Abstract
The process of wound healing is a dynamic event that starts with inflammation, proliferation, and cell migration of various types of fibroblast cells. Therefore, identification of potential molecules which may increase the wound healing capacity of fibroblast cells is crucial. A novel hydroalcoholic formulation of belladonna (SKRIN), was developed and characterized by GC-MS/MS, DLS, TEM, and AFM and was found to contain atropine and scopolamine exhibit in aggregated nanosized particles. SKRIN-mediated fibroblast cell survival was elucidated in the presence of H2O2 by MTT and flow cytometry based assays. With an EC50 of 4.41 μg/mL, SKRIN treatment showed significant increase in cell survival that was evident from a 1.11-fold increase (p < 0.0122) in the live cell population and 4.21-fold (p < 0.0001) and 2.59-fold (p < 0.0001) reductions in the early and late apoptotic cell populations, respectively. SKRIN-mediated wound healing was measured by cell scratch assay and cell cycle analysis. During the wound closure phenomenon, SKRIN increases repairing fibroblast cell proliferation by 1.24-fold (p = 0.0481) and increases the count of G2/M phase cells by 1.76-fold (p = 0.0002) which was confirmed by increased PCNA and reduced p21 protein expressions probably mediated by molecular interactions of PCNA–p21 complex with alkaloids present in SKRIN. Relative gene expression analysis further showed that SKRIN increases the PI3K, Akt, and NF-κB expression. Our data suggests that SKRIN exhibits wound healing property by increasing cell survival and repairing fibroblast proliferation via activation of the PI3K–Akt–NF-κB pathway probably mediated by inhibition of PCNA–p21 complex interaction.
Keywords: SKRIN, wound healing, fibroblast, proliferation, cell survival, PCNA−p21 protein complex
1. Introduction
Wound healing is a dynamic and complex process where cell survival and proliferation are the two crucial factors. The process of wound healing involves three overlapping stages, explicitly, inflammation, proliferation, and remodeling.1 Fibroblast cells are one of the most abundant cell types in connective tissues which are involved in all the three stages of the wound healing process. Under normal physiological conditions these cell types are responsible for tissue homeostasis and collagen and elastin synthesis.2 Immediately after injury, the first phase of homeostasis begins with fibrin clot formation and vascular constriction.3 As a part of the inflammatory response, immune cells secrete various factors, cytokines, and chemokines including platelet-derived growth factor (PDGF), tumor necrosis factor-alpha (TNF-α), and interleukin-1 beta (IL-1β).4 These factors act as chemoattractant for fibroblast that induces its recruitment toward the wound site by binding to its cell surface receptors. As the inflammatory phase ends, proliferation starts within 24–48 h after injury, and fibroblasts start to appear at the site of injury. Various growth factors including fibroblast growth factor (FGF), PDGF, transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), and keratinocyte growth factor (KGF) have been shown to stimulate the fibroblast migration, differentiation, proliferation and cell survival.5
Fibroblast migration toward the wound site, known as contact guidance, is dependent upon fibronectin, tenascin-C, and orientation of collagen protein.6 Fibroblast cells infiltrate at the site of injury to produce matrix metalloproteinases (MMPs) and other proteases for degradation of the fibrin clot following formation of an extracellular matrix (ECM) that comprises of glycoproteins, collagen I–IV and XVIII, fibronectin, proteoglycans, glycosaminoglycans (GAGs), thrombospondin, laminin, hyaluronic acid, and heparin sulfate.7 To facilitate wound closure upon tissue injury, fibroblasts play a crucial role by breaking down the fibrin clot and differentiate into myofibroblasts that result in active synthesis of ECM proteins leading to wound contracting. Fibroblast and myofibroblasts both provide a contractile force that brings edges of wound together, resulting in wound closure.8 After wound closure, the level of fibroblasts decreases to a normal level by around 2 weeks. As the similar tensile strength is achieved to the surrounding tissue, the actively dividing cells undergo apoptosis to maintain the homeostasis.9
Fibroblast cell proliferation and differentiation is regulated by a potent cyclin-dependent kinase (CDK) inhibitor, p21Waf1/Cip1 (p21), which mediates G1/S cell cycle arrest and induces cellular senescence. Inhibition of p21 expression has been found to increase the rate of the wound healing process.10 The ability of p21 protein to interact with proliferating cell nuclear antigen (PCNA) makes it distinct from other CDK inhibitors. The binding of p21 with PCNA causes G1 and G2 cell cycle arrest and thereby reduces the cell cycle progression and proliferation.11 Moreover, several of the signaling pathways have been shown to involve different stages of the wound healing process. Nuclear factor kappa B (NF-κB)12 and phosphoinositide 3-kinases (PI3K)–protein kinase B (PKB also known as Akt) (PI3K–Akt)13 pathways play a crucial role in the wound healing mechanism by increasing fibroblast cell survival and proliferation.
Various molecules and factors have been shown to exhibit wound healing property. Human PDGF has been approved for clinical uses, whereas TGF-β and FGF failed to exhibit regenerative capacity in clinical settings, most likely due to their short half-life.14 Complementary and alternative medicine (CAM) is being used in association with conventional medicines for wound healing that comprises a variety of remedies based on nutritional supplements, botanicals, and others.15 Belladonna is a medicinal plant that exhibits diverse pharmacological properties in various disciplines of medicines including homeopathy, ayurveda, and conventional medicine.16,17 Atropine, scopolamine, and hyoscyamine are the principal tropane alkaloids present in Atropa belladonna. An aqueous extract of belladonna has been shown to increase the number of fibroblasts at the site of a wound.18 However, the mechanism of regeneration mediated by belladonna has not been well understood. Therefore, in the present study, we have prepared a hydroalcoholic formulation of belladonna (SKRIN) and elucidated its wound healing efficacy in fibroblast cells and deciphered its plausible mechanism of action.
2. Results
2.1. Biophysical Characterization of SKRIN
To characterize SKRIN, we first identify the molecules present in the formulation using GC-MS/MS. Our analysis demonstrated the presence of atropine and scopolamine at the retention times of 14.02 and 14.74, respectively, in SKRIN using the National Institute of Standards and Technology (NIST) Mass Spectral Library. Resolution of both compounds was distinct, and differences in the retention times made the identification significant (Figure 1A). The m/z ratios of atropine and scopolamine were observed at 289 and 304 (Figure 1B). To observe the particle size distribution profile of SKRIN, we carried out dynamic light scattering (DLS). We observed particles that were in the size range of 10–100 nm. The variations in the size range of particles detected in the solutions represent a polydisperse particle size (Figure 1C) and showed the delays time of time of 1.5 and 7 μs (Figure 1D). In addition, we observed the morphology of the particles using transmission electron microscopy (TEM). Dark spots were observed which indicates individual particles forming aggregated structures. We have found that SKRIN comprises aggregated nanosized particles (Figure 1E). To validate the particle size, we further performed atomic force microscopy (AFM) which showed the particles in varied size ranges. Most of the particles were in the size range of 100–200 nm with a height of 5–6 nm (Figure 1F).
Figure 1.
Biophysical characterization of SKRIN. (A) Chromatogram generated by GC-MS/MS, showing the peaks of SKRIN atropine and scopolamine at 14.02 and 14.74 retention times, respectively. (B) The m/z ratios of atropine and scopolamine at 289 and 304 were observed. (C) Particle size as observed by dynamic light scattering (DLS) which showed particles in the size range between 10 and 100 nm. (D) Delays time of particle size shows the delays time of 1.5 and 7 μS. (E) TEM was performed which showed dark spots, indicated by white arrows, showing aggregated nanosized particle structures. (F) AFM showed the particles in varied size ranges mostly in size range of 100–200 nm with a height of 5–6 nm.
2.2. SKRIN Increases Fibroblast Cell Survival
To elucidate the effect of SKRIN on fibroblast cell survival, median cytotoxic concentration (CC50) and median effective concentration (EC50) was determined. Nonlinear regression analysis suggests that the CC50 of SKRIN for fibroblast cells is 41.70 μg/mL (Figure 2A). H2O2 (1 mM) was used to measure the EC50 of SKRIN, as it acts as a positive inducer of fibroblast cell death. Fibroblast cells were stimulated with H2O2 and treated with below CC50 concentration of SKRIN (1–10 μg/mL) and cell survival assay was performed after 24 h of treatment. Nonlinear regression analysis suggests that the EC50 of SKRIN for fibroblast cells is 4.41 μg/mL (Figure 2B). The percentage cell survival data suggested that SKRIN was nontoxic to the fibroblast cells at lower doses and increases fibroblast cell survival. Moreover, we determined the cell survival effect of SKRIN at different time points in cells stimulated with H2O2 and treated with EC50 of SKRIN. Cell survival assay was performed at 24, 48, and 72 h post-treatment. As compared with cells stimulated with H2O2, H2O2 stimulated SKRIN-treated fibroblasts showed 1.12-fold (p = 0.0496), 1.30-fold (p = 0.0030), and 1.32-fold (p = 0.0026) increases in fibroblast cell survival at 24, 48, and 72 h respectively (Figure 2C). The fibroblast cell survival boost exhibited by SKRIN was highest at 48 h post-treatment. These data suggest that SKRIN exhibits a fibroblast cell survival property at lower concentrations and may have various therapeutic applications in wound healing where fibroblast cell survival and proliferation are crucial to achieve.
Figure 2.
Fibroblast cell survival during SKRIN treatment. (A) Percentage of fibroblast cell viability graph showing the median cytotoxic concentration (CC50) of SKRIN is 41.70 μg/mL. (B) Cells were stimulated with H2O2 and treated with SKRIN, and the median effective concentration (EC50) of SKRIN was found to be 4.41 μg/mL. (C) The cell survival effect of SKRIN was monitored at different time points showing SKRIN treatment increases the fibroblast cell survival by 1.12-fold (p = 0.0496), 1.30-fold (p = 0.0030), and 1.32-fold (p = 0.0026) increase in cell survival at 24, 48, and 72 h, respectively. (D) Image flow cytometry showing different cellular phases [live (1), early apoptotic (2), late apoptotic (3), and necrotic cells (4)] were observed in various channels [bright-field (a), Annexin V-FITC (b), propidium iodide (PI) (c), and merged (d)] representing the extent of apoptosis. (E) SKRIN-treated fibroblast cells were acquired after annexin V-FITC/PI staining by image flow cytometry. (F) As compared with H2O2, SKRIN-treatment increases the live cell population by 1.11-fold (p < 0.0122) and (G) decreases the early apoptotic cell population by 4.21-fold (p < 0.0001) and late apoptotic cell population by 2.59-fold (p < 0.0001). The data is expressed as the mean ± standard error of mean (n = 3) where p values are represented as the difference between H2O2 stimulated cells and SKRIN-treated cells.
2.3. SKRIN Reduces Fibroblast Cell Apoptosis
To further investigate the role of SKRIN on fibroblast cell survival, H2O2 was used to induce cell death, and apoptosis assay was performed using image flow cytometry by acquiring 10 000 events. The extent of cell death was observed in different apoptotic phases. Healthy live cells were identified with only bright-field images with no signals of annexin V-FITC and propidium idodide (PI), whereas early apoptotic cells were identified as discrete signals of annexin V-FITC signals on the cell periphery and a low signal of PI. Late apoptotic cells were identified as the cells showing highest signals of annexin V-FITC and PI. In addition, necrotic cells were identified with the lowest signals of annexin V-FITC due to loss of membrane integrity and high signals of PI (Figure 2D). Dot plots were generated to compare the percentage of cells in each phase of apoptosis (Figure 2E). The percentage cell population analysis suggests that SKRIN increases the fibroblast cell survival in H2O2 stimulated cells. As compared with the H2O2, we have found that SKRIN treatment increases the live cell population by 1.11-fold (p < 0.0122) (Figure 2F). In addition, we found that SKRIN treatment decreases the percentage of the early apoptotic cell population by 4.21-fold (p < 0.0001) and that of the late apoptotic cell population by 2.59-fold (p < 0.0001) (Figure 2G).
2.4. SKRIN Increases Wound Closure Capacity of Fibroblast Cells by Inducing Cell Proliferation
The increased cell survival property of SKRIN influenced us to elucidate the wound healing effect of SKRIN on fibroblast cells. A wound healing assay was carried out in SKRIN-treated fibroblast cells, and wound closure was monitored at 6, 12, and 24 h post-treatment. The percentage of wound area was measured to elucidate wound healing property of SKRIN. We have found that SKRIN treatment reduces the wound area by increasing the proliferative capacity of fibroblasts cells (Figure 3A). As compared to control, SKRIN treatment showed 1.5-fold reduction (p = 0.0007) in the wound area at 24 h post-treatment, suggesting its role in wound healing by increasing the fibroblast proliferation (Figure 3B). To confirm the proliferation of fibroblast cells during wound closure, we further performed BrdU assay using flow cytometry in H2O2-stimulated SKRIN-treated cells upon cell scratch (Figure 3C). Our data suggests that SKRIN increases the incorporation of BrdU in the fibroblast cells during the wound closure phenomenon by 1.24-fold (p = 0.0481) (Figure 3D). These data suggests that SKRIN increases the cell proliferation of repairing fibroblast monolayers during wound closure.
Figure 3.
SKRIN-mediated fibroblast cell proliferation. (A) Cell scratch assay showing the wound healing effect of SKRIN at different time points. Horizontal arrows showing the width of wound reduction at different time points. (B) Percentage area of wound graph showing that as compared with control, SKRIN treatment reduces the wound area by 1.5-fold (p = 0.0007). (C) BrdU assay was performed to analyze the SKRIN-mediated repairing fibroblast cell proliferation during wound closure. (D) SKRIN increases the incorporation of BrdU in the fibroblast cells during wound closure by 1.24-fold (p = 0.0481).
2.5. SKRIN Induces the Cell Cycle Shift in Repairing Fibroblast Monolayers
To further observe fibroblast cell proliferation mediated via SKRIN during wound closure, cell cycle analysis was performed in repairing fibroblast monolayers using image flow cytometry (Figure 4A). The distribution of cell cycle phases during the wound healing process was analyzed after cell scratch assay in SKRIN-treated cells. We have found that SKRIN treatment significantly increases the distribution of the G2/M phase of the cell cycle. The morphological differences in cells were also identified during cell cycle progression. Fibroblast cells in the sub-G1 phase were considered apoptotic/necrotic cells and were identified by their irregular shape with the lowest PI signal. The cells shown to be in the G0/G1 phase were identified as healthy cells with round nuclei. The cells shown to be in the S phase were identified as healthy cells with elongated nuclei. The cells shown to be in the G2/M phase appeared as healthy cells with dividing nuclei identified in PI channel (Figure 4B). As compared with the control, SKRIN treatment increases the distribution of cells in the G2/M phase by 1.76-fold (p = 0.008) (Figure 4C). This data suggests that SKRIN treatment induces a cell cycle shift in repairing fibroblast monolayers by increasing the number of cells in the G2/M phase of the cell cycle.
Figure 4.
Effect of SKRIN on fibroblast cell cycle and PCNA–p21 expression. (A) Flow cytometry based cell cycle analysis was performed in control and SKRIN-treated cells by measuring the cell count vs DNA content (PI). (B) Morphological differences in bright-field (BF) and PI channels were observed for each cell cycle phases. (C) As compared with the control, SKRIN-treated cells showed a 1.47-fold increase (p = 0.0008) in the G2/M phase of the cell cycle. (D) SKRIN-mediated fibroblast cell proliferation was determined by Western blot analysis. (E) Densitometric analysis of Western blotting showing that SKRIN reduces the expression of p21 protein by 1.30-fold (p = 0.0031) and increases the PCNA expression by 1.18-fold (p = 0.0134) and expression level of proteins was normalized to GAPDH. The data is expressed as the mean ± standard error of mean (n = 3) where p values are represented as the difference between control and SKRIN-treated cells.
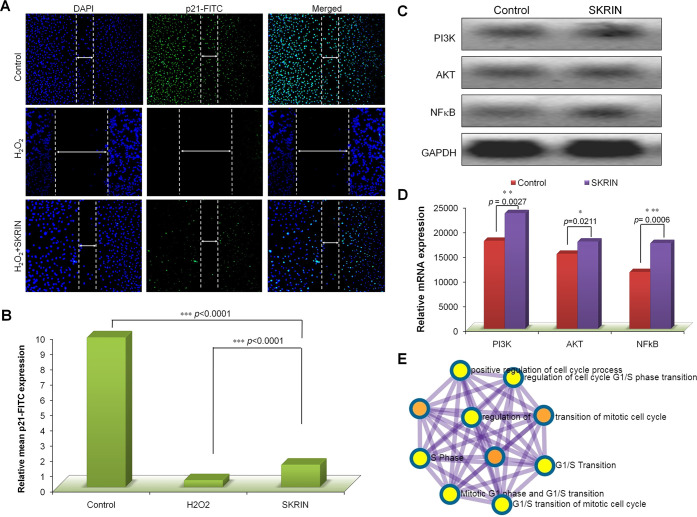
2.6. SKRIN Increases the PCNA Expression in Repairing Fibroblast Cells
To further monitor the effect of SKRIN on fibroblast cell proliferation, the expression of the PCNA–p21 (p21Cip1/waf1) complex was observed in the repairing fibroblast monolayers by Western blot analysis (Figure 4D). p21 is a member of the family of cyclin dependent kinase (CDK) inhibitors which blocks the function of proliferating cell nuclear antigen (PCNA). We have found that SKRIN reduces the expression of p21 protein by 1.30-fold (p = 0.0031), suggesting that SKRIN treatment increases the repairing fibroblast cell proliferation and thereby increases their wound healing capacity. Interestingly, we found an increased expression of PCNA in SKRIN-treated cells by 1.18-fold (p = 0.0134) (Figure 4E).
2.7. SKRIN Targets PCNA–p21 Complex
The SKRIN-mediated reduction of p21 and increase in PCNA protein expression instigated us to elucidate the interaction of atropine and scopolamine with PCNA–p21 complex by molecular docking (Figure 5A). The molecules were docked with the human PCNA–p21 complex, and the results of the best dockings were compared based on their MolDock scores, H-bond energy, interacting residues of active site, and other crucial noncovalent interactions. Our data suggests that atropine and scopolamine both exhibited significant binding affinity toward crucial residues of the PCNA–p21 complex and thereby interfere with protein–protein interaction. More specifically, we have found that atropine interacts with Pro 129, Tyr 133, Ile 228, Glu 238, Lys 248, and Tyr 250 residues with an interaction energy of −105.43 kJ/mol (Figure 5B,C), and scopolamine interacts with Pro 129, Glu 130, Gln 131, Tyr 133, Ile 228, and Lys 248 residues with an interaction energy of −90.54 kJ/mol (Figure 5D,E), suggesting that SKRIN increases the fibroblast cell proliferation by interfering with the PCNA–p21 protein complex. Molecular docking analysis suggests that atropine and scopolamine interact with the crucial residues of PCNA–p21 protein complex and thereby reduce its expression and increase the fibroblast proliferation.
Figure 5.
Molecular docking of alkaloids present in SKRIN with the p21–PCNA protein complex. (A) Backbone ribbon model showing the p21 and PCNA complex. (B) Two dimensional view of atropine showing Pro 129, Tyr 133, Ile 228, Glu 238, Lys 248, and Tyr 250 are the interacting residues. (C) 3D view of atropine with p21–PCNA complex protein. (D) 2D view of scopolamine showing Pro 129, Glu 130, Gln 131, Tyr 133, Ile 228, and Lys 248 are the interacting residues. (E) 3D view of scopolamine with protein complex. (F) Immunofluorescence showing the expression of PCNA labeled with FITC where horizontal arrows showing the width of wound reduction in control and SKRIN-treated groups. (G) SKRIN increases repairing fibroblast cell proliferation during wound closure by 2.78-fold (p < 0.0001) as compared with H2O2-treated cells.
2.8. SKRIN Affects the PCNA–p21 Expression
To observe the expression of PCNA and p21 expression in repairing fibroblast cells, we determined the expression of PCNA and p21 protein expression in the repairing fibroblast cells during the wound closure phenomenon using immunofluorescence (Figure 5F). We have observed a higher expression of PCNA in control cells which was marked by increased FITC expression and cell proliferation in the wound closure phenomenon. Our data suggests that SKRIN increases the fibroblast cell proliferation in H2O2-stimulated cells during wound closure which was observed by increased expression of PCNA-FITC expression by 2.78-fold (p < 0.0001) as compared with H2O2 treated cells (Figure 5G). Similarly we monitored the expression of p21 protein expression in repairing fibroblast cells by immunofluorescence (Figure 6A). Our data suggests that H2O2-stimulated cells showed decreased levels of p21 expression and cell proliferation which was obvious in apoptotic cells. However, we found that SKRIN treatment increases fibroblast cell proliferation in H2O2-stimulated cells during wound closure. As observed via Western blot, we found that SKRIN reduces p21 expression as compared with that in the control in order to increase the cell proliferation in repairing fibroblast cells by 6.71-fold (p < 0.0001) which was observed by decreased expression of p21-FITC expression (Figure 6B).
Figure 6.
Relative mRNA expression and mechanism. (A) Immunofluorescence showing the expression of p21 labeled with FITC where horizontal arrows showing the width of wound reduction in control and SKRIN-treated groups (B) SKRIN reduce the p21 expression in repairing fibroblast cells by 6.71-fold (p < 0.0001). (C) Gel electrophoresis showing the relative gene expression involved in SKRIN-mediated fibroblast cell survival and proliferation. (D) Densitometric analysis of relative mRNA expression showing a 1.31-fold increase (p = 0.0027) in PI3K, a 1.16-fold increase (p = 0.0211) in Akt, and a 1.511-fold increase (p = 0.0006) in NF-κB expression. (E) Gene network of showing the involvement of the crucial pathways during SKRIN-mediated wound healing. The data is expressed as the mean ± standard error of mean (n = 3) where p values are represented as the difference between control and SKRIN-treated cells.
2.9. SKRIN-Mediated Activation of PI3K–Akt–NF-κB Pathway
Considering the crucial role of the PI3K–Akt–NF-κB pathway in wound healing, gene expression analysis was performed in SKRIN-treated fibroblast cells (Figure 6C). We have found that SKRIN treatment activates the Akt pathway that results in SKRIN-mediated fibroblast cell survival via activation of the NF-κB pathway. As compared with the control, SKRIN treatment showed a 1.31-fold increase (p = 0.0027) in PIK3 expression, a 1.16-fold increase (p = 0.0211) in Akt expression, and a 1.51-fold increase (p = 0.0006) in NF-κB expression (Figure 6D). The activation of the PI3K–Akt–NF-κB pathway causes fibroblast cell survival and results in inhibition of p21 that increases the fibroblast cell proliferation via releasing the PCNA. Increased level of SKRIN-mediated proliferation has been shown to be associated with the cancer progression. Interestingly, we have found that atropine and scopolamine both are noncarcinogenic in nature and therefore can be used for wound healing (Table-1). The mechanism of SKRIN-mediated fibroblast cell survival and proliferation has been presented in Figure 7.
Table 1. Prediction of Chemical Carcinogenicitya.
| method | CDK | CDKExt | CDKGraph | KR | KRC | MACCS | Pubchem | average | class |
|---|---|---|---|---|---|---|---|---|---|
| Atropine | |||||||||
| RF | 0.07 | 0.06 | 0.14 | 0.09 | 0.07 | 0.12 | 0.11 | 0.09 | noncarcinogenic |
| SVM | 0.15 | 0.20 | 0.25 | 0.22 | 0.22 | 0.22 | 0.24 | 0.21 | noncarcinogenic |
| XGBoost | 0.07 | 0.19 | 0.47 | 0.45 | 0.34 | 0.63 | 0.76 | 0.41 | noncarcinogenic |
| Scopolamine | |||||||||
| RF | 0.06 | 0.07 | 0.17 | 0.09 | 0.07 | 0.16 | 0.14 | 0.11 | noncarcinogenic |
| SVM | 0.18 | 0.21 | 0.25 | 0.22 | 0.20 | 0.22 | 0.24 | 0.22 | noncarcinogenic |
| XGBoost | 0.07 | 0.19 | 0.47 | 0.45 | 0.29 | 0.63 | 0.76 | 0.41 | noncarcinogenic |
The chemical carcinogenicity was predicted using CarcinoPred-EL.42
Figure 7.
Mechanism of SKRIN-mediated fibroblast cell survival and proliferation. SKRIN treatment increases the fibroblast cell proliferation via targeting PCNA–p21 complex. SKRIN treatment increases the expression of PI3K which activates PDK1. The activation of PDK1 results in the activation of Akt that further activates NF-κB which results in fibroblast cell survival. Activation of Akt also inhibits the p21 expression and thereby increasing the fibroblast cell proliferation via increasing PCNA expression. RTK: receptor tyrosine kinase; PIP2: phosphatidylinositol 4,5-bisphosphate; PIP3: phosphatidylinositol (3,4,5)-trisphosphate; PDK1: phosphoinositide-dependent kinase-1; Akt: protein kinase B; IκBα/β: IkappaB kinase; NF-κB: nuclear factor kappa B; p21Cip/Waf: cyclin-dependent kinase inhibitor 1; PCNA: proliferating cell nuclear antigen.
3. Discussion
During skin or tissue injury, the process of wound healing is a dynamic event that starts with inflammation, proliferation, and cell migration of various types of fibroblast cells. Fibroblasts play a crucial role from late inflammatory phase to epithelial formation during the process of wound healing by producing growth factors, collagens, cytokines, and ECM components. During this process, fibroblast cell survival, migration, and proliferation are the most crucial phenomenon.19 Therefore, the ideal wound healing therapy should focus on fibroblast cell survival and its proliferation. Various growth factors, FGF, PDGF, TGF-β, VEGF, and KGF, have been shown to stimulate fibroblast migration, differentiation, proliferation, and cell survival. However, only PDGF has been approved as a therapy for the treatment of diabetic foot ulcers, whereas TGF-β and FGF failed to exhibit a regenerative capacity in clinical settings, most likely due to their short half-life.14 Therefore, there is a need to explore natural sources for the development of effective wound healing therapeutic molecules.
Atropine has been shown to exhibit wound contraction properties,20 and scopolamine exhibits antimicrobial activity against a range of pathogens.21 For the first time, we have found that SKRIN treatment increases fibroblast cell survival. To confirm this, the fibroblast cells were stimulated with H2O2, which acts as a positive inducer of fibroblast cell death. In addition, treatment with H2O2 on fibroblast cells has been shown to reduce cell proliferation, G0/G1 cell cycle arrest, and apoptosis.22 We have found that SKRIN treatment increases the live cell population and decreases the apoptotic cell population in H2O2 treated cells. Consequently, the cell scratch assay was carried out to observe the effect of SKRIN on wound healing. Development of a wound healing therapy targeting fibroblast cell proliferation is crucial to achieve.23,24
Duplication of DNA is a key step in cell division and proliferation which is controlled at different stages of cell cycle. Therefore, to monitor the role of SKRIN on fibroblast cell proliferation, we analyzed cell cycle progression in repairing cells during wound closure. Our analysis suggests that SKRIN treatment shifted the cell cycle of the repairing fibroblast from the G0/G1 phase to the G2/M phase, suggesting increased proliferation capacity of SKRIN-treated cells. To confirm fibroblast cell proliferation, we further observed the expression of one of the major players of cellular proliferation is CDK inhibitor 1, p21, which is a critical regulator of cellular differentiation, proliferation and senescence. SKRIN treatment showed significant reduction in p21 protein expression, suggesting that p21 inhibition bypasses cell cycle arrest and increases fibroblast cell proliferation. In addition, the ability of p21 protein to interact with PCNA makes it distinct from other CDK inhibitors. The binding of p21 with PCNA causes G1 and G2 cell cycle arrest and thereby reduces the cell cycle progression and proliferation. We have found that SKRIN treatment significantly increases the PCNA expression that increases the fibroblast cell proliferation. Gene network analysis revealed the involvement of the crucial pathways during SKRIN-mediated wound healing involving positive regulation of the cell cycle and regulation of the G1/S cell cycle phase transition (Figure 6E).
Furthermore, SKRIN-mediated cell proliferation was confirmed by BrdU incorporation assay which showed increased repairing fibroblast cell proliferation. We have found the increased expression of PCNA in repairing fibroblast cells which has been shown to be overexpressed during wound healing activity.25 Moreover, inhibition of p21 expression has been shown to ameliorate delayed wound healing in aged mice. Therefore, targeting p21 may act as potential clinical avenues to promote wound healing.10 Moreover; we found that H2O2-stimulated cells undergo apoptosis and thereby showed low levels of p21 expression and cell proliferation. Interestingly, our data suggests that SKRIN reduces p21 expression compared with that in the control cells and increase its expression as compared with that in the H2O2-stimulated cells. This data clearly indicates that SKRIN increases the repairing fibroblast cell proliferation during wound closure.
To confirm the cell proliferation effect of SKRIN, we further analyzed the molecular interactions of atropine and scopolamine with PCNA–p21 complex. The binding of p21 protein with PCNA is crucial and therefore targeted using molecular docking. Tyr 133 is a highly conserved residue which involves in the interaction of PCNA–p21 complex.27 Our molecular docking data suggests that atropine and scopolamine both interact with Tyr 133 residues and thereby interfere with PCNA–p21 protein interaction. Our data suggests that SKRIN interacts with the crucial residues of PCNA–p21 protein complex and thereby reduces its expression and increases the fibroblast proliferation.
To investigate the underlying mechanism of action, the expression of NF-κB was investigated as related to fibroblast cell survival. We have found that SKRIN treatment activates the NF-κB pathway and resulted in fibroblast cell survival. The activation of NF-κB pathway has been shown as critical signaling pathways during the process of wound healing mediated by silk fibroin. In addition, the extracts of Periplaneta americana have been shown to exhibit skin wound healing via activation of NF-κB pathway.28 Furthermore, we investigated PI3K/Akt pathways which involve fibroblast migration and proliferation. Our data suggests that SKRIN treatment activates the PI3K/Akt pathway that resulted in inhibition of p21 protein and increases the fibroblast cell proliferation during the wound closure process. Activation of PI3K/Akt pathway has been reported during Calendula officinalis tincture mediated wound healing by inducing fibroblast migration and proliferation.29 Similarly, periplocin has been shown to exhibit wound healing properties via activation of PI3K/Akt pathways.30 These results demonstrate that SKRIN increases the wound healing capacity of fibroblast cells via activation of the PI3K/Akt pathway, which results in fibroblast cell survival with increased level of proliferation. Increased level of SKRIN-mediated proliferation has been shown to be associated with the cancer progression. Interestingly, we have found that atropine and scopolamine both are noncarcinogenic in nature and therefore can be used for the wound healing.
4. Conclusions
Collectively, for the first time our data suggests that SKRIN treatment exhibits a wound healing property by significantly increasing the fibroblast cell survival and proliferation via activation of PI3K–Akt–NF-κB pathway probably mediated by interfering with the PCNA–p21 complex interaction. This study provides various scopes for the development of novel wound healing therapy. However, more precise study with various wound healing models is in progress to further explore its role in medicine.
5. Materials and Methods
5.1. Preparation of SKRIN
SKRIN was prepared from a stock of belladonna tincture. Belladonna tincture (Atropa belladonna extract in ethanol, was prepared as per the Pharmacopoeia of India, 1971). Whole plant extract of 1 g was dissolved in 10 mL of 47% ethanol to prepare the hydroalcoholic stock solution. To achieve desired concentrations of SKRIN, the stock was serially diluted in the same percentage of ethanol with high-pressure homogenization.
5.2. Gas Chromatograph–Triple Quadrupole Mass Spectrometer (GC-MS/MS)
SKRIN was analyzed by GC-MS/MS as mentioned elsewhere.31 Briefly, SKRIN was derivatized using BSTFA+TMCS (99:1) (N,O-bis (trimethylsilyl)trifluoro acetamide, trimethylchlorosilane) and analyzed by GC-MS/MS [Thermo Fisher 1300 GC coupled with TSQ 8000 Mass spectrometer] with a column TraceGOLD TG-5MS GC Columns (Thermo Fisher Scientific). Helium was used as a carrier gas with the flow rate of 1 mL/min. The MS transfer line temperature was set at 290 °C, and the source temperature was set at 250 °C. The ramping temperature program set from 70 °C for 1 min of hold time, and the temperature was increased at the rate of 15 °C/min until 280 °C with the total run time of 24 min. TSQ 8000 Triple Quadrupole MS detector was used for data analysis and total ion count (TIC) was used for evaluation of data for compound identification. The NIST library was used for comparison of mass spectra of the components.
5.3. DLS Particle Size Distribution Analysis
Size determination of the particles in the samples was performed through DLS. DLS measures the light scattering caused by the Brownian motion of the particles. The samples were vortexed to provide a homogeneous solution and transferred to a 1.5 mL quartz cuvette for analysis using the HORIBA nanoparticle analyzer SZ-100. The instrument was set to record 3 measurements with a total of 10 scans each. The distribution curve was set to be polydisperse with broad and standard distribution range.
5.4. TEM
To detect the size and morphology of the molecules/nanoparticles present in SKRIN, TEM analysis was performed as performed previously.32 TEM uses electromagnetic lenses to focus electrons into a very thin beam which is transmitted through an ultrathin specimen, interacting with it as the electron beam passes through. The samples were dropped onto the carbon-coated TEM grids, vacuum-dried, and then observed under a JEOL 3010 TEM at 300 kV.
5.5. AFM
Surface imaging of the particles in SKRIN was performed through AFM. First, 10 μL of SKRIN was dropped onto a clean glass slide and allowed to evaporate in a clean and sterile environment. The measurements of the sample were made using a commercial AFM (Hydra SPM MV4000) attached to an inverted optical microscope. Data analysis was performed in WSXM 5.0 Develop 1.1 software.
5.6. Fibroblast Cell Cultures
Fibroblast cells (BHK-21) were cultured and maintained in 1× DMEM medium supplemented with 10% (v/v) fetal bovine serum (FBS), 2 g/L sodium bicarbonate, 4.5 g/L glucose, and antibiotics (60 μg/mL penicillin G sodium, 50 μg/mL streptomycin sulfate) at 37 °C in 5% CO2.
5.7. Fibroblast Cell Survival Assay, CC50, and EC50
Fibroblast cells were seeded in 96-well Corning cell culture plates at the density of 0.1 × 105 cells per well. To determine the cell survival property of SKRIN, cells were treated with a range of concentration (1–100 μg/mL) to measure the median cytotoxic concentration (CC50) at 24 h post-treatment. Consequently, cells were stimulated with 1 mM of H2O2 and incubated for 24 h. Following incubation, cells were treated with below CC50 concentration of SKRIN to measure the median effective concentration (EC50) for cell survival after 24 h of incubation. The EC50 concentration was used to measure the fibroblast cell survival property of SKRIN at 24, 48, and 72 h post-treatment. Cell survival assay was performed by adding 5 mg/mL solution of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) (Duchefa Biochemie, Amsterdam).310 Following the addition of MTT solution, plates were incubated for 4 h to appear the purple formazan crystals which were dissolved by 100 μL of dimethyl sulfoxide (DMSO).33 The plate was wrapped completely with aluminum foil and incubated for 12 h. After incubation, the absorbance was measured at 570 nm using SpectraMax M5e (Molecular Devices) where DMSO was used as a blank. Cell survival was determined using the following formula, and the fold change was calculated accordingly.
where A stands for absorbance, and At, Ab, and Ac stands for Atreatment, Ablank and Acontrol, respectively.
The median effective concentration (EC50) was further used to evaluate the SKRIN-mediated cell survival at 24, 48, and 72 h in both cell types. CC50 and EC50 of the SKRIN were measured by nonlinear regression analysis using GraphPad Prism, a powerful and an efficient biostatistics application which is useful for the biologists to analyze and evaluate large data sets.
5.8. Apoptosis Assay
To perform the apoptosis assay, cells were cultured in 6-well cell culture plates with the density of 0.3 × 106 per well. To induce apoptosis, cells were stimulated with 1 mM H2O2 (Sigma) and incubated for 24 h.34 Cells were treated with EC50 of SKRIN and incubated for 48 h. Before acquisition, cells were trypsinized and washed with 1X PBS followed by washing with 1X binding buffer. Annexin V-FITC and PI staining (Sc-4252-Santa Cruz Biotechnology, Inc.) of the cells were performed according to the manufacturer’s instructions. We counted 10 000 cells for each sample by Amnis ImagestreamX Imaging flow cytometry, and analysis of apoptosis was performed by IDEAS software.35
5.9. Wound Healing Assay
To observe the wound healing effect of SKRIN, cell migration studies were performed in fibroblast cells. Cells were seeded in 6-well cell culture plates with the density of 0.3 × 106 cells per well. The monolayer of the cells were manually scratched with a 200 μL pipet tip by gliding the tip across the monolayer of cells in straight line direction36 and were treated with EC50 of SKRIN. An inverted microscope (Nikon, Japan) equipped with a digital camera was used to capture the images at 10× magnification at different time points. SKRIN-mediated wound closure was monitored and expressed as the percentage of wound area.
5.10. Cell Cycle Analysis
Cells were cultured in 6-well cell culture plates with the density of 0.3 × 106 per well. A cell scratch was carried out, and the cells were treated with EC50 of SKRIN. After 48 h of incubation, cells were trypsinized, washed with 1× PBS, and fixed with absolute ethanol. For cell cycle analysis in repairing fibroblast monolayers, fixed cells were treated with PI (Sigma) according to the manufacturer’s instructions.37 We have acquired 10 000 cells for each sample via Amnis ImagestreamX Imaging flow cytometry, and cell cycle analysis was performed to measure the percentage cell cycle distribution in each phase (sub-G1, G0/G1, S, and G2/M) of the cell cycle by IDEAS software.
5.11. Bromodeoxyuridine Cell Proliferation Assay
Cells were cultured in 6-well plate with the density of 0.3 × 106 per well. Cell scratch was performed, and the cells were treated with EC50 of SKRIN and stimulated with 1 mM of H2O2 as the negative control. Immediately, medium containing 10 μM bromodeoxyuridine (BrdU, Sigma), low glucose, and 2% FBS was added for 1 h.38 To monitor the incorporation of BrdU in repairing fibroblast or proliferating cells during the wound closure, cells were trypsinized, washed, and stained with anti-BrdU FITC-labeled antibody (BioLegend, USA). A total of 50 000 events were acquired using BD-FACS Aria III flow cytometer, and the data was analyzed using Flowing software 2.39
5.12. Western Blotting
Fibroblast cells were cultured and treated with SKRIN as mentioned earlier. NP-40 buffer was used to prepare the whole cell lysate. Proteins were separated on a 10% SDS-PAGE separating gel. Separated proteins were transferred to nitrocellulose blotting membrane (Amersham Hybond ECL Nitrocellulose Membrane) using Trans-Blot Turbo (BIO-RAD) at 25 V for 7 min. Bovine serum albumin of 5% was used to block the membrane for 1 h at 4 °C. Monoclonal anti-p21WAF1/Cip1 antibody (1:2000) was used to determine p21 expression. Similarly, a monoclonal anti-PCNA antibody was used to determine the PCNA expression. Sheep anti-mouse IgG-HRP-conjugated secondary antibody (RPN4201-GE Healthcare) was used to see the expression of p21 and PCNA using Pierce ECL Western Blotting substrate (Thermo Scientific). GAPDH antibody (SC-365062-Santa Cruz Biotechnology, Inc.) was used as the control.
5.13. Immunofluorescence of Repairing Fibroblast Cells
To observe the expression of PCNA and p21 protein in repairing fibroblast cells during SKRIN-mediated wound closure, we cultured the cells in a 4-well Lab-Tek Chamber Slide with the density of 0.015 × 106 cells per well. Upon formation of monolayers of fibroblast cells, a cell scratch was performed, and cells were stimulated with 1 mM of H2O2 as the negative control for 12 h. After incubation, cells were treated with EC50 of SKRN and incubated further for 12 h. After incubation, cells were fixed with 100% chilled methanol and stained with anti-PCNA antibody (Sigma, USA) for 2 h. After incubation, cells were washed with PBS and stained with secondary antibody labeled with FITC. Similarly, another Lab-Tek Chamber Slide was grown, stimulated with 1 mM of H2O2, and treated EC50 of SKRN. Cells were fixed and stained with anti-p21 antibody (Sigma, USA) for 2 h. After incubation, cells were washed with PBS and stained with secondary antibody labeled with FITC. Fluorescence microscopy was performed using Nikon upright fluorescence microscope. The intensity of fluorescence signals was determined using fluorescence in ImageJ (Fiji) software.
5.14. Molecular Docking
The Protein Data Bank (PDB) structure of human PCNA complex with p21 protein (PDB ID: 5E0U) was retrieved from Research Collaboratory for Structural Bioinformatics PDB protein data bank.40 The 3D structures of atropine (CID: 174174) and scopolamine (CID: 3000322) were downloaded in from PubChem (https://pubchem.ncbi.nlm.nih.gov/) in structure-data file (SDF) format. PubChem provides free access for chemicals and molecules with information about their physicochemical properties, safety, toxicity and biological activities. Molecular docking of atropine and scopolamine with PCNA–p21 complex was performed using a protein–ligand docking simulation program, Molegro Virtual Docker (MVD), which performs docking simulations and generated MolDock scores in a completely integrated computational package.41 The MolDock score is an extension of the piecewise linear potential (PLP) which includes hydrogen bonding and electrostatic terms. Both 2D and 3D molecular interactions were visualized by Discovery Studio 2016 client which provides a stereo graphics options with inbuilt depth cueing, shading, and blur capabilities that help user to identify the molecular interaction effectively in docking studies.
5.15. Gene Expression by Real-Time PCR
To decipher the mechanism of SKRIN-mediated fibroblast cell survival and proliferation, gene expression analysis was performed by real-time PCR (RT-PCR). Cells were treated as mentioned above and total RNA was isolated using TRIzol. Approximately 200 ng of isolated RNA was used to perform the cDNA synthesis using Reverse Transcription System (Promega). PCR was performed for each gene where GAPDH was used as the control. The amplified products were run on 0.8% agarose gel. Gene network involve in SKRIN-mediated wound healing was performed using Metascape server.
5.16. Prediction of Chemical Carcinogenicity
Due to the associated risk of cell proliferation in various types of cancer progression, we determine the carcinogenicity of atropine and scopolamine for safe use for the management of wound healing using CarcinoPred-EL.42 This software is based on the three novel ensemble classification models and was developed to predict carcinogenicity of chemicals using seven types of machine learning methods and molecular fingerprints.
5.17. Statistical Analysis
The statistical difference among more than two independent groups was performed by one-way analysis of variance. Experiments were carried out in triplicate, and the values are represented as the mean ± standard error of the mean. Significance levels between appropriate control and SKRIN-treated cells was denoted by ***, **, and * for p < 0.001, p < 0.01, and p < 0.05, respectively.
Acknowledgments
The authors are grateful to the Vice Chancellor, King George’s Medical University (KGMU), Lucknow, India for the encouragement for this work.
Glossary
Abbreviations
- PDGF
platelet-derived growth factor
- TNF-α
tumor necrosis factor-alpha
- IL-1β
interleukin-1 beta
- FGF
fibroblast growth factor
- TGF-β
transforming growth factor-β
- VEGF
vascular endothelial growth factor
- KGF
keratinocyte growth factor
- MMPs
matrix metalloproteinases
- GAGs
glycosaminoglycans
- ECM
extracellular matrix
- p21
p21Waf1/Cip1
- PCNA
proliferating cell nuclear antigen
- NF-κB
nuclear factor kappa B
- PKB/Akt
phosphoinositide 3-kinases (PI3K)–protein kinase B
- CAM
complementary and alternative medicine
- SKRIN
belladonna formulation
- GC-MS/MS
gas chromatography–mass spectrometry
- DLS
dynamic light scattering
- TEM
transmission electron microscopy
- AFM
atomic force microscopy
- CC50
median cytotoxic concentration
- EC50
median effective concentration
- FITC
fluorescein isothiocyanate
- PI
propidium iodide
- CDK
cyclin dependent kinase
- H2O2
hydrogen peroxide
- BrdU
bromodeoxyuridine
Author Contributions
¶ S.K.S., S.K., and V.K.M. contributed equally as first authors. S.K.S. conceived the idea. S.K. and S.K.S. collected the data, devised the initial draft, and reviewed the final draft. S.K.S., S.K., V.K.M., S.V.C., R.K., K.S., D.N., A.K., R.K.M., S.G., and V.K. finalized the draft for submission. All authors read and approved the final version of the manuscript.
We have not received any specific funding for this work. S.K.S. is supported by CCRH, Government of India.
The authors declare no competing financial interest.
References
- Blair M. J.; Jones J. D.; Woessner A. E.; Quinn K. P. (2020) Skin structure-function relationships and the wound healing response to intrinsic aging. Adv. Wound Care (New Rochelle). 9, 127–143. 10.1089/wound.2019.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge P. (2013) Wound healing and the role of fibroblasts. J. Wound Care. 22, 407–412. 10.12968/jowc.2013.22.8.407. [DOI] [PubMed] [Google Scholar]
- Serra M. B.; Barroso W. A.; da Silva N. N.; Silva S.; Borges A.; Abreu I. C.; Borges M. (2017) From inflammation to current and alternative therapies involved in wound healing. Int. J. Inflammation 2017, 3406215. 10.1155/2017/3406215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behm B.; Babilas P.; Landthaler M.; Schreml S. (2012) Cytokines, chemokines and growth factors in wound healing. J. Eur. Acad. Dermatol. Venereol. 26, 812–820. 10.1111/j.1468-3083.2011.04415.x. [DOI] [PubMed] [Google Scholar]
- Nardini M.; Perteghella S.; Mastracci L.; Grillo F.; Marrubini G.; Bari E.; Formica M.; Gentili C.; Cancedda R.; Torre M. L.; Mastrogiacomo M. (2020) Growth factors delivery system for skin regeneration: an advanced wound dressing. Pharmaceutics 12, 120. 10.3390/pharmaceutics12020120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland J.; Denyer M.; Britland S. (2005) Contact guidance in human dermal fibroblasts is modulated by population pressure. J. Anat. 206, 581–587. 10.1111/j.1469-7580.2005.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatak S.; Maytin E. V.; Mack J. A.; Hascall V. C.; Atanelishvili I.; Moreno Rodriguez R.; Markwald R. R.; Misra S. (2015) Roles of proteoglycans and glycosaminoglycans in wound healing and fibrosis. Int. J. Cell Biol. 2015, 834893. 10.1155/2015/834893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B.; Wang J. H. (2011) Fibroblasts and myofibroblasts in wound healing: force generation and measurement. J. Tissue Viability. 20, 108–120. 10.1016/j.jtv.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton J. E.; Unger J. G.; Rohrich R. J. (2013) The role of wound healing and its everyday application in plastic surgery: a practical perspective and systematic review. Plast. Reconstr. Surg. Glob. Open. 1, e10–e19. 10.1097/GOX.0b013e31828ff9f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D.; de Vries J. C.; Muschhammer J.; Schatz S.; Ye H.; Hein T.; Fidan M.; Romanov V. S.; Rinkevich Y.; Scharffetter-Kochanek K. (2020) Local and transient inhibition of p21 expression ameliorates age-related delayed wound healing. Wound Repair Regen. 28, 49–60. 10.1111/wrr.12763. [DOI] [PubMed] [Google Scholar]
- Mansilla S. F.; de la Vega M. B.; Calzetta N. L.; Siri S. O.; Gottifredi V. (2020) CDK-independent and PCNA-dependent functions of p21 in DNA replication. Genes 11, 593. 10.3390/genes11060593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y. R.; Sultan M. T.; Park H. J.; Lee J. M.; Ju H. W.; Lee O. J.; Lee D. J.; Kaplan D. L.; Park C. H. (2018) NF-κB signaling is key in the wound healing processes of silk fibroin. Acta Biomater. 67, 183–195. 10.1016/j.actbio.2017.12.006. [DOI] [PubMed] [Google Scholar]
- Li G.; Li Y. Y.; Sun J. E.; Lin W. H.; Zhou R. X. (2016) ILK-PI3K/AKT pathway participates in cutaneous wound contraction by regulating fibroblast migration and differentiation to myofibroblast. Lab. Invest. 96, 741–751. 10.1038/labinvest.2016.48. [DOI] [PubMed] [Google Scholar]
- Park J. W.; Hwang S. R.; Yoon I. S. (2017) Advanced growth factor delivery systems in wound management and skin regeneration. Molecules 22, 1259. 10.3390/molecules22081259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira R. F.; Bártolo P. J. (2016) Traditional Therapies for Skin Wound Healing. Adv. Wound Care (New Rochelle) 5, 208–229. 10.1089/wound.2013.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurya V. K.; Kumar S.; Kabir R.; Shrivastava G.; Shanker K.; Nayak D.; Khurana A.; Manchanda R. K.; Gadugu S.; Kar S. K.; Verma A. K.; Saxena S. K. (2020) Dark classics in chemical neuroscience: an evidence-based systematic review of belladonna. ACS Chem. Neurosci. 11, 3937. 10.1021/acschemneuro.0c00413. [DOI] [PubMed] [Google Scholar]
- Kumar S.; Maurya V. K.; Kabir R.; Nayak D.; Khurana A.; Manchanda R. K.; Gadugu S.; Shanker K.; Saxena S. K. (2020) Antiviral activity of belladonna during Japanese encephalitis virus infection via inhibition of microglia activation and inflammation leading to neuronal cell survival. ACS Chem. Neurosci. 11, 3683–3696. 10.1021/acschemneuro.0c00603. [DOI] [PubMed] [Google Scholar]
- Gál P.; Toporcer T.; Grendel T.; Vidová Z.; Smetana K. Jr; Dvoránková B.; Gál T.; Mozes S.; Lenhardt L.; Longauer F.; Sabol M.; Sabo J.; Backor M. (2009) Effect of Atropa belladonna L. on skin wound healing: biomechanical and histological study in rats and in vitro study in keratinocytes, 3T3 fibroblasts, and human umbilical vein endothelial cells. Wound Repair Regen. 17, 378–386. 10.1111/j.1524-475X.2009.00475.x. [DOI] [PubMed] [Google Scholar]
- Addis R.; Cruciani S.; Santaniello S.; Bellu E.; Sarais G.; Ventura C.; Maioli M.; Pintore G. (2020) Fibroblast proliferation and migration in wound healing by phytochemicals: evidence for a novel synergic outcome. Int. J. Med. Sci. 17, 1030–1042. 10.7150/ijms.43986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P.; Jagetia G. C. (2000) Effect of topical atropine on wound contraction in Swiss albino mice: A preliminary report. Indian J. Plast Surg. 33, 86–89. [Google Scholar]
- Ozçelik B.; Kartal M.; Orhan I. (2011) Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 49, 396–402. 10.3109/13880209.2010.519390. [DOI] [PubMed] [Google Scholar]
- Park W. H. (2013) H2O2 inhibits the growth of human pulmonary fibroblast cells by inducing cell death, GSH depletion and G1 phase arrest. Mol. Med. Rep. 7, 1235–1240. 10.3892/mmr.2013.1303. [DOI] [PubMed] [Google Scholar]
- Yeh C. J.; Chen C. C.; Leu Y. L.; Lin M. W.; Chiu M. M.; Wang S. H. (2017) The effects of artocarpin on wound healing: in vitro and in vivo studies. Sci. Rep. 7, 15599. 10.1038/s41598-017-15876-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M.; Gutknecht D.; Simon J. C.; Schulz J. N.; Eckes B.; Anderegg U.; Saalbach A. (2015) Controlling the balance of fibroblast proliferation and differentiation: impact of Thy-1. J. Invest. Dermatol. 135, 1893–1902. 10.1038/jid.2015.86. [DOI] [PubMed] [Google Scholar]
- Fu X.; Fang L.; Li H.; Li X.; Cheng B.; Sheng Z. (2007) Adipose tissue extract enhances skin wound healing. Wound Repair Regen. 15, 540–548. 10.1111/j.1524-475X.2007.00262.x. [DOI] [PubMed] [Google Scholar]
- Duffy C. M.; Hilbert B. J.; Kelch B. A. (2016) A Disease-Causing Variant in PCNA Disrupts a Promiscuous Protein Binding Site. J. Mol. Biol. 428, 1023–1040. 10.1016/j.jmb.2015.11.029. [DOI] [PubMed] [Google Scholar]
- Song Q.; Gou Q.; Xie Y.; Zhang Z.; Fu C. (2017) Periplaneta americana extracts promote skin wound healing via nuclear factor kappa B canonical pathway and extracellular signal-regulated kinase signaling. Evid. Based Complement Alternat. Med. 2017, 5821706. 10.1155/2017/5821706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinda M.; Dasgupta U.; Singh N.; Bhattacharyya D.; Karmakar P. (2015) PI3K-mediated proliferation of fibroblasts by Calendula officinalis tincture: implication in wound healing. Phytother. Res. 29, 607–616. 10.1002/ptr.5293. [DOI] [PubMed] [Google Scholar]
- Chen L.; Jiang P.; Li J.; Xie Z.; Xu Y.; Qu W.; Feng F.; Liu W. (2019) Periplocin promotes wound healing through the activation of Src/ERK and PI3K/Akt pathways mediated by Na/K-ATPase. Phytomedicine 57, 72–83. 10.1016/j.phymed.2018.12.015. [DOI] [PubMed] [Google Scholar]
- Hong P., and Pat McConville P. (2013) Conversion of a USP gas chromatography method to convergence chromatography: analysis of Atropa belladonna, Waters Corporation, Milford, MA.
- Kumar S.; Maurya V. K.; Nayak D.; Khurana A.; Manchanda R. K.; Gadugu S.; Bhatt M.; Saxena S. K. (2020) Calcarea carbonica treatment rescues lipopolysaccharide-induced inflammatory response in human mononuclear cells via downregulation of inducible cyclooxygenase pathway. J. Integr. Med. 18, 441–449. 10.1016/j.joim.2020.06.001. [DOI] [PubMed] [Google Scholar]
- van Meerloo J.; Kaspers G. J. L.; Cloos J. (2011) Cell sensitivity assays: the MTT assay. Methods Mol. Biol. 731, 237–245. 10.1007/978-1-61779-080-5_20. [DOI] [PubMed] [Google Scholar]
- Kumar P.; Nagarajan A.; Uchil P. D. (2018) Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, pdb.prot095505. 10.1101/pdb.prot095505. [DOI] [PubMed] [Google Scholar]
- Burdon R. H.; Gill V.; Alliangana D. (1996) Hydrogen peroxide in relation to proliferation and apoptosis in BHK-21 hamster fibroblasts. Free Radical Res. 24, 81–93. 10.3109/10715769609088004. [DOI] [PubMed] [Google Scholar]
- Czop M.; Bogucka-Kocka A.; Kubrak T.; Knap-Czop K.; Makuch-Kocka A.; Galkowski D.; Wawer J.; Kocki T.; Kocki J. (2019) Imaging flow cytometric analysis of stilbene-dependent apoptosis in drug resistant human leukemic cell lines. Molecules 24, 1896. 10.3390/molecules24101896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bopage N. S.; Kamal Bandara Gunaherath G. M.; Jayawardena K. H.; Wijeyaratne S. C.; Abeysekera A. M.; Somaratne S. (2018) Dual function of active constituents from bark of Ficus racemosa L in wound healing. BMC Complementary Altern. Med. 18, 29. 10.1186/s12906-018-2089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamchun S.; Thongboonkerd V. (2018) Cell cycle shift from G0/G1 to S and G2/M phases is responsible for increased adhesion of calcium oxalate crystals on repairing renal tubular cells at injured site. Cell Death Discovery 4, 106. 10.1038/s41420-018-0123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielas S. L.; Silhavy J. L.; Brancati F.; Kisseleva M. V.; Al-Gazali L.; Sztriha L.; Bayoumi R. A.; Zaki M. S.; Abdel-Aleem A.; Rosti R. O.; et al. (2009) Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat. Genet. 41, 1032–1036. 10.1038/ng.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahraneshin Samani F.; Moore J. K.; Khosravani P.; Ebrahimi M. (2014) Features of free software packages in flow cytometry: a comparison between four non-commercial software sources. Cytotechnology 66, 555–559. 10.1007/s10616-013-9609-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy C. M.; Hilbert B. J.; Kelch B. A. (2016) A disease-causing variant in PCNA disrupts a promiscuous protein binding site. J. Mol. Biol. 428, 1023–1040. 10.1016/j.jmb.2015.11.029. [DOI] [PubMed] [Google Scholar]
- Thomsen R.; Christensen M. H. (2006) MolDock: a new technique for high-accuracy molecular docking. J. Med. Chem. 49, 3315–3321. 10.1021/jm051197e. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Ai H.; Chen W.; Yin Z.; Hu H.; Zhu J.; Zhao J.; Zhao Q.; Liu H. (2017) CarcinoPred-EL: Novel models for predicting the carcinogenicity of chemicals using molecular fingerprints and ensemble learning methods. Sci. Rep. 7, 2118. 10.1038/s41598-017-02365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]