Abstract

Contemporary literature documents extensive research on common causative mechanisms, pathogenic pathways and dual effective remedies for Alzheimer’s disease (AD) and Type 2 diabetes mellitus (T2DM). Tolbutamide (TBM), chlorpropamide (CPM), and glyburide (GLY) are three sulfonylurea antidiabetic drugs of different generations. All these drugs were found to exhibit moderate to strong inhibitory efficiency on the neurotransmitter degrading enzyme acetylcholinesterase (AChE) with GLY (IC50 = 0.74 ± 0.02 μM) being the most potent, followed by CPM (IC50 = 5.72 ± 0.24 μM) and TBM (IC50 = 28.9 ± 1.60 μM). Notably, the inhibition efficiency of GLY is even comparable with the FDA approved AD drug, donepezil (DON). The larger size of GLY spans almost the full gorge of AChE ranging from catalytic active site (CAS) to the peripheral active site (PAS) with relatively strong binding affinity (6.0 × 105 M–1) and acts as a competitive inhibitor for AChE. On the other hand, while they show relatively weak binding ((2–6) × 104 M–1), both CPM and TBM act as noncompetitive binders. While these two drugs can bind to PAS, MD simulation results predict an alternative noncompetitive inhibition mechanism for CPM. These results open the possibility of repurposing the antidiabetic drugs, particularly GLY, in the treatment of AD. The consequential side effect of excess acetylcholine production, due to the administration of these drugs to AD-unaffected patients, can be rectified by using colloidal gold and silver nanofluids as potential AChE activity boosters.
Keywords: sulfonylurea drugs, acetylcholinesterase, Alzheimer’s disease, dual therapy, MD simulation, nanofluid
Introduction
The cholinergic hypothesis of Alzheimer’s disease (AD) considers that the deficiency of the neurotransmitter acetylcholine (ACh) is responsible for cognition impairment and places importance on the vital role of the enzyme acetylcholinesterase (AChE) in the process.1,2 AChE hydrolyzes ACh into acetate and choline, which triggers the termination of neurotransmission in brain synapses.3 In normal conditions, this breakdown is necessary to impede overstimulation by ACh that would otherwise give rise to attention deficit hyperactivity disorder (ADHD) causing symptoms such as anxiety, delirium, depression, convulsions, nausea, vomiting, diarrhea, etc.(4) However, a lowered ACh level is believed to be the primary cause for the malfunction of cognizance pattern in the case of AD.5 Many of the AD drugs enhance the cholinergic action by suppressing the activity of AChE and therefore, the availability of ACh increases in the synapses of the forebrain regions.6 The structural features of AChE, which has an extraordinarily high catalytic efficiency, comprise a catalytic active site (CAS) situated near the bottom and a peripheral active site (PAS) located near the entrance of a narrow but deep (∼20 Å) gorge.7 There are a multitude of AChE inhibitors developed with the potential to bind at the CAS, the PAS, or both. However, these drugs provide only symptomatic relief to a patient and can slow down the progression of AD only up to certain extent, but they fall short of resolving the multifarious etiology of AD entirely.8,9
Like AD, diabetes mellitus is also a very widespread malaise and both of them manifest prominently with the onset of aging process.10,11 Extensive epidemiological studies have provided definitive evidence for a major linkage between Type 2 diabetes mellitus (T2DM) and AD. Apart from common risk factors such as oxidative stress, dysmetabolism, higher cholesterol level, cardiovascular disease, and degeneration and deposition of amyloid-β (Aβ), several common pathological processes are also found both in AD and T2DM.12 Extensive research on T2DM has further unraveled the pivotal role of insulin in brain damage and demonstrated that the primary brain insulin resistance as well as insulin deficiency mediate cognitive impairment and AD.13,14 It is pointed out that insulin participates modestly in neurological function by stimulating the expression of choline acetyltransferase, the enzyme responsible for acetylcholine synthesis.15,16 Suboptimal insulin levels as well as poor insulin receptor sensitivity, which are the primary causes of T2DM, can therefore contribute to a decrease in acetylcholine, further delineating a possible biochemical link between diabetes and AD.17 Also in one of the earlier reports, the role for glycated end products, which is quite common for T2DM, has been hypothesized in the pathogenesis of AD.18 The connection between the two diseases has been found to be so profound that AD is even referred to as Type 3 diabetes.19,20 However, in spite of nearly two decades of vigorous research, the attempt to interlink AD and T2DM under a single primary molecular pathogenic mechanism is still elusive. Though well-documented evidence of the link has resulted in various debated theories, it has led to an intensive search of drugs that could act as dual therapeutic agents for both of these diseases.
A recent study shows the effect of insulin on memory deficit and AChE activity in brain areas of scopolamine-induced amnesic mice.21 The results of the study corroborated that the antiamnesic effect of insulin may be facilitated through enhancement of cholinergic activity. Many antidiabetic drugs have been tested for their effect on cholinesterase activity. For example, metformin was found to moderately inhibit the activity of AChE in a mixed-type inhibition with an IC50 value of 2.35 mM.22 Another study also demonstrated metformin to lower AChE activity in a human-based trial.23 Another sulfonamide prodrug containing an eight-carbon alkyl chain presented inhibitory potency against AChE with IC50 = 890 nM.22 Recent computational studies have also shown that the antidiabetic drug, invokana (canagliflozin) acts as a dual inhibitor of AChE and sodium glucose cotransporter 2 with an estimated inhibition constant (Ki) of 128.59 nM against AChE.24 Affirmative effects are also observed in other antidiabetic drugs of gliflozin class. A report signifying the interaction of AChE with ertugliflozin reveals Ki value to be 31.9 μM, while sotagliflozin displayed a more efficient interaction with Ki value of 5.6 μM.25 On the other hand, dapagliflozin is found to bind with AChE with an estimated inhibition constant of 25.02 μM.26
Sulfonylureas (SU) are a class of antidiabetic drugs that are frequently used in the management of T2DM. The drugs act through an elevated release of insulin from the pancreatic β-cells.27 Out of several SU antidiabetic drugs available, tolbutamide (TBM) and chlorpropamide (CPM) are the “first-generation” drugs and have been commercially available for many years. On the other hand, glyburide (GLY) is developed more recently and belongs to “second-generation” class of drugs. It is more potent than the first-generation drugs and has been known to have fewer side effects.28 Among the first-generation drugs, CPM exhibited better and more prolonged efficiency as an antidiabetic drug in comparison to TBM.29 However, the administration of these drugs are debated since various side effects including confusion, dizziness, headaches, weakness, anxiety, nausea, vomiting, etc. are experienced, which, incidentally matches with the effects those are symptomatic of excess ACh production.30 In a recent study, glimepiride, an SU drug, is reported to be an effective competitive inhibitor against AChE with IC50 = 235 μM.31
Taking a cue from these available literature reports about the sturdy albeit perplexing relationship between AD and T2DM, in this study three antidiabetic drugs TBM, CPM, and GLY (structures are given in Scheme 1) have been tested in vitro and thorough in silico simulation for their efficacies as AChE inhibitors. Although both CPM and TBM are first generation antidiabetic drugs and presently not in use anymore, they are still incorporated in the present study considering them being the first SU class of drugs used in the treatment of T2DM and, therefore, prove to be excellent prototypes in the study of this class of compounds as AChE inhibitors. Further, the common side effects of CPM and TBM (like sweating, nausea, diarrhea, weakness, etc.) are also the consequences of excess ACh production and signify their possible use as AChE inhibitors. All the three drugs used in the present study indeed show positive response as inhibitory modulators of AChE activity and signifies a direct implication of their probable use as AD drugs. Also, parallel experiments are performed to check if either glucose or insulin had any effect as modulatory medium on the AChE inhibition of these drugs. Importantly, possible use of AChE activity enhancers has also been suggested as a mechanism to counter the toxic effect of these antidiabetic drugs toward non-AD diabetic patient.
Scheme 1. Structures of the Antidiabetic Drugs Tolbutamide (TBM), Chlorpropamide (CPM), and Glyburide (GLY).

The molecular weights of the drugs are mentioned in parentheses.
Materials and Methods
Chemicals
All the tested antidiabetic drugs were of analytical grade and purchased from Sigma-Aldrich with TBM, CPM, and GLY having product codes T0891, C1290, and G2539, respectively. Electrophorus electricus (electric eel) acetylcholinesterase (cat. No. C2888) and acetylthiocholine iodide (cat no. A5751) were also obtained from Sigma-Aldrich. Dithiobis 2-nitrobenzoic acid (DTNB, the Ellman reagent) was purchased from Sisco Research Laboratories (SRL), India (product no. 32363). The experiments were performed in phosphate buffer of pH 8.0 using analytical grade type II Millipore water from Elix 10 water purification system. The pH of buffer solutions was further checked by Systronics μ-pH system 361. The aliquots prepared were stored at a temperature of 253 K and settled at the experimental temperature (298 K) for 10 min prior each experiment.
Kinetics of AChE Activity
AChE activity was measured using Ellman method with slight modification,32 details of which are described in the Supporting Information. The enzymatic parameters were estimated using Michalis–Menten mechanism using nonlinear regression of the kinetic data with OriginPro2017 (OriginLab Corp., USA).
The initial rate of enzyme hydrolysis (V0) was calculated by using eq 1.
| 1 |
where ε represents the molar absorptivity of the yellow anion (13600 M–1 cm–1),33l = 0.442 is the path length, and the slope is in the unit of absorbance change per second.
Michaelis–Menten (MM) constant, Km, and maximum hydrolysis rate, Vmax, were calculated for the normal and inhibited enzymatic reaction cases by eqs 2a and 2b, respectively. In these experiments, the substrate concentration ranged from 0.0 to 2.0 mM at two different inhibitor concentrations of 10 and 20 μM.
| 2a |
| 2b |
where Vmax′ = Vmax/α′, Km′ = (α/α′) × Km or Km′ = α × Km, and α = 1 + ([EI]/[E]) and α′ = 1 + ([IES]/[ES]), respectively.
The modified Hill relation (eq 3) was utilized for estimating the magnitude of K0.5, which is analogous to IC50 within pharmacological limits (see supplementary section for details), for all the inhibitors.34,35 In this set of experiments, the substrate concentration was kept fixed at the saturated reaction condition of AChE catalysis reaction (∼2.0 mM);, whereas the inhibitors concentrations were varied from zero until the saturation inhibition condition, which is 50, 140, and 5 μM for CPM, TBM, and GLY, respectively. Interestingly, the maximum therapeutic concentration of these drugs measured in the blood samples of the diabetic patients with prescribed permissible dosage are well within the concentration range of inhibitors used in the present study.
| 3 |
where ΔV is the decrease in initial velocity in the presence of certain inhibitor concentration [I], ΔVmax represents the maximum decrease in initial velocity, and nH is the Hill coefficient.
Fluorescence Studies
Steady state fluorescence measurements were carried out with Quanta Master (QM-40) apparatus obtained from Photon Technology International (PTI). For TBM and CPM, the quenching experiments were carried out by monitoring the intrinsic tryptophan fluorescence of AChE ([AChE] = 1 μM, λexc = 282 nm) with increasing concentration of drugs (0–10 μM for TBM and 0–3.5 μM for CPM). However, since the absorption peak of GLY (λmax = 310 nm) overlaps with the fluorescence range of AChE (λmax = 325 nm), a reverse titration protocol was followed in the case of highly fluorescing GLY, to minimize the self-absorption. In this case, the drug concentration was kept fixed at 1 μM and the AChE concentration was varied within the range 0–3.5 μM. The samples were incubated for 5 min for equilibration before fluorescence titration. The fluorescence spectral intensity in each case was corrected for any possible inner filter effect using eq 4.36
| 4 |
where A represent the absorption of the sample at the excitation (λE) and emission (λF) wavelength, respectively. The binding constant (KB) values were calculated using the fluorescence intensity data at 330 nm for AChE fluorescence (in the case of TBM and CPM) and 350 nm emission of GLY. All the experiments were performed in triplicate, and the results are reported as mean values (±standard error) in each case.
The time-domain fluorescence experiments were done by using 295 nm LED excitation (fwhm = 1.10 ns) in a PicoMaster time-correlated single photon counting (TCSPC) lifetime fluorimeter (PM-3) supplied by PTI. The sample fluorescence decay traces, F(t), were collected at magic angle (54.7°) and analyzed with reference to the instrument response function (IRF) obtained from scattering of dilute colloidal solution of dried nondairy coffee whitener. The analysis involved expressing F(t) as a sum of decaying exponentials (eq 5) and use of a nonlinear least-squares iterative convolution method based on Levenberg–Marquardt algorithm37 as implemented in the data analysis software (Felix GX version 4.0.3) from PTI.
| 5 |
αi and τi are the associated amplitude and fluorescence lifetime of the ith component, such that ∑αi = 1. The quality of fit was verified by several statistical checks like numerical values of reduced χ2, Durbin–Watson (DW) parameter, and simultaneous visual inspection for the random distribution of the weighted residuals.36
Circular Dichroism (CD) Studies
The far-UV (secondary structure)) CD measurements were performed with J-1500 CD spectrometer (JASCO, Japan) using a 0.1 cm cuvette. Three scans were averaged for each spectrum. The measurements of AChE in the absence and presence of the drugs were taken in the range 200–240 nm. The bandwidth was 5 nm, and the response time was 1 s. The sample spectrum was corrected by subtracting the baseline spectrum recorded for the buffer solution containing the same concentration of each drug.
Molecular Docking Studies
The Glide module of Schrodinger Suite (ver. 2020.2)38 was used to perform rigid molecular docking of the inhibitor DON molecule and the antidiabetic drugs (GLY, CPM, and TBM) into the gorge containing the catalytic active site of AChE. The crystal structure of AChE used for validation of the docking protocol was retrieved from the protein data bank (PBD ID: 6O4W). The ligand binding poses of DON, as found in the crystal structure, was reproduced in our docking protocol. The receptor grid was defined on the basis of the functionally important residues in the gorge as identified in previous studies. Various conformations of the ligand were generated by the LigPrep module of Schrodinger, and the best docked structures were selected on the basis of the Glide XP score (optimized to mimic the binding affinity).
Molecular Dynamics (MD) Simulation Study
The Gromacs software suite (ver. 2019.6) was used to carry out atomistic MD simulation of the protein–ligand complex. The Charmm36 force field was used to model the protein.39 The interaction parameters for the ligand molecules were generated using CGenFF server.40 The protein–drug complex simulations were initiated from the structures obtained from the docking protocol described above. All the systems were confined in a cubic box such that the minimum distance between the box boundary and protein was 1 nm. The system was solvated using the TIP3P water model.41 A sufficient number of counterions was added to neutralize the system. The standard protonation states of the amino acid residues were used across all systems; i.e., Asp and Glu residues were negatively charged, Arg and Lys were positively charged, and His residues were neutral. The high energy close contacts were removed using the steepest descent energy minimization. Position restrained equilibration at constant temperature (300 K) and at constant pressure (1 bar) for each of the systems was done for 2 ns using a V-rescale thermostat and a Berendsen barostat.42 After equilibration, the production run was carried out for 200 ns using Leap-frog integrator algorithm with a time interval of 2 fs. A Parrinello–Rahman barostat was used during the production run. The coordinates were saved every 10 ps.
A separate set of simulations was carried out to study the nature of noncompetitive inhibition for CPM and TBM (based on the experimental findings). In these two simulations, the ligand molecules were not docked inside the gorge. Instead, five ligand molecules were randomly inserted into the solution medium around the protein, and 500 ns long trajectories were generated in each case to explore the allosteric interaction of other binding sites.
Results and Discussion
Characterization of Anti-AChE Activity of the Drugs
All the antidiabetic drugs, studied herein, inhibit AChE activity to a different extent. A detailed kinetic analysis of the enzyme catalysis is performed to check the inhibitory effect of the drugs on AChE (Figure 1). In the case of TBM and CPM, the Michaelis Menten constant (Km) remain unchanged, whereas a notable shift in the magnitude of Vmax is observed. The values of α and α′ are rendered equal (Table 1). In the Lineweaver–Burke (LB) plots, shown in the lower panel of Figure 1, a considerable change is observed only in the intercept. These results are indicative of a noncompetitive type of binding, where the inhibitor possesses the ability to bind to both the enzyme (E) as well as the enzyme–substrate complex (ES) with similar affinity. In noncompetitive inhibition, the inhibitor does not interfere with the substrate binding site and is associated with binding at an alternative allosteric site.
Figure 1.
(Above) hydrolysis curve (scattered points) for AChE activity and its inhibition in the presence of different concentrations of TBM(a), CPM(b), GLY(c), and DON(d) (circle = 0 μM, square = 10 μM, triangle = 20 μm) and in phosphate buffer solution of pH = 8.0. The solid line represents nonlinear regression of the experimental data points. [AChE] = 0.079 units/mL. (Below) Lineweaver–Burk (LB) plot for the AChE hydrolysis data in the absence and presence of various drugs.
Table 1. Kinetic Data of Acetylcholinesterase (AChE) Hydrolysis and the Effect of Different Concentration of Inhibitors on Various Parameters in Aqueous Buffera.
| from
Michaelis–Menten equation |
from
Hill plot |
|||||
|---|---|---|---|---|---|---|
| systems | Km/μM | Vmax/nM s–1 | α | α′ | IC50/μM | nH |
| (i) AChE in buffer | 166 ± 13 | 803 ± 32.3 | 1.0 | 1.0 | ||
| (ii) AChE in buffer +10 μM TBM | 168 ± 17 | 669 ± 23.9 | 1.2 | 1.2 | 28.9 ± 1.60 | 1.40 ± 0.11 |
| (iii) AChE in buffer +20 μM TBM | 172 ± 19 | 570 ± 15.3 | 1.4 | 1.4 | ||
| (iv) AChE in buffer +10 μM CPM | 170 ± 27 | 605 ± 30.5 | 1.3 | 1.3 | 5.72 ± 0.24 | 1.37 ± 0.08 |
| (v) AChE in buffer +20 μM CPM | 166 ± 29 | 477 ± 21.7 | 1.7 | 1.7 | ||
| (vi) AChE in buffer +10 μM GLY | 374 ± 17 | 743 ± 23.9 | 2.4 | 1.1 | 0.74 ± 0.02 | 1.32 ± 0.11 |
| (vii) AChE in buffer +20 μM GLY | 786 ± 19 | 747 ± 15.3 | 5.1 | 1.1 | ||
| (viii) AChE in buffer +5 nM DON | 160 ± 36 | 550 ± 48 | 1.4 | 1.4 | 0.074 ± 0.005 | 1.45 ± 0.1 |
| (ix) AChE in buffer +10 nM DON | 138 ± 13 | 484 ± 15 | 1.5 | 1.6 | ||
TBM: tolbutamide. CPM: chlorpropamide. GLY: glyburide. DON: donepezil (control drug).
However, a completely different enzyme kinetic scheme is observed in the case of GLY. Since the values of Km changes but Vmax remains unaltered in this case (Table 1 and Figure 1), the inhibition mechanism is inferred to be of competitive type. More evidently, the value of α remains same, whereas α′ increases with an increase in inhibitor concentration. Consistent with this proposition, the intercept of the corresponding LB plot does not undergo any variation, while the slope increases. This mechanism follows only the inhibition path where the inhibitor binds at the active site of the enzyme. Since the inhibitor competes with the substrate for the active site, it reduces the accessibility of the substrate to the enzyme.43 Since in competitive inhibition the inhibitor binds at the same site as the substrate, whereas noncompetitive inhibitors bind at an allosteric site, the binding locations in AChE are expected to differ for the investigated drugs. This is further ascertained through the molecular dynamic calculations results discussed later in the Article.
The IC50 values (given in Table 1) obtained from the Hill plot (Figure 2) reveal moderate to good AChE inhibition potency for all the three antidiabetic drugs. TBM shows the lowest efficiency (with an IC50 value of 28.90 ± 1.60 μM), whereas CPM acts as a moderate inhibitor (IC50 = 5.72 ± 0.24 μM) and GLY (IC50 = 0.74 ± 0.02 μM) exhibits the highest inhibition effect on AChE activity. The FDA approved cholinergic drug donepezil (DON), which has an IC50 value of 0.074 ± 0.005 μM, has been used as a control in these experiments. The kinetic details of DON inhibition under similar experimental conditions is also incorporated in Figure 1 and Table 1. Interestingly, GLY is very close to DON with regard to the inhibitory efficacy. We further note that the relative trend on AChE inhibition activity of the investigated systems correlates with their effectiveness as antidiabetic drugs, which further confirms on the pharmacological implications in the linkage between AD and T2DM.
Figure 2.
Modified Hill analysis of AChE inhibition in the presence of various drugs.
Treading further on this line, glucose and insulin are two very important parameters to quantify T2DM since the disease is usually marked by a higher amount of blood glucose and insulin resistance.22 Administration of insulin or stimulation of insulin secretion are often prescribed as common therapeutic avenues for diabetic patients. Since the antidiabetic drugs are found to act as AChE inhibitors, to validate a more definitive linkage, it is imperative to test the direct in vitro effect of insulin and glucose on AChE activity.
Apparently, neither an excess amount of glucose nor insulin has any effect on the catalytic efficiency or kinetic parameters of AChE activity, as seen from the substrate hydrolysis curves depicted in Supporting Information (Figure S1). Also, overnight incubation of the reaction medium with insulin or glucose (10 μM concentration) does not exert any influence on the potency of the drugs. To verify if the unaltered inhibition potency in these two mediums is restricted to antidiabetic drugs only or is a characteristic of all the AD drugs, DON is used as a model in this case too. The hydrolysis of DON inhibited AChE does not undergo any change in the presence of either of these mediums. The results imply that the direct presence of excess glucose does not play any modulatory role in AChE activity leading to AD. Similarly, insulin also is not considered alone as a cholinergic therapeutic avenue for AD, as perceived from these independent experiments.
Interaction of AChE with the Drugs
Steady State Fluorescence Experiments
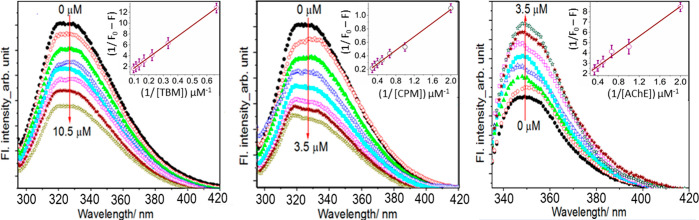
AChE shows a distinct fluorescence emission maximum at 330 nm on excitation at 282 nm. The contribution to the intrinsic fluorescence of AChE mainly comes from the tryptophan residue alone, since phenylalanine has a very low quantum yield, and when ionized, tyrosine fluorescence emission is nearly or entirely quenched. The intrinsic emission intensity of AChE is found to quench regularly in the presence of increasing concentrations of both TBM and CPM (Figure 3).
Figure 3.
Quenching of emission intensity of AChE in the presence of increasing concentrations of TBM (left) and CPM (middle). (Right) shows the quenching of GLY emission intensity in the presence of increasing concentrations of AChE. The inset shows the Benesi–Hildebrand plots.
For a more quantitative comprehension of the quenching mechanism, the Stern–Volmer (SV) relation (eq 6) is used.37
| 6 |
where F0 and F are the fluorescence intensities in the absence and presence of quencher (Q) and KSV is the Stern–Volmer quenching constant. The KSV values, obtained from the linear SV plots (Figure S2), are found to be (1.2 ± 0.07) × 104 and (5.0 ± 0.34) × 104 M–1 for TBM and CPM, respectively, implying that while both the drugs bind with AChE with moderate affinity, the interactions are almost 4 times more with CPM in comparison with TBM. On the other hand, in the case of GLY, it is seen that the addition of increasing concentrations of AChE regularly enhanced its fluorescence (Figure 3, extreme right), implying definitive interaction between the two.
To compare the extent of interaction of these drugs with AChE, the binding constant value in each case is calculated from fluorescence intensity variation data using Benesi–Hildebrand (BH) relation.44
| 7 |
Here, KB is the binding constant, F0 represents the fluorescence intensity in the absence of quencher or enhancer, and Fα is the emission intensity in the presence of highest concentration of drug or AChE. KB values are found to be (1.9 ± 0.02) × 104, (6.7 ± 0.58) × 104, and 6.0 ± 0.51) × 105 M–1 for TBM, CPM, and GLY, respectively. The results imply a greater magnitude of interaction of AChE with GLY, whereas TBM had the least affinity to bind with AChE. These predictions are further confirmed through molecular dynamic simulation, results of which are given below. It is to be noted that the binding strength of these drugs with AChE correlates well with their AChE inhibition potency, obtained from the enzymatic assay experiment.
Time-Resolved Measurements and Thermodynamics of Interaction
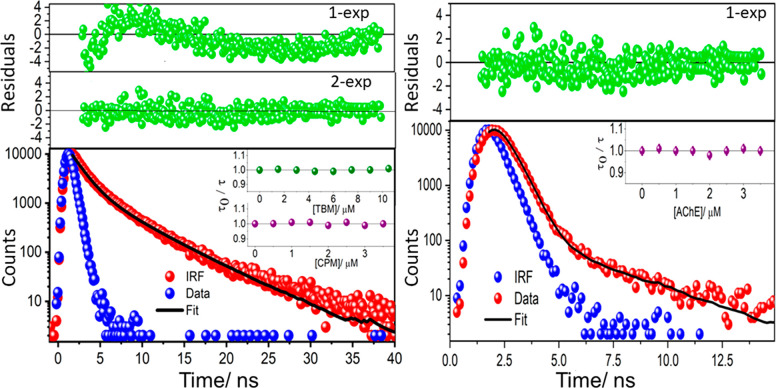
The decrease in fluorescence intensity of AChE on addition of CPM and TBM can be due to the result of either static or dynamic quenching mechanism. A ground state complex formation between AChE and the drugs corresponds to the static quenching, whereas dynamic collisions occur between the excited fluorophore and the quencher in the latter case. A definitive test for identifying the quenching mechanism is to measure the fluorophore decay time with varying concentration of the quencher. As static quenching is a ground state interaction, the fluorescence lifetime would not show any change in the presence of quencher. Typical representation of this situation is that the τ0/τ (=1) plot is independent of quencher concentration, where τ0 and τ are the fluorescence lifetimes, in the absence and presence of the quencher. On the other hand, due to the collision between the excited fluorophore with the quencher in dynamic quenching mechanism, the fluorescence lifetime decreases steadily and τ0/τ would vary with quencher concentration like that given for intensity variation in eq 6.37
A two-exponential decay analysis is required to fit the AChE fluorescence decay data, as confirmed from the distribution from weighted residuals (Figure 4). The average lifetime, calculated from the fractional contribution of each decay time to the steady state intensity with the relation τ = (α1τ12 + α2τ22)/(α1τ1 + α2τ2), is found to be 2.35 ± 0.20 ns. Interestingly, this lifetime value does not show any significant change in the presence of either TBM or CPM (Figure 4, inset), ruling out the possibility of any dynamic processes. Similarly, the GLY fluorescence lifetime (measured at 350 nm) is found to be 0.45 ± 0.10 ns, where a single exponential fitting is enough to reproduce the experimental data (Figure 4). On addition of AChE, the fluorescence lifetime remains unaltered in this case too, implying the binding to be static in nature.
Figure 4.
Time-resolved fluorescence decay traces and exponential fitted curves of AChE (left) and GLY (right). Inset: variation of τ0/τ with different concentrations of TBM and CPM (left) and AChE (right).
To get a wider perspective on its fluorescence intensity, the interaction of GLY is also studied with model water-soluble protein bovine serum albumin (data not shown). Similar enhancement of emission intensity is observed in this case too, implying that the increase in fluorescence intensity of GLY on addition of the protein is due to the sequestration of the ligand inside the macromolecular cavity. The relative fluorescence quantum yield of GLY increases from 0.23 in aqueous buffer to ca. 0.41 in the medium that contains 3.5 μM AChE. Calculation of total nonradiative decay rate (∑κnr) from the known fluorescence yield (ϕf) and decay time (τ) using the relation ∑κnr = (1 – ϕf)/τ, results a decrease in the nonradiative decay rate from ∼1.71 to ∼1.31 ns–1 when bound with AChE. Hence, the increase in fluorescence intensity of GLY in the presence of AChE can be related with the decrease in total nonradiative processes, mostly due to the drug being trapped in the macromolecular environment.
The far-UV CD spectra of AChE upon interaction with the investigated drugs at 298 K are shown in the Supporting Information (Figure S3). AChE shows a characteristic peak at 208 nm (π–π* transition) and 222 nm, which shows the α-helix structure of the protein. The results show that TBM and CPM do not cause any significant change in the intrinsic helical structure of the protein. In the case of GLY, the helical structure of AChE is seen to decrease a little, but the overall spectral pattern remains same, which indicates that the secondary conformation of AChE remains intact in the presence of all the drugs. The results confirm that the activity decrease of AChE in the presence of the drugs is mainly caused by AChE–drug binding and not by the conformation change of the enzyme.
From the binding constant values obtained from B–H analysis, the standard Gibbs free energy change (ΔG°) can be calculated using the following relation.
| 8 |
The corresponding values are found to be −24.41, −27.53, and −32.96 kJ mol–1 for TBM, CPM, and GLY, respectively. While the negative ΔG° values indicate spontaneous binding of all three drugs with AChE, the interaction is found to be the strongest in the case GLY. These results are consistent with the molecular modeling calculation discussed below.
Molecular Docking Calculations
AChE consists of a catalytic active site (CAS) and a peripheral active site (PAS) connected by a narrow, yet deep (20 Å) gorge.45−47 CAS is situated near the bottom of the gorge and the PAS resides near the enzyme surface. Since PAS is situated on the enzyme surface, the inhibitors binding at this site directly block the entry of substrates and the exit of catalytic products from the active site of the gorge. PAS inhibitors have been reported to inhibit the catalysis by causing steric hindrance to the entry and exit of the ligand, as well as by allosteric alteration of the conformation of catalytic triad and its efficiency.48 On the other hand, CAS inhibitors occupy the substrate binding site and cause inhibition by reducing the accessibility of the active site to substrates. Inhibitors that bind at both the sites; i.e., gorge spanning ligands are more effective than single site binding ligands.
Using the Glide module of the Schrodinger suite, several putative bindings poses of the inhibitor molecules are identified. While the receptor is kept rigid, several conformations are generated for the docking protocol due to the ligand flexibility. We have selected the binding pose with best Glide XP score, which is an empirical scoring function optimized to correlate with the binding affinity of the inhibitors. The glide scores of the best binding poses for different ligands are −178.92, −182.32, and −255.51 kJ mol–1 for TBM, CPM, and GLY, respectively. Based on these results, the expected trend for the binding affinity of the investigated antidiabetic drugs is GLY > CPM ∼ TBM. Interestingly, the Glide score of GLY is even better than the FDA approved cholinergic drug DON (−240.74 kJ mol–1). Thus, GLY is expected to have comparable or even relatively better performance as a competitive inhibitor to AChE as compared to some of the well-known AD drug (DON). On the other hand, CPM and TBM have significantly weaker binding affinity, possibly due to the smaller size of these molecules.
The nature and number of different binding interactions of AChE (like hydrophobic interaction, hydrogen bonding, π–π stacking, etc.) with the investigated drugs are given in Table 2. It is clear that GLY shows maximum interactions with the active site gorge residues of the protein, closely mimicking the reference drug DON. On the other hand, CPM and TBM give fewer interactions with AChE and are consistent with the lower values of the glide score in these cases.
Table 2. Molecular Docking Calculation Results for the Binding Interaction of the Investigated Sulfonylurea Class of Antidiabetic Drugs GLY, CPM, and TBM with AChEa.
| ligands | hydrogen bondb | hydrophobic interactions | π–π/π-cation stacking interactionsb |
|---|---|---|---|
| GLY | PHE 295:1 (2.98 Å) | TYR 72, TYR 124, TRP 286, TYR 337, PHE 338, VAL 294, PHE 297, TYR 341, GLY 120, GLY 121, TYR 133, GLY 448, GLU 202, TRP 86, HIS 447 | TRP 86:2 (π–π) |
| TYR 341:1 (π–π) | |||
| CPM | ASP 74:1 (2.83 Å) | ASP 74, TYR 124, TYR 337, PHE 338, TYR 341, GLY 121, TRP 86 | TRP 86:2 (π–π) |
| TYR 124:1 (3.00 Å) | |||
| TBM | PHE 295:1 (3.17 Å) | TYR 124, TYR 337, PHE 338, VAL 294, PHE 295, PHE 297, TYR 341, TRP 286 | TRP 286:1 (π–π) |
| DON | PHE 295:1 (3.06 Å) | TYR 72, ASP 74, TYR 124, TRP 286, TYR 337, PHE 338, PHE 295, PHE 297, TYR 341, GLY 120, GLY 121, TRP 86, GLU 202, SER 293, HIS 447 | TRP 86:2 (π–π) |
| TRP 286:1 (π–π) | |||
| TRP 86:1 (π-cat) |
The calculated parameters for the model drug DON are also included for comparison.
The number against each amino acid residue indicates the number of corresponding interactions.
Figure 5a depicts the interaction map of the neighboring residues involved in interactions with the inhibitors. In all the cases, significant hydrophobic interactions with the neighboring aromatic residues are observed. GLY, being a larger molecule, spans almost the full gorge ranging from CAS to the PAS and also has a few specific interactions with protein residues, e.g., PHE295, TRP286, TRP86, TYR341, etc. These interactions are almost similar to that of the DON, the control drug. On the other hand, both CPM and TBM, being smaller molecules, cannot span the whole gorge region. CPM is found to be closer to the CAS, whereas TBM is closer to the PAS. For CPM, the aromatic ring interacts with TRP86; however, it interacts with TRP286 in the PAS for TBM. There are several hydrogen bonded interactions as well, marked by the arrows, e.g., with residues TYR124, ASP74, GLU202, PHE295, etc.
Figure 5.
Interaction map of the inhibitor molecules based on the docked structures (a) and most populated cluster from the MD trajectory (b). All specific interactions, e.g., hydrogen bonding and aromatic/π-stacking interactions are marked. The solvation shell of the ligand is included as well.
Thus, molecular docking analysis clearly reveals that TBM does not bind to the CAS. Hence, it can be classified as a noncompetitive inhibitor in agreement with the experimental findings, whereas GLY is seen to be an effective gorge-spanning competitive inhibitor. Although CPM seems to interact with the CAS region in the docked structure with moderate affinity, our subsequent MD simulation studies demonstrate an alternative mechanism of action for CPM as a noncompetitive inhibitor.
Molecular Dynamics (MD) Simulation Study
General Simulation Study
Although molecular docking is a very powerful method for virtual screening and identification of the binding poses in protein–ligand interaction, there are several limitations of a rigid docking protocol. These limitations include (i) the lack of flexibility and dynamics of the receptor, (ii) the lack of water interactions and dynamics, and (iii) the absence of entropic effects. These limitations can be circumvented to some extent using atomistic MD simulation, although at a significantly higher computational cost. In order to explore the stability and interactions of the inhibitor molecules in a realistic dynamic system, we have carried out 200 ns long MD simulations for each of the protein–ligand complex structures obtained from the docking exercise. It is observed that the ligands are stable around the docked structure throughout the duration of the trajectory. The molecules undergo intermittent conformational changes while maintaining the overall position. Quite a few water molecules exist in the gorge area and participate in stabilization of the ligand. Table 3 summarizes the average interaction energy of the three antidiabetic drugs in the AChE environment along with the results for the control drug (DON). These include all nonbonded (electrostatic and van der Waal’s) interactions with protein/water/ions within 2 nm cutoff distance from the ligand. We have also computed a similar average interaction energy for the ligand in water (solvation energy), and the difference between them would give a relative estimate of the internal energy part (ΔE) of the binding free energy (ΔG).
Table 3. Average Interaction Energies of Different Inhibitors in the AChE Environment, Aqueous Environment, and the Change in Moving from the Aqueous Environment to the Protein Environment, i.e., Binding Energy (ΔE).
| ligand | protein (Epro) (kJ mol–1) | water (Ewat) (kJ mol–1) | binding energy (ΔE = Epro – Ewat) (kJ mol–1) |
|---|---|---|---|
| tolbutamide | –290.79 ± 9.62 | –244.76 ± 0.42 | –46.03 ± 9.62 |
| chlorpropamide | –295.81 ± 2.93 | –237.65 ± 0.44 | –58.16 ± 2.93 |
| glyburide | –453.55 ± 5.86 | –369.87 ± 0.42 | –83.68 ± 5.86 |
| donepezil | –727.18 ± 9.20 | –655.63 ± 0.84 | –71.55 ± 9.20 |
Based on this thermodynamic information, it is inferred that GLY has a strong favorable interaction with the receptor (analogous to that in the FDA approved cholinergic drug DON) in comparison with CPM and TBM. It is to be noted here that DON, being a charged molecule, has significant solvation energy in water. Hence the binding energy (ΔE) becomes comparable to GLY due to the desolvation penalty. Figure 5b demonstrates the interaction pattern around the inhibitor obtained from the most populated cluster of the respective MD simulation trajectories. Here the solvating water molecules are included as well. Clearly, being smaller molecules and relatively weak binding, CPM and TBM have higher solvent exposure.
Noncompetitive Inhibition Mechanism for CPM and TBM
Given the experimental results that CPM and TBM interact with AChE as noncompetitive inhibitors, the docking results are unable to explain this observation for CPM. It is to be noted here that the MD simulation results discussed above are based on the docked structure, where the drugs are buried in the gorge to begin with. Hence, in an alternative approach, instead of docking the drugs inside the gorge, the simulation starts with five inhibitor molecules randomly inserted around the protein in the solution phase. The simulations are run for 500 ns duration for both CPM and TBM to explore whether any alternative binding pockets exist on the AChE surface. Figure 6a shows the root-mean square deviation (RMSD) of the catalytic triad residues (SER203, GLU334, and HIS447) for all the trajectories. The RMSD has been computed with respect to the initial structure in the respective cases (after short equilibration). It is noted that for the free sampling of CPM, the RMSD shoots up very high (>0.4 nm), indicating significant structural distortion of the catalytic triad residues. With visual examination of the structural changes in Figure 6b, it is observed that the HIS447 residue undergoes a large conformational change pointing toward the “back-door” opening, where the ligand molecule binds. Hence, this provides an irrevocable proof of the noncompetitive mechanism of action for CPM.
Figure 6.
(a) Root mean square displacement (RMSD) of the catalytic triad residues (SER203, GLU334, and HIS447) for all trajectories: (i) DON, GLY, CPM, and TBM in docked configuration and (ii) CPM and TBM (five molecules each) in free configuration. (b) Representative snapshot demonstrating the major structural change in HIS447 (pointing downward) as compared to the crystal structure (pointing upward). The crystal structure and CPM-bound structures are marked by yellow and green ribbons, respectively. The CPM molecule is shown in a VDW sphere representation. It induces a stable salt-bridge formation near the binding site leading to the major conformational change.
Neurotoxicity of Antidiabetic Drugs toward Non-AD Subjects: Countering Excess ACh Stimulation
The in vitro AChE activity study reveals that the usage of these antidiabetic drugs would prove to be therapeutically beneficial for a person suffering from both AD and T2DM. However, the situation may be completely different for a diabetic patient without any significant symptoms of AD. This becomes particularly relevant since the concentration range of the inhibitors showing significant modulatory effect on AChE activity, estimated in the present study, are well within the maximum therapeutic concentration of these drugs in blood. For example, in one of the earliest studies, using a selective and sensitive gas chromatographic technique, the mean concentrations of TBM and CPM are estimated to be about 103 and 270 μM in blood serum samples of diabetic patients.49 On the other hand, reverse phase high performance liquid chromatographic measurement indicates about 3 μM of GLY distribution in the blood sample of patients, at the maximum recommended dosage of 20 mg per day.50 Due to the substantial reduction in AChE activity at this concentration range, continuous uptake of these drugs for a diabetic patient with sound mental health has the potential to cause several side effects corresponding to neurodegenerative disorders related to hyperexcitation of the neuronal network. In fact, the general side effects exhibited by these drugs have already been documented, which are analogous to the symptoms faced by excess ACh simulation, presumably caused by AChE inhibition in a normal (non-AD) person. Therefore, to counter possible neurodegenerative toxicity to a diabetic patient without any symptom of AD, it would be beneficial to use an AChE activity booster as a delivery medium for these antidiabetic drugs.
Recently, nanostructured materials have found huge applications in diverse fields.51,52 It is well documented that adsorption of enzymes on nanostructured solid support leads to enhanced performance in terms of activity.53,54 The phenomenon of boosting the enzyme activity is attributed to various factors like the large surface area of nanomaterials and multipoint attachment of enzyme molecules to nanomaterials, resulting in a decrease in protein unfolding, a phenomenon that results in many diseases including neurodegenerative disorders.55 This further results in the improved stability of the enzyme attached to the nanoparticles (NPs) and, in turn, advances the enzyme–substrate interaction by avoiding the potential aggregation of the free enzyme. In fact, literature reports specifically show affirmative results for both Ag and Au NPs, which are found to increase the activity of AChE.56
In this study, the relevance of these colloidal NPs as drug delivery media is monitored by checking the effect of the most potent cholinergic agent among the investigated antidiabetic drugs, i.e., GLY, on AChE, when the reaction medium was changed from buffer to colloidal NPs. Colloidal gold and silver nanoparticles (AuNP and AgNP, respectively) with average size distribution of o.d. = 14 ± 2 and 8 ± 2 nm, respectively, are synthesized and characterized following the procedure described elsewhere.52 As can be seen from Figure 7, the activity of AChE, which was considerably lowered in the presence of 20 μM GLY, is enhanced in the presence of both Au and Ag NPs. The Km and Vmax values of AChE activity in the presence of 20 μM GLY in buffer (786 ± 19 μM and 747 ± 15.3 nM s–1, respectively) further change to 269 ± 7.8 μM and 768 ± 11.9 nM s–1 in the presence of 1 mM AgNP. The corresponding values are found to be 575 ± 12.1 μM and 755 ± 16.1 nM s–1 in the presence of 1 mM AuNP. Interestingly, both parameters move closer to those of AChE in aqueous buffer medium without any inhibitor (166 ± 17 μM and 803 ± 32.3 nM s–1, respectively); however, AgNP clearly shows more the pronounced effect between the two. These results imply that if these antidiabetic drugs are used in nanofluidic formulation, the neurotoxic side effects of these drugs, discussed above, can be remedied.
Figure 7.

Effect of colloidal Au and Ag NPs on the activity of AChE in the absence and presence of GLY.
Although the exact reason for the increase in AChE activity when adsorbed on NP surfaces is not clear so far, it may be due to the formation of AChE-NP bioconjugate. Formation of this conjugate structure is evidenced from the increased average size of the NPs in TEM measurement (Figure S4). For example, the size of AuNPs increases from 14 ± 2 to 20 ± 2 nm, whereas the increase is more pronounced for AgNP (from 8 ± 2 to 18 ± 2 nm). The increase in size occurs due to the adsorption of proteins around NPs and can also be confirmed from the AFM images (Figure S5). Interestingly, in a recent report, the high catalytic efficiency of AChE has been related with the “breathing motion” of certain key aromatic residues that modulate the size of the gorge.46 The adsorption of AChE on the NP surface might further facilitate this motion and certainly influence the internal structure as well as the overall dynamics of the protein motion in a significant way, leading to the enhanced AChE activity. Furthermore, the relatively pronounced enzymatic activity increase observed for AgNP in comparison with that in AuNP is possibly due to stronger interaction of AChE with more labile surface electrons in the former. Indeed, the size and surface chemistry of NPs along with the density and orientation of the immobilized enzymes are decisive factors in the enhanced performance of enzyme–nanoparticle conjugates. Extended experimental work and simulation studies of AChE interaction with NPs of different types and sizes are perhaps necessary to resolve this issue unambiguously.
Conclusions
The present study investigates three different sulfonyl urea antidiabetic drugs of different generations, viz., tolbutamide (TBM), chlorpropamide (CPM), and glyburide (GLY), for their possible use as cholinergic inhibitors. All of these drugs cause significant inhibition of AChE activity with the IC50 values 0.74 ± 0.02, 5.72 ± 0.24, and 28.9 ± 1.60 μM for GLY, CPM, and TBM, respectively. Both in vitro and in silico studies verify that GLY acts as a gorge spanning competitive inhibitor to AChE with the binding constant almost an order of magnitude higher than those of the other two drugs. On the other hand, although the inhibition mechanism for CPM and TBM is noncompetitive in nature, their mode of interaction with AChE is distinctly different. Interestingly, the relative AChE inhibition efficiency of these drugs matches with effectiveness as antidiabetic agents. Further, GLY is even identified to show stronger binding affinity and comparable AChE inhibition efficiency than DON, an FDA approved AD drug. These results demonstrate the dual therapeutic properties of these drugs as pharmacological agents for the two important diseases like T2DM and AD and further validate the linkage between the two.
The findings also open up new avenues in the field of drug repurposing, which has been gaining momentum across the diseases. However, this dual therapeutic effectiveness has an underlying consequence, which until now had been ignored completely. While the antidiabetic drugs undoubtedly benefit a person suffering from both diabetes and AD, these drugs would adversely affect an AD-unaffected diabetic patient. The sulfonylureas (SUs), particularly GLY, despite being quite potent antidiabetic drugs can have side effects due to excess ACh production. Apparently, this neurotoxic side effect of GLY can be significantly reduced by some concomitant AChE activity boosters like Au and Ag NPs. It is to be noted that the effect of NPs has been tested only in GLY, which, among a series of SU drugs, shows better AChE inhibition efficiency even than DON. However, whether this hypothesis can be extended to other class of antidiabetic drugs needs further investigation. Additionally, optimization of NP composition (like type, size, shape, etc.) to be used as drug delivery media would pose a new challenge and needs in-depth research. The implications of this study reveal that by a judicious choice of the drugs to be administered, the pharmacology of these two diseases and the related therapeutic possibilities can be understood well toward overall increase in the quality of life for both AD and diabetes patients.
Acknowledgments
Thanks are due to Prof. S. Ghosh and Dr. P. Dey of Indian Association for the Cultivation of Science (IACS), Kolkata, for their help in performing AFM experiments. P.B. thanks NEHU for providing a doctoral fellowship to her. A.D. and S.C. thank the supercomputing facility of SNBNCBS.
Glossary
ABBREVIATIONS
- AD
Alzheimer’s disease
- T2DM
Type 2 diabetes mellitus
- TBM
tolbutamide
- CPM
chlorpropamide
- GLY
glyburide
- AChE
acetylcholinesterase
- DON
donepezil
- CAS
catalytic active site
- PAS
peripheral active site
- ACh
acetylcholine
- ADHD
attention deficit hyperactivity disorder
- SU
sulfonylureas
- MM
Michalis–Menten
- TCSPC
time-correlated single photon counting
- IRF
instrument response function
- DW
Durbin–Watson
- LB
Lineweaver–Burke
- RMSD
root-mean square deviation
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.0c00168.
Protocols describing the kinetic characterization of AChE activity and modified Hill analysis, AChE hydrolysis curve in the presence of insulin and glucose (Figure S1), Stern–Volmer plot for AChE fluorescence quenching in the presence of TBM and CPM (Figure S2), CD spectra (Figure S3), TEM and AFM images of AChE-NP conjugates (Figures S4 and S5, respectively). (PDF)
Author Contributions
P.B. has performed the experiments, analyzed the data, and drafted the manuscript. A.D. and S.C. performed the MD simulation experiment and drafted the manuscript. D.P. did the CD experiment and analyzed the data. K.A. and S.M. were involved in designing the research, data analysis, finalizing the manuscript, and funding arrangements.
Partial financial support from Department of Biotechnology (BT/232/NE/TBP/2011) and Department of Science and Technology (SR/FST/CSI-194/2008), Govt. of India, are gratefully acknowledged. S.C. thanks SERB/DST, India, for funding (ECR/2018/002903).
The authors declare no competing financial interest.
Supplementary Material
References
- Martyn J. A. J.; Fagerlund M. J.; Eriksson L. I. (2009) Basic Principles of Neuromuscular Transmission. Anaesthesia 64, 1–9. 10.1111/j.1365-2044.2008.05865.x. [DOI] [PubMed] [Google Scholar]
- Craig L. A.; Hong N. S.; McDonald R. J. (2011) Revisiting the Cholinergic Hypothesis in the Development of Alzheimer’s Disease. Neurosci. Biobehav. Rev. 35, 1397–1409. 10.1016/j.neubiorev.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Wimo A.; Guerchet M.; Ali G. C.; Wu Y. T.; Prina A. M.; Winblad B.; Jönsson L.; Liu Z.; Prince M. (2017) The Worldwide Costs of Dementia 2015 and Comparisons with 2010. Alzheimer's Dementia 13, 1–7. 10.1016/j.jalz.2016.07.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S.; Sharma N. (2000) Neurological syndromes following organophosphate poisoning. Neurol India 48, 308–311. [PubMed] [Google Scholar]
- Adeyinka A., and Kondamudi N. P. (2018) Cholinergic Crisis, StatPearls Publishing, Treasure Island, FL. [PubMed] [Google Scholar]
- Colovic M. B.; Krstic D. Z.; Lazarevic-Pasti T. D.; Bondzic A. M.; Vasic V. M. (2013) Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Current Neuropharmacology. 11, 315–335. 10.2174/1570159X11311030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martis E. A. F.; Chandarana R. C.; Shaikh M. S.; Ambre P. K.; D’Souza J. S.; Iyer K. R.; Pissurlenkar R. R. S. (2015) Quantifying ligand–receptor interactions for gorge spanning acetylcholinesterase inhibitors for the treatment of Alzheimer’s disease. J. Biomol. Struct. Dyn. 33, 1107–1125. 10.1080/07391102.2014.931824. [DOI] [PubMed] [Google Scholar]
- Yiannopoulou K. G.; Papageorgiou S. G. (2013) Current and future treatments for Alzheimer’s disease. Ther. Adv. Neurol. Disord. 6, 19–33. 10.1177/1756285612461679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godyń J.; Jończyk J.; Panek D.; Malawska B. (2016) Therapeutic strategies for Alzheimer’s disease in clinical trials. Pharmacol. Rep. 68, 127–138. 10.1016/j.pharep.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Llorente M. D.; Urrutia V. (2006) Diabetes, psychiatric disorders, and the metabolic effects of antipsychotic medications. Clin Diabetes. 24, 18–24. 10.2337/diaclin.24.1.18. [DOI] [Google Scholar]
- Luchsinger J. A.; Tang M. X.; Stern Y.; Shea S.; Mayeux R. (2001) Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am. J. Epidemiol. 154, 635–641. 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- Haan M. N. (2006) Therapy Insight: type 2 diabetes mellitus and the risk of late-onset Alzheimer’s disease. Nat. Clin. Pract. Neurol. 2, 159–166. 10.1038/ncpneuro0124. [DOI] [PubMed] [Google Scholar]
- Qiu W. Q.; Folstein M. F. (2006) Insulin, insulin-degrading enzyme and amyloid-beta peptide in Alzheimer’s disease: review and hypothesis. Neurobiol. Aging 27, 190–198. 10.1016/j.neurobiolaging.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Exalto L.; Whitmer R.; Kappele L.; Biessels G. (2012) An update on type 2 diabetes, vascular dementia and Alzheimer’s disease. Exp. Gerontol. 47, 858–864. 10.1016/j.exger.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Apostolatos A.; Song S.; Acosta S. (2012) Insulin promotes neuronal survival via the alternatively spliced protein kinase C delta II isoform. J. Biol. Chem. 287, 9299–9310. 10.1074/jbc.M111.313080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco M.; Ferreira S. T.; De Felice F. G. (2015) Neuronal stress signaling and eIF2a phosphorylation as molecular links between Alzheimer’s disease and diabetes. Prog. Neurobiol. 129, 37–57. 10.1016/j.pneurobio.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Rönnemaa E.; Zethelius B.; Sundelöf J. (2008) Impaired insulin secretion increases the risk of Alzheimer disease. Neurology 71, 1065–1071. 10.1212/01.wnl.0000310646.32212.3a. [DOI] [PubMed] [Google Scholar]
- Kroner J. (2009) The Relationship between Alzheimer’s disease and Diabetes: Type 3 Diabetes?. Altern Med. Rev. 14, 373–379. [PubMed] [Google Scholar]
- Steen E.; Terry B. M.; Rivera E. J. (2005) Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease – is this type 3 diabetes?. J. Alzheimer's Dis. 7, 63–80. 10.3233/JAD-2005-7107. [DOI] [PubMed] [Google Scholar]
- Rivera E. J.; Goldin (2005) Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J. Alzheimer's Dis. 8, 247–268. 10.3233/JAD-2005-8304. [DOI] [PubMed] [Google Scholar]
- Agrawal R.; Tyagi E.; Shukla R.; Nath C. (2008) Effect of insulin and melatonin on acetylcholinesterase activity in the brain of amnesic mice. Behav. Brain Res. 189, 381–386. 10.1016/j.bbr.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Markowicz-Piasecka M.; Sikora J.; Mateusiak L.; Mikiciuk-Olasik E.; Huttunen K. M. (2017) Metformin and Its Sulfenamide Prodrugs Inhibit Human Cholinesterase. Oxid. Med. Cell. Longevity 2017, 1–11. 10.1155/2017/7303096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinglos M. N.; Lebovitz H. E. (1980) Sulfonylurea treatment of insulin-independent diabetes mellitus. Metab., Clin. Exp. 29, 488–94. 10.1016/0026-0495(80)90175-4. [DOI] [PubMed] [Google Scholar]
- Rizvi S. M. D.; Shakil S.; Biswas D.; Shakil S.; Shaikh S.; Bagga P.; Kamal M. A. (2014) Invokana (Canagliflozin) as a Dual Inhibitor of Acetylcholinesterase and Sodium Glucose Co-Transporter 2: Advancement in Alzheimer’s Disease- Diabetes Type 2 Linkage via an Enzoinformatics Study. CNS Neurol. Disord.: Drug Targets 13, 447–451. 10.2174/18715273113126660160. [DOI] [PubMed] [Google Scholar]
- Shakil S. (2017) Molecular interaction of anti-diabetic drugs with Acetylcholinesterase and Sodium Glucose Co-Transporter 2. J. Cell. Biochem. 118, 3855–3865. 10.1002/jcb.26036. [DOI] [PubMed] [Google Scholar]
- Shaikh S.; Rizvi S. M. D.; Shakil S.; Riyaz S.; Biswas D.; Jahan R. (2016) Forxiga (dapagliflozin): Plausible role in the treatment of diabetes-associated neurological disorders. Biotechnol. Appl. Biochem. 63, 145. 10.1002/bab.1319. [DOI] [PubMed] [Google Scholar]
- Sola D.; Rossi L.; Schianca G. P.; Maffioli P.; Bigliocca M.; Mella R.; Corlianò F.; Fra G. P.; Bartoli E.; Derosa G. (2015) Sulfonylureas and their use in clinical practice. Arch. Med. Sci. 11, 840–848. 10.5114/aoms.2015.53304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda-Olson A. M.; Patch R. K.; Leibson C. L.; Vella A.; Frye R. L.; Weston S. A.; Roger V. L. (2009) Effect of second-generation sulfonylureas on survival in patients with diabetes mellitus after myocardial infarction. Mayo Clin. Proc. 84, 28–33. 10.4065/84.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West K. M.; McCampbell S. R. (1959) Relative potencies of chlorpropamide and tolbutamide in man. Ann. N. Y. Acad. Sci. 74, 473–477. 10.1111/j.1749-6632.1959.tb39571.x. [DOI] [PubMed] [Google Scholar]
- Sola D.; Rossi L.; Schianca G. P. C.; Maffioli P.; Bigliocca M.; Mella R.; Derosa G. (2015) Sulfonylureas and their use in clinical practice. Arch. Med. Sci. 11, 840–848. 10.5114/aoms.2015.53304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi S. M. D.; Shaikh S.; Naaz D.; Shakil S.; Ahmad A.; Mohd; Haneef A.; Abuzenadah A. N. (2016) Kinetics and Molecular Docking Study of an Anti-diabetic Drug Glimepiride as Acetylcholinesterase Inhibitor: Implication for Alzheimer’s Disease-Diabetes Dual Therapy. Neurochem. Res. 41, 1475–1482. 10.1007/s11064-016-1859-3. [DOI] [PubMed] [Google Scholar]
- Ellman G. L.; Courtney K. D.; Andres V.; Featherstone R. M. (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 7, 88–95. 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Pheifer J. H.; Briggs D. E. (1995) The estimation of thiols and disulphides in barley. J. Inst. Brew. 101, 5–10. 10.1002/j.2050-0416.1995.tb00843.x. [DOI] [Google Scholar]
- Copeland R. A. (2018) Evaluation of Enzyme Inhibitors in Drugs Discovery: A Guide for Medicinal Chemists and Pharmacologists, second ed., Chapter 5, John Wiley & Sons, Inc., New York. [PubMed] [Google Scholar]
- Chen X.; Wehle S.; Kuzmanovic N.; Merget B.; Holzgrabe U.; König B.; Sotriffer C. A.; Decker M. (2014) Acetylcholinesterase inhibitors with photoswitchable inhibition of β- amyloid aggregation. ACS Chem. Neurosci. 5, 377–389. 10.1021/cn500016p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M. M.; Sonu V. K.; Gashnga P. M.; Moyon N. S.; Mitra S. (2016) Caffeine and Sulfadiazine Interact Differently with Human Serum Albumin: A Combined Fluorescence and Molecular Docking Study. Spectrochim. Acta, Part A 152, 23–33. 10.1016/j.saa.2015.07.051. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. R. (1999) Principles of Fluorescence Spectroscopy, 2nd ed., Kluwer Academic/Plenum Publishers, New York. [Google Scholar]
- Friesner R. A.; Murphy R. B.; Repasky M. P.; Frye L. L.; Greenwood J. R.; Halgren T. A.; Sanschagrin P. C.; Mainz D. T. (2006) Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein-Ligand Complexes. J. Med. Chem. 49, 6177–6196. 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- Huang J.; MacKerell A. D. Jr (2013) CHARMM36 all-atom additive protein force field: validation based on comparison to NMR data. J. Comput. Chem. 34, 2135–2145. 10.1002/jcc.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanommeslaeghe K.; Hatcher E.; Acharya C.; et al. (2010) CHARMM General Force Field (CGenFF): A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 31, 671–690. 10.1002/jcc.21367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen W. L.; Chandrasekhar J.; Madura J. D.; Impey R. W.; Klein M. L. (1983) Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935. 10.1063/1.445869. [DOI] [Google Scholar]
- Bussi G.; Donadio G.; Parrinello M. (2007) Canonical sampling through velocity rescaling. J. Chem. Phys. 126, 014101. 10.1063/1.2408420. [DOI] [PubMed] [Google Scholar]
- Basha S. J.; Mohan P.; Yeggoni D. P.; Babu Z. R.; Kumar P. B.; Rao A. D.; Subramanyam R.; Damu A. G. (2018) New Flavone-Cyanoacetamide Hybrids with a Combination of Cholinergic, Antioxidant, Modulation of β-Amyloid Aggregation, and Neuroprotection Properties as Innovative Multifunctional Therapeutic Candidates for Alzheimer’s Disease and Unraveling Their Mechanism of Action with Acetylcholinesterase. Mol. Pharmaceutics 15, 2206–2223. 10.1021/acs.molpharmaceut.8b00041. [DOI] [PubMed] [Google Scholar]
- Kuntz I. D.; Gasparro F. P.; Johnston M. D. Jr; Taylor R. P. (1968) Molecular interactions and the Benesi-Hildebrand equation. J. Am. Chem. Soc. 90, 4778–4781. 10.1021/ja01020a004. [DOI] [Google Scholar]
- Nawaz S. A.; Ayaz M.; Brandt W.; Wessjohann L. A.; Westermann B. (2011) Cation-π and π-π Stacking Interactions Allow Selective Inhibition of Butyrylcholinesterase by Modified Quinine and Cinchonidine Alkaloids. Biochem. Biophys. Res. Commun. 404, 935–940. 10.1016/j.bbrc.2010.12.084. [DOI] [PubMed] [Google Scholar]
- Cheng S.; Song W.; Yuan X.; Xu Y. (2017) Gorge Motions of Acetylcholinesterase Revealed by Microsecond Molecular Dynamics Simulations. Sci. Rep. 7, 3219. 10.1038/s41598-017-03088-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoni G.; de Sousa J.; de la Mora E.; Dias J.; Jean L.; Sussman J. L.; Silman I.; Renard P.; Brown R. C. D.; Weik M.; Baati R.; Nachon F. (2018) Structure-Based Optimization of Nonquaternary Reactivators of Acetylcholinesterase Inhibited by Organophosphorus Nerve Agents. J. Med. Chem. 61, 7630–7639. 10.1021/acs.jmedchem.8b00592. [DOI] [PubMed] [Google Scholar]
- Bennion B. J.; Essiz S. G.; Lau E. Y.; Fattebert J. L.; Emigh A.; Lightstone F. C. (2015) A wrench in the works of human acetylcholinesterase: soman induced conformational changes revealed by molecular dynamics simulations. PLoS One 10, 0121092. 10.1371/journal.pone.0121092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melander A.; Sartor G.; Wåhlin E.; Scherstén B.; Bitzén P. O. (1978) Serum tolbutamide and chlorpropamide concentrations in patients with diabetes mellitus. Br. Med. J. 1 (6106), 142–144. 10.1136/bmj.1.6106.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran S. D.; Philip B. K.; Gopinath R.; Suresh B. (2007) RPHPLC method for the estimation of glibenclamide in human serum. Indian J. Pharm. Sc. 69, 796–799. 10.4103/0250-474X.39436. [DOI] [Google Scholar]
- Pelaz B.; Alexiou P.; Alvarez-Puebla R. A. (2017) Diverse Applications of Nanomedicine. ACS Nano 11, 2313–2381. 10.1021/acsnano.6b06040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonu V. K.; Rajkumar I.; Bhattacharjee K.; Joshi S. R.; Mitra S. (2019) Interaction of caffeine and sulfadiazine with lysozyme adsorbed at colloidal metal nanoparticle interface: influence on drug transport ability and antibacterial activity. J. Biomol. Struct. Dyn. 37, 321–335. 10.1080/07391102.2018.1426497. [DOI] [PubMed] [Google Scholar]
- Misson M.; Zhang H.; Jin B. (2015) Nanobiocatalyst advancements and bioprocessing applications. J. R. Soc., Interface 12, 20140891. 10.1098/rsif.2014.0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahab R. A.; Elias N.; Abdullah F.; Ghoshal S. K. (2020) On the taught new tricks of enzymes immobilization: An all-inclusive overview. React. Funct. Polym. 152, 104613. 10.1016/j.reactfunctpolym.2020.104613. [DOI] [Google Scholar]
- Wong S. W.; Khan F.; Micklefield J. (2009) Selective Covalent Protein Immobilization: Strategies and Applications. Chem. Rev. 109, 4025–4053. 10.1021/cr8004668. [DOI] [PubMed] [Google Scholar]
- Abbas S. A. R. (2011) The Effects of Gold and Silver Nanoparticles on Choline Estrase and Monoamino Oxidase Enzymes Activities. Int. J. Chem. 3, 61–68. 10.5539/ijc.v3n4p61. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.