Abstract
Objective:
Children with ADHD often have sleep complaints and cognitive deficits. The aim of this pilot study was to determine whether sleep extension improves inhibitory control, a primary cognitive deficit in ADHD.
Method:
Children with (n = 11) and without (n = 15) ADHD participated in a within-subject sleep extension intervention that targeted nocturnal sleep duration. Sleep was assessed with actigraphy and polysomnography. Inhibitory control was assessed with a Go/No-Go task.
Results:
For children without ADHD, there was a significant main effect of time, such that morning inhibitory control was 10% greater than evening inhibitory control. However, inhibitory control did not differ between the baseline and extension conditions in this group. For children with ADHD, although morning inhibitory control did not differ from evening inhibitory control, sleep extension improved inhibitory control by 13% overall.
Conclusion:
These results suggest that a sleep extension intervention improves inhibitory control in children with ADHD.
Keywords: ADHD, cognitive function, inhibitory control, sleep, sleep duration
ADHD is a neurobehavioral condition, affecting an esti-mated 7% of children 18 years of age and younger (Thomas, Sanders, Doust, Beller, & Glasziou, 2015). Symptoms of ADHD that manifest during childhood persist into adoles-cence and adulthood, and are linked to heightened risk for maladaptive outcomes (Harpin, 2005; Wilens, Faraone, & Biederman, 2004). As such, opportunities for early diagnosis and intervention are needed.
Theoretical models of ADHD suggest that symptoms emerge as a consequence of impairments in inhibitory control (Barkley, 1997; Doyle, 2006; Nigg, 2000; Oosterlaan, Logan, & Sergeant, 1998). Inhibitory control is the ability to voluntarily withhold a prepotent response (Cavanagh & Frank, 2014; Durston et al., 2002). Accumulating evidence in typically developing (TD) children indicates that inhibitory control is compromised by insufficient sleep (Gruber, Cassoff, Frenette, Wiebe, & Carrier, 2012). Impaired sleep is also common in disorders that are comorbid with ADHD such as anxiety and autism spectrum disorder (ASD; Mayes, Calhoun, Bixler, & Vgontzas, 2009). Relatedly, cognitive impairments have been associated with sleep restriction in samples with ADHD (Owens, 2005; Weiss, Craig, Davies, Schibuk, & Stein, 2015; Yoon, Jain, & Shapiro, 2012). For example, when instructed to delay their bedtime by 1 hr for six consecutive nights, children with ADHD subsequently experienced reduced vigilance and attention (Gruber et al., 2011).
One recent study suggests that sleep-targeted interventions may improve outcomes in children with ADHD. Hiscock and colleagues (2015) found that ADHD symptoms were reduced, and cognitive outcomes (i.e., working memory performance) improved, in a sample of school-aged children following a 6-month sleep hygiene intervention (Hiscock et al., 2015). Although this study was well powered, and the findings robust, whether sleep was directly improved from the intervention is not clear. The study employed the Children’s Sleep Habits Questionnaire which indicated improvements at both 3- and 6-month follow-ups. A between-subjects comparison of actigraphy in a subset of participants suggested that sleep time increased by 10 min for the intervention compared with control group.
Sleep hygiene interventions aim to improve sleep by educating caregivers on factors that affect sleep time and quality. Such interventions have been shown to extend sleep duration in TD children (Gruber et al., 2012; Sadeh, Gruber, & Raviv, 2003; Vriend, Davidson, Shaffner, Corkum, & Rusak, 2013). For example, Gruber and colleagues (2012) found that children (7 to 11 years of age) increased sleep time by an average of 27 min by advancing bedtime by 1 hr for five consecutive nights. Caregiver report of children’s daytime sleepiness was reduced and teacher reported inhibitory control was improved following with sleep extension. Thus, interventions that increase sleep duration can also successfully improve behavior.
A recent study by Becker and colleagues (2019) used a similar protocol to assess the effects of sleep extension on out-comes in adolescents, 14 to 17 years of age, with ADHD. Following five nights of sleep extension when 9.5 hr were spent in bed, daytime sleepiness and attention problems were improved. Importantly, however, these changes were observed relative to an equivalent period of sleep restriction in which participants adjusted their bedtime to spend only 6.5 hr in bed. Although behavioral differences between the extension and restriction periods are clinically relevant, the authors did not test how sleep extension may have impacted outcomes relative to the child’s habitual or typical sleep duration. A direct comparison between extended sleep and baseline sleep is needed to determine whether or not prolonged sleep duration causally affects outcomes in this population. This study also lacked a control group of TD adolescents, which makes it difficult to understand specificity to ADHD. Relatedly, the impact of sleep extension on inhibitory control—a primary impairment in ADHD—was unexplored. Understanding causal relations between sleep and primary deficits of this dis-order may better inform sleep-based intervention strategies.
The goal of this pilot study was to determine whether a sleep extension intervention would improve inhibitory control in 6- to 9-year-old children with ADHD. To test this, we adapted Gruber et al.’s (2012) protocol to measure the effects of prolonged sleep duration on inhibitory control. Based on the beneficial effects of sleep extension reported in TD children (Gruber et al., 2012) and adolescents with ADHD (Becker et al., 2019), it was hypothesized that inhibitory control would be improved by sleep extension in children with ADHD.
Method
Participants
Participants were 11 children with ADHD (2F; Mage = 8.27, SD = 1.10 years; Table 1) and 15 TD children (5F; Mage = 8.23 years, SD = 1.10 years). One additional participant was recruited but did not complete the experimental proto-col due to illness. Children were recruited via phone/email from a previous study (n = 7), the campus Child Studies Database, advertisements in child-oriented establishments, and community events in Amherst, MA, USA. Group sample sizes were estimated based on similar studies (Gruber et al., 2011).By approximately 7 years of age, maturation of the frontal lobe supports development of cognitive processes such as inhibitory control (Anderson, 2002; Tao, Wang, Fan, & Gao, 2014). Furthermore, ADHD is typically diagnosed around 7 years of age (Applegate et al., 1997). As such, we specifically recruited children between 6 to 9 years of age. Eligible participants slept ≤10 hr and had a bedtime after 8 p.m. on average weeknights. The National Sleep Foundation recommends that 6- to 13-year-old children obtain 9 to 11 hr of sleep per night (Hirshkowitz et al., 2015). Provided this recommendation, it is likely that children sleeping >10 hr at night would have difficulty extending sleep duration further. As the intervention in this study targeted bedtime (rather than wake time; see Procedure), the requirement for children to have a bedtime after 8 p.m. was intended to pre-vent the sleep extension manipulation from interfering with evening activities (e.g., dinner).Children in the ADHD group were required to have a current diagnosis of ADHD. Caregivers were asked who diagnosed their child, when that diagnosis was assigned, and whether their child was currently taking medication for ADHD. Caregivers of children in the TD group were explicitly asked to confirm that their child did not have a current or former diagnosis of ADHD. Exclusion criteria for both groups included the following: (a) current diagnosis or his-tory of intellectual disabilities or developmental delay, (b) current diagnosis or history of a sleep disorder, and (c) uncorrected hearing or visual impairments.
Table 1.
Participant demographics.
| ADHD Mean (SD) |
TD Mean (SD) |
p-value | |
|---|---|---|---|
| Age (years) | 8.27 (1.10) | 8.23 (1.10) | .92 |
| Gender (females: males) | 2:9 | 5:10 | .39 |
| Ethnicity (%) | |||
| Non-Hispanic | 63.63 | 93.33 | |
| Hispanic | 27.27 | 6.67 | .12 |
| Missing | 9.09 | - | |
| Annual household income (%) | |||
| $5,001 to $10,000 | - | 6.67 | |
| $10,001 to $20,000 | 18.18 | - | |
| $40,001 to $70,000 | 36.36 | 33.33 | |
| $70,001 to $100,000 | 9.09 | 13.33 | .45 |
| $100,001 to $150,000 | 27.27 | 20.00 | |
| More than $150,000 | 9.09 | 26.67 | |
| Hyperactive symptoms | 15.09 (6.70) | 3.13 (3.80) | ≤.001 |
| Inattentive symptoms | 13.36 (5.33) | 2.00 (2.70) | ≤.001 |
| ODD symptoms | 2.18 (2.32) | - | - |
| Anxiety problems | 2.36 (2.34) | 0.92 (1.56) | .09 |
| Body mass index | 19.37 (3.88) | 17.11 (4.12) | .17 |
Notes: In the ADHD group, n = 11. In the TD group, n = 15 (with the exception of anxiety problems where n = 12).
ADHD = attention-deficit/hyperactivity disorder; TD = typically developing; SD = standard deviation; ODD = Oppositional Defiant Disorder
Procedure
Procedures were approved by the Institutional Review Board at the University of Massachusetts Amherst. Accordingly, researchers described the procedures and caregiver written consent was obtained at an in-home visit. During this initial visit, the sleep diary was given to the caregiver. Child assent was obtained and the Actiwatch was fitted to child’s nondominant wrist. Children and caregivers were shown how to use the Actiwatch and an instruction sheet was provided for future reference. Caregivers were asked to oversee the child’s use of the Actiwatch and complete the sleep diary each day. Children and caregivers were instructed that the child must maintain their habitual wake time across the two 5-day testing periods. For consistency, maintaining habitual wake time was intended to ensure that sleep extension was a product of earlier sleep onset time and not delayed wake time. Children’s wake time is also often constrained by bus schedules and school start times so this enhances translational impact.
There were two conditions, a baseline condition and a sleep extension condition (Figure 1). There was approximately 1 week between the baseline and extension conditions. The order of conditions was counterbalanced across participants with random assignment to condition order. For most children, the conditions were matched for the day of the week they took place and were instructed to maintain a consistent bedtime and waketime regardless of day of the week. During the baseline condition, children followed their normal bedtime routine for five consecutive nights. During the extension condition, caregivers were instructed to put their child to bed 90 min (1.5 hr) earlier than their habitual bedtime for five consecutive nights. Caregivers were provided a list of tips to aid in implementing the earlier bedtime (see Text S1).
Figure 1. Go/No-Go stimulus presentation order.
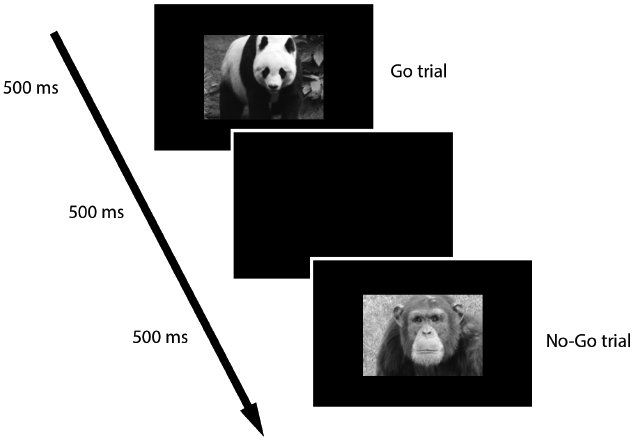
Go trials are those in which images of animals including a giraffe, elephant, and panda (shown above) were presented. No-Go trials are those in which an image of a chimpanzee (shown above) was presented.
On the last night of the baseline and extension conditions, children, accompanied by a caregiver, arrived at the sleep laboratory approximately 1 hr before their habitual (baseline condition) or extended (extension condition) bed-time. After settling in, children completed a baseline assessment of the Go/No-Go task. Children were then fitted with polysomnography (PSG). Children and caregivers slept in the lab (in separate beds in the same room) overnight. The following morning, children woke at their habitual wake time. The PSG equipment was removed, and children were given time to complete their normal morning routine. Children then completed the morning assessment of the Go/No-Go task. This concluded the overnight visit. At the end of each in-lab visit, the Actiwatch and sleep diaries were collected. Caregivers were provided monetary compensation and children chose an age-appropriate prize for their participation.
Sleep Measures
Actigraphy. Actiwatch Spectrum wristwatches (Spectrum 2; Philips Respironics) were used to measure sleep and wake onset times and confirm that experimental manipulations were followed (Acebo et al., 2005). The Actiwatch has off-wrist detection and a triaxial accelerometer that samples activity at 32 Hz, with a sensitivity of <0.01 g. Activity was stored in 15-s epochs. Actigraphy is a reliable index of time spent asleep and awake in developmental populations (Sit-nick, Goodlin-Jones, & Anders, 2008).Children and their caregivers were instructed to press an event marker on the Actiwatch at bedtime and when the child woke up (i.e., discontinued the sleep bout) each day. Event markers and sleep diary entries were used to confirm the start and end time of each sleep bout. Actiware software (Philips Respironics) was then used to differentiate intervals of sleep and wake. Sleep onset time was set at the first of three consecutive minutes of sleep and wake onset time was set at the last of five consecutive minutes of sleep (Acebo et al., 2005). Total sleep time was defined as the total minutes scored as sleep between sleep onset and final wake onset.
PSG. PSG electrode caps (EasyCap) were used to record overnight sleep physiology. Data were collected from 24 electroencephalography (EEG) electrodes, two electro-oculography (EOG) electrodes, and two electromyography (EMG) electrodes (affixed to the chin) referenced to a mid-forehead ground (FPz) and referenced to Cz and contralateral mastoids. PSG was scored according to the revised American Academy of Sleep Medicine manual (Iber, Ancoli-Israel, Chesson, & Quan, 2007) by a trained researcher.
Sleep diary. Sleep diaries were used to validate scoring of actigraphy data. Caregivers logged their child’s sleep latency, sleep onset time, and morning wake onset time each day of the experimental protocol.
Cognitive and Behavioral Measures
Go/no-go task. A developmentally appropriate Go/No-Go task was used to assess inhibitory control (Cremone, Lugo-Candelas, Harvey, McDermott, & Spencer, 2017; Lamm, White, McDermott, & Fox, 2012). In Go trials (75% of trials), color images of various animals (e.g., giraffe, elephant, panda) were presented. In No-Go trials (25% of trials), a chimpanzee was presented (Figure 2). Displayed images were 3 in. in height and 4 in. in width, centered on a 14-in. computer screen positioned approximately 15 in. from participants.
Figure 2. Outline of experimental protocol.
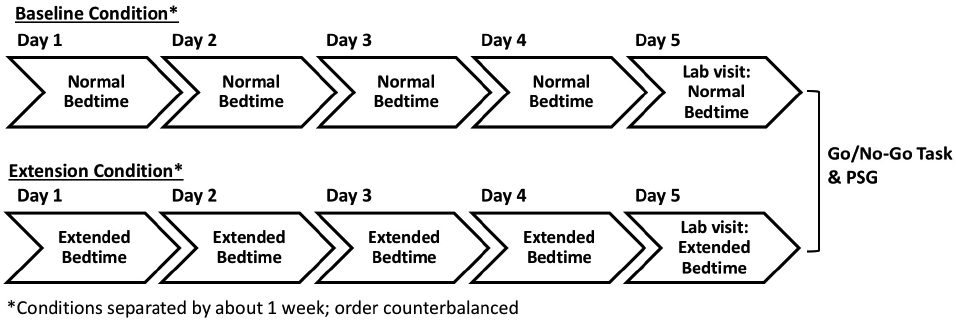
Each child completed a 5-day baseline condition and a 5-day extension condition. At the end of each condition, each child participated in an in-lab overnight visit. The order of conditions was counterbalanced across participants.
Each trial began with the presentation of an animal image for 500 ms. Children were instructed to respond, via a button press on a computer mouse, for all of the animals (Go trials) except for the chimpanzee for which they were to inhibit their response (No-Go trials). A blank screen was presented for 500 ms between trials. Children were given 12 practice trials to ensure they understood task instructions. Subsequently, test trials were presented in two blocks of 60 trials each. Two pseudorandom trial orders were used for all participants (for evening and morning sessions). Trial order was counterbalanced across sessions, conditions, and participants.
Questionnaires. The ADHD section of the disruptive behavior rating scales (DBRS; caregiverreport) is a valid and reliable assessment of ADHD symptomology in school-aged children (internal consistency: α = .86 to .92, test–retest reliability, r = .49 to .61; Pelham, Fabiano, & Massetti, 2005) and was used to evaluate symptomology in the ADHD and TD groups, via symptom counts (Barkley & Murphy, 2006). As Oppositional Defiant Disorder (ODD) is highly comorbid with childhood ADHD (Waschbusch, 2002), the ODD scale of the DBRS was also used to evalu-ate ODD in the ADHD group.
Statistical Analyses
Statistical analyses were performed in SPSS. For primary analyses (actigraphy, Go/No-Go), an alpha of .05 was used to determine significance. To correct for multiple comparisons, an alpha of .01 was used for exploratory analyses (PSG, correlations).
Sleep
Actigraphy. Actigraphy data were used to assess the efficacy of sleep extension in extending sleep duration from baseline. Data could not be retrieved from one TD participant’s Actiwatch. Therefore, actigraphy data in the TD group were averaged across 14 TD children (5F; Mage = 8.18, SD = 1.13 years).A repeated-measures analysis of variance (ANOVA) was used to compare sleep-timing variables (i.e., sleep onset and total sleep time) between the baseline and extension conditions across groups. In these models, sleep-timing variables were independently entered as outcome variables. Condition (baseline and extension) was entered as a within-subjects factor and group (ADHD and TD) was entered as a between-subjects factor.
PSG. PSG data were collected to assess differences in sleep quantity and quality between groups and conditions. Due to recording error, five participants in the TD group did not have usable PSG data. Consequently, data for sleep physiology were averaged across 10 TD children (2F, Mage = 7.95 years, SD = 0.96 years). Usable PSG data were obtained from all children in the ADHD group.
Repeated-measures ANOVAs were used to compare PSG outcome variables (i.e., total sleep time and time spent in distinct sleep stages) between conditions and groups. Here too, condition was entered as a within-subjects factor and group as a between-subjects factor.
Inhibitory Control
To determine whether sleep extension improved inhibitory control in children with and without ADHD, a repeated-measures ANOVA was used. In this model, inhibitory con-trol, as measured by accuracy (% correct) on No-Go trials (O’Connell et al., 2009), was entered as the outcome vari-able. Time (evening and morning) and condition were entered as within-subject factors and group was entered as a between-subjects factor. Separate repeated-measures ANOVAs were then run independently for each group to assess group-specific changes in inhibitory control in the sleep extension condition.
Results
Participant demographics are provided in Table 1. At the group level, children in both groups met the sleep criteria of sleep less than 10 hr, on average, with bedtimes after 8 p.m. Child age, t(24) = 0.11, p = .92, gender, X2(1, N = 26) = .74, p = .39, ethnicity, X2(1, N = 25) = 2.43, p = .12, and annual household income, X2(1, N = 26) = 4.74, p = .45, did not differ between groups. As expected, based on DBRS scores, children in the ADHD group had significantly more symptoms of ADHD than children in the TD group, t(24) = 7.03, p ≤ .001, 95% confidence interval (CI) = [16.47, 30.17].
Sleep
Descriptive statistics from actigraphy and PSG are provided in Table 2 and Supplemental Table 1, respectively.
Table 2.
Differences in sleep timing between conditions and groups (actigraphy; mean across 5-days).
| ADHD Mean (SD) |
TD Mean (SD) |
p-value (Condition) |
p-value (Group) |
|
|---|---|---|---|---|
| Total sleep time (minutes) | ||||
| Baseline | 579.57 (46.02) | 588.94 (26.02) | ≤.001 | .34 |
| Extension | 627.53 (46.46) | 644.51 (30.48) | ||
| Sleep onset time (military time, minutes) | ||||
| Baseline | 21:21 (39.05) | 21:01 (33.60) | ≤.001 | .18 |
| Extension | 20:15 (55.49) | 19:52 (30.06) | ||
| Wake onset time (military time, minutes) | ||||
| Baseline | 7:06 (54.00) | 6:50 (25.35) | ≤.01 | .57 |
| Extension | 6:43 (45.03) | 6:37 (20.73) | ||
| Wake after sleep onset (minutes) | ||||
| Baseline | 42.58 (23.50) | 48.58 (15.40) | ≤.001 | .17 |
| Extension | 56.87 (17.33) | 70.93 (20.63) | ||
| Sleep efficiency (%) | ||||
| Baseline | 89.05 (3.67) | 87.70 (2.91) | ≤.001 | .08 |
| Extension | 86.87 (2.99) | 83.99 (2.76) | ||
Notes: In ADHD group, n = 11. In TD group, n = 14.
ADHD = attention-deficit/hyperactivity disorder; TD = typically developing; SD = standard deviation
Actigraphy. In the repeated-measures ANOVA comparing sleep onset time between conditions and groups, the main effect of condition was significant, F(1,23) = 258.71, p ≤ .001, ηp2 = .92; Table 2. Children advanced sleep onset time from an average time of 9:11 p.m. during the baseline condition to 8:04 p.m. during the extension condition. The main effect of group and the condition by group interaction were not significant (ps ≥ .18).
Average wake onset time was significantly earlier during the extension condition relative to the baseline condition, main effect of condition: F(1,23) = 10.34, p ≤ .01, ηp2 = .31. Children woke earlier in the sleep extension condition (Table 2). The main effect of group and the condition by group interaction were not significant (ps ≥ .57).
When comparing total sleep time, the main effect of condition was again significant: overall, children slept 52 min longer during the extension condition relative to the baseline condition, F(1,23) = 59.76, p ≤ .001, ηp2 = .72; Table 2. The main effect of group and the condition by group interaction were not significant (ps ≥ .34).
After falling asleep, children woke up significantly more during the extension condition relative to the baseline condition, main effect of condition: F(1,23) = 35.42, p ≤ .001, ηp2 = .61; Table 2. Similarly, sleep efficiency (the ratio of time spent asleep compared with time in the sleep bout, as detected by the scoring software) was reduced during the extension condition, main effect of condition: F(1,23) = 37.67, p ≤ .001, ηp2 = .62. The main effects of group and condition by group interactions were not significant (ps ≥ .08). Taken together, these findings suggest that although children fell sleep earlier and slept longer during the extension condition, they woke more often during the night as would be expected with reduced sleep pressure relative to the baseline condition (Borbély, 1982). Importantly, however, sleep efficiency values were high (≥ 84%) across groups and conditions (Table 2) and comparable with previous reports in this age group (Gruber et al., 2014; Paavonen et al., 2010).
PSG. Analysis of the PSG data support the results from actigraphy such that total sleep time was significantly longer in the extension condition compared with the baseline condition, main effect of condition: F(1,19) = 17.26, p = .001, ηp2 = .48; Table S1. Sleep efficiency was also margin-ally reduced during the extension condition, main effect of condition: F(1, 19) = 4.98, p = .04, ηp2 = .21; Table S1. The main effect of group and the condition by group inter-action were not significant (ps ≥ .12). Time spent in distinct sleep stages did not differ between conditions or groups after controlling for multiple comparisons (α = .01), although children with ADHD tended to have a greater pro-portion of slow wave sleep (SWS) relative to TD children, main effect of group: F(1, 19) = 5.10, p = .04, ηp2 =.21; Table S1.
Collectively, these data indicate that the sleep extension condition was effective in extending sleep duration for both TD children and children with ADHD. Thus, we next examined whether extending sleep improved inhibitory control.
Inhibitory Control
A repeated-measures ANOVA was used to compare sleep-dependent changes in inhibitory control between times, conditions, and groups. Expectedly, inhibitory control was greater in TD children, main effect of group: F(1,24) = 4.32, p = .05, ηp2 = .15; Figure 3. The main effect of time was also significant, as inhibitory control was greater in the morning relative to the evening in both groups, F(1,24) = 8.39, p = .01, ηp2 = .26. The main effect of condition was not significant, F(1,24) = 2.78, p = .11. These results indicate that there was sleep-dependent improvement of inhibitory control across children, regardless of diagnostic status or sleep extension.
Figure 3. Difference in inhibitory control between conditions and groups.
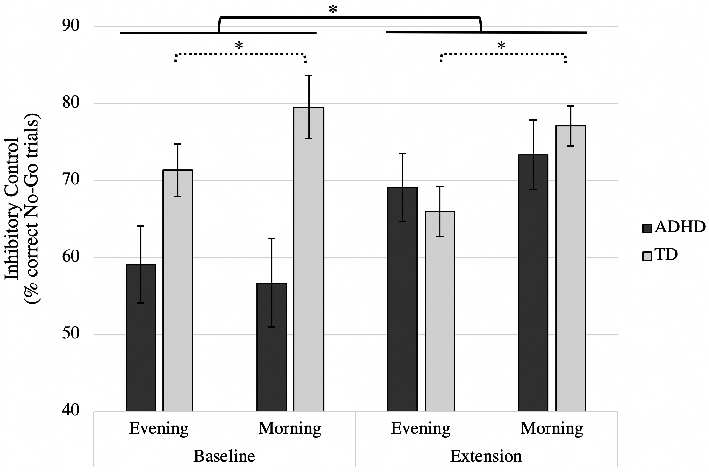
The solid brackets represent the significant main effect of condition in the ADHD group (motivated by the significant 2-way interaction between condition and group in the omnibus ANOVA). The dotted brackets represent the significant main effect of time in the TD group (motivated by the significant 2-way interaction between time and group in the omnibus ANOVA). Error bars represent standard error; *p’s ≤ .05.
The condition by group, F(1,24) = 9.23, p = .01, ηp2 = .28, and time by group interactions, F(1,24) = 5.75, p = .03, ηp2 = .19, were significant, suggesting that sleep-dependent improvements in inhibitory control differed between children with and without ADHD. Follow-up ANOVAs were used to assess interactions within each group, independently. The two-way interaction between condition and time and the three-way interaction between condition, time, and group were not significant (p ≥ .22).
ADHD group. Consistent with the results of Cremone and colleagues (2017), the main effect of time was not significant in the ADHD group, suggesting that inhibitory control did not improve following an interval of sleep, F(1,10) =.11, p = .75. However, the main effect of condition was significant, F(1,10) = 7.20, p = .02, ηp2 = .42: children with ADHD had greater inhibitory control dur-ing the extension condition relative to the baseline condition. The condition by time interaction was not significant, F(1,10) = 2.79, p = .13.
TD group. The main effect of condition was not significant, F(1,14) = 1.46, p = .25; Figure 3. However, the main effect of time was significant, F(1,14) = 16.80, p = .001,η p2 = .55. Consistent with Cremone et al. (2017), for TD children, inhibitory control was significantly greater in the morning, relative to the evening. This benefit of sleep on subsequent performance did not differ for the baseline and extension conditions as the condition by time interaction was not significant, F(1,14) =.26, p = .62.
Sleep and Inhibitory Control
An exploratory bivariate correlation was run to assess relations between the change in total sleep time (extension–base-line) and the change in inhibitory control (extension–baseline; collapsed across the evening and morning assessments). However, these correlations were not significant in either group (ps ≥ .48).
Discussion
This pilot study examined the efficacy of a sleep extension intervention in children with and without ADHD. Both groups of children were able to extend overnight sleep duration by 52 min, on average, when instructed to advance bed-time by 90 min (1.5 hr). Inhibitory control improved more than 13% from baseline when children with ADHD extended overnight sleep duration. Although, inhibitory control was not improved by sleep extension in the TD group, their morning inhibitory control was 10% greater than evening inhibitory control, consistent with the results of Cremone et al. (2017).
Sleep
In accordance with previous studies (Gruber et al., 2012), TD children extended sleep duration by 56 min, on average, (range between 24 and 87 min) when bedtime was advanced. Importantly, this study successfully demonstrated sleep extension in children with ADHD—a population consistently reported to have insufficient sleep marked by reduced sleep duration and bedtime resistance (Owens, Maxim, Nobile, McGuinn, & Msall, 2000; Weiss et al., 2015). Specifically, when instructed to advance their bedtime by 90 min, children with ADHD extended sleep duration by 48 min, on average (range between 38 and 91 min)1. This find-ing was consistent with a recent study by Becker and col-leagues (2019) who reported that adolescents with ADHD (14 to 17 years of age) were able to successfully extend sleep duration by 66 min (relative to a stabilization period) when instructed to increase time in bed to 9.5 hr.
Inhibitory Control
Deficits in inhibitory control contribute to primary symptoms of ADHD (Barkley, 1997; Doyle, 2006; Nigg, 2000; Oosterlaan et al., 1998) and secondary cognitive impairments (Barkley, 1997). As inhibitory control was improved by sleep extension in this sample, these findings suggest that extending sleep duration may improve symptoms and cognitive function in children with ADHD. If such is the case, this method of symptom management may be preferred over pharmacological treatments, as sleep-based interventions have not been linked to adverse side effects.
Moreover, the beneficial effect of sleep extension was robust, and the effect size comparable with those of many stimulants used to treat ADHD (Faraone, Biederman, Spencer, & Aleardi, 2006), suggesting that sleep-based interventions may be an effective means of managing impair-ments in inhibitory control. An effect size of this magnitude is particularly noteworthy given that this intervention assessed changes in behavior after only 5 days of experimental manipulation. However, as interventions with this population typically span several weeks (Herbert, Harvey, Roberts, Wichowski, & Lugo-Candelas, 2013), and measure symptom expression across context and at various points in the day, it is important to assess prolonged changes to behavior with multiple outcome variables in future studies. We hypothesize that these effects would be more robust over time as children and caregivers adjust to the earlier bedtime.
In contrast to the findings in the ADHD group, sleep extension did not alter behavior in TD children. The lack of improvement in this group may be a consequence of the outcome targeted. Inhibitory control was intact in the TD sample tested (see Figure 3). As such, there may have been little room for sleep-related enhancement of inhibitory functioning.
Sleep and Inhibitory Control
Collectively, these results suggest that sleep extension improves inhibitory control among children with ADHD. Notably, however, the change in sleep duration was not correlated with the improvements in inhibitory control in either group. It is possible that behavioral improvements were a consequence of stabilization of the sleep routine rather than changes to sleep duration per se. Future studies should include measures of stability and family functioning to identify distal factors affected by the sleep manipulation that may be related to improved cognitive function in children with ADHD. In addition, a larger sample of children with usable PSG data is needed to determine whether differences in sleep physiology contribute to differences in inhibitory control mechanistically.
Limitations and Future Directions
In summary, the results of this pilot study suggest sleep extension improved inhibitory control in children with ADHD. Counter to our hypothesis, however, overnight sleep did not improve inhibitory control in children with ADHD albeit a benefit of sleep extension (i.e., lack of a significant main effect of time or three-way interaction between condition, time, and group). It is possible that the intervention used in this study had effects beyond sleep extension that contributed to improved behavior. For example, the sleep extension condition may have improved “household chaos”—the level of routine and structure in a home—that is associated with ADHD symptomology (Auerbach, Zilberman-Hayun, Atzaba-Poria, & Berger, 2017). Additional studies with multi-informant or multi-setting observations of inhibition and other outcomes (i.e., household chaos) are needed to explore this hypothesis. Similarly, statistical outcomes (i.e., the three-way interaction between condition, time, and group) may also be altered in a larger sample.
It is also important to acknowledge that six out of 11 children in the ADHD sample were taking medications for ADHD symptom management. Because discontinuation of these medications may reduce their efficacy, children were not asked to discontinue taking medications during our pro-longed experimental protocol. However, as these medications may alter sleep (Konofal, Lecendreux, & Cortese, 2010), future studies should assess the effects of sleep extension on cognitive outcomes in medication naïve children or children who are able to discontinue medication use during experimental testing. Notably, Becker and colleagues (2019) report that adolescents with ADHD extended sleep duration when stimulant mediation use was discontinued during a 3-week sleep protocol during the summer, suggesting that the beneficial effects of sleep extension reported in the current study may transfer to a sample of medication-free school-aged children with the disorder.
Future studies should also evaluate the efficacy of sleep-based interventions in broader samples of children with ADHD. Recent evidence indicates sleep is more impaired among school-aged females with ADHD, relative to age-matched males (Becker, Cusick, Sidol, Epstein, & Tamm, 2018). Therefore, a replication study with a larger proportion of female participants is needed to assess the generalizability of these findings across gender.
The current study is also limited in that we did not evaluate clinical sleep disorders or developmental comorbidities. Clinical sleep disorders, such as sleep apnea and periodic limb movement disorder (PLMD), are common in children with ADHD (Konofal et al., 2010) and may alter the efficacy of sleep-based interventions across children. Anxiety and ASDs are also prevalent in children with ADHD (Larson, Russ, Kahn, & Halfon, 2011) and are independently associated with heightened rates of sleep problems (Mayes et al., 2009). Moreover, recent evidence suggests that a cognitive behavioral therapy for anxiety improved sleep in children with comorbid ADHD and anxiety, suggesting that sleep–hygiene interventions may have similar outcomes in this population (Bériault et al., 2018). Thus, additional studies with larger, more diverse samples are needed to evaluate the efficacy of sleep-based interventions in more representative samples of children with ADHD.
Conclusion
Taken together, these results indicate that children with and without ADHD are capable of extending overnight sleep duration when instructed to advance their bedtime. In children with ADHD, the extension of overnight sleep duration improved inhibitory control—a primary deficit in this population that is strongly associated with symptom severity. Conversely, inhibitory control was improved by overnight sleep, regardless of sleep duration, in TD children. Collectively, these findings suggest that targeting sleep improves cognitive function in young children with and without ADHD.
Supplementary Material
Acknowledgments
The authors would like to thank the children and families who participated in this research.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by NIH R01 HL111695 (Rebecca M. C. Spencer), a Dissertation Research Grant from the Graduate School at University of Massachusetts Amherst (Amanda Cremone-Caira), and Experiment.com (Amanda Cremone-Caira).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
One child in the ADHD group slept 69 min longer during the baseline condition, relative to the extension condition, on average. This child exhibited significant variability in sleep duration during both conditions. Importantly, how-ever, behavioral results were unchanged when this child was excluded from analyses.
References
- Acebo C, Sadeh A, Seifer R, Tzischinsky O, Hafer A, & Carskadon MA (2005). Sleep/wake patterns derived from activity monitoring and maternal report for healthy 1- to 5-year-old children. Sleep, 28, 1568–1577. [DOI] [PubMed] [Google Scholar]
- Anderson P (2002). Assessment and development of Executive Function (EF) during childhood. Child Neuropsychology, 8, 71–82. doi: 10.1076/chin.8.2.71.8724 [DOI] [PubMed] [Google Scholar]
- Applegate B, Lahey BB, Hart EL, Biederman J, Hynd GW, Barkley RA, . . . Shaffer D (1997). Validity of the age-of-onset criterion for ADHD: A report from the DSM-IV field trials. Journal of the American Academy of Child & Adolescent Psychiatry, 36, 1211–1221. doi: 10.1097/00004583-199709000-00013 [DOI] [PubMed] [Google Scholar]
- Auerbach JG, Zilberman-Hayun Y, Atzaba-Poria N, & Berger A (2017). The contribution of maternal ADHD symptomatology, maternal DAT1, and home atmosphere to child ADHD symptomatology at 7 years of age. Journal of Abnormal Child Psychology, 45, 415–427. doi: 10.1007/s10802-016-0230-0 [DOI] [PubMed] [Google Scholar]
- Barkley RA (1997). Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin, 121, 65–94. doi: 10.1037/0033-2909.121.1.65 [DOI] [PubMed] [Google Scholar]
- Barkley RA, & Murphy KR (2006). Attention-deficit hyperactivity disorder: A clinical workbook. New York, NY: Guilford Press. [Google Scholar]
- Becker SP, Cusick CN, Sidol CA, Epstein JN, & Tamm L (2018). The impact of comorbid mental health symptoms and sex on sleep functioning in children with ADHD, 27, 353–365. doi: 10.1038/ng.3564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker SP, Epstein JN, Tamm L, Tilford AA, Tischner CM, Isaacson PA, . . . Beebe DW (2019). Shortened sleep duration causes sleepiness, inattention, and opposition-ality in adolescents with ADHD: Findings from a crossover sleep restriction/extension study. Journal of the American Academy of Child & Adolescent Psychiatry, 58, 433–442. doi: 10.1016/j.jaac.2018.09.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bériault M, Turgeon L, Labrosse M, Berthiaume C, Verreault M, Berthiaume C, & Godbout R (2018). Comorbidity of ADHD and anxiety disorders in school-age children: Impact on sleep and response to a cognitive-behavioral treatment. Journal of Attention Disorders, 22, 414–424. doi: 10.1177/1087054715605914 [DOI] [PubMed] [Google Scholar]
- Borbély AA (1982). A two process model of sleep regulation. Human Neurobiology, 1, 195–204 [PubMed] [Google Scholar]
- Cavanagh JF, & Frank MJ (2014). Frontal theta as a mechanism for cognitive control. Trends in Cognitive Sciences, 18, 414–421. doi: 10.1016/j.tics.2014.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremone A, Lugo-Candelas CI, Harvey EA, McDermott JM, & Spencer RMC (2017). REM theta activity enhances inhibitory control in typically developing children but not children with ADHD symptoms. Experimental Brain Research, 235, 1491–1500. doi: 10.1007/s00221-017-4906-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle AE (2006). Executive functions in attention-deficit/hyperactivity disorder. Journal of Clinical Psychiatry, 67(8), 21–26. [PubMed] [Google Scholar]
- Durston S, Thomas KM, Yang Y, Ulug AM, Zimmerman RD, & Casey BJ (2002). A neural basis for the develop-ment of inhibitory control. Developmental Science, 5, F9–F16. [Google Scholar]
- Faraone SV, Biederman J, Spencer TJ, & Aleardi M (2006). Comparing the efficacy of medications for ADHD using meta-analysis. Medscape General Medicine, 8(4), Article 4. [PMC free article] [PubMed] [Google Scholar]
- Gruber R, Cassoff J, Frenette S, Wiebe S, & Carrier J (2012). Impact of sleep extension and restriction on children’s emotional lability and impulsivity. Pediatrics, 130, e1155–e1161. doi: 10.1542/peds.2012-0564 [DOI] [PubMed] [Google Scholar]
- Gruber R, Somerville G, Enros P, Paquin S, Kestler M, & Gillies-Poitras E (2014). Sleep efficiency (but not sleep duration) of healthy school-age children is associated with grades in math and languages. Sleep Medicine, 15, 1517–1525. doi: 10.1016/j.sleep.2014.08.009 [DOI] [PubMed] [Google Scholar]
- Gruber R, Wiebe S, Montecalvo L, Brunetti B, Amsel R, & Carrier J (2011). Impact of sleep restriction on neurobehavioral functioning of children with attention deficit hyperactivity disorder. Sleep, 34, 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpin VA (2005). The effect of ADHD on the life of an indi-vidual, their family, and community from preschool to adult life. Archives of Disease in Childhood, 90, i2–i7. doi: 10.1136/adc.2004.059006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert SD, Harvey EA, Roberts JL, Wichowski K, & Lugo-Candelas CI (2013). A randomized controlled trial of a parent training and emotion socialization program for families of hyperactive preschool-aged children. Behavior Therapy, 44, 302–316. doi: 10.1016/j.beth.2012.10.004 [DOI] [PubMed] [Google Scholar]
- Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, . . . Adams Hillard PJ (2015). National sleep foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health, 1, 40–43. doi: 10.1016/j.sleh.2014.12.010 [DOI] [PubMed] [Google Scholar]
- Hiscock H, Sciberras E, Mensah F, Gerner B, Efron D, Khano S, & Oberklaid F (2015). Impact of a behavioural sleep intervention on symptoms and sleep in children with attention deficit hyperactivity disorder, and parental mental health: Randomised controlled trial. BMJ, 350(1), Article h68. doi: 10.1136/bmj.h68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson AL, & Quan SF (2007). The AASM manual for the scoring of sleep and associated events: Rules, terminology, and technical specifications. Darien, IL: American Academy of Sleep Medicine. [Google Scholar]
- Konofal E, Lecendreux M, & Cortese S (2010). Sleep and ADHD. Sleep Medicine, 11, 652–658. doi: 10.1016/j.sleep.2010.02.012 [DOI] [PubMed] [Google Scholar]
- Lamm C, White LK, McDermott JM, & Fox NA (2012). Neural activation underlying cognitive control in the context of neutral and affectively charged pictures in children, 79, 181–187. doi: 10.1016/j.bandc.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson K, Russ SA, Kahn RS, & Halfon N (2011). Patterns of comorbidity, functioning, and service use for US children With ADHD, 2007. Pediatrics, 127, 462–470. doi: 10.1542/peds.2010-0165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes SD, Calhoun S, Bixler EO, & Vgontzas AN (2009). Sleep problems in children with autism, ADHD, anxiety, depression, acquired brain injury, and typical development. Sleep Medicine Clinics, 4, 19–25.doi: 10.1016/j.jsmc.2008.12.004 [DOI] [Google Scholar]
- Nigg JT (2000). On inhibition/disinhibition in developmental psychology: Views from cognitive and personality psychology and a working inhibition taxonomy. Psychological Bulletin, 126, 220–246. [DOI] [PubMed] [Google Scholar]
- O’Connell RG, Dockree PM, Bellgrove MA, Turin A, Ward S, Foxe JJ, & Robertson IH (2009). Two types of action error: Electrophysiological evidence for separable inhibitory and sustained attention neural mechanisms producing error on go/no-go tasks. Journal of Cognitive Neuroscience, 21, 93–104. doi: 10.1162/jocn.2009.21008 [DOI] [PubMed] [Google Scholar]
- Oosterlaan J, Logan GD, & Sergeant JA (1998). Response inhibition in AD/HD, CD, comorbid AD/HD+CD, anxious, and control children: A meta-analysis of studies with the stop task. Journal of Child Psychology and Psychiatry, 39, 411–425. doi: 10.1111/1469-7610.00336 [DOI] [PubMed] [Google Scholar]
- Owens JA (2005). The ADHD and sleep conundrum: A review. Journal of Developmental & Behavioral Pediatrics, 26, 312–322. [DOI] [PubMed] [Google Scholar]
- Owens JA, Maxim R, Nobile C, McGuinn M, & Msall M (2000). Parental and self-report of sleep in children with attention-deficit/hyperactivity disorder. Archives of Pediatrics and Adolescent Medicine, 154, 549–555. [DOI] [PubMed] [Google Scholar]
- Paavonen EJ, Räikkönen K, Pesonen A, Lahti J, Komsi N, Heinonen K, . . . Porkkaheiskanen T (2010). Sleep qual-ity and cognitive performance in 8-year-old children. Sleep Medicine, 11, 386–392. doi: 10.1016/j.sleep.2009.09.009 [DOI] [PubMed] [Google Scholar]
- Pelham W, Fabiano G, & Massetti G (2005). Evidence-based assessment of attention deficit hyperactivity disorder in children and adolescents. Journal of Clinical Child & Adolescent Psychology, 34, 559–568. doi: 10.1207/s15374424jccp3403 [DOI] [PubMed] [Google Scholar]
- Sadeh A, Gruber R, & Raviv A (2003). The effects of sleep restriction and extension on school-age children: What a difference an hour makes. Child Development, 74, 444–455. doi: 10.1111/1467-8624.7402008 [DOI] [PubMed] [Google Scholar]
- Sitnick SL, Goodlin-Jones BL, & Anders TF (2008). The use of actigraphy to study sleep disorders in preschoolers: Some concerns about detection of nighttime awakenings. Sleep, 31, 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao T, Wang L, Fan C, & Gao W (2014). Development of self-control in children aged 3 to 9 years: Perspective from a dual-systems model. Scientific Reports, 4, Article 7272. doi: 10.1038/srep07272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R, Sanders S, Doust J, Beller E, & Glasziou P (2015). Prevalence of attention-deficit/hyperactivity disor-der: A systematic review and meta-analysis. Pediatrics, 135, e994–e1001. doi: 10.1542/peds.2014-3482 [DOI] [PubMed] [Google Scholar]
- Vriend J, Davidson F, Shaffner S, Corkum P, & Rusak B (2013). Manipulating sleep duration alters cognitive and emo-tional functioning in children. Sleep Medicine, 12(10), S14–S15. doi: 10.1016/S1389-9457(11)70050-8 [DOI] [Google Scholar]
- Waschbusch DA (2002). A meta-analytic examination of comorbid hyperactive-impulsiveattention problems and con-duct problems. Psychological Bulletin, 128, 118–150. doi: 10.1037//0033-2909.128.1.118 [DOI] [PubMed] [Google Scholar]
- Weiss MD, Craig SG, Davies G, Schibuk L, & Stein M (2015). New research on the complex interaction of sleep and ADHD. Current Sleep Medicine Reports, 1, 114–121. doi: 10.1007/s40675-015-0018-8 [DOI] [Google Scholar]
- Wilens TE, Faraone SV, & Biederman J (2004). Attention-deficit/hyperactivity disorder in adults. JAMA, 292, 619–623. [DOI] [PubMed] [Google Scholar]
- Yoon SYR, Jain U, & Shapiro C (2012). Sleep in attention-deficit/hyperactivity disorder in children and adults: Past, present, and future. Sleep Medicine Reviews, 16, 371–388. doi: 10.1016/j.smrv.2011.07.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


