Abstract
Bacterial vaginosis (BV) affects reproductive-age women and can lead to pelvic inflammatory disease, postpartum endometritis, and preterm labor/delivery and predisposes the infection of sexually transmitted diseases. Typically, BV diagnosis involves the analysis of vaginal swab samples via microscopy operated by highly skilled personnel. Hence, novel approaches for BV diagnosis are an existing need. In response, the first immunosensing platform targeting sialidase, a BV biomarker, is reported. The nanophotonic operational principle of this biosensing platform allows for a cheaper, faster, and simpler analysis when compared with an indirect enzyme-linked immunosorbent assay (ELISA). The clinical evaluation of such a nanotechnology is highlighted, where 162 vaginal swab samples were analyzed with high sensitivity and specificity (96.29%, respectively). The resulting nanoimmunosensing platform offers a resourceful approach to perform a timely BV diagnosis.
Keywords: bioassay, applied nanotechnology, infectious diseases, applied biotechnology, graphene oxide, quantum dots
Introduction
Bacterial vaginosis (BV) is an infectious disease that involves the alteration of healthy vaginal microbiota by anaerobic bacteria colonizing the vagina. BV mainly affects reproductive-age women, and its global prevalence has been estimated to account from 20 to 30%, even reaching around 50–60% in high risk population groups, such as sex workers.1 Nearly 50% of BV cases are asymptomatic;2 however, some symptoms include moderate white-grayish vaginal secretion after sexual intercourse, fish-like smelling vaginal discharge (generated by cadaverine, putrescine, and trimethylamine production), and the less common dysuria and dyspareunia.3 The lack of symptoms could be related to a chronic disease, which is associated with the development of pelvic inflammatory disease, preterm premature rupture, low weight in the newborn, miscarriage, and amniotic fluid infection that can persist (even after birth) and lead to the death of the newborn.4 Besides, BV predisposes the infection of sexually transmitted diseases caused by Clamidya trachomatis, Neisseria gonorrhea, Mycoplasma genilium, Trichomonas vaginalis,5,6 human papillomavirus,7 and human immunodeficiency virus.8
Typically, the clinical diagnosis of BV is based on the evaluation of the following Amsel criteria: white or grayish vaginal discharge, vaginal pH > 3.5, amine production, and the presence of “clue cells”. The appearance of at least three of these parameters is considered indicative of BV infection.9 Moreover, the Nugent score is based on the classification of bacterial morphotypes in normal microbiota (score of 0–3), mixed (score 4–6), and bacterial vaginosis (score 7–10).10 Consequently, both methods depend on observations performed by highly skilled personnel, even trained during several years.11
Several microbiological agents provoke the development of BV, including Prevotella, Bacteroides, Mobiluncus, and Gardnerella.12 However, all of them produce hydrolytic enzymes such as sialidase (SLD). The main function of SLD is the removal of sialic acid in cervico-vaginal mucus, and its degradation in sialoglycans is then employed as a nutrient source for bacterial growth.13,14 Besides, the enzymatic activity of SLD is crucial during the colonization and establishment of BV infection, and it is related to the host immune response evasion and clinical outcomes of BV in pregnant women.15,16 This close relationship between SLD production and BV development suggests the potential role of SLD as a biomarker of BV infection.17
In spite of the potential clinical value of SLD as a biomarker of BV, analytical platforms targeting SLD have been scarcely developed. One of the most common techniques for the detection of SLD in vaginal swab samples was proposed by Cauci et al.18 This technique is based on the enzymatic hydrolysis of methoxyphenyl acetyl muramic acid via SLD, thereby promoting the formation of methoxyphenol, which is then measured. Generally, in this technique, methoxyphenol concentrations greater than 5.1 nM are considered as BV positive.19 To the best of our knowledge, only one commercially available test targeting SLD exists, which is called OSOM BVBLUE (Sekisui Diagnostics, Burlington, MA). However, this test offers a multistep qualitative colorimetric result for the determination of SLD, which eventually leads to different levels of specificity and sensitivity.20−24
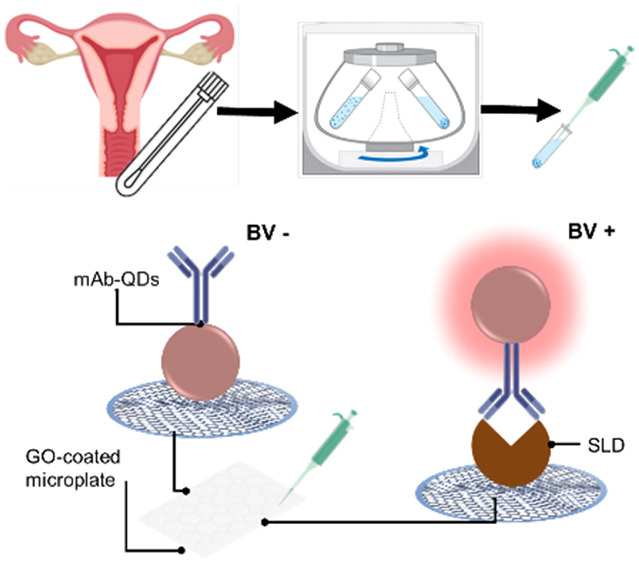
In this context, our research team recently developed a novel monoclonal antibody (mAb) against SLD, which has been validated via several immunological methods and demonstrated to recognize SLD with high specificity.25 Taking advantage of this mAb, we designed a single-step quantitative biosensing system for targeting SLD. This biosensing concept has been recently reported to be technically sound as a virtually universal wash-free and single-step immunosensing platform.26,27 Particularly, this biosensing system targeting SLD relies on mAb-decorated quantum dots (mAb-QDs) and graphene oxide-coated microwells. The nanoconjugates (mAb-QDs) operate as a fluorescent probe interrogating the presence of SLD, where those nanoconjugates capturing SLD via immunoreactions will offer a strong fluorescence and those nanoconjugates that did not capture SLD will be quenched by the graphene oxide-coated microwells via nonradiative energy transfer. Herein, we report on the performance of our biosensing platform for the detection of SLD (nanoBV) in vaginal swab samples as a potential tool for BV diagnosis. The overall approach is depicted in Figure 1. The straightforward operational principle of nanoBV is depicted in Figure 1B.
Figure 1.
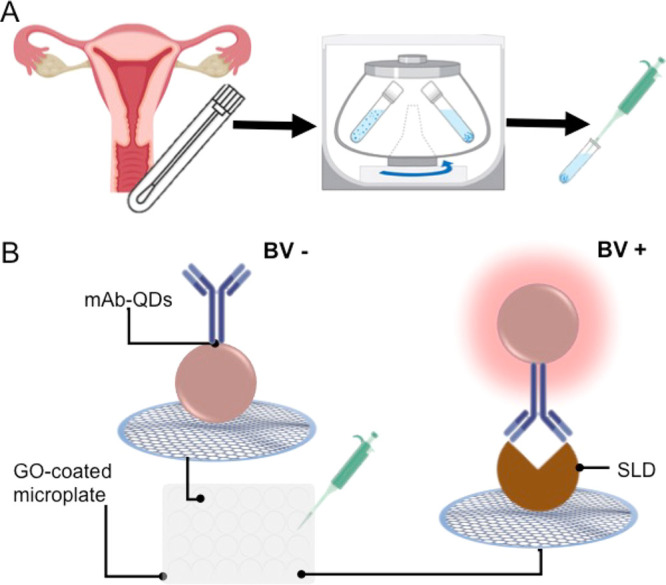
Schematic representation of the overall approach. (A) Sample collection, storage, and preparation. The sample is collected from the vaginal sac fundus with a sterile swap and placed in sodium chloride saline solution to be stored. The sample is then centrifuged to remove cellular waste, and the supernatant is aspirated to be analyzed. (B) Biosensing platform targeting SLD (nanoBV). The clinical sample is diluted (1:4) and mixed with the nanoconjugate (mAb-QDs) in a GO-coated microwell. Typically, negative samples (BV−) exhibit quenched nanoconjugates via nonradiative energy transfer due to the affinity between mAb-QDs and the GO-coated microwell. Generally, positive samples (BV+) exhibit a strong fluorescence, which can be quantified. This occurs because of the complex SLD/mAb-QDs has no affinity with the GO-coated microwell.
Results and Discussion
Optimization of the SLD Immunosensing Platform
The analytical sensitivity of our immunosensing platform can be optimized using different concentrations of both GO and the nanoconjugate.26 In view hereof, we first assessed the analytical performance of nanoBV by preparing GO-coated microwells using a constant concentration of 1200 μg mL–1 and different concentrations of the nanoconjugate in terms of mAb load, i.e., final QDs concentration of 0.20 nM combined with several final concentrations of mAb, including 0.375, 0.750, and 1.125 μg mL–1 (this QD concentration was systematically selected on the basis of previous experiments dealing with the capabilities of GO-coated microwells to quench different concentrations of QDs).26,27 Different concentrations of SLD from 31.25 to 2000 ng mL–1 and blank samples were assayed following the experimental procedures. As mentioned in the Experimental Section, fluorescence quenching was quantitatively assessed using the (If/I0) ratio given by the corresponding fluorescence at time f (If) divided by the respective fluorescence at time 0 (I0). Among these conditions, the nanoconjugate incorporating the mAb concentration of 0.375 μg mL–1 was observed to display the expected SLD-dependent concentration analytical behavior (see Figure S1 included in the Supporting Information (SI)). We then explored the analytical performance of nanoBV using GO-coated microwells fabricated at several GO concentrations (1200, 1300, and 1400 μg mL–1) and the previously selected concentration of the nanoconjugate (final mAb concentration at 0.375 μg mL–1, and final QDs concentration at 0.20 nM). Figure S2 (SI) shows the results of this series of experiments. Calibration plots were performed with the If/I0 ratios resulting at 120 min of the assay, where the highest determination coefficient (R2 = 0.994) was observed at this point with those GO-coated microwells fabricated with GO at 1400 μg mL–1 (see Figures S3 and S4 and Table S1, detailed in the SI). Hence, the immunosensing platform operating with this specific configuration was chosen as optimal, that is GO-coated microwells fabricated with [GO] at 1400 μg mL–1, as well as the nanoconjugate configured with a final [mAb] at 0.375 μg mL–1 and a final [QDs] at 0.20 nM.
Considering the aforementioned optimization, we further investigated the analytical behavior of nanoBV in a different dynamic range, that is from 1.56 to 100 ng mL–1. As shown in Figure S5 (see the SI), we performed calibration plots, with data resulting at 5, 30, 60, 90, and 120 min of the assay. We assessed the corresponding calibration plots in terms of R2, limit of detection (LOD), and coefficient of variation (CV) (see Tables S2 and S3, SI). Thereby, nanoBV was observed to achieve the lowest LOD at 120 min (see Table S2 and Figure S4), with CVs from 0.14 to 2.19% (see Table S3, assay number 1). This LOD accounted for ca. 0.012 ng mL–1. The optimized analytical performance of nanoBV is shown in Figure 2.
Figure 2.
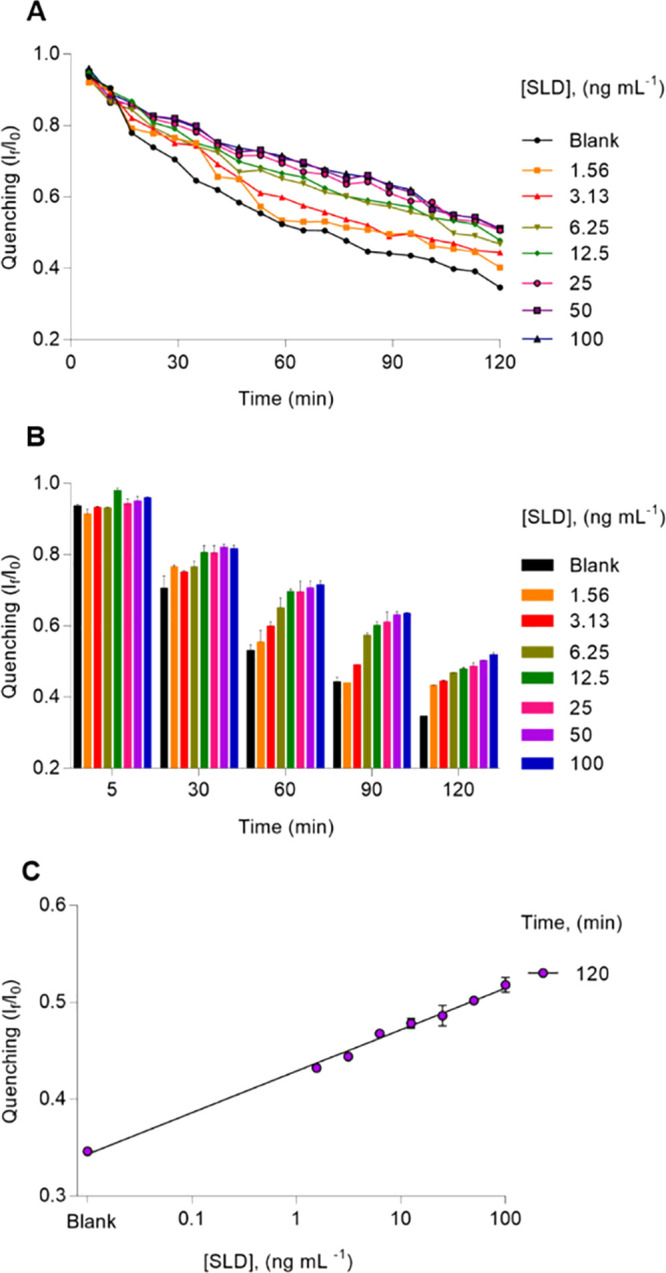
Analytical performance of the optimized SLD nanoimmunosensing system. (A) Kinetics of the fluorescence quenching corresponding to different concentrations of SLD. (B) Performance of nanoBV at specific times. (C) Calibration plot resulting at 120 min of the assay. The error bars represent the standard deviation of three parallel experiments.
We also compared the optimized LOD of nanoBV with that of an indirect enzyme-linked immunosorbent assay (ELISA) previously reported by our team,25 whose limit of detection accounted for 0.031 ng mL–1 (see Figure S6, SI); hence, nanoBV was observed to be 2.5-fold more sensitive than the indirect ELISA in terms of limit of detection. The overall cost of both detection platforms targeting SLD was also compared. The indirect ELISA test had an estimated cost of 0.107 USD, whereas nanoBV had an estimated cost of 0.086 USD (see Table S4, SI); thus, nanoBV was ca. 20% cheaper than the indirect ELISA—at laboratory scale. Furthermore, the indirect ELISA requires more than 6 h to get a final result, whereas nanoBV needs around 2 h and its results can be monitored in real-time (see Table S5, SI). Consequently, nanoBV exhibited not only advantages in terms of cost but also in terms of technical and analytical features when compared with an indirect ELISA.
Clinical Evaluation of nanoBV
Prompted by the aforementioned advantageous scenario, following the procedures detailed in the Experimental Section, we performed the analysis of 162 samples of vaginal swabs previously determined as BV positive or normal microbiota (NM) via the Amsel criteria, respectively.9 Fifty-four samples were found to be BV positive, whereas 108 samples exhibited NM. The corresponding SLD content was estimated by performing seven assays across different days (in different microwell plates). To this end, each assay/microwell plate got an associated calibration plot (see Figures S7 and S8, SI). We also assessed both the intra-assay precision and the inter-assay precision of nanoBV in terms of CV. Within these seven assays, nanoBV displayed intra-assay CVs from 0.14 to 3.53% (see Table S3, SI); whereas the inter-assay CVs ranged from 5.82 to 7.94% (see Table S6, SI). Hence, the evaluated precision resulted to be acceptable for immunosensing.28 Moreover, Table S7 displays the LOD resulting in each assay.
Figure S9 (SI) displays the fluorescence quenching kinetics resulting from each vaginal swab sample analyzed in this research. The SLD content of the analyzed samples was quantified by interpolating the corresponding If/I0 values obtained at 120 min of the assay into the respective calibration plot. Typically, a reference range of normal levels measured by a diagnostic tool covers four standard deviations accordingly, and provided results are normally distributed.29 Since the SLD levels resulting from the explored NM group have no normal distribution (see Figure S10, SI), we proposed the respective mean plus 2 times the corresponding 95% confidence interval as a threshold to discriminate between BV positive cases and BV negative cases. The resulting threshold is ca. 25.194 ng mL–1. Thereby, using this threshold, we estimated the clinical sensitivity and specificity of nanoBV. Given the limits of the explored dynamic range (see Figure S4C, SI), interpolated values exceeding 2000 ng mL–1 were classified as ≥2000 ng mL–1 in the corresponding analysis (see Figures S7, S8, and S10, SI). Figure S10 (SI) summarizes the SLD content of all the samples analyzed via nanoBV, where two samples previously classified as BV positive by Amsel criteria appear below the recommended threshold (that is, two false negatives), whereas four samples previously classified as NM by Amsel criteria appear above the suggested threshold (that is, four false positives). This leads to a clinical sensitivity of 96.29% and a clinical specificity of 96.29%, which is also depicted by the corresponding receiver operating characteristic (ROC) curve in Figure 3C. Besides, the positive predictive value accounted for 92.86% and the negative predictive value was 98.11%.
Figure 3.
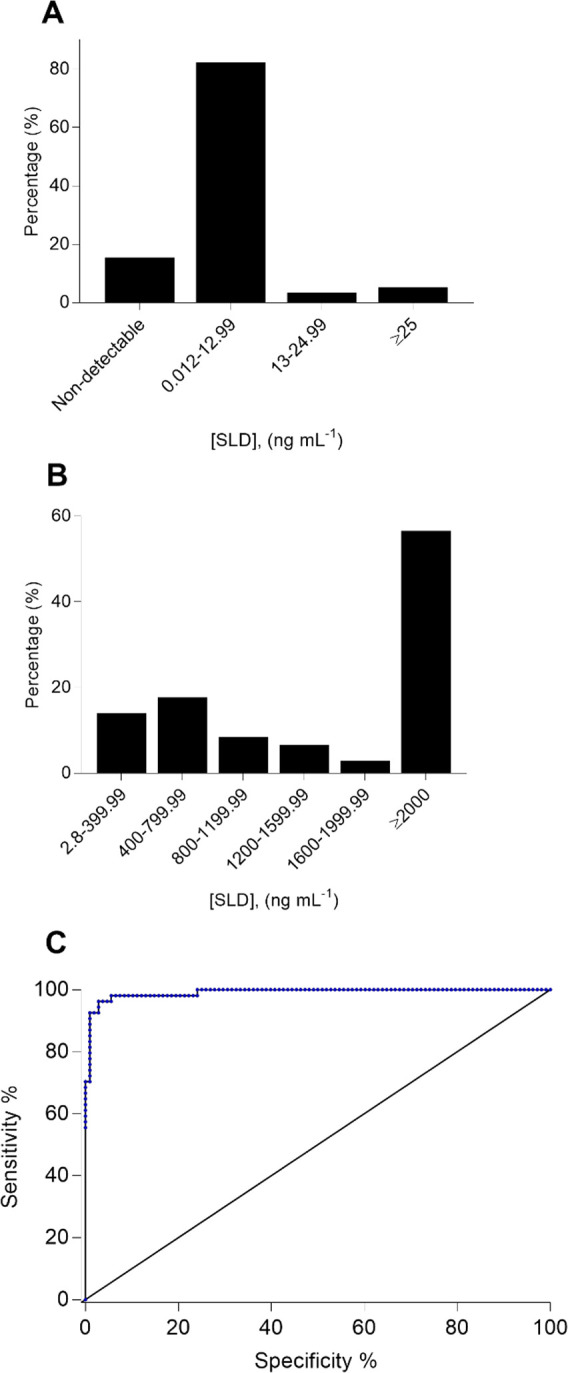
Analysis of SLD levels in 162 clinical samples using nanoBV. The vaginal swab samples were classified in two groups according to Amsel criteria: bacterial vaginosis positive (BV+, n = 54 samples) and normal microbiota (NM, n = 108 samples). (A) Histogram of the resulting SLD levels in NM samples. (B) Histogram of the resulting SLD levels in BV+ samples. (C) Corresponding ROC curve depicting the high sensitivity and specificity of nanoBV. The area under the curve is 0.99.
According to nanoBV results, ca. 14% of the samples classified as NM by Amsel criteria displayed nondetectable SLD levels and ca. 81% of the same group of samples had SLD levels from 0.012 to 12.99 ng mL–1 (see Figure 3A). In contrast, nearly 56% of the samples classified as BV positive via Amsel criteria exhibited a SLD level ≥2000 ng mL–1 and ca. 17% of BV positive samples accounted from 400 to 800 ng mL–1 in terms of SLD levels (see Figure 3B). These findings are expected to be further investigated to offer useful information on the evaluation of the other risks interrelated with BV, the development of BV, and the patient status. To the best of our knowledge, nanoBV is the first immunosensing technology reporting SLD levels in vaginal swab samples. Existing quantitative approaches targeting SLD are based on the catalytic activity of this enzyme,17,23 which can be affected by variation in temperature/pH or any process leading to denaturation upon sample collection.30 Conversely, nanoBV does not measure the catalytic activity of SLD but the enzyme directly, even in denatured conditions—provided the corresponding binding sites can still be recognized by the developed mAb.25 We hypothesize that this is the origin of the SLD levels reported by nanoBV, which are not in agreement with those SLD levels resulting from the aforementioned method based on enzymatic hydrolysis of methoxyphenyl acetyl muramic acid via SLD,18 since this method generally reports SLD levels lower than 600 ng mL–1.16,31 Consequently, our research also opens a debate on the SLD levels found in vaginal swab samples depending on the employed analytical platform.
Experimental Section
Materials and Equipment
A synthetic peptide of SLD was designed by our team and synthesized by Pepmic (Gusu District, Zushou, China). Full details related on this peptide were previously reported.25 Dimethyl sulfoxide (DMSO), biotin N-hydroxysuccinimide ester, Tween 20, phosphate-buffered saline (PBS), and sodium bicarbonate (NaHCO3) were acquired from Sigma-Aldrich (St. Louis, MO); BSA (employed in the inmunobuffer) was purchased from ToCris Bioscience (Bristol, United Kingdom). The employed monolayer GO exhibits an average lateral size around 500 nm and a carbon/oxygen ratio around 1 (characterization provided by the manufacturer, Angstron Materials, Dayton, OH). CdSe@ZnS quantum dots (QD) (Invitrogen Carlsbad, CA), with an average size of 14 ± 2 nm and a maximum emission wavelength at 665 nm, were employed in this research. Ninety-six-well microplates Costar 3603 were purchased from Corning Incorporated (Corning, NY). Fluorescence analysis was performed using a Cytation 5 (Biotek, Winooski, VT). Figure S11 displays a picture of the employed microwell plates reader.
Sample Collection and Conventional BV Diagnostic
Vaginal swab samples were collected by “Servicio de Diagnóstico Integral en la detección Oportuna del Cáncer Cérvico Uterino” at Universidad Autónoma de Guerrero (Chilpancingo, Guerrero, Mexico). The study included women aged from 16 to 65 years. All patients signed an informed consent based on the Helsinki declaration (2013), and the study was previously approved by the Ethics Committee at Universidad Autónoma de Guerrero (Guerrero, Mexico). Samples were collected in Stuart transport medium and saline solution. The saline samples were then inspected to find “clue cells”, whereas samples in Stuart medium were used for the quantification of bacterial morphotypes in Gram staining and for G. vaginalis culture in Columbia Agar supplemented with 10% of human blood (from a healthy donor with previous consent) and a SR119RE selective supplement (OXOID; Thermo Fisher Scientific Cat# 1441974). A collection of 162 samples of vaginal swabs was included in this research. The samples were classified as BV positive (BV+) or normal microbiota (NM), respectively, according to the aforementioned Amsel criteria.9 Fifty-four samples were found to be BV positive, whereas 108 samples exhibited NM.
mAb Production and Validation
Full details on the production and validation of mAb were recently reported by our research team.25
Enzyme-Linked Immunosorbent Assay (ELISA) Targeting SLD
mAb-based indirect ELISA targeting SLD was performed following recently reported procedures.25
Biotinylation of mAb
The mAb concentration was adjusted to 1 mg mL–1 and dialyzed overnight in carbonate buffer 0.1 M pH 9.6. We then prepared a solution containing 2 mg of biotin N-hydroxysuccinimide ester (Sigma-Aldrich Cat# H1759) and 1 mL of DMSO (dimethyl sulfoxide) and added 200 μL to the antibody solution. The sample was then incubated during 4 h at room temperature in the dark and dialyzed overnight in PBS (pH 7. 2) to eliminate nonconjugated biotin.
Production of Microwells Coated with Graphene Oxide (GO)
In optimized conditions, 100 μL of a suspension of graphene oxide (1400 μg mL–1) was added to each microwell to be employed and incubated overnight at 600 rpm and 23 °C. GO suspension was then aspirated and washed three times with Milli-Q water to eliminate any excess of GO.
mAb (anti-SLD) Coupling with QDs
Streptavidin-coated QDs were conjugated with biotinylated mAb in a standard PBS-Tween buffer containing 1% bovine serum albumin (immunobuffer). QDs were prepared at a final concentration of 8 nM (diluted in 200 μL of immunobuffer). mAb was prepared at a final concentration of 15 μg (diluted in 200 μL of immunobuffer). Both of them were mixed for 45 min at 600 rpm.
SLD Detection
Typically, the SLD peptide concentration ranged from 1.56 to 100 ng mL–1. Fifty microliters of each antigen dilution and 50 μL of the nanoconjugate mAb-QD (final concentrations of 0.375 μg mL–1 and 0.20 nM mL–1, respectively) were added in the respective GO-coated microwell. Blank samples were also analyzed. All the samples were studied in triplicate. A kinetic analysis of fluorescence quenching was performed during 120 min. It is worth mentioning that previous experiments of the overall biosensing platform suggest that the involved operational mechanism displays its best performance within 120 min.26,27 Fluorescence quenching was quantitatively assessed using the (If/I0) ratio given by the corresponding fluorescence at time f (If) divided by the respective fluorescence at time 0 (I0). The limit of detection (LOD) was estimated by interpolating the value of the blank plus 3 times its standard deviation within the respective calibration curve.
Clinical Sample Analysis
Vaginal swab samples were centrifuged and diluted 1:4, and their SLD content was detected according to the aforementioned procedure. The SLD concentration levels in real samples were estimated using the corresponding calibration curve.
Statistical Analysis
The vaginal swab samples were classified in two groups according to Amsel criteria: bacterial vaginosis positive (BV, n = 54 samples) and normal microbiota (NM, n = 108 samples). All the box plots included in the SI show the median and the extreme values of the respective distribution. In Figure S10, a χ2 test was used to obtain a p value accounting for <0.0001. As previously discussed, since the SLD levels resulting from the explored NM group have no normal distribution (see Figure S10, SI), we proposed the respective mean plus 2 times the corresponding 95% confidence interval as a threshold to discriminate between BV positive cases and BV negative cases. The clinical sensitivity was determined by dividing the number of true positives by the number of true positives plus the number of false negatives. The clinical specificity was determined by dividing the number of true negatives by the number of true negatives plus the number of false positives. A positive predictive value was determined by dividing the number the number of true positives by the number of true positives plus the number of false positives. A negative predictive value was determined by dividing the number of true negatives by the number of true negatives plus the number of false negatives.
Conclusions
Taking advantage of a new antibody and a conceptually innovative immunosensing platform, a novel nanotechnology targeting SLD was successfully demonstrated by analyzing 162 clinical samples. nanoBV facilitates the simple and cost-effective detection of SLD when compared with an indirect ELISA. Using Amsel criteria as a reference for the determination of BV, which relies on observations performed by highly trained personnel, SLD content in vaginal swab samples were detected with high clinical specificity and sensitivity (96.42%, respectively) via nanoBV. The market offers qualitative kits for the determination of SLD; however, as a complementary tool, the quantitative character of nanoBV is amenable to facilitating suitable triage of patients undergoing VB, as well as effective therapy monitoring and timely evaluation of other risks related to BV.
Acknowledgments
The authors acknowledge financial support by CONACYT (Mexico, Grant Nos. 312271 and 376135).
Glossary
Abbreviations
- BV
bacterial vaginosis
- SLD
sialidase
- NM
normal microbiota
- mAb
monoclonal antibody
- QD
quantum dots
- GO
graphene oxide
- ELISA
enzyme-linked immunosorbent assay
- LOD
limit of detection
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.0c00211.
Figures of optimization of nanoBV in terms of antibody and GO concentration, calibration curves, analytical performance of an indirect ELISA targeting SLD, calibration plots, fluorescence quenching kinetics, estimated SLD levels, and overall biosensing platform images and tables of limit of detection and determination coefficient of nanoBV, evaluation of the analytical performance of nanoBV across time, evaluation of the precision of nanoBV in terms of CV, estimation of cost of the indirect ELISA and the nanoimmunosensing system targeting SLD, technical comparison between indirect ELISA and nanoBV, and evaluation of the LOD of nanoBV across different assays (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare the following competing financial interest(s): Pending patent. Mexico patent application: MX/A/2019/010242.
Supplementary Material
References
- Bautista C. T.; Wurapa E. K.; Sateren W. B.; Morris S. M.; Hollingsworth B. P.; Sanchez J. L. (2017) Association of Bacterial Vaginosis With Chlamydia and Gonorrhea Among Women in the U.S. Army. Am. J. Prev. Med. 52 (5), 632–639. 10.1016/j.amepre.2016.09.016. [DOI] [PubMed] [Google Scholar]
- Kerubo E. N.; Laserson K. F.; Otecko N.; Odhiambo C.; Mason L.; Nyothach E.; Oruko K. O.; Bauman A.; Vulule J.; Zeh C.; Phillips-Howard P. A. (2016) Prevalence of Reproductive Tract Infections and the Predictive Value of Girls’ Symptom-Based Reporting: Findings from a Cross-Sectional Survey in Rural Western Kenya. Sex Transm Infect 92 (4), 251–256. 10.1136/sextrans-2015-052371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulshrestha S.; Soni P.; Parihar R. S.; Khatri P. K.; Chandora A K..; Soni L. K. (2015) Abnormal vaginal discharge: comparison of clinical and microbiological criteria for the diagnosis of bacterial vaginosis in western rajasthan, india. Int. J. Biol. Med. Res. 6, 5090–5094. [Google Scholar]
- Lamont R. F. (2015) Advances in the Prevention of Infection-Related Preterm Birth. Front. Immunol. 6, 566. 10.3389/fimmu.2015.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista C. T.; Wurapa E.; Sateren W. B.; Morris S.; Hollingsworth B.; Sanchez J. L. (2016) Bacterial Vaginosis: A Synthesis of the Literature on Etiology, Prevalence, Risk Factors, and Relationship with Chlamydia and Gonorrhea Infections. Mil Med. Res. 3, 4. 10.1186/s40779-016-0074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipitsyna E.; Khusnutdinova T.; Budilovskaya O.; Krysanova A.; Shalepo K.; Savicheva A.; Unemo M. (2020) Bacterial Vaginosis-Associated Vaginal Microbiota Is an Age-Independent Risk Factor for Chlamydia Trachomatis, Mycoplasma Genitalium and Trichomonas Vaginalis Infections in Low-Risk Women, St. Petersburg, Russia. Eur. J. Clin. Microbiol. Infect. Dis. 39 (7), 1221–1230. 10.1007/s10096-020-03831-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suehiro T. T.; Malaguti N.; Damke E.; Uchimura N. S.; Gimenes F.; Souza R. P.; Sela da Silva V. R.; Consolaro M. E. L. (2019) Association of Human Papillomavirus and Bacterial Vaginosis with Increased Risk of High-Grade Squamous Intraepithelial Cervical Lesions. Int. J. Gynecol Cancer 29, 242–249. 10.1136/ijgc-2018-000076. [DOI] [PubMed] [Google Scholar]
- Thurman A. R.; Kimble T.; Herold B.; Mesquita P. M. M.; Fichorova R. N.; Dawood H. Y.; Fashemi T.; Chandra N.; Rabe L.; Cunningham T. D.; Anderson S.; Schwartz J.; Doncel G. (2015) Bacterial Vaginosis and Subclinical Markers of Genital Tract Inflammation and Mucosal Immunity. AIDS Res. Hum. Retroviruses 31 (11), 1139–1152. 10.1089/aid.2015.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsel R.; Totten P. A.; Spiegel C. A.; Chen K. C.; Eschenbach D.; Holmes K. K. (1983) Nonspecific Vaginitis. Diagnostic Criteria and Microbial and Epidemiologic Associations. Am. J. Med. 74 (1), 14–22. 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- Nugent R. P.; Krohn M. A.; Hillier S. L. (1991) Reliability of Diagnosing Bacterial Vaginosis Is Improved by a Standardized Method of Gram Stain Interpretation. J. Clin. Microbiol. 29 (2), 297–301. 10.1128/JCM.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Munckhof E. H. A.; van Sitter R. L.; Boers K. E.; Lamont R. F.; Te Witt R.; le Cessie S.; Knetsch C. W.; van Doorn L.-J.; Quint W. G. V.; Molijn A.; Leverstein-Van Hall M. A. (2019) Comparison of Amsel Criteria, Nugent Score, Culture and Two CE-IVD Marked Quantitative Real-Time PCRs with Microbiota Analysis for the Diagnosis of Bacterial Vaginosis. Eur. J. Clin. Microbiol. Infect. Dis. 38 (5), 959–966. 10.1007/s10096-019-03538-7. [DOI] [PubMed] [Google Scholar]
- Coudray M. S.; Madhivanan P. (2020) Bacterial Vaginosis—A Brief Synopsis of the Literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 245, 143–148. 10.1016/j.ejogrb.2019.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis W. G.; Robinson L. S.; Gilbert N. M.; Perry J. C.; Lewis A. L. (2013) Degradation, Foraging, and Depletion of Mucus Sialoglycans by the Vagina-Adapted Actinobacterium Gardnerella Vaginalis. J. Biol. Chem. 288 (17), 12067–12079. 10.1074/jbc.M113.453654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson L. S.; Schwebke J.; Lewis W. G.; Lewis A. L. (2019) Identification and Characterization of NanH2 and NanH3, Enzymes Responsible for Sialidase Activity in the Vaginal Bacterium Gardnerella Vaginalis. J. Biol. Chem. 294 (14), 5230–5245. 10.1074/jbc.RA118.006221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govinden G.; Parker J. L.; Naylor K. L.; Frey A. M.; Anumba D. O. C.; Stafford G. P. (2018) Inhibition of Sialidase Activity and Cellular Invasion by the Bacterial Vaginosis Pathogen Gardnerella Vaginalis. Arch. Microbiol. 200 (7), 1129–1133. 10.1007/s00203-018-1520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi C.; Donders G. G. G.; Bellen G.; Brown D. R.; Parada C. M. G. L.; Silva M. G. (2013) Sialidase Activity in Aerobic Vaginitis Is Equal to Levels during Bacterial Vaginosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 167 (2), 205–209. 10.1016/j.ejogrb.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Wu S.; Lin X.; Hui K. M.; Yang S.; Wu X.; Tan Y.; Li M.; Qin A.-Q.; Wang Q.; Zhao Q.; Ding P.; Shi K.; Li X. J. (2019) A Biochemiluminescent Sialidase Assay for Diagnosis of Bacterial Vaginosis. Sci. Rep 9, 20024. 10.1038/s41598-019-56371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauci S.; Driussi S.; Monte R.; Lanzafame P.; Pitzus E.; Quadrifoglio F. (1998) Immunoglobulin A Response against Gardnerella Vaginalis Hemolysin and Sialidase Activity in Bacterial Vaginosis. Am. J. Obstet. Gynecol. 178 (3), 511–515. 10.1016/S0002-9378(98)70430-2. [DOI] [PubMed] [Google Scholar]
- Ombrella A. M.; Belmonte A.; Nogueras M. G.; Ruiz Abad I.; Sutich E. G.; Dlugovitzky D. G. (2006) Actividad sialidasa en mujeres con vaginosis bacteriana. Medicina (B. Aires) 66 (2), 131–134. [PubMed] [Google Scholar]
- Sekisui . OSOM® BVBLUE® Test. https://www.sekisuidiagnostics.com/products-all/osom-bvblue-test/ (accessed 2020-05-16).
- Madhivanan P.; Krupp K.; Li T.; Ravi K.; Selezneva J.; Srinivas V.; Arun A.; Klausner J. D. (2014) Performance of BVBlue Rapid Test in Detecting Bacterial Vaginosis among Women in Mysore, India. Infect. Dis. Obstet. Gynecol. 2014, 908313. 10.1155/2014/908313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw C. S.; Morton A. N.; Garland S. M.; Horvath L. B.; Kuzevska I.; Fairley C. K. (2005) Evaluation of a Point-of-Care Test, BVBlue, and Clinical and Laboratory Criteria for Diagnosis of Bacterial Vaginosis. J. Clin. Microbiol. 43 (3), 1304–1308. 10.1128/JCM.43.3.1304-1308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter S.; Satter H.; Tarafder S.; Miah R. A.; Sharmin S.; Ahmed S. (2011) Rapid Detection of Bacterial Vaginosis (BV) by BVBlue Test. Bangladesh Journal of Medical Microbiology 4 (1), 24–27. 10.3329/bjmm.v4i1.8465. [DOI] [Google Scholar]
- Sumeksri P.; Koprasert C.; Panichkul S. (2005) BVBLUE Test for Diagnosis of Bacterial Vaginosis in Pregnant Women Attending Antenatal Care at Phramongkutklao Hospital. J. Med. Assoc Thai 88 (Suppl 3), S7–S13. [PubMed] [Google Scholar]
- Cortés-Sarabia K.; Rodríguez-Nava C.; Medina-Flores Y.; Mata-Ruíz O.; López-Meza J. E.; Gómez-Cervantes M. D.; Parra-Rojas I.; Illades-Aguiar B.; Flores-Alfaro E.; Vences-Velázquez A. (2020) Production and Characterization of a Monoclonal Antibody against the Sialidase of Gardnerella Vaginalis Using a Synthetic Peptide in a MAP8 Format. Appl. Microbiol. Biotechnol. 104, 6173–6183. 10.1007/s00253-020-10691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Riaño E. J.; Avila-Huerta M. D.; Mancera-Zapata D. L.; Morales-Narváez E. (2020) Microwell Plates Coated with Graphene Oxide Enable Advantageous Real-Time Immunosensing Platform. Biosens. Bioelectron. 165, 112319. 10.1016/j.bios.2020.112319. [DOI] [PubMed] [Google Scholar]
- Avila-Huerta M. D.; Ortiz-Riaño E. J.; Mancera-Zapata D. L.; Morales-Narváez E. (2020) Real-Time Photoluminescent Biosensing Based on Graphene Oxide-Coated Microplates: A Rapid Pathogen Detection Platform. Anal. Chem. 92, 11511–11515. 10.1021/acs.analchem.0c02200. [DOI] [PubMed] [Google Scholar]
- Morales-Narváez E.; Montón H.; Fomicheva A.; Merkoçi A. (2012) Signal Enhancement in Antibody Microarrays Using Quantum Dots Nanocrystals: Application to Potential Alzheimer’s Disease Biomarker Screening. Anal. Chem. 84 (15), 6821–6827. 10.1021/ac301369e. [DOI] [PubMed] [Google Scholar]
- Phillips P. (2009) Pitfalls in Interpreting Laboratory Results. Aust. Prescr. 32, 43–46. 10.18773/austprescr.2009.022. [DOI] [Google Scholar]
- Grahame D. A. S., Bryksa B. C., and Yada R. Y. (2015) 2 - Factors Affecting Enzyme Activity in Improving and Tailoring Enzymes for Food Quality and Functionality (Yada R. Y., Ed.) pp 11–55, Woodhead Publishing. 10.1016/B978-1-78242-285-3.00002-8 [DOI] [Google Scholar]
- Ferreira C. S. T.; Marconi C.; Parada C. M. d. L. G.; Duarte M. T. C.; Goncalves A. P. O.; Rudge M. V. C.; Silva M. G. d. (2015) Bacterial Vaginosis in Pregnant Adolescents: Proinflammatory Cytokine and Bacterial Sialidase Profile. Cross-Sectional Study. Sao Paulo Medical Journal 133 (6), 465–470. 10.1590/1516-3180.2014.9182710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


