating the appropriate liquid medium with colonies from a fresh agar plate and incubation at 37 °C with shaking at 180 rpm for approximately 18 h. Where necessary, cultures were cryo-preserved in a sterile suspension of 25% glycerol and 75% Mueller–Hinton broth (v/v) at −80 °C.
Preparation and Storage of 14+
Compound 14+ was synthesized as previously described.46 Stocks solutions of 14+ were made to a concentration of 5 mg mL–1 in sterile deionized water and were stored at room temperature protected from light.
Disc Diffusion Assay
Disk diffusion assays were performed in accordance with European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines.53 After incubation, the diameter of the zones of inhibition were measured and compared to EUCAST breakpoints.54,55
Determination of Minimal Inhibitory Concentrations
MICs of 14+ were determined via the standard broth-dilution method in 96-well microtiter plates in GDMM. The MIC was evaluated using 2-fold decreasing concentrations of the compound between 50 and 0.09 μM against starter cultures diluted to an OD600 = 0.05–0.1 (equivalent to a 0.5 McFarland standard). Plates were incubated at 37 °C for 16–20 h after which the presence or absence of growth in each well was observed to determine the MIC of 14+ for each of the strains tested.
Bacterial Growth and Viability Measurements
Cultures were incubated in 250 mL conical flasks from 2.5% starter cultures of E. coli EC958 in 30 mL of GDMM media at 37 °C with shaking at 150 rpm. Once cultures reached an early exponential growth phase (OD600 ∼ 0.4), 14+ was added at the appropriate final concentrations, and growth was monitored at regular intervals for the subsequent 5 h. Viability was measured for samples harvested at the relevant time points after serial dilution in phosphate-buffered saline (PBS) by plating 10 μL aliquots on Mueller–Hinton agar and incubation overnight at 37 °C.
Structured Illumination Microscopy
Samples were prepared and analyzed as previously described.46 Briefly, E. coli cultures were grown in GDMM to an OD600 = 0.3–0.4, and 14+ was added to a final concentration of 0.8 μM. Samples were incubated for 0, 20, 60, and 120 min before washing with nitric acid and fixation with paraformaldehyde (4%). Localization of 14+ in E. coli EC958 cells was visualized through structured illumination microscopy using a 1516× oil objective and SlowFade Gold Antifade Mountant. Cell fluorescence was measured using ImageJ by selecting a cell and measuring area, integrated density, and mean gray value. Background fluorescence was determined and corrected for in each image. Four cells were measured per image. The corrected total cell fluorescence was calculated by CTCF = integrated density – {(area of selected cell) × (mean fluorescence of background readings)}.
Evolution of Resistance Assays
After determination of the MIC for 14+, a 1% inoculum of starter culture incubated in GDMM was added to 10 mL of GDMM. Compound 14+ was then added where appropriate to the culture at 0.5 × MIC, allowing growth of the strain in sub-MIC exposure. Control cultures were set up in the same manner in the absence of 14+. Cultures were incubated at 37 °C and 180 rpm for 24 h, after which a 1% inoculum from this culture was used to inoculate a fresh tube of GDMM +/– 14+. This process was repeated every 24 h of the lifetime of the experiment. Each week, cultures were cryo-preserved and retested for MIC with the amount of compound added to subsequent cultures adjusted as required to maintain a 0.5 × MIC.
Quantitative PCR Analysis
Cells were grown as described above in GDMM. At the designated times pre- and post- injection of a final concentration of 1.5 μM 14+, 5 mL samples of culture were removed into 10 mL volumes of RNA Protect (Qiagen, UK) in triplicate for RNA stabilization. RNA was extracted using the RNeasy Mini Kit from Qiagen according to manufacturer’s instructions, and qPCR was carried out as previously described82 using a QuantStudio 3 Real Time PCR System (Applied Biosystems). Primers used for PCR can be found in the Supporting Information.
Kinetic Turbidimetric Solubility Assays
Aqueous solubility was measured using a high throughput turbidimetric assay. A 10 mM stock of each compound (nicardipine hydrochloride and 14+) were made up in DMSO. This stock was diluted in PBS (pH 7.4) to give the following concentrations (μM): 0.4, 2, 4, 20, 40, 100, and 200. The final DMSO concentration was 1%. Each concentration was plated out in triplicate. The solutions were incubated for 2 h at 37 °C, and the absorbance was read on a plated reader at 620 nm.
Statistical Analyses
GraphPad Prism v7.05 software was used for statistical analysis tests, including one-way ANOVA, two-way ANOVA with Turkey’s multiple comparison tests, and Welch’s t-test.
Acknowledgments
K.L.S. is grateful to the BBRSC for a Ph.D. studentship through the White Rose Structural Biology DTP. A.M.V. is grateful to Nottingham Trent University for a Ph.D. studentship through the Vice Chancellors Award competition. We thank our reviewers for insightful comments that have greatly improved the quality of this report. We are grateful to Dr. Jack C. Leo for providing omp mutant strains for testing.
Glossary
Abreviations
- UPEC
uropathogenic Escherichia coli
- PCR
polymerase chain reaction
- qPCR
quantitative polymerase chain reaction
- DMSO
dimethyl sulfoxide
- FDA
Food and Drug Administration
- EUCAST
European committee on antimicrobial susceptibility tests
- MIC
minimal inhibitory concentration
- SEM
scanning electron microscopy
- ANOVA
analysis of variance
- WT
wild type
- GDMM
glucose defined minimal medium
- ATP
adenosine triphosphate
- KPC
Klebsiella pneumoniae carbapenemase
- NCTC
National Collection of Type Cultures
- PBS
phosphate buffered saline
- TMP
3,4,7,8-tetramethyl-1,10-phenanthroline
- TPPHZ
tetrapyridophenazine
- DMF
dimethylformamide
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.0c00159.
Chemistry methods for [Ru(3,4,7,8-tetramethyl-1,10-phenanthroline)2Cl2]2+, [{Ru(TMP)2}2(tpphz)]4+, anion metathesis, primer sequences designed for transcriptomic analysis, kinetic turbidimetric solubility plate readings, variation in colony size of Escherichia coli after exposure to 14+, and MIC and MBC assays of Escherichia coli porin mutants compared to the wildtype (PDF)
Author Contributions
§ A.M.V. and K.L.S. contributed equally to this manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Richter M. F.; Hergenrother P. J. (2019) The Challenge of Converting Gram-Positive-Only Compounds into Broad-Spectrum Antibiotics. Ann. N. Y. Acad. Sci. 1435 (1), 18–38. 10.1111/nyas.13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacconelli E.; Carrara E.; Savoldi A.; Harbarth S.; Mendelson M.; Monnet D. L.; Pulcini C.; Kahlmeter G.; Kluytmans J.; Carmeli Y.; et al. (2018) Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 18 (3), 318–327. 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- Nordmann P.; Dortet L.; Poirel L. (2012) Carbapenem Resistance in Enterobacteriaceae: Here Is the Storm!. Trends Mol. Med. 18 (5), 263–272. 10.1016/j.molmed.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Paterson D. L.; Harris P. N. A. (2016) Colistin Resistance: A Major Breach in Our Last Line of Defence. Lancet Infect. Dis. 16 (2), 132–133. 10.1016/S1473-3099(15)00463-6. [DOI] [PubMed] [Google Scholar]
- Poirel L.; Jayol A.; Nordmann P. (2017) Crossm. Clin. Microbiol. Rev. 30 (2), 557–596. 10.1128/CMR.00064-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X.; Doi Y.; Zeng L.; Lv L.; Liu J.-H. (2016) Carbapenem-Resistant and Colistin-Resistant Escherichia Coli Co-Producing NDM-9 and MCR-1. Lancet Infect. Dis. 16 (3), 288–289. 10.1016/S1473-3099(16)00057-8. [DOI] [PubMed] [Google Scholar]
- Richter M. F.; Drown B. S.; Riley A. P.; Garcia A.; Shirai T.; Svec R. L.; Hergenrother P. J. (2017) Predictive Compound Accumulation Rules Yield a Broad-Spectrum Antibiotic. Nature 545 (7654), 299–304. 10.1038/nature22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deresinski S. (2005) Methicillin-Resistant Staphylococcus Aureus: An Evolutionary, Epidemiologic, and Therapeutic Odyssey. Clin. Infect. Dis. 40 (4), 562–573. 10.1086/427701. [DOI] [PubMed] [Google Scholar]
- Lowy F. D. (2003) Antimicrobial Resistance: The Example of Staphylococcus Aureus. J. Clin. Invest. 111 (9), 1265–1273. 10.1172/JCI18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piddock L. J. V. (2016) Reflecting on the Final Report of the O’Neill Review on Antimicrobial Resistance. Lancet Infect. Dis. 16, 767–768. 10.1016/S1473-3099(16)30127-X. [DOI] [PubMed] [Google Scholar]
- Kaper J. B.; Nataro J. P.; Mobley H. L. T. (2004) Pathogenic Escherichia Coli. Nat. Rev. Microbiol. 2 (2), 123–140. 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- Ronald A. (2002) The Etiology of Urinary Tract Infection: Traditional and Emerging Pathogens. Am. J. Med. 113, 14–19. 10.1016/S0002-9343(02)01055-0. [DOI] [PubMed] [Google Scholar]
- Kucheria R.; Dasgupta P.; Sacks S. H.; Khan M. S.; Sheerin M. S. (2005) Urinary Tract Infections: New Insights into a Common Problem. Postgrad. Med. J. 81 (952), 83–86. 10.1136/pgmj.2004.023036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olorunmola F. O.; Kolawole D. O.; Lamikanra A. (2013) Antibiotic Resistance and Virulence Properties in Escherichia Coli Strains from Cases of Urinary Tract Infections. African J. Infect. Dis. 7 (1), 1–7. 10.4314/ajid.v7i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. R.; Anderson J. T.; Clabots C.; Johnston B.; Cooperstock M. (2010) Within-Household Sharing of a Fluoroquinolone-Resistant Escherichia Coli Sequence Type ST131 Strain Causing Pediatric Osteoarticular Infection. Pediatr. Infect. Dis. J. 29 (5), 473–475. 10.1097/INF.0b013e3181c89bd7. [DOI] [PubMed] [Google Scholar]
- Johnson J. R.; Menard M.; Johnston B.; Kuskowski M. A.; Nichol K.; Zhanel G. G. (2009) Epidemic Clonal Groups of Escherichia Coli as a Cause of Antimicrobial-Resistant Urinary Tract Infections in Canada, 2002 to 2004. Antimicrob. Agents Chemother. 53 (7), 2733–2739. 10.1128/AAC.00297-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantón R.; Coque T. M. (2006) The CTX-M B-Lactamase Pandemic. Curr. Opin. Microbiol. 9 (5), 466–475. 10.1016/j.mib.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Totsika M.; Beatson S. A.; Sarkar S.; Phan M. D.; Petty N. K.; Bachmann N.; Szubert M.; Sidjabat H. E.; Paterson D. L.; Upton M.; Schembri M. A. (2011) Insights into a Multidrug Resistant Escherichia Coli Pathogen of the Globally Disseminated ST131 Lineage: Genome Analysis and Virulence Mechanisms. PLoS One 6 (10), e26578. 10.1371/journal.pone.0026578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novais Â.; Pires J.; Ferreira H.; Costa L.; Montenegro C.; Vuotto C.; Donelli G.; Coque T. M.; Peixe L. (2012) Characterization of Globally Spread Escherichia Coli ST131 Isolates (1991 to 2010). Antimicrob. Agents Chemother. 56 (7), 3973–3976. 10.1128/AAC.00475-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlowsky J. A.; Hoban D. J.; DeCorby M. R.; Laing N. M.; Zhanel G. G. (2006) Fluoroquinolone-Resistant Urinary Isolates of Escherichia Coli from Outpatients Are Frequently Multidrug Resistant: Results from the North American Urinary Tract Infection Collaborative Alliance-Quinolone Resistance Study. Antimicrob. Agents Chemother. 50 (6), 2251–2254. 10.1128/AAC.00123-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ender P. T.; Gajanana D.; Johnston B.; Clabots C.; Tamarkin F. J.; Johnson J. R. (2009) Transmission of an Extended-Spectrum-Beta-Lactamase-Producing Escherichia Coli (Sequence Type ST131) Strain between a Father and Daughter Resulting in Septic Shock and Emphysematous Pyelonephritis. J. Clin. Microbiol. 47 (11), 3780–3782. 10.1128/JCM.01361-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. R.; Johnston B.; Clabots C.; Kuskowski M. A.; Castanheira M. (2010) Escherichia Coli Sequence Type ST131 as the Major Cause of Serious Multidrug-Resistant E. Coli Infections in the United States. Clin. Infect. Dis. 51 (3), 286–294. 10.1086/653932. [DOI] [PubMed] [Google Scholar]
- Foxman B.; Barlow R.; D’Arcy H.; Gillespie B.; Sobel J. D. (2000) Urinary Tract Infection: Self-Reported Incidence and Associated Costs. Ann. Epidemiol. 10 (8), 509–515. 10.1016/S1047-2797(00)00072-7. [DOI] [PubMed] [Google Scholar]
- Foxman B. (2010) The Epidemiology of Urinary Tract Infection. Nat. Rev. Urol. 7 (12), 653–660. 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- Dwyer F. P.; Gyarfas E. C.; Rogers W. P.; Koch J. H. (1952) Biological Activity of Complex Ions. Nature 170 (4318), 190–191. 10.1038/170190a0. [DOI] [PubMed] [Google Scholar]
- Dwyer F. P.; Reid I. K.; Shulman A.; Laycock G. M.; Dixson S. (1969) The Biological Actions of 1,10-Phenanthroline and 2,2′-Bipyridine Hydrochlorides, Quaternary Salts and Metal Chelates and Related Compounds. Aust. J. Exp. Biol. Med. Sci. 47 (2), 203–218. 10.1038/icb.1969.21. [DOI] [PubMed] [Google Scholar]
- Howson S. E.; Bolhuis A.; Brabec V.; Clarkson G. J.; Malina J.; Rodger A.; Scott P. (2012) Optically Pure, Water-Stable Metallo-Helical ‘Flexicate’ Assemblies with Antibiotic Activity. Nat. Chem. 4 (1), 31–36. 10.1038/nchem.1206. [DOI] [PubMed] [Google Scholar]
- Li F.; Collins J. G.; Keene F. R. (2015) Ruthenium Complexes as Antimicrobial Agents. Chem. Soc. Rev. 44 (8), 2529–2542. 10.1039/C4CS00343H. [DOI] [PubMed] [Google Scholar]
- Southam H. M.; Butler J. A.; Chapman J. A.; Poole R. K. (2017) The Microbiology of Ruthenium Complexes. Adv. Microb. Physiol. 71, 1–96. 10.1016/bs.ampbs.2017.03.001. [DOI] [PubMed] [Google Scholar]
- Viganor L.; Howe O.; McCarron P.; McCann M.; Devereux M. (2017) The Antibacterial Activity of Metal Complexes Containing 1,10- Phenanthroline: Potential as Alternative Therapeutics in the Era of Antibiotic Resistance. Curr. Top. Med. Chem. 17 (11), 1280–1302. 10.2174/1568026616666161003143333. [DOI] [PubMed] [Google Scholar]
- Frei A.; Zuegg J.; Elliott A. G.; Baker M.; Braese S.; Brown C.; Chen F.; Dowson C. G.; Dujardin G.; Jung N.; et al. (2020) Metal Complexes as a Promising Source for New Antibiotics. Chem. Sci. 11 (10), 2627–2639. 10.1039/C9SC06460E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketikidis I.; Banti C. N.; Kourkoumelis N.; Tsiafoulis C. G.; Papachristodoulou C.; Kalampounias A. G.; Hadjikakou S. K. (2020) Metal Complexes, an Untapped Source of Antibiotic Potential?. Antibiotics 9, 25–90. 10.3390/antibiotics9010025. [DOI] [Google Scholar]
- Ramos A. I.; Braga T. M.; Braga S. S. (2012) Ru(II)-Based Antimicrobials: Looking Beyond Organic Drugs. Mini-Rev. Med. Chem. 12 (3), 227–235. 10.2174/1389557511209030227. [DOI] [PubMed] [Google Scholar]
- Gill M. R.; Garcia-Lara J.; Foster S. J.; Smythe C.; Battaglia G.; Thomas J. A. (2009) A Ruthenium(II) Polypyridyl Complex for Direct Imaging of DNA Structure in Living Cells. Nat. Chem. 1 (8), 662–667. 10.1038/nchem.406. [DOI] [PubMed] [Google Scholar]
- Sreedharan S.; Gill M. R.; Garcia E.; Saeed H. K.; Robinson D.; Byrne A.; Cadby A.; Keyes T. E.; Smythe C.; Pellett P.; Bernardino de la Serna J.; Thomas J. A. (2017) Multimodal Super-Resolution Optical Microscopy Using a Transition-Metal-Based Probe Provides Unprecedented Capabilities for Imaging Both Nuclear Chromatin and Mitochondria. J. Am. Chem. Soc. 139 (44), 15907–15913. 10.1021/jacs.7b08772. [DOI] [PubMed] [Google Scholar]
- Gill M. R.; Cecchin D.; Walker M. G.; Mulla R. S.; Battaglia G.; Smythe C.; Thomas J. A. (2013) Targeting the Endoplasmic Reticulum with a Membrane-Interactive Luminescent Ruthenium(II) Polypyridyl Complex. Chem. Sci. 4 (12), 4512–4519. 10.1039/c3sc51725j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill M. R.; Derrat H.; Smythe C. G. W.; Battaglia G.; Thomas J. A. (2011) Ruthenium(II) Metallo-Intercalators: DNA Imaging and Cytotoxicity. ChemBioChem 12 (6), 877–880. 10.1002/cbic.201000782. [DOI] [PubMed] [Google Scholar]
- Ramu V.; Gill M. R.; Jarman P. J.; Turton D.; Thomas J. A.; Das A.; Smythe C. (2015) A Cytostatic Ruthenium(II)-Platinum(II) Bis(Terpyridyl) Anticancer Complex That Blocks Entry into S Phase by Up-Regulating P27 KIP1. Chem. - Eur. J. 21 (25), 9185–9197. 10.1002/chem.201500561. [DOI] [PubMed] [Google Scholar]
- Gill M. R.; Jarman P. J.; Halder S.; Walker M. G.; Saeed H. K.; Thomas J. A.; Smythe C.; Ramadan K.; Vallis K. A. (2018) A Three-in-One-Bullet for Oesophageal Cancer: Replication Fork Collapse, Spindle Attachment Failure and Enhanced Radiosensitivity Generated by a Ruthenium(II) Metallo-Intercalator. Chem. Sci. 9 (4), 841–849. 10.1039/C7SC03712K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarman P. J.; Noakes F.; Fairbanks S.; Smitten K.; Griffiths I. K.; Saeed H. K.; Thomas J. A.; Smythe C. (2019) Exploring the Cytotoxicity, Uptake, Cellular Response, and Proteomics of Mono- and Dinuclear DNA Light-Switch Complexes. J. Am. Chem. Soc. 141 (7), 2925–2937. 10.1021/jacs.8b09999. [DOI] [PubMed] [Google Scholar]
- Walker M. G.; Jarman P. J.; Gill M. R.; Tian X.; Ahmad H.; Reddy P. A. N.; McKenzie L.; Weinstein J. A.; Meijer A. J. H. M.; Battaglia G.; Smythe C. G. W.; Thomas J. A. (2016) A Self-Assembled Metallomacrocycle Singlet Oxygen Sensitizer for Photodynamic Therapy. Chem. - Eur. J. 22 (17), 5996–6000. 10.1002/chem.201600852. [DOI] [PubMed] [Google Scholar]
- Saeed H. K.; Jarman P. J.; Archer S.; Sreedharan S.; Saeed I. Q.; Mckenzie L. K.; Weinstein J. A.; Buurma N. J.; Smythe C. G. W.; Thomas J. A. (2017) Homo- and Heteroleptic Phototoxic Dinuclear Metallo-Intercalators Based on Ru-II(Dppn) Intercalating Moieties: Synthesis, Optical, and Biological Studies. Angew. Chem., Int. Ed. 56 (41), 12628–12633. 10.1002/anie.201707350. [DOI] [PubMed] [Google Scholar]
- Archer S. A.; Raza A.; Dröge F.; Robertson C.; Auty A. J.; Chekulaev D.; Weinstein J. A.; Keane T.; Meijer A. J. H. M.; Haycock J. W.; MacNeil S.; Thomas J. A. (2019) A Dinuclear Ruthenium(Ii) Phototherapeutic That Targets Duplex and Quadruplex DNA. Chem. Sci. 10, 3502–3513. 10.1039/C8SC05084H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza A.; Archer S. A.; Fairbanks S. D.; Smitten K. L.; Botchway S. W.; Thomas J. A.; MacNeil S.; Haycock J. W. (2020) A Dinuclear Ruthenium(II) Complex Excited by Near-Infrared Light through Two-Photon Absorption Induces Phototoxicity Deep within Hypoxic Regions of Melanoma Cancer Spheroids. J. Am. Chem. Soc. 142 (10), 4639–4647. 10.1021/jacs.9b11313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed H. K.; Sreedharan S.; Jarman P. J.; Archer S. A.; Fairbanks S. D.; Foxon S. P.; Auty A. J.; Chekulaev D.; Keane T.; Meijer A. J. H. M.; Weinstein J. A.; Smythe C. G. W.; Bernardino de la Serna J.; Thomas J. A. (2020) Making the Right Link to Theranostics: The Photophysical and Biological Properties of Dinuclear RuII–ReI Dppz Complexes Depend on Their Tether. J. Am. Chem. Soc. 142 (2), 1101–1111. 10.1021/jacs.9b12564. [DOI] [PubMed] [Google Scholar]
- Smitten K. L.; Southam H. M.; de la Serna J. B.; Gill M. R.; Jarman P. J.; Smythe C. G. W.; Poole R. K.; Thomas J. A. (2019) Using Nanoscopy To Probe the Biological Activity of Antimicrobial Leads That Display Potent Activity against Pathogenic, Multidrug Resistant, Gram-Negative Bacteria. ACS Nano 13 (5), 5133–5146. 10.1021/acsnano.8b08440. [DOI] [PubMed] [Google Scholar]
- Smitten K. L.; Fairbanks S. D.; Robertson C. C.; Bernardino de la Serna J.; Foster S. J.; Thomas J. A. (2020) Ruthenium Based Antimicrobial Theranostics – Using Nanoscopy to Identify Therapeutic Targets and Resistance Mechanisms in Staphylococcus Aureus. Chem. Sci. 11 (1), 70–79. 10.1039/C9SC04710G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski C. A. (2000) Drug-like Properties and the Causes of Poor Solubility and Poor Permeability. J. Pharmacol. Toxicol. Methods 44 (1), 235–249. 10.1016/S1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]
- Di L.; Fish P. V.; Mano T. (2012) Bridging Solubility between Drug Discovery and Development. Drug Discovery Today 17 (9–10), 486–495. 10.1016/j.drudis.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Waring M. J.; Arrowsmith J.; Leach A. R.; Leeson P. D.; Mandrell S.; Owen R. M.; Pairaudeau G.; Pennie W. D.; Pickett S. D.; Wang J.; Wallace O.; Weir A. (2015) An Analysis of the Attrition of Drug Candidates from Four Major Pharmaceutical Companies. Nat. Rev. Drug Discovery 14 (7), 475–486. 10.1038/nrd4609. [DOI] [PubMed] [Google Scholar]
- Veber D. F.; Johnson S. R.; Cheng H. Y.; Smith B. R.; Ward K. W.; Kopple K. D. (2002) Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 45 (12), 2615–2623. 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- Amidon G. L.; Lennernäs H.; Shah V. P.; Crison J. R. (1995) A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution and in Vivo Bioavailability. Pharm. Res. 12 (3), 413–420. 10.1023/A:1016212804288. [DOI] [PubMed] [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing . (2017) EUCAST Disk Diffusion Manual, version 6.0, https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/2017_manuals/Manual_v_6.0_EUCAST_Disk_Test_final.pdf.
- European Committee on Antimicrobial Susceptibility Testing . (2019) Breakpoint tables for interpretation of MICs and zone diameters, version 9.0, https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_9.0_Breakpoint_Tables.pdf.
- European Committee on Antimicrobial Susceptibility Testing . (2018) Breakpoint tables for interpretation of MICs and zone diameters, version 8.0, https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_8.0_Breakpoint_Tables.pdf.
- Zhou K.; Zhou L.; Lim Q.; Zou R.; Stephanopoulos G.; Too H. P. (2011) Novel Reference Genes for Quantifying Transcriptional Responses of Escherichia Coli to Protein Overexpression by Quantitative PCR. BMC Mol. Biol. 12, 18. 10.1186/1471-2199-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J.; Papenfort K. (2006) Small Non-Coding RNAs and the Bacterial Outer Membrane. Curr. Opin. Microbiol. 9 (6), 605–611. 10.1016/j.mib.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Kishii R.; Takei M. (2009) Relationship between the Expression of OmpF and Quinolone Resistance in Escherichia Coli. J. Infect. Chemother. 15 (6), 361–366. 10.1007/s10156-009-0716-6. [DOI] [PubMed] [Google Scholar]
- Meuskens I.; Michalik M.; Chauhan N.; Linke D.; Leo J. C. (2017) A New Strain Collection for Improved Expression of Outer Membrane Proteins. Front. Cell. Infect. Microbiol. 7, 464. 10.3389/fcimb.2017.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull F.; Koldewey P.; Humes J. R.; Radford S. E.; Bardwell J. C. A. (2016) Substrate Protein Folds While It Is Bound to the ATP-Independent Chaperone Spy. Nat. Struct. Mol. Biol. 23 (1), 53–58. 10.1038/nsmb.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S. K.; Lambadi P. R.; Ghosh T.; Pathania R.; Navani N. K. (2014) Genetic Regulation of Spy Gene Expression in Escherichia Coli in the Presence of Protein Unfolding Agent Ethanol. Gene 548 (1), 142–148. 10.1016/j.gene.2014.07.003. [DOI] [PubMed] [Google Scholar]
- McLean S.; Begg R.; Jesse H. E.; Mann B. E.; Sanguinetti G.; Poole R. K. (2013) Analysis of the Bacterial Response to Ru(CO)3Cl(Glycinate) (CORM-3) and the Inactivated Compound Identifies the Role Played by the Ruthenium Compound and Reveals Sulfur-Containing Species as a Major Target of CORM-3 Action. Antioxid. Redox Signaling 19 (17), 1999–2012. 10.1089/ars.2012.5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinverni J. C.; Silhavy T. J. (2009) An ABC Transport System That Maintains Lipid Asymmetry in the Gram-Negative Outer Membrane. Proc. Natl. Acad. Sci. U. S. A. 106 (19), 8009–8014. 10.1073/pnas.0903229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa M.; Miyakawa M.; Matsumura Y.; Tsuchido T. (2002) Escherichia Coli Small Heat Shock Proteins, IbpA and IbpB, Protect Enzymes from Inactivation by Heat and Oxidants. Eur. J. Biochem. 269 (12), 2907–2917. 10.1046/j.1432-1033.2002.02958.x. [DOI] [PubMed] [Google Scholar]
- Deng K.; Wang S.; Rui X.; Zhang W.; Tortorello M. L. (2011) Functional Analysis of YcfR and YcfQ in Escherichia Coli O157:H7 Linked to Outbreaks of Illness Associated with Fresh Produce. Appl. Environ. Microbiol. 77 (12), 3952–3959. 10.1128/AEM.02420-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. S., and Le J. (2018) Urinary Tract Infections, in PSAP 2018 Book 1-Infectious Diseases, pp 7–28, American College of Clinical Pharmacy. [Google Scholar]
- Jansåker F.; Frimodt-Møller N.; Sjögren I.; Knudsen J. D. (2014) Clinical and Bacteriological Effects of Pivmecillinam for ESBL-Producing Escherichia Coli or Klebsiella Pneumoniae in Urinary Tract Infections. J. Antimicrob. Chemother. 69 (3), 769–772. 10.1093/jac/dkt404. [DOI] [PubMed] [Google Scholar]
- Chopra I.; Roberts M. (2001) Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 65 (2), 232–260. 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rather P. N.; Munayyer H.; Mann P. A.; Hare R. S.; Miller G. H.; Shaw K. J. (1992) Genetic Analysis of Bacterial Acetyltransferases: Identification of Amino Acids Determining the Specificities of the Aminoglycoside 6′-N- Acetyltransferase Ib and IIa Proteins. J. Bacteriol. 174 (10), 3196–3203. 10.1128/JB.174.10.3196-3203.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez M. S.; Nikolaidis N.; Tolmasky M. E. (2013) Rise and Dissemination of Aminoglycoside Resistance: The aac(6′)-Ib Paradigm. Front. Microbiol. 4, 1–13. 10.3389/fmicb.2013.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw K. J.; Rather P. N.; Hare R. S.; Miller G. H. (1993) Molecular Genetics of Aminoglycoside Resistance Genes and Familial Relationships of the Aminoglycoside-Modifying Enzymes. Microbiol. Rev. 57 (1), 138–163. 10.1128/MMBR.57.1.138-163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didelot X.; Walker A. S.; Peto T. E.; Crook D. W.; Wilson D. J. (2016) Within-Host Evolution of Bacterial Pathogens. Nat. Rev. Microbiol. 14 (3), 150–162. 10.1038/nrmicro.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde B. M.; Ben Zakour N. L.; Stanton-Cook M.; Phan M.-D.; Totsika M.; Peters K. M.; Chan K. G.; Schembri M. A.; Upton M.; Beatson S. A. (2014) The Complete Genome Sequence of Escherichia Coli EC958: A High Quality Reference Sequence for the Globally Disseminated Multidrug Resistant E. Coli O25b:H4-ST131 Clone. PLoS One 9 (8), e104400–13. 10.1371/journal.pone.0104400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonoyan L.; Fleming G. T. A.; Friel R.; O’Flaherty V. (2019) Continuous Culture of Escherichia Coli, under Selective Pressure by a Novel Antimicrobial Complex, Does Not Result in Development of Resistance. Sci. Rep. 9 (1), 2401. 10.1038/s41598-019-38925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toprak E.; Veres A.; Michel J.-B.; Chait R.; Hartl D. L.; Kishony R. (2012) Evolutionary Paths to Antibiotic Resistance under Dynamically Sustained Drug Selection. Nat. Genet. 44 (1), 101–105. 10.1038/ng.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamus-Białek W.; Wawszczak M.; Arabski M.; Majchrzak M.; Gulba M.; Jarych D.; Parniewski P.; Głuszek S. (2019) Ciprofloxacin, Amoxicillin, and Aminoglycosides Stimulate Genetic and Phenotypic Changes in Uropathogenic Escherichia Coli Strains. Virulence 10 (1), 260–276. 10.1080/21505594.2019.1596507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaktaji R. P.; Heidari F. (2013) Study the Expression of ompf Gene in Esherichia Coli Mutants. Indian J. Pharm. Sci. 75 (5), 540–544. 10.4103/0250-474X.122849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver L. L. (2011) Challenges of Antibacterial Discovery. Clin. Microbiol. Rev. 24 (1), 71–109. 10.1128/CMR.00030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K.; Ishihama A. (2006) Characterization of Copper-Inducible Promoters Regulated by CpxA/CpxR in Escherichia Coli. Biosci., Biotechnol., Biochem. 70 (7), 1688–1695. 10.1271/bbb.60024. [DOI] [PubMed] [Google Scholar]
- Yamamoto K.; Ogasawara H.; Ishihama A. (2008) Involvement of Multiple Transcription Factors for Metal-Induced Spy Gene Expression in Escherichia Coli. J. Biotechnol. 133 (2), 196–200. 10.1016/j.jbiotec.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Oyedotun K. S.; Lemire B. D. (2004) The Quaternary Structure of the Saccharomyces Cerevisiae Succinate Dehydrogenase: Homology Modeling, Cofactor Docking, and Molecular Dynamics Simulation Studies. J. Biol. Chem. 279 (10), 9424–9431. 10.1074/jbc.M311876200. [DOI] [PubMed] [Google Scholar]
- Flatley J.; Barrett J.; Pullan S. T.; Hughes M. N.; Green J.; Poole R. K. (2005) Transcriptional Responses of Escherichia Colito S-Nitrosoglutathione under Defined Chemostat Conditions Reveal Major Changes in Methionine Biosynthesis. J. Biol. Chem. 280 (11), 10065–10072. 10.1074/jbc.M410393200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.