Abstract
Background.
After 25 years without poliomyelitis cases caused by wild poliovirus (WPV) circulation in Israel, sewage sampling detected WPV type 1 (WPV1) in April 2013, despite high vaccination coverage with only inactivated poliovirus vaccine (IPV) since 2005.
Methods.
We used a differential equation-based model to simulate the dynamics of poliovirus transmission and population immunity in Israel due to past exposure to WPV and use of oral poliovirus vaccine (OPV) in addition to IPV. We explored the influences of various immunization options to stop imported WPV1 circulation in Israel.
Results.
We successfully modeled the potential for WPVs to circulate without detected cases in Israel. Maintaining a sequential IPV/OPV schedule instead of switching to IPV-only in 2005 would have kept population immunity high enough in Israel to prevent WPV1 circulation. The Israeli response to WPV1 detection prevented paralytic cases; a more rapid response might have interrupted transmission more quickly.
Conclusions.
IPV-based protection alone might not provide sufficient population immunity to prevent poliovirus transmission after an importation. As countries transition to IPV in immunization schedules, they may need to actively manage population immunity and consider continued OPV use to avoid the potential circulation of imported live polioviruses until globally-coordinated cessation of OPV use.
Keywords: polio, eradication, population immunity, OPV, IPV
INTRODUCTION
Salk inactivated poliovirus vaccine (IPV) became available in the late 1950s and Sabin oral poliovirus vaccines (OPV) in the 1960s. Challenge studies indicate that IPV-induced immunity significantly reduces oral-oral poliovirus transmission but reduces participation in fecal-oral transmission much less than OPV-induced immunity[1, 2]. Israel introduced Salk IPV in early 1957 using mass campaigns (supplemental immunization activities [SIAs]), followed by introduction routine immunization (RI) in 1958. This led to a transition from hundreds of paralytic cases caused by wild polioviruses (WPVs) each year during 1950-1956 to a period of limited transmission with episodic outbreaks.[3] Israel introduced oral poliovirus vaccine (OPV) beginning with monovalent OPV types 1 and 2 (mOPV1 and mOPV2) in SIAs in 1961 and type 3 (mOPV3) in 1962. Israel stopped mOPV SIAs when it switched from IPV to trivalent OPV (tOPV) for RI starting in 1963 (3 doses, 6 weeks apart and a booster dose 6 months later).[4,5] Continued polio cases among the non-Jewish population during 1974-1979 led Israel to conduct annual national 2-week (May-June) SIAs during 1979-1982 with mOPV1 targeting children under age 3 years, including all non-Jews and Jews living near non-Jewish settlements.[4,6]
While most of the country continued to use tOPV for RI without any SIAs after 1982, two subdistricts (Hadera and Ramla) used enhanced-potency IPV for RI at 2, 4, and 12–16 months of age from 1982 to 1988.[6] Annual mOPV1 SIAs for non-Jewish children in Hadera and all children in Ramla in 1982, 1983, and 1984 targeted children under the age of 3 years at the time of the SIA.[6] In 1988, a type 1 WPV outbreak (WPV1) occurred, cases occurring predominantly in Hadera, after an importation most likely from northern Egypt in 1987.[7,8] To control the outbreak, Hadera (and Ramla) conducted an outbreak response SIA with tOPV in September 1988 and Israel conducted a national SIA in October 1988.
Similar to other countries that successfully eliminated WPVs, in 1990 Israel switched to a sequential IPV-OPV RI schedule to avoid possible cases of vaccine-associated paralytic polio (VAPP). The schedule included 3 IPV doses followed by 3 tOPV doses administered by age 12 months, with tOPV booster doses at ages 6 and 16 years.[7] In 2005, 3 years after the World Health Organization (WHO) certified the European Region (including Israel) as polio-free,[9] Israel implemented an IPV-only RI schedule, with doses given at age 2, 4, 6, and 12-16 months and a booster dose at age 7 years.
Environmental surveillance began in northern Israel during the 1987-1988 outbreak and expanded nationally to cover 30-40% of the population with routine sewage sampling in multiple locations in 1989.[10] Israel reported no WPV-associated polio cases after 1989; however, the environmental surveillance system isolated WPV1 in 1995 and type 2 vaccine-derived polioviruses (VDPV) on multiple occasions since 1998 from two sampling locations.[7] On April 7, 2013, WPV1 isolated in environmental samples taken from Beer Sheva and Rahat led to a retrospective analysis of earlier samples, which suggested introduction of WPV1 in early February 2013.[10-12] Repeated environmental sampling revealed evidence of WPV1 transmission based on positive samples collected from multiple sites in the South over extended periods of time, but limited transmission in the rest of Israel [10]. In response to the detected WPV1, Israel conducted a national IPV catch-up SIA campaign targeting all children under age 6 years with incomplete vaccination, combined with a booster dose for adults aged >18 years in Rahat (a South district town at the epicenter [11]). Israel then conducted a national bivalent types 1 and 3 OPV (bOPV) SIA during August-October 2013 for children born since 2005,[11] and added 2 bOPV doses given at ages 6 and 18 months to the national RI schedule in January 2014.[13]
Prior modeling results suggested that as the world approaches the eradication of WPVs and countries increasingly shift from OPV to IPV, national health leaders will need to (1) recognize the heterogeneity in population immunity might create pockets of susceptible individuals and (2) develop immunization strategies to protect the entire population.[14] Because of the relatively lower population immunity to transmission induced by IPV compared with OPV, the observation of WPV1 transmission in Israel without detected polio cases confirms the possibility for live polioviruses (LPVs) to circulate in the context of high IPV-only coverage (in the absence of WPVs).[15] This paper models population immunity for Israel to provide insights about potential LPV transmission in a country using IPV for national RI and the development of immunization strategies to prevent transmission following LPV importations.
METHODS
We collected the best available information about RI and SIAs histories (summarized in Table 1). Table 2 shows the inputs we used in our differential equation based model with developed country settings and assumptions related to demographics, poliovirus transmissibility and seasonality.[1,2,16,17] We divided the population of Israel into 13 age groups, with three preferentially mixing age groups (i.e., 0–4, 5–14, and 15 or more years). Considering the different immunization schedules in the 1980s and the epidemiology of the 1988 WPV1 outbreak, we divided the Israeli population into two preferentially mixing regions for Hadera and the rest of the country before 1994. From 1994, when population immunity in both regions became similar, we changed the geographic division of the population to the South district and the rest of Israel to capture differences in hygiene and crowding conditions. Each region also includes a preferentially mixing subpopulation that represents historically undervaccinated non-Jews for which Israel separately reports polio incidence and vaccination coverage.[3-7,18,19] Thus, the model includes four subpopulations representing Jews and non-Jews in different parts of the country before 1994 (i.e., Hadera and the rest of Israel) and from 1994 (i.e., South district and the rest of Israel).
Table 1.
Summary of the Timing and Vaccine Used in the Routine Immunization (RI) schedule and for Supplementary Immunization Activities (SIAs) in Israel
| Vaccination input | Best estimate | Source | Notes |
|---|---|---|---|
| Relative RI coverage in non-Jewish subpopulations between 1958–1993 | |||
| - Before 1979 | 0.30 | ||
| - 1979 | 0.40 | ||
| - 1980 | 0.50 | ||
| - 1981 | 0.60 | ||
| - 1982–1988 | 0.70 | ||
| - 1989–1990 | 0.80 | ||
| - 1991–1992 | 0.90 | ||
| - After 1993 | 1.00 | ||
| Relative SIA coverage in non-Jewish subpopulations | |||
| - Before 1979 | 0.40 | ||
| - 1979 | 0.50 | ||
| - 1980 | 0.60 | ||
| - 1981 | 0.70 | ||
| - 1982–1984 | 0.80 | ||
| - 2013 | 1.00 | ||
| Characterization of Salk IPV SIAs during 1957–1958 | [3] | ||
| - First day of first SIA, 1957 | 15 (Jan 15) | Children aged 6 months–2 years | |
| - First day of second SIA, 1957 | 46 (Feb 15) | Children aged 6 months–2 years | |
| - First day of third SIA, 1957 | 74 (March 15) | Children aged 3–29 years | |
| - First day of fourth SIA, 1957 | 105 (April 15) | Children aged 3 years | |
| - First day of fifth SIA, 1957 | 135(May 15) | Children aged 4–5 months | |
| - First day of sixth SIA, 1957 | 166 (June 15) | Children aged 4–5 months | |
| - First day of seventh SIA, 1958 | 15 (Jan 15) | Children aged 2–4 years | |
| - Duration of SIAs (days) | 14 | ||
| - True coverage in Jewish subpopulations | 0.93 | ||
| - Repeated missed probability | 0.80 | ||
| Characterization of IPV RI during 1958-1961 | [3] | The first 2 doses at 4 and 6 months of age. | |
| - Primary doses by age 6 months | 2 | ||
| - Primary doses at age 12 months | 1 | ||
| - Coverage in Jewish subpopulations | Time series | ||
| Characterization of mOPV1 SIAs during 1961-1962 | [4,5] | Children younger than 5 years | |
| - First day of first SIA, 1961 | 166 (June 15) | ||
| - First day of second SIA, 1962 | 32 (Feb 1) | ||
| - First day of third SIA, 1962 | 91 (April 1) | ||
| - Duration of SIAs (days) | 14 | ||
| - True coverage in Jewish subpopulations | 0.70 | ||
| - Repeated missed probability | 0.80 | ||
| Characterization of mOPV2 SIA in 1961 | [4,5] | Children younger than 5 years | |
| - First day of SIA | 319 (Nov 15) | ||
| - Duration of SIA (days) | 14 | ||
| - True coverage in Jewish subpopulations | 0.70 | ||
| - Repeated missed probability | 0.80 | ||
| Characterization of mOPV3 SIA in 1962 | [4,5] | Children younger than 5 years | |
| - First day of SIA | 152 (June 1) | ||
| - Duration of SIA (days) | 14 | ||
| - True coverage in Jewish subpopulations | 0.75 | ||
| - Repeated missed probability | 0.80 | ||
| Characterization of tOPV SIA in 1963 | [4,5] | Children younger than 5 years | |
| - First day of SIA | 32 (Feb 1) | ||
| - Duration of SIA (days) | 14 | ||
| - True coverage in Jewish subpopulations | 0.80 | ||
| - Repeated missed probability | 0.80 | ||
| Characterization of OPV RI during 1963–1988 | [4, 19] | The first 3 doses at 2, 4 and 6 months of age except Hadera between1982–1988 | |
| - Primary doses by age 6 months | 3 | ||
| - Primary doses at age 12 months | 1 | ||
| - Coverage in Jewish subpopulations | Time series | ||
| Characterization of IPV RI during 1982–1988 | [6,7, 20] | The first 2 doses at 2 and 4 months of age. Only in Hadera | |
| - Primary doses by age 6 months | 2 | ||
| - Primary doses at age 12 months | 1 | ||
| - Coverage in Jewish subpopulations | Time series | ||
| Characterization of mOPV1 SIAs during 1979–1982 | [4, 6] | Children younger than 3 years, mostly non-Jewish living in poor sanitary conditions with adjacent Jewish settlements | |
| - Frequency | Annual | ||
| - First day of SIA | 140 (May 25) | ||
| - Duration of SIA (days) | 14 | ||
| - True coverage in Jewish subpopulations | 0.80 | ||
| - Repeated missed probability | 0.80 | ||
| Characterization of mOPV1 SIAs during 1982–1984 | [6] | Only in Hadera among non-Jewish kids aged 0–2 years and born before 1982 | |
| - Frequency | Annual | ||
| - First day of SIA | 140 (May 25) | ||
| - Duration of SIA (days) | 14 | ||
| - True coverage in Jewish subpopulations | 1.00 | ||
| - Repeated missed probability | 0.80 | ||
| Characterization of tOPV outbreak response in 1988 in Hadera subpopulations | [6] | All people aged 2 months–29 years | |
| - First day of response (2013) | 263 (Sept 20) | ||
| - Duration of response (days) | 10 | ||
| - True coverage in Jewish subpopulations | 0.80 | ||
| - Repeated missed probability | 0.80 | ||
| Characterization of tOPV outbreak response in 1988 in Non-Hadera subpopulations | [6] | All people aged 2 months–29 years | |
| - First day of response (2013) | 283 (Oct 10) | ||
| - Duration of response (days) | 18 | ||
| - True coverage in Jewish subpopulations | 0.80 | ||
| - Repeated missed probability | 0.80 | ||
| Characterization of IPV/OPV RI during 1989–2004 | [7, 19] | At 2 months IPV. | |
| - Primary doses by age 6 months | 4 | At 4 months tOPV+IPV | |
| - Primary doses at age 12 months | 2 | At 6 months tOPV. | |
| - Booster doses at age 5 years | 1 | At 12 months tOPV+IPV. | |
| - Booster doses at age 15 years | 1 | Booster doses tOPV | |
| - Coverage | Time series | ||
| Characterization of IPV RI during 2005–2013 | [7, 19] | The firs3 doses at 2, 4 and 6 months | |
| - Primary doses by age 6 months | 3 | ||
| - Primary doses at age 12 months | 1 | ||
| - Booster doses at age 10 years | 1 | ||
| - Coverage | Time series | ||
| Characterization of IPV catch up before bOPV response to WPV1 circulation in 2013 in South districts | [10,11] | All children younger than 10 years who have not received any IPV doses in the past | |
| - First day of response (2013) | 186 (July 5) | ||
| - Duration of response (days) | 30 | ||
| - True coverage in Jewish subpopulations | 0.65 | ||
| - Repeated missed probability | 0.80 | ||
| Characterization of IPV booster dose before bOPV response to WPV1 circulation in 2013 in South districts | [10,11] | Adults who have not received any IPV doses in the past | |
| - First day of response (2013) | 186 (July 5) | ||
| - Duration of response (days) | 30 | ||
| - True coverage in Jewish subpopulations | 0.10 | ||
| - Repeated missed probability | 0.80 | ||
| Characterization of IPV catch up before bOPV response to WPV1 circulation in 2013 in non-South districts | [10,11] | All children younger than 10 years who have not received any IPV doses in the past | |
| - First day of response (2013) | 199 (July 18) | ||
| - Duration of response (days) | 30 | ||
| - True coverage in Jewish subpopulations | 0.65 | ||
| - Repeated missed probability | 0.80 | ||
| Characterization of bOPV response SIA to WPV1 circulation in 2013 in South district | [10,11] | All children born after 1 January 2004, who have received at least 1 dose of IPV in the past | |
| - First day of response (2013) | 217 (Aug 5) | ||
| - Duration of response (days) | 50 | ||
| - True coverage in Jewish subpopulations | 0.80 | ||
| - Repeated missed probability | 0.80 | ||
| Characterization of bOPV response SIA to WPV1 circulation in 2013 in non-South districts | [10,11] | All children born after 1 January 2004, who have received at least 1 dose of IPV in the past | |
| - First day of response (2013) | 230 (Aug 18) | ||
| - Duration of response (days) | 50 | ||
| - True coverage in Jewish subpopulations | 0.60 | ||
| - Repeated missed probability | 0.80 | ||
| Characterization of IPV RI between 2005–2013 | [7, 19] | At age 2 and 4 months - IPV. | |
| - Primary doses by age 6 months | 4 | At age 6 months - IPV+bOPV | |
| - Primary doses at age 12 months | 1 | At age 12 months - IPV | |
| - Primary doses at age 24 months | 1 | At age 24 months – bOPV+ | |
| - Booster doses at age 10 years | 1 | Booster dose IPV | |
| - Coverage | Time series |
The formulation of IPV changed in 1982 to increase the potency of the vaccine
Abbreviations: bOPV, bivalent oral polio vaccine; IPV, inactivated polio vaccine; mOPV, monovalent OPV; mOPV1, 2, and 3, monovalent OPV types 1, 2, and 3; RI, routine immunization; SIA, supplemental immunization activity; tOPV, trivalent OPV; WPV1, wild poliovirus type 1
Table 2.
Israel-Specific Model Inputs Used in Addition to Generic Model Inputs Published Elsewhere[1,2,15-17]
| Model input (symbol) | Best estimate | Notes |
|---|---|---|
| Number of subpopulations | 4 | See text for description |
| Number of age groups | 13 | 0-1; 2; 3; 4–5; 6–11 months; 1; 2; 3; 4; 5–9; 10–14; 15–29; ≥ 30 years |
| Number of mixing age groups | 3 | 0–4; 5–14; ≥ 15 years |
| Starting year | 1950 | |
| Number of years of model burn in with no seasonality | 3 | |
| Number of years of seasonality burn in | 0 | |
| Average basic reproductive number for WPV1 (R0) | 5 | Except for non-Jewish subpopulation in the South district after 1994 |
| Average basic reproductive number for WPV1 (R0) in non-Jewish subpopulation in the South district | 6 | Applied after 1994 |
| Proportional change in R0 due to seasonality (α) | 0.15 | |
| Day of seasonal peak in R0 | 110 (Apr 20) | |
| Proportion of contacts reserved for individuals within the same mixing age group (κ) | 0.5 | |
| Proportion of transmissions via oropharyngeal route (poro) | 0.7 | Except non-Jewish subpopulation in the South district after 1994 |
| Proportion of transmissions via oropharyngeal route (poro) in non-Jewish subpopulation in the South district | 0.6 | Applied after 1994 |
| Proportion of potentially infectious contacts of individuals in the Hadera subpopulation with other individuals in the Hadera subpopulation (pwithin) | 0.95 | |
| Proportion of potentially infectious contacts of individuals in the under-vaccinated subpopulation (non-Jewish) with other individuals in the under-vaccinated subpopulation (qwithin) | 0.99 | |
| Characterization of regular WPV1 importations during 1962-1982 | Introduction to all four subpopulations | |
| - Frequency | Annual | |
| - Day of introductions | 40 (Feb 9) | |
| Characterization of regular WPV1 importations during 1983-1987 | Introductions to non-Jewish subpopulations | |
| - Frequency | Biannual | |
| - Day of introductions | 40 (Feb 9) | |
| Date of introduction for the 1995 WPV1 circulation | 40 (Feb 9) | Introductions to non-Jewish subpopulation only |
| Date of introduction for the 2013 WPV1 circulation | 34 (Feb 3) | Introductions to non-Jewish subpopulation only [10,11] |
| Per-dose take rate (tr) (PV1;PV2;PV3) | Based on [31] | |
| - tOPV | 0.65; 0.75; 0.55 | |
| - mOPV | 0.90; 0.95; 0.90 | |
| - bOPV | 0.80; NA; 0.80 | |
| - sIPV | 0.50; 0.60; 0.50 | |
| - eIPV | 0.70; 0.70; 0.70 |
Abbreviations: WPV1, wild poliovirus type 1; bOPV, bivalent oral polio vaccine; mOPV, monovalent OPV; NA, not applicable; PV1,PV2, and PV3, poliovirus types 1,2, and 3; tOPV, trivalent OPV.
We assumed an average basic reproductive number (R0 ) for WPV1 of 5 for the entire period, with seasonal variation (Table 2). However, after changing the subpopulation in 1994, we assumed a higher average basic reproductive number for WPV1 (R0 = 6) and a lower proportion of oropharyngeal transmission (poro =0.6) in the non-Jewish subpopulation in the South district based on conditions more conducive to transmission (i.e., relatively poor hygiene, crowding).
Our model considers eight immunity states: fully susceptible individuals with no successful vaccination or LPV infection; maternally immune infants born to mothers with any active, recent, or historic immunity; individuals who acquired immunity from either one or ≥2 prior LPV infections; individuals with 1, 2, or ≥3 successful IPV doses; and individuals with some combination of both ≥1 LPV infection and ≥1 IPV dose.[1,2,16,17] We assumed that LPV infection can lead to paralytic poliomyelitis only in fully susceptible individuals (including infants born with maternal immunity who lose protective maternal antibody levels); whereas individuals in all other immunity states remain completely and permanently protected from paralytic poliomyelitis. Individuals in any immunity state can get reinfected and participate asymptomatically in poliovirus transmission to varying degrees (depending on immunity state).[1,2,16,17]
Our model includes fecal-oral and oropharyngeal transmission routes, 20 stages of OPV reversion, and five stages of immunity waning. Similar to prior work,[17] we characterized population immunity to poliovirus transmission by computing the effective immune proportion adjusted for age- and sub-population-mixing (EIPM), with an effective immunity threshold EIP*=(1- 1/R0), above which infections eventually die out.[1,2,16,17] To simulate die-out of a given virus strain in the model, we set the force-of-infection for that strain to zero whenever the infectiousness-weighted prevalence of infectious individuals falls below a certain “transmission threshold” calibrated based on the experience with poliovirus die-out in diverse situations.[17] The model characterizes infections, and we estimated cases of paralysis as a function of the paralysis to infection ratios for first infections, which vary by serotypes for WPVs, VDPVs, and VAPP.[1,2,16,17]
Because of limited information about immigrant immunity status,[20] the model generally assumes the same population immunity profile for immigrants as in the Israeli population (Table 2).[17] However, the population of Israel increased rapidly in the 1950s because of major immigration of Jewish people, which probably led to net inflow of unvaccinated families. Therefore, up until 1965, we assumed 10% of immigrants under age 15 years that would enter as immunes according to the existing population immunity profile instead entered the population as fully susceptible individuals. Moreover, because of persistent WPV circulation in neighboring countries, during 1962–1982 we assumed annual effective introductions of WPV1 to all Israeli subpopulations at the threshold at which transmission can occur.[17] We continued effective WPV introductions on a biannual basis during 1983-1987 only into non-Jewish subpopulations, because we assumed they disproportionally interacted with other populations with persistent WPV circulation during that time (e.g., Egypt). We introduced WPV1 into the non-Jewish population of the South district at the beginning of 1995 as the importation first detected in Gaza and again at the beginning of 2013 as the importation that originated from Pakistan.[11, 12]
Depending on the RI schedule in a given period, we generally assumed administration of RI doses when a child aged from the 0 to 1-month into the 2-month age group, from the 3-month into the 4 to 5-month age group, from the 4 to 5-month into the 6 to 11-month age group, and from the 6 to 11-month into the 1-year age group. However, in Hadera during 1982-1988, we assumed no IPV dose administered when a child aged from the 4 to 5-month into the 6 to 11-month age group. Consistent with prior work,[21,22] we modeled the cumulative effect of IPV doses administered at the same time as OPV doses as an effective boost by 1 IPV dose. Also, we characterized each SIA by its coverage and its probability of repeatedly missing individuals targeted in the preceding SIA round (Table 1).[23]
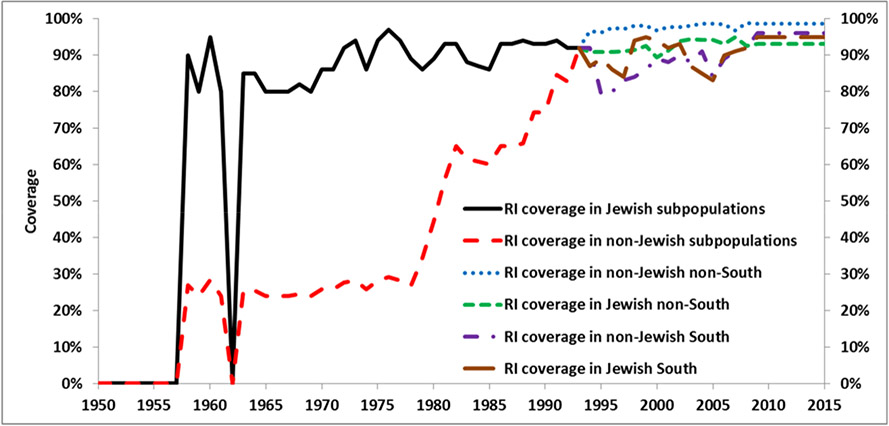
We collected information about vaccination coverage[3-7, 10,11] and used national WHO-UNICEF estimates of the third dose of polio vaccine coverage for Jewish subpopulations during 1980-1993 (Figure 1).[19] Our model assumes low relative coverage for non-Jewish subpopulations compared to Jewish subpopulations starting from the early years of vaccination, which we increased up to 1 gradually during 1979-1993. We used Israel Ministry of Health data as the basis for RI coverage estimates for Jewish and non-Jewish subpopulations for the South district and the rest of the country from 1994 forward (Figure 1). Moreover, because of cross-border population movements between Israel and the West Bank and Gaza, we accounted for the possibility of secondary OPV spread to the Israeli population from all SIAs in the West Bank and Gaza (Table 3). During these SIAs, we assumed effective OPV coverage of 0.01 for non-Jewish and 0.001 for Jewish subpopulations.
Figure 1.
Routine immunization coverage with 3 or more vaccine (OPV, IPV) doses in Israel.[4,6,7,19]
Table 3.
List of SIA activities in the West Bank and Gaza Strip (chronological order).
| Start date | Duration | Vaccine | Fraction NJ | Fraction J | TC | PRM |
|---|---|---|---|---|---|---|
| 4-1-1995 | 5 | tOPV | 0.01 | 0.001 | 1 | 0.1 |
| 5-1-1995 | 5 | tOPV | 0.01 | 0.001 | 1 | 0.1 |
| 4-6-1996 | 5 | tOPV | 0.01 | 0.001 | 1 | 0.1 |
| 5-11-1996 | 5 | tOPV | 0.01 | 0.001 | 1 | 0.1 |
| 3-1-1997 | 5 | tOPV | 0.01 | 0.001 | 1 | 0.1 |
| 4-1-1997 | 5 | tOPV | 0.01 | 0.001 | 1 | 0.1 |
| 3-1-2000 | 5 | tOPV | 0.01 | 0.001 | 1 | 0.1 |
| 4-1-2000 | 5 | tOPV | 0.01 | 0.001 | 1 | 0.1 |
| 3-1-2001 | 5 | tOPV | 0.01 | 0.001 | 1 | 0.1 |
| 4-1-2001 | 5 | tOPV | 0.01 | 0.001 | 1 | 0.1 |
| 4-6-2002 | 5 | tOPV | 0.01 | 0.001 | 1 | 0.1 |
| 5-11-2002 | 5 | tOPV | 0.01 | 0.001 | 1 | 0.1 |
| 9-28-2002 | 5 | tOPV | 0.01 | 0.001 | 1 | 0.1 |
| 10-29-2002 | 5 | tOPV | 0.01 | 0.001 | 1 | 0.1 |
| 12-08-2013 | 8 | bOPV | 0.01 | 0.001 | 1 | 0.1 |
| 01-08-2014 | 8 | bOPV | 0.01 | 0.001 | 1 | 0.1 |
Abbreviations: bOPV, bivalent oral polio vaccine; J, Jewish population; NJ, non-Jewish population; PRM, repeated missed probability; SIA, supplemental immunization activity; TC, true coverage; tOPV, trivalent OPV.
For the reference case vaccination strategy, we assumed the RI schedule included 4 IPV doses by age 12 months and 1 booster dose by age 7 years during 2005-2013. We included the IPV catch-up SIA conducted during July-August 2013 and the bOPV SIA conducted from August to October of 2013, as well as the addition of 2 bOPV doses to the RI schedule starting in January 2014. Including the 2 OPV doses given in Rahat, we assumed a SIA duration of 50 days for the reference case (i.e., the SIA reached 90% of all individuals vaccinated within 50 days.[10])
We compared the reference case with several alternatives, including the counterfactual scenario of maintenance of the sequential IPV/OPV schedule after 2005 and no response (Table 4). We considered the option of typical outbreak response 45 days after onset of the first paralytic case, with detection of the case occurring 10 days after infection,[17] to simulate a typical outbreak response in the absence of environmental surveillance. Because speed matters during a response,[24] we considered the impact of a more rapid response by shifting the SIA round to start in July (i.e., SIA 1 month earlier), or by assuming an option with the bOPV SIA conducted over only 4 days (i.e., SIA duration 4 days), or both (i.e., SIA lasting 4 days, 1 month earlier). Finally, we considered a scenario of 20% higher absolute SIA coverage in the rest of Israel (excluding the South district) (i.e., better SIA coverage).
Table 4.
Characteristics of options to improve population immunity prior to WPV1 introduction and potential 2013 SIA strategies after the detection of the WPV1 circulation.
| Number of new infections 2013–2014 |
Cumulative cases in 2013–2014 |
Reduction in WPV1 elimination time (days) |
Additional vaccine doses administered (millions) 2005–2014 |
||
|---|---|---|---|---|---|
| OPV | IPV | ||||
| Reference case | 7,700 | 0.8 | Reference* | Reference | Reference |
| Maintain sequential IPV/OPV schedule after 2005 | 8 | 0.001 | 340 | 4.30 | 0 |
| No response | 172,500 | 18 | - | −1.02 | 0 |
| Response 45 days after onset of the 1st paralytic case | 40,900 | 4 | −310 | 0 | 0 |
| SIA 1 month earlier | 4,200 | 0.4 | 43 | 0 | 0 |
| SIA duration of 4 days | 5,700 | 0.6 | 32 | 0 | 0 |
| SIA lasting 4 days, 1 month earlier | 2,800 | 0.3 | 73 | 0 | 0 |
| Better SIA coverage | 7,600 | 0.8 | 0 | 0.27 | 0 |
Reference case assumes 360 days with die-out occurring February 1, 2014.
Abbreviation: IPV, inactivated polio vaccine; OPV, oral polio vaccine; SIA, supplemental immunization activity; WPV1, wild poliovirus type 1.
RESULTS
Figure 2 displays the annual paralytic polio incidence in Israel caused by all WPVs in the model compared with the total reported for all three serotypes during 1950-2015 (broken into three periods to accommodate the dramatic changes in the y-axis scales as immunization reduced incidence). Overall, our model shows a similar pattern of “low” and “high” years during 1950-1965 (Figure 2a), although the model estimates fewer cases than reported for the 7 years before initial IPV vaccination (1950-1956). The lower model estimates compared to the reported cases reflects uncertainty about reported cases, potential inclusion of non-polio cases in early monitoring, and/or the extensive immigration of infected or susceptible individuals, especially from Eastern European countries, which we only partially considered in the model by disproportional inflow of fully susceptible individuals. The model also produces good correlation with the observed incidence during 1965-1980 (Figures 2a and 2b), when high population immunity resulted from the increase in RI coverage and four annual mOPV1 SIAs conducted during 1979-1982. Figure 2b shows the 1988 outbreak with WPV1 importation in 1987 and relatively quick elimination after the immunization response. The sequential IPV/OPV schedule adopted in 1990 maintained population immunity high enough to avert widespread circulation despite assumed periodic importations of WPV1 and VPDV2 (i.e., no reported cases after 1988 in Figures 2b and 2c). Our model mimics the behaviour of the 1995 imported WPV1 resulting in 1-month of low-level circulation followed by die-out.
Figure 2.
Annual reported wild poliovirus cases during 1950–2015, modeled annual paralytic incidence for Israel over periods with changing y-axis scales.
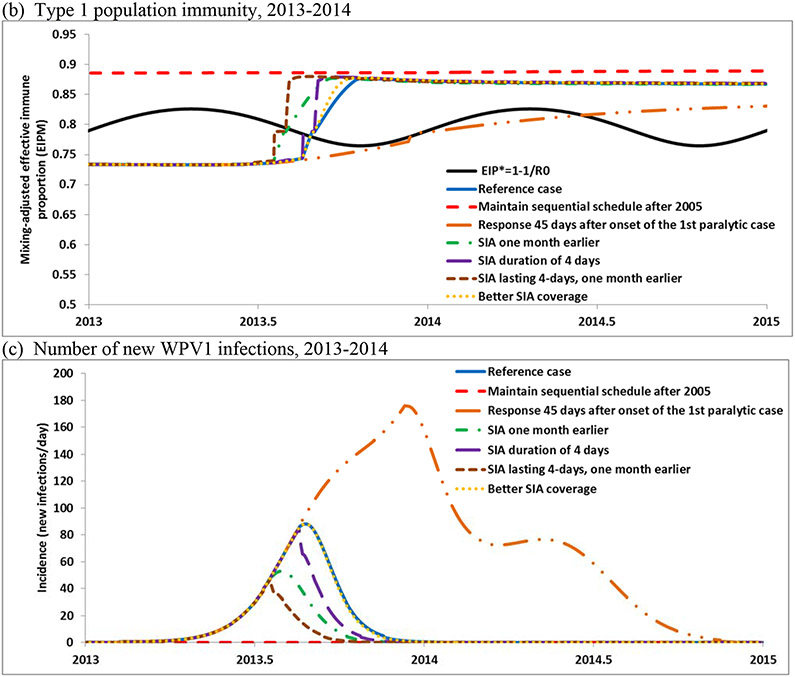
Figure 3 shows the age- and mixing-adjusted effective immune proportion (i.e., the EIPM or overall population immunity for all of Israel) and the seasonally varying threshold EIP* (i.e., the threshold above which transmission dies out) for all three serotypes. Comparison of the curves in Figure 3a shows that the RI schedule with OPV significantly increased overall population immunity to transmission over the threshold until we observed a considerable drop during 1982-1988 that corresponded to the adoption of the IPV-only schedule in Hadera. The population immunity decreased because of the lower effect of IPV vaccination on transmission compared with OPV and the reduced number of doses (i.e., 2 instead of 3 doses by age 6 months) and lack of secondary OPV spread associated with the IPV-only schedule in Hadera.[12] The increase observed in the late 1980s occurs due to the outbreak in Hadera, outbreak tOPV response SIAs including a national SIA, and subsequent use of a sequential IPV/OPV schedule nationally. After the 2005 change in the RI schedule to all-IPV, Figure 3a shows that population immunity for type 1 started to decrease and over time dropped significantly below the EIP*. However, it dropped relatively more slowly than during 1982-1988 because of better coverage and more doses in the schedule. For the 2013 introduction of WPV1 the model estimates 360 days of circulation with 85% of infections in the South and 0.8 paralytic cases assuming a paralysis-to-infection ratio of 1/200[16,17] (see Figure 2c). After the IPV catch-up campaigns, bOPV SIA response, and the addition of 2 bOPV doses to the RI schedule in 2014, population immunity for serotype 1 for the reference case returned to high levels similar to those observed before the 2005 change in the RI schedule (Figure 3a).
Figure 3.
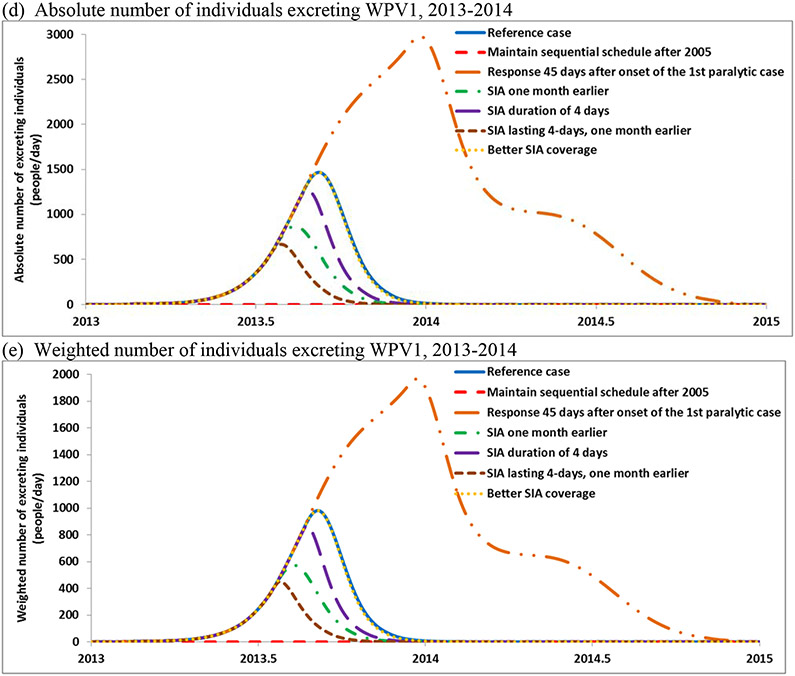
Population immunity in Israel for serotype 1 for the reference case compared with the threshold (EIP*) (a), and for the options that may improve population immunity before introduction of and after detection of circulating WPV1 in 2013 (b), number of new infections (c) and excreting individuals (d,e)
Abbreviations: EIPM, effective immune proportion adjusted for age- and sub-population-mixing; EIP*, effective immune proportion threshold [equal to (1- 1/R0)], above which infections eventually die out.
Figures 3b-e show the results of the reference case and the options for maintaining population immunity by responding to the signal of silent circulation of WPV1 from environmental surveillance during 2013–2014. Figure 3b shows the population immunity at the beginning of 2013 significantly below the EIP* (see run up in Figure 3a), and Figure 3c shows the estimated number of daily new infections (including in partially infectible individuals who benefit from immunity to paralysis because of prior infection or vaccination with IPV).
Table 4 displays the estimated numbers of infections and cases associated with the different options. Maintaining the sequential IPV/OPV schedule prevents the circulation of the imported WPV or other LPVs. The model estimates 18 cases in 2014 with no response to interrupt transmission of the imported WPV or four cases if outbreak response started 45 days after onset of the first paralytic case. The results also suggest that when circulation occurs, earlier and more rapid response result in shorter times required to interrupt transmission (Figure 3c).
Table 4 also shows the model estimates of WPV1 elimination times relative to the reference case and the number of vaccine doses used. The model uses a threshold of five effective infections per million people (i.e., weighted to account for differential infectiousness of individuals in different immunity states) as a requirement to sustain transmission. Thus, once the number of infections drops below this threshold in all age groups and subpopulations, the force of infection drops to zero and no new infections occur. For the reference case the model estimated onset of the last new infection around January 31, 2014. Infected individuals continue to excrete poliovirus until recovery (e.g., average duration of approximately 30 days for fully susceptible individuals). Continued excretion implies that environmental surveillance may detect positive samples for some time after the onset of the last infection. To account for the possibility of the detection of poliovirus in sewage after the die-out of transmission, Figures 3d and 3e show the absolute number of individuals excreting and the weighted number of individuals excreting (weighted by relative infectiousness, which accounts for a lower contribution from partially infectible individuals).
The model results imply WPV detection by a sensitive surveillance system in early March 2014, consistent with the actual experience. The laboratory collected the last sample found to contain viable WPV1 on April 3, 2014, with prior positive samples collected from the same site on March 6 and March 20, and the last isolation at any other site occurring in a sample collected February 13, 2014. With weekly sampling, the isolation of live virus in successive sewage samples from the same site might represent virus excreted by the same individual(s) rather than virus from new infections due to continued person-to-person transmission (particularly toward the end of the event). Passage and pooling of virus in the sewage system may continue beyond the time of excretion, depending on the many factors, including flow rates, temperature, and concentrations of organic matter.[25,26]
DISCUSSION
Maintaining high population immunity to poliovirus transmission will protect countries from LPV transmission after an importation.[27] The recent Israeli experience[10-12] suggests that even with high IPV coverage, developed countries may still face the risk for transmission after importation of LPVs.[14,15] Our results for Hadera also demonstrate that drops in population immunity in one sufficiently large region can significantly affect national population immunity and vulnerability to transmission (Figure 3a).
The strategy of not giving bOPV to children without prior IPV doses reflected concern about exposing previously unimmunized children to the risk for VAPP. This choice leaves these children fully susceptible to the uncertain, small (non-zero) risk for exposure to a WPV that causes paralysis at a significantly higher rate than OPV (i.e., >1,000-fold), compared with giving the child OPV, which comes with a certain, small (again non-zero) risk for VAPP. Our model suggests that targeting all children would have increased population immunity enough to stop transmission more than a month earlier. However, this transmission occurred with no reported cases, and concern about VAPP represented an important health communication challenge. Given the unpredictable nature of paralytic cases, we focused on expected cases, but in reality, each infection in a fully susceptible individual either will cause paralysis or not.
Maintaining a sequential schedule in our model appears to keep population immunity above the threshold required to prevent the transmission of imported WPVs. The Israeli experience and these model insights might help to inform decisions about switching to an IPV-only RI schedule. Further studies should continue to explore the dynamics of population immunity and OPV cessation.[21,22,28,29] Our results remain limited and depend on the assumptions we used. For example, our results depend on the assumption that IPV-induced immunity reduces participation in fecal-oral poliovirus transmission much less than immunity induced from a LPV infection, which remains numerically uncertain, but consistent with the data from challenge studies.[1, 2] Also, because die-out is a stochastic process, the model approximates with uncertainty the real events that we cannot observe.
These results underscore the value of response to an early warning signal from environmental surveillance, which prevented at least four expected cases. Our model results suggest advantages in rapid response to WPV circulation rather than the actual more drawn-out campaign. Conducting an OPV campaign comes with logistical and risk communication challenges, when implemented in a country with IPV-only RI and in the absence of cases.[30] The Israeli experience shows the importance of effectively communicating the risks associated with a silently circulating WPV and the risks and benefits of OPV.[30] As concerns about WPV1 circulation subside, Israel will face choices about continuing bOPV in RI, which might range from no change, to cessation, to replacement with tOPV. National health leaders everywhere should appreciate the importance of managing population immunity to transmission even in well-vaccinated populations and that they may need to consider continued use of OPV after introduction of IPV until global coordinated cessation of OPV use.
Acknowledgments.
We thank Drs. Stephen L. Cochi, Fran Kopel, Yossi Manor, and Ella Mendelson for helpful discussions; Yael Alfasy, Nesia Cohen, Yael Glazer, Ruslan Gosinov, and the staff members of the Division of Epidemiology, Public Health Services, Israeli Ministry of Health for epidemiological data assistance; and Musa Hindiyeh, Danit Sofer, Merav Weil, and members of the Israeli National Centers for Virus in the Environment and Polio and non-Polio Enteroviruses and the Molecular Virology Section for laboratory data assistance..
Financial support. Three authors (DAK, RJDT, and KMT) thank the U.S. Centers for Disease Control and Prevention (CDC) for supporting this work under Cooperative Agreement U66IP000519. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the CDC or the Israel Ministry of Health.
Footnotes
Conflicts of Interest. None.
References
- 1.Duintjer Tebbens RJ, Pallansch MA, Chumakov KM, Halsey NA,Hovi T,Minor PD,Modlin JF, Patriarca PA, Sutter RW, Wright PF, Wassilak SGF, Cochi SL, Kim J-H, Thompson KM. Expert review on poliovirus immunity and transmission. Risk Anal 2013; 33(4):544–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duintjer Tebbens RJ, Pallansch MA, Chumakov KM, Halsey NA, Hovi T, Minor PD, Modlin JF, Patriarca PA, Sutter RW, Wright PF, Wassilak SGF, Cochi SL, Kim J-H, Thompson KM. Review and assessment of poliovirus immunity and transmission: Synthesis of knowledge gaps and identification of research needs. Risk Anal 2013; 33(4):606–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies AM, Marberg K, Goldblum N, Levine S, Yekutiel P. Epidemiology of poliomyelitis in Israel, 1952–59* - with evaluation of Salk vaccination during a three-year period. BullWHO 1960; 23(1): 53–72. [PMC free article] [PubMed] [Google Scholar]
- 4.Goldblum N, Swartz TA. The Israeli experience in the control of poliomyelitis during a quarter af a century, 1957-1982. Rev Infect Dis 1984; 6 (2):S313–17. [DOI] [PubMed] [Google Scholar]
- 5.Goldblum N, Gerichter CB, Tulchinsky TH, Melnick JL. Poliomyelitis control in Israel, the West Bank and Gaza Strip: changing strategies with the goal of eradication in an endemic area. Bull WHO 1994; 72(4): 783–79. [PMC free article] [PubMed] [Google Scholar]
- 6.Slater PE, Orenstein WA, Morag A, Avni A, Handsher R, Green MS, Costin C, Yarrow A, Rishpon S, Havkin O, Ben-Zvi T. Kew OM, Rey M, Epstein I, Swartz TA, Melnick JL. Poliomyelitis outbreak in Israel in 1988: a report with two commentaries. Lancet 1990; 335:1192–98. [DOI] [PubMed] [Google Scholar]
- 7.Swartz TA. The Epidemiology of polio in Israel. A historical perspective. Israel Center for Disease Control (ICDC), Ministry of Health; 2008. [Google Scholar]
- 8.Shulman LM, Handsher R, Yang CF, Yang SJ, Manor J, Vonsover A, Grossman Z, Pallansch MA, Mendelson E, Kew OM. Resolution of the pathways of poliovirus type 1 transmission during an outbreak. J Clin Microbiol 2000; 38(3):945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Progress towards global eradication of poliomyelitis, 2002. Wkly Epidemiol Rec 2003; 78:138–44. [PubMed] [Google Scholar]
- 10.Shulman LM, Mendelson E, Anis E, Bassal R, Gdalevich M, Hindiyeh M, Kaliner E, Kopel E, Manor Y, Moran-Gilad J, Ram D, Sofer D, Somekh E, Tasher D, Weil M, Gamzu R, Grotto I. Laboratory Challenges in Response to Silent Introduction and Sustained Transmission of Wild type 1 Poliovirus into Israel in 2013. J Infect Dis 2014; 210(S1):S304–S314. [DOI] [PubMed] [Google Scholar]
- 11.Anis E, Kopel E, Singer S, Kaliner E, Moerman L, Moran-Gilad J, Sofer D, Manor Y, Shulman L, Mendelson E, et al. : Insidious reintroduction of wild poliovirus into Israel, 2013. Euro Surveillance 2013; 18:pii=20586. [DOI] [PubMed] [Google Scholar]
- 12.Manor Y, Shulman LM, Kaliner E, Hindiyeh M, Ram D, Sofer D, Moran-Gilad J, Lev B, Grotto I, Gamzu R, Mendelson E. Intensified environmental surveillance supporting the response to wild poliovirus type 1 silent circulation in Israel, 2013. Euro Surveillance 2014; 19:pii=20708 [DOI] [PubMed] [Google Scholar]
- 13.State of Israel. Ministry of Health. Immunization of Babies and Children. Available at: http://www.health.gov.il/English/Topics/Pregnancy/Vaccination_of_infants/Pages/default.aspx, accessed at April 16, 2014.
- 14.Thompson KM, Pallansch MA, Duintjer Tebbens RJ, Wassilak SGF, Cochi SL. Modeling population immunity to support efforts to end the transmission of LPVs. Risk Anal 2013; 33(4):647–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson KM, Wallace GS, Duintjer Tebbens RJ, Smith PJ, Barskey AE, Pallansch MA, Gallagher KM, Alexander JP, Armstrong GL, Cochi SL, Wassilak SGF. Trends in the risk of U.S. polio outbreaks and poliovirus vaccine availability for response. Public Health Rep 2012; 127(1):23–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duintjer Tebbens RJ, Pallansch MA, Kim J-H, Burns CC, Kew OM, Oberste S, Diop O, Wassilak SGF, Cochi SL, Thompson KM. Oral poliovirus vaccine evolution and insights relevant to modeling the risks of circulating vaccine-derived polioviruses. Risk Anal 2013;33(4):680–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duintjer Tebbens RJ, Pallansch MA, Kalkowska DA, Wassilak SGF, Stephen L. Cochi SL, Thompson KM. Characterizing poliovirus transmission and evolution: Insights from modeling experiences with wild and vaccine-related polioviruses. Risk Anal 2013; 33(4):703–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swartz TA, Ben-Porath E, Kanaaneh H, Leitner L, Goldblum N. Comparison of inactivated poliovirus vaccine and oral poliovirus vaccine programs in Israel. Rev Infect Dis 1984; 6(S2):S556–561. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. WHO/UNICEF estimated coverage time series. WHO and UNICEF, 2013. Available at: http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tswucoveragepol3.html, accessed at March 1, 2014. [Google Scholar]
- 20.Grotto I, Handsher R, Gdalevich M, Mimouni D, Huerta M, Green MS, Mendelson E, Shpilberg O. Decline in immunity to polio among young adults. Vaccine 2001; 19:4162–6. [DOI] [PubMed] [Google Scholar]
- 21.Kalkowska DA, Duintjer Tebbens RJ, Thompson KM. Modeling strategies to increase population immunity and prevent poliovirus transmission in the high-risk area of northwest Nigeria. J Infect Dis 2014; 210(S1): S398–411. [DOI] [PubMed] [Google Scholar]
- 22.Kalkowska DA, Duintjer Tebbens RJ, Thompson KM. Modeling strategies to increase population immunity and prevent poliovirus transmission in two high-risk areas in northern India. J Infect Dis 2014; 210(S1): S412–423. [DOI] [PubMed] [Google Scholar]
- 23.Duintjer Tebbens RJ, Kalkowska DA, Wassilak SGF, Pallansch MA, Cochi SL, Thompson KM. The potential impact of expanding target age groups for polio immunization campaigns. BMC Infect Dis 2014; doi: 10.1186/1471-2334-14-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson KM, Duintjer Tebbens RJ, Pallansch MA. Evaluation of response scenarios to potential polio outbreaks using mathematical models. Risk Anal 2006; 26(6):154–1556. [DOI] [PubMed] [Google Scholar]
- 25.Dowdle W, van der Avoort H, de Gourville E, Delpeyroux F, Desphande J, Hovi T, Martin J, Pallansch M, Kew O, and Wolff C. Containment of polioviruses after eradication and OPV cessation: characterizing risks to improve management. Risk Anal 2006; 26:1449–69. [DOI] [PubMed] [Google Scholar]
- 26.Hovi T, Stenvik M, Partanen H, and Kangas A. Poliovirus surveillance by examining sewage specimens. Quantitative recovery of virus after introduction into sewerage at remote upstream location. Epidemiol Infect 2001; 127:101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson KM, Duintjer Tebbens RJ. The case for cooperation in managing and maintaining the end of poliomyelitis: Stockpile needs and coordinated OPV cessation. The Medscape J Med 2008; 10 (8):190 Available at: http://www.medscape.com/viewarticle/578396. [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson KM, Duintjer Tebbens RJ. Modeling the dynamics of oral poliovirus vaccine cessation. J Infect Dis 2014; 210(S1): S475–484 [DOI] [PubMed] [Google Scholar]
- 29.Duintjer Tebbens RJ, Thompson KM. Modeling the potential role of inactivated poliovirus vaccine to manage the risks of oral poliovirus vaccine cessation. J Infect Dis 2014; 210(S1): S485–497. [DOI] [PubMed] [Google Scholar]
- 30.Kaliner E, Moran-Gilad J, Grotto I, Somekh E, Kopel E, Gdalevich M, Shimron E, Amikam Y, Leventhal A, Lev B, Gamzu R. Silent reintroduction of wild-type poliovirus of Israel, 2013 – risk communication challenges in an argumentative atmosphere. Euro Surveillance 2014; 19(7):pii=20703. [DOI] [PubMed] [Google Scholar]
- 31.Thompson KM, Pallansch MA, Duintjer Tebbens RJ, Wassilak SGF, Cochi SL. Pre-eradication national vaccine policy options for poliovirus infection and disease control. Risk Anal 2013;33(4):516–543. [DOI] [PMC free article] [PubMed] [Google Scholar]