Abstract
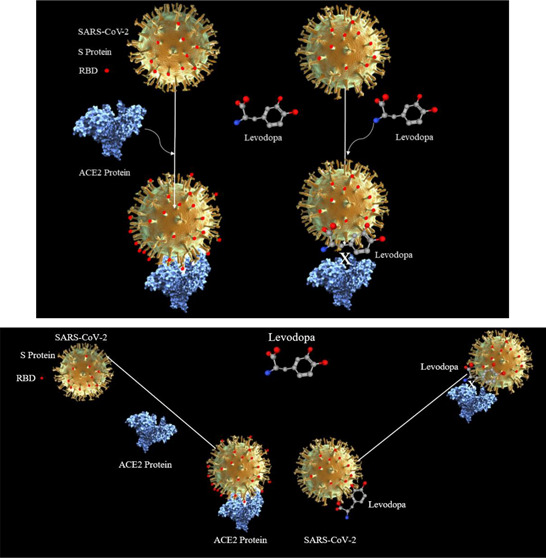
Levodopa is a prodrug that is converted into dopamine, which replenishes the deficient dopamine in the brain of patients suffering from Parkinsonism. We hypothesize that levodopa may interact with the receptor binding domain of the SARS-CoV-2 and may act as a physical impediment to the viral entry into the host cell.
Keywords: SARS-CoV-2, COVID-19, Wuhan coronavirus outbreak, viral pandemics, SARS virus, MERS virus, bat virus, zoonotic infections, biological agents, BSL 4, vaccine and antibody against SARS-CoV-2
Levodopa
Chemistry
Levodopa (accession number: DB01235), originally marketed as an anti-Parkinsonism drug, is a 3,4-dihydroxy-l-phenylalanine of the class of prodrugs that need to be converted into an active compound inside the body to exert its pharmacological actions. Levodopa (also called l-DOPA or l-3,4-dihydroxyphenylalanine), the metabolic precursor of dopamine (DA), is the single most effective agent in the treatment of Parkinson’s disease (PD).1
Pharmacology of Levodopa
Approved by the FDA, the use of levodopa and carbidopa (a peripheral dopa decarboxylase inhibitor) in a combined product called Sinemet was approved on May 2, 1975. Levodopa is indicated for the treatment of postencephalitic parkinsonism and symptomatic parkinsonism following carbon monoxide intoxication or manganese intoxication.2 Its mechanism of action involves replenishing of DA by conversion into this molecule by the action of an enzyme, dopa decarboxylase.1,2 When given orally, levodopa is absorbed speedily from the small intestine by the transport system that involves transporters for aromatic amino acids. Levels of the drug in plasma usually peak on average 1 h after an oral dose. The half-life in plasma is about 1–3 h.1 The DA produced is responsible for the therapeutic effectiveness of the drug in PD. Following its release, it is either transported back into dopaminergic terminals by the presynaptic uptake mechanism or metabolized by the actions of monoamine oxidases (MAO) and catechol-o-methyltransferase (COMT).1
Receptor Binding Domain of the SARS-CoV-2 Spike Protein
The spike (S) protein of SARS-CoV-2 is expressed on the cell surface and only a specific segment of which is the receptor binding domain (RBD) that plays a dynamic role in host cell recognition by interacting with the host cell protein angiotensin-converting enzyme 2 (ACE2).3−5 A cascade follows the initial binding of RBD of SARS-CoV-2 to the ACE2, involving TMPRSS2 and furin, which results in the entry of the virus into the cell resulting in the fusion of the membrane of the SARS-CoV-2 with the host cell.4,6 Subsequently, the SARS-CoV-2 takes over the host cellular transcriptional and translation process, and the subsequent series of events results in the budding of a complete virus from the host cell resulting in cell death. Of the several proteins that can be potential drug targets in treatment of SARS-CoV-2, the RBD of the S-protein is a pivotal protein in the pathogenesis of SARS-CoV-2 infection. The use of bioinformatics computational tools was expected to identify susceptibilities in the RBD of SARS-CoV-2 that can be exploited for therapeutic gains.4 With the interest in drug discovery by the repurposing of existing drugs, molecular docking of FDA-approved drugs that could show induced-fit docking prediction by interacting with the RBD of SARS-CoV-2 led us to study levodopa. It was hypothesized that drugs that had the potential to engage with the RBD with strong bonds could, at their best, neutralize or physically interfere with RBD–ACE2 interaction, thereby blocking SARS-CoV-2 entry into the host cell. After studying the ligand-binding attributes of S-protein, a diverse list of FDA-approved drugs and their structural analogs were selected for molecular docking and in future the subsequent in vitro testing in SARS-CoV-2. With the unattainability of clinical isolates of the virus as an alternative to direct in vitro testing on clinical isolates of SARS-CoV-2, we first deliberated to strengthen our rationale of docking selected drugs over the RBD by performing molecular dockings and studying their interaction with this segment of the S-protein. In-depth analysis of the assembly of the amino acids in the RBD of SARS-CoV-2 that interact with human ACE2, hinted at by the chemistry of the ligand-binding attributes of the RBD of SARS-CoV-2, we selected levodopa and its analogs for molecular docking predictions and ligand-binding plotting.
Docking Predictions of Levodopa over the RBD of SARS-CoV-2
The 3D chemical structure of levodopa and related compounds (ligands) were retrieved from PubChem and DrugBank. The structure of the RBD of Spike glycoprotein (S-protein) of SARS-CoV-2 uncovered in recent publications with Protein Data Bank (PDB) IDs 6vsb.1, 6m0j, and 6vw1(3,5,6) were downloaded from PDB. The RBD structures were first analyzed using a Discovery Studio (DS) modeling visualizer.
The GalaxyWeb online server was used for docking purpose.7 The structures of levodopa and RBD of SARS CoV-2, i.e., 6vw1, 6m17, and 6m0j, were submitted as inputs in the PDB format. The binding pocket of the receptor was retrieved from the RBD of SARS-CoV-2 models,3,5,6 and the amino acids that bind with ACE2 were considered the active site. After obtaining the docking results, the docked model was downloaded and analyzed using BIOVIA Discovery Studio Visualizer. The constellation of amino acids that interact with the human ACE2 receptors was identified as described previously.6 The amino acids that were recognized were studied for their interaction with the levodopa binding pocket in the RBD of SARS-CoV-2. Amino acids on the RBD that were directly involved in interacting with the ACE2 receptor and the levodopa in a 3D format were visualized to ascertain the interaction of amino acids. We recognized the structure of binding atoms and the interactions between the bonding atoms of levodopa and similar drugs with RBD.
Docking Attributes of Levodopa on RBD of SARS-CoV-2
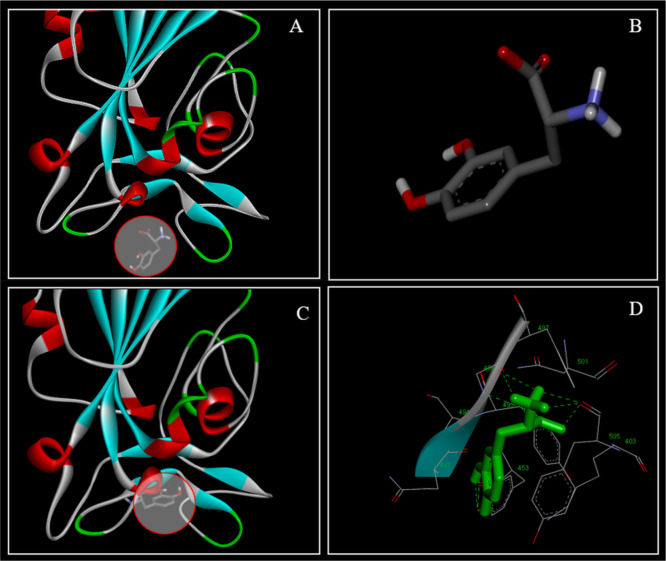
The docking results were visualized using DS Visualizer (Figure 1). The levodopa (Figure 1A, circled and Figure 1B) and its docking area on RBD of SARS-CoV-2 are shown with the contact area zoomed where the levodopa docked on the RBD (Figure 1C). Interaction of levodopa without receptor surface of the RBD and atoms (Figure 1C) and with receptor surface and interacting atoms (Figure 1D) showed the interaction of levodopa within the binding pocket.
Figure 1.
Drug docking details for levodopa in the RBD of S-protein of SARS-CoV-2. Levodopa (circle in A, structure in B) in an unbound state and interacting within the docking pocket (C, circled area) is shown. Levodopa (B) interacting with amino acids without a visible receptor surface on RBD (C, circle). The receptor surface atoms on the RBD (sticks) are shown with levodopa (green) engaging amino acids within the RBD (D).
Attributes of the Docking Prediction of Levodopa on RBD of SARS-CoV-2
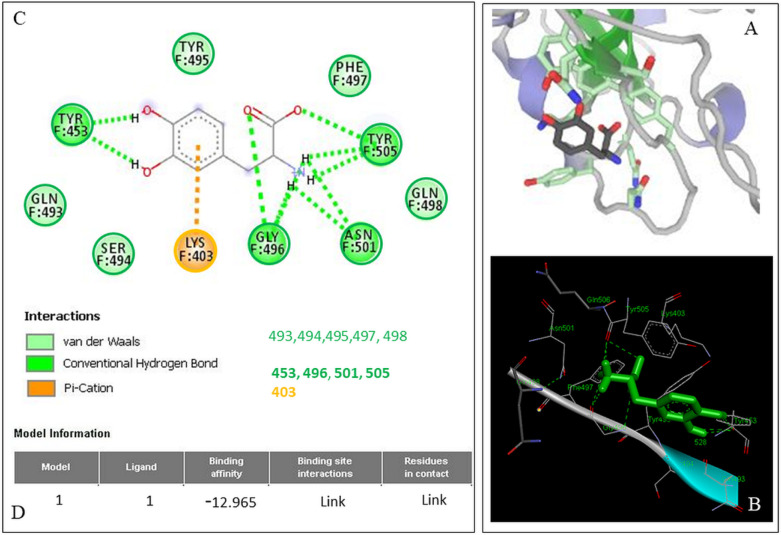
Docking results showed that levodopa has the potential to bind with all the models of RBD mentioned above. The binding energy for the receptor–ligand interaction obtained for the model 6vw1 was −12.965 and similar values were obtained for the models (data not shown). Amino acids that were predicted to be involved in ligand–receptor interaction are Lys403, Gln493, Ser494, Tyr495, Gly496, Phe497, Gln498, Asn501, Tyr505, and Tyr453. Of the above amino acids that interact, there were 2 categories found: those with direct binding to levodopa in noncovalent interactions and others which served as supporting moieties. Interestingly, the docking prediction and ligand plot results show (Figure 2A) that levodopa interacted with the amino acids within the RBD7 of model 6vw1 that come in direct contact with amino acids of ACE23 (Figure 2B,C) that enables the enzymatic cascade needed for SARS-CoV-2 to enter the host cell.4 One of the target amino acids, Lys-403, is within the pocket of the RBD shown to be involved in bonding, with a π–cation interaction between it and levodopa (Figure 2C). Lys and Arg are assumed to be almost always in a protonated state and hence are cationic. The cation−π interface is a stabilizing electrostatic interaction of a cation with the polarizable π–electron cloud of an aromatic ring (Figure 2, Lys-403 and the dotted ring of levodopa). Out of curiosity, we also studied the docking predictions for DA which is the active derivative of levodopa, to see the similarities in docking prediction and if the docking scores of DA were better than levodopa. This was also deemed necessary because if repurposed for COVID-19; we wanted to see if the prodrug levodopa and the active metabolite, DA, both can engage with the RBD of the S-protein of SARS-CoV-2. Results obtained a binding energy value for the receptor—DA interaction for the model 6vw1 was −11.462, and it engaged with eight amino acids with π–π, T-shaped, hydrogen, and van der Waal bonds (data not shown).
Figure 2.
Nature of the bonds and amino acids engaged in levodopa binding to the RBD of SARS-CoV-2 S-protein. (A) Levodopa ligand plot detailing the amino acids the RBD that are engaged along with the nature of the bonding of levodopa with them. (B) 3D model of 6vw1 with the levodopa docked on the RBD. (C) Amino acid sequences in the RBD chains of the model 6vw1 are shown with their interacting atoms (sticks) that can be matched with the constellation shown in the ligand plot. (D) Binding energy values for the receptor–ligand interaction. Note the π–cation interaction between the aromatic ring of levodopa and Lys403; the rest of the interactions are shown in light and dark green.
Discussion
The ongoing pandemic of COVID-19 has proven to be a mammoth problem, and SARS-CoV-2 has driven the research to discover drugs, vaccines,4 and monoclonal antibodies to combat pandemic-related morbidity and mortality. Many drugs like remdesivir, dexamethasone, famotidine, and hydroxyquinoline, and a few antidepressants have recently been given to patients with COVID-19 in trials with mixed results. As the synthesis of a SARS-CoV-2 specific drug remains a remote possibility, there is a need to discover drugs that could engage the RBD of the S-protein to prevent its interaction with the human ACE2 receptor. Finding chemical agents from the already existing pool of FDA-approved compounds and drugs that engage with the RBD with strong bonds is expected to prevent the aforesaid interaction and the consequent prevention of the SARS-CoV-2 entry into the cells. Also, the drug molecule can act as a physical impediment due to its presence over the RBD of SARS-CoV-2. We selected levodopa, used clinically to minimize the symptoms of PD and proven to be efficacious when used with the drug carbidopa.1,2 The interaction of levodopa with the RBD of the SARS-CoV-2 has not been reported yet, nor has the drug been used in clinical trials of COVID-19. The fact that levodopa crosses the blood–brain barrier gives it an edge over other drugs to combat neuro-COVID that has acute and long-term disabling consequences in COVID-19. The latter is in particular true in “long-haulers” with chronic COVID syndrome. We for the first time hypothesized that in addition to its known mechanism of actions, as a prodrug that enzymatically gets converted into DA, there was a possibility that the interaction of levodopa with the amino acids of the RBD could play a role in preventing the SARS-CoV-2 entry into host cells via ACE2 interaction. Though the nature of the interaction of levodopa with the RBD is mostly in form of hydrogen bonds, van der Waal, and π–cationic interactions (Figure 2B,C), the exact effects of levodopa binding to the RBD of the SARS-CoV-2 are not known yet, but we infer that the levodopa binding with the π–cationic interactions of its aromatic ring to the Lys403 residues in the RBD (Figure 2C) could act as a physical impediment and therefore could weaken the interaction of the RDB with the ACE2 receptor. Another notable finding is regarding the hydrogen bonds and van der Waal interactions, which tend to involve amino acid residues in continuity (493–498) and such interactions could enforce levodopa as a physical obstacle in the open state of RBD when it is about to interact with the ACE2 receptor of the host cells. Not shown in the results is that the active derivative of levodopa, DA, which also docked onto 8 amino acids of the RBD of 6vw1 with a docking binding free energy of −11.462, making levodopa useful even if the drug gets converted by the liver into DA in patients affected by COVID-19.
Future Directions
In vitro testing of levodopa in SARS-CoV-2 with validation of the RBD bound to levodopa, coupled with fluorescent anti-ACE2 antibodies tested in tissue treated with levodopa, is expected to broaden the significance of levodopa and its analogs in COVID-19 affected patients. If levodopa and its analog DA minimize tissue infectivity by SARS-CoV-2 in vitro assays, then they can be tested in clinical trials to determine their efficacy in the treatment of COVID-19.
The authors declare no competing financial interest.
References
- Goodman L. S., Brunton L. L., Chabner B., and Knollmann B. C. (2011) Goodman & Gilman’s Pharmacological Basis of Therapeutics, McGraw-Hill, New York. [Google Scholar]
- Law V.; Knox C.; Djoumbou Y.; Jewison T.; Guo A. C.; Liu Y.; Maciejewski A.; Arndt D.; Wilson M.; Neveu V.; Tang A.; Gabriel G.; Ly C.; Adamjee S.; Dame Z. T.; Han B.; Zhou Y.; Wishart D. S. (2014) DrugBank 4.0: shedding new light on drug metabolism. Nucleic Acids Res. 42 (1), D1091–7. 10.1093/nar/gkt1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J.; Ye G.; Shi K.; Wan Y.; Luo C.; Aihara H.; Geng Q.; Auerbach A.; Li F. (2020) Structural basis of receptor recognition by SARS-CoV-2. Nature 581 (7807), 221–224. 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig A. M.; Khaleeq A; Syeda H. (2020) Elucidation of cellular targets and exploitation of the receptor-binding domain of SARS-CoV-2 for vaccine and monoclonal antibody synthesis. J. Med. Virol. 92, 2792. 10.1002/jmv.26212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R.; Zhang Y.; Li Y.; Xia L.; Guo Y.; Zhou Q. (2020) Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 367 (6485), 1444–1448. 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J.; Ge J.; Yu J.; Shan S.; Zhou H.; Fan S.; Zhang Q.; Shi X.; Wang Q.; Zhang L.; Wang X. (2020) Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 581 (7807), 215–220. 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- Ko J.; Park H.; Heo L.; Seok C. (2012) GalaxyWEB server for protein structure prediction and refinement. Nucleic Acids Res. 40 (W1), W294–W297. 10.1093/nar/gks493. [DOI] [PMC free article] [PubMed] [Google Scholar]