Abstract
Di(2-ethylhexyl) phthalate (DEHP) is a synthetic chemical and widely used as a plasticizer. Humans can be exposed to DEHP through direct contact or environmental contamination. Lycopene (Lyc) has been discussed as a potential effector in the prevention and therapy of various diseases. 140 male mice were assigned into control, vehicle control, Lyc (5 mg/kg BW/d), DEHP (500 and 1000 mg/kg BW/d, respectively), and DEHP + Lyc groups and treated with an oral gavage that lasted 28 d. The ultrastructural results showed that DEHP induced pathological changes and mitochondrial injuries. We further revealed that DEHP exposure destroyed the Fe2+ imbalance homeostasis and, consequently, increases of lipid peroxidation and inhibition of cysteine/glutamate antiporter, all of which were involved in the process of ferroptsis. Moreover, the supplementation of Lyc significantly inhibited the ferroptsis changes mentioned above. Altogether, these results indicated that DEHP exposure triggered splenic cell death via ferroptosis; meanwhile, they also shed new evidence on a potential clue for the intervention and prevention of DEHP-related diseases.
Keywords: di(2-ethylhexyl) phthalate, lycopene, ferroptosis, iron ion homeostasis, spleen
Phthalates are well-documented plasticizers and are widely used in a lot of consumable products, such as food containers, medical devices, children toys.1 Among the family, di(2-ethylhexyl) phthalate (DEHP) is the most produced; thus, its content is more abundant in our environment.2 Because DEHP is relatively stable and not chemically bound to the plastics, it is easily into foodstuff via the food chain.3 Studies have shown that DEHP accumulation in animals may be due to chronic exposure to contaminated water and food, which can threaten animal and human health.4,5 Many studies have demonstrated that DEHP has some potentially adverse effects on spleen, the reproductive tract, liver, heart, kidney, and lung (in primate and rodent).6−8 The spleen is a secondary lymphoid tissue, occupies the core role in the immune system, and is highly sensitive to damage by xenobiotics. Over the years, there was a study that has confirmed the theory that DEHP-induced splenic toxicity has mainly caused the massive injury of spleen cells.7 However, it is still unclear what the detailed mechanisms of DEHP-induced spleen damage are.
Ferroptosis, a novel form of cell death, is characterized by an iron-dependent accumulation of lipid hydroperoxides to lethal levels.9 It is remarkably unlike apoptosis, necroptosis, and autophagy in its morphological, biochemical, and genetic features.10,11 For example, during ferroptosis, the mitochondrial shrinkage led to greater inner membrane density and outer membrane rupture; meanwhile, mitochondria cristae fragmentation occurred at the ultrastructural level.10,12 Moreover, among these distinct features, the core mechanism of ferroptosis is iron-mediated oxidative stress.13 Recently, because ferroptosis was putatively involved in numerous pathophysiological processes, it has attracted considerable concern. Studies have reported that some small molecules, experimental compounds, or drugs, such as erastin, sorafenib, arsenite, etc., could trigger ferroptosis in many types of cells.14,15 Although mounting evidence showed that DEHP induces cell death mainly by apoptosis,16 oxidative stress,17 or autophagy18 in mice, whether DEHP could induce ferroptosis in a spleen remains poorly understood. There were investigations that have revealed that DEHP induced lipid accumulation and peroxidation in the tissues or cells.19,20 Chronic DEHP exposure could significantly lead to mitochondrial dysfunction and morphological damage.17,21 These findings together imply that DEHP may be a potential way to induce cell death via induction of ferroptosis.
Lycopene (Lyc) is a natural pigment synthesized exclusively by plants and microorganisms. The presence of Lyc and other carotenoids make many kinds of fruits display a red color. Tomatoes are the largest contributor to the dietary intake of Lyc in humans.22 As one of the most effective antioxidants found in plants, Lyc is widely used for protection against oxidative stress-mediated tissue injury. Some of the inhibitory mechanisms of Lyc have been recognized, for instance, antioxidants, cell progression inhibitor, and apoptotic induction.23,24 Epidemiological studies indicated that food products containing Lyc have chemo-preventive effects against chemical toxicants.25 A previous study reported that Lyc has a protective effect against oxidative stress causing tissue toxicity. Simultaneously, the ability of Lyc to reduce lipid peroxidation and protein carbonylation was also observed.26 Moreover, there were studies that indicated that a suppressed formation of lipid peroxidation products or a reduced intracellular iron accumulation could inhibit ferroptosis, which may protect organisms from related pathologies induced by environmental pollutants.11,15 However, the Lyc protective role against DEHP-induced splenic damage has not been elucidated; hence, whether Lyc can inhibit ferroptosis induced by DEHP in a spleen might be worth exploring. The current study has addressed the question of a Lyc protective effect on ferroptosis using an in vivo model of the mouse. The results provide new information and a possible route for people to reveal the mechanisms of DEHP splenic toxicity. Therefore, as a specific agent or drug to inhibit ferroptosis, Lyc may also act as a potential therapy or prevention against DEHP toxicity effects in the future.
Materials and Methods
Antibodies and Reagents
DEHP was provided by Shanghai Aladdin Biochemical Technology Co., Ltd.; Lyc was purchased from North China Pharmaceutical Group; the antibodies to glutathione peroxidase4 (GPX4), cystine/glutamate antiporter (SLC7A11), Acyl-CoA synthetase long-chain family member 4 (ACSL4), solute carrier family 3 member 2 (SLC3A2), β-actin, and secondary antibodies were all obtained from Bioss Biological Technology Co. Ltd. Transferrin receptor 1(TFR1) kit, tissue iron assay kit, arachidonic acid (AA) kit, glutathione (GSH), and GSH/GSSG assay kit were obtained from RuiXin Tech Co. Ltd.
Experimental Animals
Three-week-old specific pathogen-free ICR (Institute of Cancer Research) male mice (weights of 18–22 g) were provided by Liaoning Changsheng Biotech Co. Ltd. The mice were housed under conditions at 22 ± 2 °C with 35–65% humidity and a light/dark cycle of 12 h/12 h in the cage. The animals were quarantined for a week before formal experiments, then randomly divided into seven groups: vehicle control group (Vcon), control group (Con), 5 mg/kg BW/d Lyc group (Lyc), 500 and 1000 mg/kg BW/d DEHP group (D5 and D10, respectively), DEHP + Lyc group (DL5 and DL10, respectively) (n = 20). The animals were exposed to DEHP via oral gavage, which lasted for 28 d, and then sacrificed after being anesthetized. The protocols were approved by the Northeast Agricultural University Institutional Animal Care and Use Committee (Haerbin, China), and all the treatments were performed according to the recommendations of the Guide for the Care and Use of Laboratory Animals of the China National Institute of Health.
Hematoxylin and Eosin (H&E) Staining
H&E staining was used to visualize the morphological changes of the spleen. In brief, the separated spleen tissues were fixed in 10% formalin, routinely embedded in paraffin, sectioned into 5 μm slices, deparaffinized in xylene, rehydrated with ethanol, and then stained with H&E. After they were dehydrated with graded ethanol and cleared in xylene, the sections were mounted with a neutral balsam for observation by light microscopy (Nikon, Japan).
Ultrastructural Observation
Transmission electron microscopy was used to observe the mitochondrial ultrastructure. Briefly, fresh spleen tissues were cut to 1 mm3 in size and prefixed with glutaraldehyde at 4 °C for more than 24 h. After they were washed with 0.1 mol/L phosphate buffer, the hardened spleen tissues were fixed with 1% osmic acid for 2 h. Next, the fixed specimen was dehydrated in a series of concentrations of ethanol and embedded with epoxy resin. Finally, sections were mounted, stained with saturated acetate uranium, and observed under transmission electron microscopy. Moreover, all slides were observed at the same magnification under the light microscope. Randomly chosen four areas from seven groups were assessed for the number of mitochondria per cell.
Measurement of Fe2+, AA, TFR1, GSH, and GSH/GSSG
The Fe2+ and AA commercial kits were used to determine the Fe2+ and AA contents according to the protocols provided by the manufacturer. The TFR1 content in the spleen was evaluated using the enzyme-linked immunosorbent assay (ELISA) kit following the instructions. The contents of GSH and GSH/GSSG were measured using the GSH and GSH/GSSG kits according to the protocols. A method to avoid interassay was used in which all samples were run in one assay, and the assay was repeated at least three times.
RT-qPCR Analysis
Total RNA isolation was performed using RNAout reagent (3070, Tiandz, Inc.). The quality of the RNA was measured via using the Thermo Scientific NanoDrop 2000 spectrophotometer, with the SYBR Premix Ex TaqII (Tli RNase HPlus) to measure the quantitative real-time polymerase chain reaction (RT-qPCR). All primer sequences of target genes were obtained from TSINGKE (S-2). The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and β-actin mRNA levels were used to calculate and normalize the mRNA relative abundance of target genes.
Western Blot Analysis
Proteins in the spleen tissues were separated on 12% sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (PAGE) and transferred to poly(vinylidene fluoride) membranes (pore size 0.45 μM). The detailed process of western blotting referred to here has been previously described.2 The signals of the target protein were visualized via using chemiluminescence reagents in the Amersham Imager 600 (GE). The Density of each band was normalized with ImageJ software (National Institutes of Health). The β-actin, an internal protein, acted as the normalized loading variation.
Statistical Analysis
All data were expressed as the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used for comparison among the different groups. The spearman correlation coefficients were used to determine the association between the parameters (IBM SPSS, Armonk). A p-value less than 0.05 was considered significant.
Results
Histopathological Analysis
As shown in Figure 1, the spleen cells had been well-organized and intact in the experimental D10 group, indicating that DEHP exposure damaged the cellular structure, which leads to the white pulp shrinking, becoming disorganized, and even disappearing in the high dose of the DEHP group. Moreover, compared with the Vcon group, the number of lymphocytes was not significantly changed in the Lyc and DL5 groups, but it was dramatically increased in the D5, D10, and DL10 groups. However, the number of lymphocytes was significantly lower in the DEHP combined with Lyc group than in the groups exposed to DEHP alone.
Figure 1.
Histopathology of spleen tissue. Red arrow shows the lymphocyte, scale bar = 50 μm.
Ultrastructural Analysis
As shown in Figure 2A, the mitochondrial ultrastructural damages mainly include the condensed and uniform round-shaped mitochondria and mitochondrial membrane rupture; meanwhile, mitochondrial cristae broke and even disappeared in the groups exposed to DEHP alone. In addition, the number of mitochondria per cell significantly declined in the D10 group compared with the Vcon group. However, these changes were cured or relieved due to the Lyc supplementation (Figure 2B).
Figure 2.
Effects of DEHP on the ultrastructural morphology in the mouse spleen. (A) The ultrastructural changes of spleen. Mitochondrial vacuolization and cristae fracture (red arrow); (N) nuclear. (B) The number of mitochondria per cell. Data are mean ± SD. Octothorpe (#) indicates the significance differences between the vehicle control group and another group: #P < 0.05, ##P < 0.01, ###P < 0.001. Asterisks (*) indicate the significant differences between the DEHP alone treatment groups and the Lyc + DEHP combined treatment groups: *P < 0.05, **P < 0.01, ***P < 0.001.
Lyc Could Ameliorate Chronic DEHP Exposure Leading to Iron Metabolic Disorder
On the basis of the mitochondrial damages being similar to the classical morphological changes in ferroptotic cell death, we determined the concentration of iron in the spleen. As shown in Figure 3A, the Fe2+ content was particularly increased in the D5, D10, and DL10 group compared with the Vcon group. In addition, compared with the DEHP-treated alone groups, the Fe2+ content was significantly decreased in the DEHP combined with Lyc groups (P < 0.05). These results indicated that Lyc protected the spleen against chronic DEHP exposure that caused Fe2+ accumulation.
Figure 3.
Lyc could ameliorate chronic DEHP exposure that leads to iron metabolism disorder. (A) The iron content. (B–E) The mRNA expression of related genes. Data are presented as the mean ± SD. Octothorpe (#) indicates the significant differences between the vehicle control group and another group: #P < 0.05, ##P < 0.01, ###P < 0.001. Asterisks (*) indicate the significant differences between the DEHP alone treatment groups and the Lyc + DEHP combined treatment groups: *P < 0.05, **P < 0.01, ***P < 0.001.
To assess the relative involvement of genes in the iron metabolism in DEHP-induced splenic toxicity and the function of Lyc, we measured the mRNA expressions of ferroptosis-related signal pathway indicators in the spleen (Figure 3B–E). Our data showed that the content of iron ion transporter-related gene (TFR1) and the mRNA expressions of iron storage-related genes (TFRC, FTH1, and FTL) had an uptrend in the D5 group compared with the Vcon group, while these indicators were significantly increased in the D10 group (P < 0.01 or P < 0.001). Although the expressions of TRF1, FTL, and FTRC were still upregulated in the DL10 group compared with the Vcon group, they had significantly decreased (P < 0.001) compared with the DEHP-treated alone groups. These results together suggested that DEHP exposure could affect iron ion homeostasis-related gene expressions, but the addition of Lyc could alleviate the above changes, implying that Lyc could improve a DEHP-induced iron metabolism disorder.
Lyc Could Antagonize against DEHP-Induced Lipid Oxidation Metabolic Pathway Related Factors Upregulation
On the basis of another important biological process that induces ferroptosis, namely, the formation of lipid peroxidation products, a study was conducted to determine the mRNA and protein expressions of related signal pathway indicators. As shown in Figure 4A–C, compared with the Vcon group, the mRNA expressions of ACSL4, Lpcat3, and PTGS2 had dramatically increased in the groups treated with DEHP alone (P < 0.01 or P < 0.001). Additionally, the Lpcat3 and PTGS2 mRNA expression levels were still higher in the DL10 group than they were in the Vcon group; whereas, compared with the DEHP-treated alone groups, these factors had extremely decreased in the DEHP and Lyc combined group, indicating Lyc could reverse the trends of the above genes. Meanwhile, the result of the ACSL4 protein expression was similar to its mRNA expression (Figure 4E,F). Additionally, compared with the Vcon group, the AA content was dramatically upregulated (P < 0.001) in the D5, D10, and DL10 groups. The AA content was significantly decreased (P < 0.01 or P < 0.001) in the DEHP and Lyc combined group compared with the D5 and D10 groups (Figure 4D). Above all, our data suggested that DEHP exposure could activate lipid oxidation metabolism pathway-related factors, thereby triggering the ferroptotic cell death in mouse spleen. However, the addition of Lyc significantly improved the above changes.
Figure 4.
Lyc could antagonize against DEHP-induced lipid oxidation metabolism pathway-related factors. (A–C) The mRNA expression of related factors. (D) The content of AA. (E–F) The protein expression of related factors. Data are presented as the mean ± SD. Octothorpe (#) indicates the significant differences between the vehicle control group and another group: #P < 0.05, ##P < 0.01, ###P < 0.001. Asterisks (*) indicate the significant differences between the DEHP alone treatment groups and the Lyc + DEHP combined treatment groups: *P < 0.05, **P < 0.01, ***P < 0.001.
Lyc Could Decrease Chronic DEHP-Induced System xc–/GPX4 Pathway-Related Genes Expression Upregulation
To test the relative involvement of genes of the glutathione metabolic pathway in the DEHP-induced splenic toxicity and the function of Lyc, we measured the mRNA and protein expressions of related signal pathway indicators in the spleen. As depicted in Figure 5A–C, compared with the Vcon group, the mRNA expressions of SLC3A2, SLC7A11, and GPX4 were upregulated in the Lyc group, but the changes were not significant, while these indicators were extremely decreased in the groups treated with DEHP alone (P < 0.05, P < 0.01, or P < 0.001). Moreover, the GSH content and GSH/GSSG ratio were lower in the D10 group than they were in the Vcon group (Figure 5D,E). But the addition of Lyc could alleviate the above changes; the mRNA expression of these factors was still returned to a normal level in the DEHP and Lyc combination groups as compared with the groups treated with DEHP alone (P < 0.05 or P < 0.01). Similar trends were also found in the protein expressions of SLC3A2, SLC7A11, and GPX4 (Figure 5F–I). Altogether, our results indicated that chronic exposure of DEHP could cause system xc–/GPX4 pathway damage, but the impairment could be reversed by Lyc in mouse spleen.
Figure 5.
Lyc could affect chronic DEHP-induced system xc–/GPX4 pathway-related gene expressions. (A–C) The mRNA expression of SLC7A11, SLC3A2, and GPX4. (D) The content of GSH. (E) The ratio of GSH/GSSG. (F–I) The protein expressions of SLC7A11, SLC3A2, and GPX4. Data are presented as the mean ± SD. Octothorpe (#) indicates the significant differences between the vehicle control group and another group: #P < 0.05, ##P < 0.01, ###P < 0.001. Asterisks (*) indicate the significant differences between the DEHP-alone treatment groups and the Lyc + DEHP combined treatment groups: *P < 0.05, **P < 0.01, ***P < 0.001.
Correlation Analysis
We calculated the Pearson’s correlation coefficients of the parameters measured. As shown in Figures 6, S-3, and S-4, there were strongly positive correlations between the lipid oxidation metabolism pathway-related factors (ACSL4) and the iron metabolism-related genes (FTH1) (P < 0.01); moreover, the system xc–/GPX4 pathway-related genes (GPX4) also had a strongly positive correlation with the lipid oxidation metabolism pathway-related factors (PTGS2) in the groups exposed to DEHP alone. On the contrary, iron metabolism-related genes (FTRC) had a strongly negative correlation with system xc–/GPX4 pathway-related genes (SLC3A2, SLC7A11, and GPX4) in Lyc antagonism groups (P < 0.05, P < 0.01), and the supplementation of Lyc changed the degree of correlation between the system xc–/GPX4 pathway and the lipid oxidation metabolism pathway-related genes. Together, these data indicated that Lyc could inhibit DEHP-induced ferroptosis via regulating related gene expressions.
Figure 6.
Correlation analysis of DEHP and/or Lyc exposure-induced ferroptosis-related pathway genes of mouse spleen on day 28. (A) The result of a correlation analysis in the DEHP-treated alone groups. (B) The result of a correlation analysis in the DEHP combined with Lyc groups. *P < 0.05; **P < 0.01.
Discussion
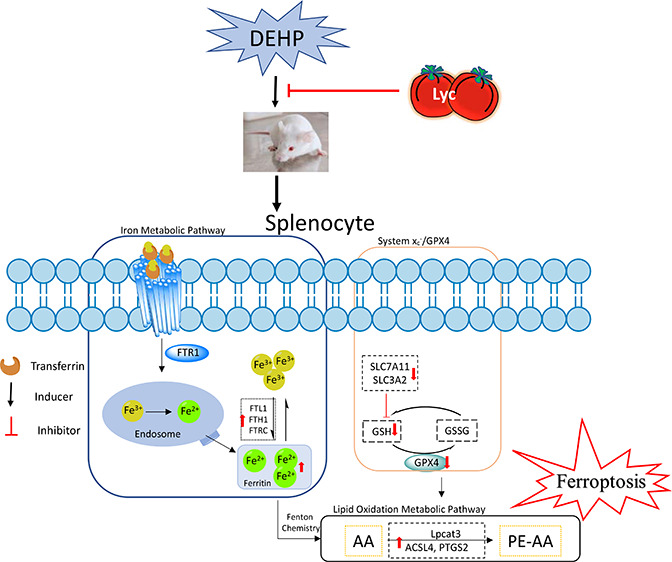
Because of the manufacturing demand worldwide, DEHP production grows annually by an estimated amount of 5%, making DEHP one of the highest-volume chemicals produced.27 DEHP has a huge consumption, and the stability of its chemical structure causes its content to increase in soil, water, and atmospheric dust particles. Moreover, as an environmental persistent organic pollutant, the potential impact of DEHP on human health has gotten more attention around the world.28 Ferroptosis is a newly characterized form of regulated cell death, which highly depends on the concentration of iron and the production of lipid peroxidation.11,12 Today, the study was conducted to find whether DEHP-induced ferroptotic cell death occurs in the spleen and the detailed underlying mechanisms. Our main findings showed that DEHP exposure induced the indexes of ferroptosis-related signal pathways, which were dramatically changed. Typical morphological changes of ferroptotic cell death were observed in the spleen of DEHP-treated mice. Our results also showed that DEHP could lead to a disruption of iron metabolism, increases of lipid peroxidation, a depletion of glutathione and adenosine triphosphate, and an inhibition of cysteine/glutamate antiporter. These results together suggested that DEHP-induced ferroptosis led to spleen cell death, but the addition of Lyc could significantly improve spleen injuries induced by DEHP, implying that Lyc may be a potential strategy for reducing the splenic toxicity of DEHP (Figure 7).
Figure 7.
Schematic diagram illustrating the proposed mechanism of iron ion homeostasis regulation on lycopene antagonizing DEHP-induced ferroptosis in splenocyte.
The balance between iron absorption, output, utilization, and storage decides the homeostasis of intracellular iron.29 The optimal intracellular iron level is maintained via iron regulatory proteins in cells, which can posttranscriptionally regulate the expression of a set of genes such as TFR1 for iron uptake and ferritin for iron storage (FTL1, FTH1, and FTRC).9 Ferric iron (Fe3+) enters the endosome via the membrane protein TFR1, and it is reduced to ferrous iron (Fe2+) by iron reductase, then released into the labile iron pool in the cytoplasm. However, excess iron ions are either stored in ferritin heteropolymers in the form of Fe3+ or released into the extracellular matrix by the member protein ferroportin. Previous studies have shown that erastin (a classic ferroptosis initiator) exposure could significantly increase FTL1 and FTH1 expressions, increase intracellular Fe2+ concentration, and inhibit the production of lipid reaction oxygen species in various tissues and cells,15,30 indicating that ferritin regulation and iron metabolic homeostasis may be important regulatory points of ferroptosis mechanism. In the study, the data showed that DEHP exposure upregulated the expression of absorb-related genes (TFR1) and storage-related genes (TFRC, FTH1, and FTL1) following the increasing concentration of the iron ion in groups exposed to DEHP alone. However, the addition of Lyc successfully reversed the iron status in the spleen. These findings indicated that Lyc may be an effective strategy for the prevention of DEHP-induced splenic toxicity via the regulation of ferroptosis.
Excessive reactive oxygen species induced by ferroptotic cells initiate lipid peroxidation upregulation via Fenton chemistry, which is another crucial biochemical feature of ferroptosis. The extents of lipid peroxidation and ferroptosis are determined by the abundance and location of intracellular oxidizable substrates of lipid peroxidation. Acyl-CoA synthetase (ACSL4 for synthesizing phosphatidylethanolamines (PEs)) and Lpcat3 are two enzymes related to ferroptotic lipid metabolism, which is for lipid remodeling. The ACSL family has five isoforms (ACSL1, ACSL3–6) that have been identified in humans and rodents, but only ACSL4 is involved in ferroptotic cell death.31 Sebastian et al. have results that also showed that ACSL4 is a critical determinant of ferroptosis sensitivity.32 Furthermore, ACSL4 as lipid metabolic intermediates can facilitate fatty acid metabolism and membrane modifications. The ACSL4 expression upregulation will accelerate the accumulation of lipid peroxides leading to cell death. A previous study reported that ACSL4 was overexpressed in promoting xenobiotics-induced ferroptosis.33 Lpcat3 is a protein that catalyzes the reacylation of lysophospholipids to phospholipids. Lpcat3 is highly expressed in the intestine and liver and also shows moderate but significant levels of expression in macrophages.34 In this study, when compared with a control group, it was learned that DEHP exposure could promote ACSL4 and Lcapt3 overexpression, indicating that DEHP may induce ferroptosis in mouse spleen. However, the Lyc supplementation could adversely affect the above phenomenon. Moreover, Lpcat3 and ACSL4 can promote AA acylated into membrane phospholipids.35 AA is one of the lipids most susceptible to oxidation, and its level is directly related to lipid accumulation in cells.35 Meanwhile, lipidomic analyses indicate that phosphatidylethanolamines containing AA are key membrane phospholipids.12 PEs are involved in a multitude of physiological functions, such as apoptotic and ferroptotic cell-death pathways, and activate oxidative phosphorylation.36 In our study, the AA content had increased after DEHP exposure, indicating that DEHP exposure may initiate the lipid accumulation process in mouse spleen. In other words, our results demonstrated that the upregulation of ACSL4 may be responsible for ferroptosis-associated AA metabolism during DEHP exposure in spleen. Besides, AA synthesized prostaglandin (PG) is regulated by PTGS2, a key rate-limiting enzyme, and its expression level is closely related to lipid accumulation in cells. It was worth noting that the PTGS2 expression increased in mice during the ferroptosis process.37 In our study, the results showed that the PTGS2 expression was upregulated with the increase of AA content and ACSL4 level in groups exposed to DEHP alone, but the Lyc supplementation alleviated these results. Our findings were consistent with previous results that an ACSL4 expression is required for PEGS2 metabolism in HepG2 and HL60 cells,33 indicating DEHP exposure-induced ferroptosis in mouse spleen and Lyc could alleviate DEHP-induced spleen injuries. Moreover, the upregulated ACSL4-PTGS2 pathway may be an important molecular event in DEHP-induced ferroptosis in mouse spleen.
A cystine/glutamate transporter system (xc–) can provide adequate levels of cystine (i.e., the oxidized form of cysteine) as an essential precursor for the synthesis of GSH.38 It consists of a 12-pass transmembrane protein transporter SLC7A11 and a single-pass transmembrane regulatory protein SLC3A2, functioning as a provider of GSH biosynthesis. A previous study reported that, under high-iron conditions, the decreased expressions of SLC7A11 and SLC3A2 could facilitate the onset of ferroptosis,39 which is consistent with our results. Moreover, DEHP exposure significantly decreased the content of GSH, indicating that the synthesis content of GSH was inadequate. The antioxidant function of GSH is accomplished largely by GSH peroxidase (GPx) catalyzed reactions, which reduce hydrogen peroxide and lipid peroxide as GSH is oxidized to GSSG.40 More specifically, the synthesis of GSH appears to protect cells from ferroptotic death. GPX4, an antioxidant defense enzyme, is another central regulator of ferroptosis.37 GPX4 as a GSH-dependent enzyme could decrease the level of reactive oxygen species in cells. Many reports have provided evidence that a knockdown of GPX4 could increase the lethality of ferroptosis inducers; rather, an overexpression of GPX4 causes the complete opposite effect on the induction of cell death.15,41 This study showed that DEHP exposure significantly inhibited the GPX4 mRNA and protein expressions in the spleen, which was highly associated with the increase of iron content, indicating ferroptosis may have occurred. Moreover, as it acts as a GPX4 cofactor, GSH maintains the level of GPX4, depending on the exchange of glutamate and cystine via the antiporter system xc–.13 Meanwhile, Cao et al. reported that depletion of GSH could cause GPX4 inactivation and increase intracellular lipid peroxidation, eventually leading to ferroptosis.42 Our result also showed that the GPX4 expression level dramatically decreased following the decrease of GSH content. However, the addition of the antioxidant Lyc effectively prevented the above processes. Token together, these data suggested that DEHP disrupted the GSH metabolism via the xc–/ GPX4 antioxidant system and, subsequently, caused the ferroptotic cell death, but Lyc could effectively mitigate DEHP-induced damage to the antioxidant system.
Conclusion
Overall, our results have demonstrated that ferroptosis may be a newly characterized form of spleen cell death in response to DEHP exposure, but the addition of Lyc could suppress in some degree the occurrence of this phenomenon. Our results demonstrated that the Lyc supplementation alleviated DEHP-induced ferroptosis involving iron metabolism disorder, overgeneration of lipid peroxidation products, and disruption of glutathione metabolic pathway. Together, these data may provide compelling evidence that new strategies can be developed for targeting ferroptosis in the prevention or therapy against DEHP-related diseases.
Acknowledgments
This work was supported by National Natural Science Foundation of China (Grant No. 31572586), Excellent Youth Foundation of Heilongjiang Province of China (Grant No. JC2017005), and China Agriculture Research System (Grant No. CARS-35).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.1c00001.
The primer pairs used for qRT-PCR analyses. The result of correlation analysis in the DEHP-treated alone groups. The result of correlation analysis in the DEHP combined with Lyc groups (PDF)
Author Contributions
# (X.-Y.D. and S.-Y.Z.) These authors contributed equally to this study.
The authors declare no competing financial interest.
Supplementary Material
References
- Del Pup L.; Mantovani A.; Cavaliere C.; Facchini G.; Luce A.; Sperlongano P.; Caraglia M.; Berretta M. (2016) Carcinogenetic mechanisms of endocrine disruptors in female cancers. Oncol. Rep. 36, 603–612. 10.3892/or.2016.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardelli T. C.; Albert O.; Lalancette C.; Culty M.; Hales B. F.; Robaire B. (2017) In Utero and Lactational Exposure Study in Rats to Identify Replacements for Di(2-ethylhexyl) Phthalate. Sci. Rep. 7, 3862. 10.1038/s41598-017-03979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. N.; Li H. X.; Yang T. N.; Li X. W.; Huang Y. Q.; Zhu S. Y.; Li J. L. (2020) Di-(2-ethylhexyl) phthalate induced developmental abnormalities of the ovary in quail (Coturnix japonica) via disruption of the hypothalamic-pituitary-ovarian axis. Sci. Total Environ. 741, 140293. 10.1016/j.scitotenv.2020.140293. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Ma D. X.; Wang H. G.; Li M. Z.; Talukder M.; Wang H. R.; Li J. L. (2020) Lycopene Prevents DEHP-Induced Liver Lipid Metabolism Disorder by Inhibiting the HIF-1α-Induced PPARα/PPARγ/FXR/LXR System. J. Agric. Food Chem. 68, 11468–11479. 10.1021/acs.jafc.0c05077. [DOI] [PubMed] [Google Scholar]
- Crobeddu B.; Ferraris E.; Kolasa E.; Plante I. (2019) Di(2-ethylhexyl) phthalate (DEHP) increases proliferation of epithelial breast cancer cells through progesterone receptor dysregulation. Environ. Res. 173, 165–173. 10.1016/j.envres.2019.03.037. [DOI] [PubMed] [Google Scholar]
- Chiellini F.; Ferri M.; Morelli A.; Dipaola L.; Latini G. (2013) Perspectives on alternatives to phthalate plasticized poly(vinyl chloride) in medical devices applications. Prog. Polym. Sci. 38, 1067–1088. 10.1016/j.progpolymsci.2013.03.001. [DOI] [Google Scholar]
- Yu L.; Li H. X.; Guo J. Y.; Huang Y. Q.; Wang H.; Talukder M.; Li J. L. (2019) Di (2-ethyl hexyl) phthalate (DEHP)-induced spleen toxicity in quail (Coturnix japonica) via disturbing Nrf2-mediated defense response. Environ. Pollut. 251, 984–989. 10.1016/j.envpol.2019.05.061. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Li M. Z.; Talukder M.; Luo Y.; Shen Y.; Wang H. R.; Li J. L. (2020) Effect of mitochondrial quality control on the lycopene antagonizing DEHP-induced mitophagy in spermatogenic cells. Food Funct. 11, 5815–5826. 10.1039/D0FO00554A. [DOI] [PubMed] [Google Scholar]
- Dixon S. J.; Lemberg K. M.; Lamprecht M. R.; Skouta R.; Zaitsev E. M.; Gleason C. E.; Patel D. N.; Bauer A. J.; Cantley A. M.; Yang W. S.; Morrison B. 3rd; Stockwell B. R. (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072. 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y.; Hou W.; Song X.; Yu Y.; Huang J.; Sun X.; Kang R.; Tang D. (2016) Ferroptosis: process and function. Cell Death Differ. 23, 369–379. 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli J. P. F.; Shah R.; Pratt D. A.; Conrad M. (2017) Ferroptosis inhibition: mechanisms andopportunities. Trends Pharmacol. Sci. 38, 489–498. 10.1016/j.tips.2017.02.005. [DOI] [PubMed] [Google Scholar]
- Doll S.; Conrad M. (2017) Iron and ferroptosis: A still ill-defined liaison. IUBMB Life 69, 423–434. 10.1002/iub.1616. [DOI] [PubMed] [Google Scholar]
- Stockwell B. R.; Friedmann Angeli J. P.; Bayir H.; Bush A. I.; Conrad M.; Dixon S. J.; Fulda S.; Gascon S.; Hatzios S. K.; Kagan V. E.; Noel K.; Jiang X.; Linkermann A.; Murphy M. E.; Overholtzer M.; Oyagi A.; Pagnussat G. C.; Park J.; Ran Q.; Rosenfeld C. S.; Salnikow K.; Tang D.; Torti F. M.; Torti S. V.; Toyokuni S.; Woerpel K. A.; Zhang D. D. (2017) Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 171, 273–285. 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K.; Skouta R.; Kaplan A.; Yang W. S.; Hayano M.; Dixon S. J.; Brown L. M.; Valenzuela C. A.; Wolpaw A. J.; Stockwell B. R. (2016) Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat. Chem. Biol. 12, 497–503. 10.1038/nchembio.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng P.; Zhang S.; Jiang X.; Cheng S.; Zhang J.; Cao X.; Qin X.; Zou Z.; Chen C. (2020) Arsenite induces testicular oxidative stress in vivo and in vitro leading to ferroptosis. Ecotoxicol. Environ. Saf. 194, 110360. 10.1016/j.ecoenv.2020.110360. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Lin J.; Talukder M.; Zhu S. Y.; Li M. Z.; Wang H. R.; Li J. L. (2020) Aryl Hydrocarbon Receptor as a Target for Lycopene Preventing DEHP-Induced Spermatogenic Disorders. J. Agric. Food Chem. 68, 4355–4366. 10.1021/acs.jafc.9b07795. [DOI] [PubMed] [Google Scholar]
- Ashari S.; Karami M.; Shokrzadeh M.; Ghandadi M.; Ghassemi-Barghi N.; Dashti A.; Ranaee M.; Mohammadi H. (2020) The implication of mitochondrial dysfunction and mitochondrial oxidative damage in Di (2-ethylhexyl) phthalate induced nephrotoxicity in both in vivo and in vitro models. Toxicol Mech Methods 1–27. 10.1080/15376516.2020.1758980. [DOI] [PubMed] [Google Scholar]
- Yirong C.; Shengchen W.; Jiaxin S.; Shuting W.; Ziwei Z. (2020) DEHP induces neutrophil extracellular traps formation and apoptosis in carp isolated from carp blood via promotion of ROS burst and autophagy. Environ. Pollut. 262, 114295. 10.1016/j.envpol.2020.114295. [DOI] [PubMed] [Google Scholar]
- Park C. G.; Sung B.; Ryu C. S.; Kim Y. J. (2020) Mono-(2-ethylhexyl) phthalate induces oxidative stress and lipid accumulation in zebrafish liver cells. Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol. 230, 108704. 10.1016/j.cbpc.2020.108704. [DOI] [PubMed] [Google Scholar]
- Amara I.; Timoumi R.; Graiet I.; Ben Salem I.; Adelou K.; Abid-Essefi S. (2019) Di (2-ethylhexyl) phthalate induces cytotoxicity in HEK-293 cell line, implication of the Nrf-2/HO-1 antioxidant pathway. Environ. Toxicol. 34, 1034–1042. 10.1002/tox.22774. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Li M. Z.; Shen Y.; Li J.; Wang H. R.; Talukder M.; Li J. (2019) Lycopene prevents DEHP-induced Leydig cell damage with the Nrf2 antioxidant signaling pathway in mice. J. Agric. Food Chem. 68, 2031–2040. 10.1021/acs.jafc.9b06882. [DOI] [PubMed] [Google Scholar]
- Tjahjodjati; Sugandi S.; Umbas R.; Satari M. (2020) The protective effect of lycopene on prostate growth inhibitory efficacy by decreasing insulin growth factor-1 in indonesian human prostate cancer cells. Res. Rep. Urol. 12, 137–143. 10.2147/RRU.S232745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.; Wen C.; Yang M.; Gan D.; Fan C.; Li A.; Li Q.; Zhao J.; Zhu L.; Lu D. (2019) Lycopene protects against t-BHP-induced neuronal oxidative damage and apoptosis via activation of the PI3K/Akt pathway. Mol. Biol. Rep. 46, 3387–3397. 10.1007/s11033-019-04801-y. [DOI] [PubMed] [Google Scholar]
- Lin J.; Xia J.; Zhao H. S.; Hou R.; Talukder M.; Yu L.; Guo J. Y.; Li J. L. (2018) Lycopene Triggers Nrf2-AMPK Cross Talk to Alleviate Atrazine-Induced Nephrotoxicity in Mice. J. Agric. Food Chem. 66, 12385–12394. 10.1021/acs.jafc.8b04341. [DOI] [PubMed] [Google Scholar]
- Tang L.; Guan H.; Ding X.; Wang J. S. (2007) Modulation of aflatoxin toxicity and biomarkers by lycopene in F344 rats. Toxicol. Appl. Pharmacol. 219, 10–17. 10.1016/j.taap.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Bandeira A. C. B.; da Silva T. P.; de Araujo G. R.; Araujo C. M.; da Silva R. C.; Lima W. G.; Bezerra F. S.; Costa D. C. (2017) Lycopene inhibits reactive oxygen species production in SK-Hep-1 cells and attenuates acetaminophen-induced liver injury in C57BL/6 mice. Chem.-Biol. Interact. 263, 7–17. 10.1016/j.cbi.2016.12.011. [DOI] [PubMed] [Google Scholar]
- Teng Zhang L. L.; Qin Xun-Si; Zhou Yang; Zhang Xi-Feng; Wang Lin-Qing; Felici Massimo De; Chen Hong; Qin Guo-Qing; Shen Wei (2014) Di-(2-ethylhexyl) phthalate and bisphenol a exposure impairs mouse primordial follicle assembly in vitro. Environ. Mol. Mutagen 55, 343–353. 10.1002/em.21847. [DOI] [PubMed] [Google Scholar]
- Da-Wen Gao Z.-D. W. (2016) Phthalate esters in the environment: A critical review of their occurrence, biodegradation, and removal during wastewater treatment processes. Sci. Total Environ. 541, 986–1001. 10.1016/j.scitotenv.2015.09.148. [DOI] [PubMed] [Google Scholar]
- Galaris D.; Barbouti A.; Pantopoulos K. (2019) Iron homeostasis and oxidative stress: an intimate relationship. Biochim. Biophys. Acta, Mol. Cell Res. 1866, 118535. 10.1016/j.bbamcr.2019.118535. [DOI] [PubMed] [Google Scholar]
- Tang Q.; Bai L.; Zou Z.; Meng P.; Xia Y.; Cheng S.; Mu S.; Zhou J.; Wang X.; Qin X.; Cao X.; Jiang X.; Chen C. (2018) Ferroptosis is newly characterized form of neuronal cell death in response to arsenite exposure. NeuroToxicology 67, 27–36. 10.1016/j.neuro.2018.04.012. [DOI] [PubMed] [Google Scholar]
- Chen W. C.; Wang C. Y.; Hung Y. H.; Weng T. Y.; Yen M. C.; Lai M. D. (2016) Systematic analysis of gene expression alterations and clinical outcomes for long-chain acyl-Coenzyme a synthetase family in cancer. PLoS One 11, e0155660. 10.1371/journal.pone.0155660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll S.; Proneth B.; Tyurina Y. Y.; Panzilius E.; Kobayashi S.; Ingold I.; Irmler M.; Beckers J.; Aichler M.; Walch A.; Prokisch H.; Trumbach D.; Mao G.; Qu F.; Bayir H.; Fullekrug J.; Scheel C. H.; Wurst W.; Schick J. A.; Kagan V. E.; Angeli J. P.; Conrad M. (2017) ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 13, 91–98. 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H.; Li X.; Zhang X.; Kang R.; Tang D. (2016) Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem. Biophys. Res. Commun. 478, 1338–43. 10.1016/j.bbrc.2016.08.124. [DOI] [PubMed] [Google Scholar]
- X. Rong B. W.; Dunham M. M.; Hedde P. N.; Wong J. S.; Gratton E.; Young S. G.; Ford D. A.; Tontonoz P. (2015) Lpcat3-dependent production of arachidonoyl phospholipids is a key determinant of triglyceride secretion. eLife 4, e06557. 10.7554/eLife.06557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan V. E.; Mao G.; Qu F.; Angeli J. P.; Doll S.; Croix C. S.; Dar H. H.; Liu B.; Tyurin V. A.; Ritov V. B.; Kapralov A. A.; Amoscato A. A.; Jiang J.; Anthonymuthu T.; Mohammadyani D.; Yang Q.; Proneth B.; Klein-Seetharaman J.; Watkins S.; Bahar I.; Greenberger J.; Mallampalli R. K.; Stockwell B. R.; Tyurina Y. Y.; Conrad M.; Bayir H. (2017) Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 13, 81–90. 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C.; Liu Y.; Dai R.; Ismail N.; Su W.; Li B. (2020) Ferroptosis and Its Potential Role in Human Diseases. Front. Pharmacol. 11, 239. 10.3389/fphar.2020.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. S.; SriRamaratnam R.; Welsch M. E.; Shimada K.; Skouta R.; Viswanathan V. S.; Cheah J. H.; Clemons P. A.; Shamji A. F.; Clish C. B.; Brown L. M.; Girotti A. W.; Cornish V. W.; Schreiber S. L.; Stockwell B. R. (2014) Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331. 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T.; Sugita Y.; Bannai S. (1987) Regulation of glutathione levels in mouse spleen lymphocytes by transport of cysteine. J. Cell. Physiol. 133, 330–6. 10.1002/jcp.1041330217. [DOI] [PubMed] [Google Scholar]
- Wang H.; An P.; Xie E.; Wu Q.; Fang X.; Gao H.; Zhang Z.; Li Y.; Wang X.; Zhang J.; Li G.; Yang L.; Liu W.; Min J.; Wang F. (2017) Characterization of ferroptosis in murine models of hemochromatosis. Hepatology 66, 449–465. 10.1002/hep.29117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell B. R.; Jiang X.; Gu W. (2020) Emerging Mechanisms and Disease Relevance of Ferroptosis. Trends Cell Biol. 30, 478–490. 10.1016/j.tcb.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L.-J.; Luo X.-J.; Tu H.; Chen H.; Xiong X.-M.; Li N.-S.; Peng J. (2021) Ferroptosis Occurs in Phase of Reperfusion but Not Ischemia in Rat Heart Following Ischemia or ischemia/reperfusion. Naunyn-Schmiedeberg's Arch. Pharmacol. 394, 401. 10.1007/s00210-020-01932-z. [DOI] [PubMed] [Google Scholar]
- Cao J. Y.; Dixon S. J. (2016) Mechanisms of ferroptosis. Cell. Mol. Life Sci. 73, 2195–209. 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.









