Summary
Mutations in two type-3 receptor tyrosine kinases (RTK), KIT and FLT3, are common in both acute myeloid leukemia (AML) and systemic mastocytosis (SM) and lead to hyperactivation of key signaling pathways. Fortunately, a significant number of tyrosine kinase inhibitors TKIs have been developed that target either FLT3 or KIT and significant clinical benefit has been demonstrated in multiple clinical trials. Given the structural similarity of FLT3 and KIT, it is not surprising that some of these TKIs inhibit both of these receptors. This is typified by midostaurin, which has been FDA approved for mutant FLT3-positive AML and for KIT-D816V-positive SM. Here, we compare the in vitro activities of the clinically available FLT3 and KIT inhibitors with those of midostaurin against a panel of cells expressing a variety of oncogenic FLT3 or KIT receptors, including wild-type (wt) FLT3, FLT3-ITD, FLT3-D835Y, the resistance mutant FLT3-ITD+F691L, KIT-D816V, and KIT-N822K. We also examined the effects of these inhibitors in vitro and in vivo on cells expressing mutations in c-CBL found in AML that result in hypersensitization of TK receptors such as FLT3 and KIT. The results show a wide spectrum of activity of these various mutations to these clinically available TKIs.
Keywords: Tyrosine kinase inhibitors, KIT, FLT3, BLU-285, acute myeloid leukemia
Introduction
Acute myeloid leukemia (AML) is a hematologic malignancy that is marked by a partial block in differentiation and aberrant myeloid progenitor cell proliferation. Hyperactivation of various signaling pathways stems from genetic modifications that lead to mutations in key signaling molecules. Type-3 receptor tyrosine kinases (RTK), including KIT and FLT3, are frequently mutated or otherwise hyperactivated in AML and play a significant role in transformation.
Constitutively activated FLT3 (Fms-Like Tyrosine kinase-3; STK-1, human Stem Cell Tyrosine Kinase-1; or FLK-2, Fetal Liver Kinase-2) is detected in approximately one third of AML patients and confers a poor prognosis (Stirewalt and Radich, 2003). Internal tandem duplications in the juxtamembrane (JM) domain (FLT3-ITD) represents the most prevalent form of FLT3 mutation identified up to this point, with an occurrence of up to 25% in AML and less than 5% in patients with myelodysplastic syndrome (MDS) (Nakao et al., 1996; Horiike et al., 1997).
Mutations within the tyrosine kinase domain (TKD) of FLT3 are the second most common type of FLT3 mutation in AML, identified in up to 14% of adult AML patients (Bacher et al., 2008; Thiede et al., 2002; Frohling et al., 2002). Point mutations in the “activation loop” of FLT3 kinase, which are proposed to alter the conformation of the kinase domain and in essence activate the protein, have been identified in around 7% of AML patients, with a change in the aspartic acid residue at position 835 being the most frequently occurring (Yamamoto et al., 2001). Other mutations were identified in an in vitro screen carried out to identify mutations conferring resistance to FLT3 inhibitors; this screen led to identification of mutations at four distinct residues in the ATP-binding pocket of FLT3 conferring varying levels of resistance (Asn-676, Phe-691, Ala-627, and Gly697) (Cools et al., 2004). In patients, a mutation at the ‘gatekeeper’ residue F691, as well as mutations at the D835 residue (D835H/Y), were found to be associated with treatment with, and resistance to, the wide-spectrum, first generation FLT3 inhibitor, sorafenib (DB00398; Nexavar; co-developed and co-marketed by Bayer (Whippany, NJ, USA) and Onyx Pharmaceuticals (South San Francisco, CA, USA) (Zhang et al. 2008; Baker et al., 2013), and mutations at residue D835 and the F691L mutation were also observed in association with treatment with the highly targeted second generation FLT3 inhibitor, quizartinib (AC220; Daiichi Sankyo (Basking Ridge, NJ, USA)) (Smith et al., 2012; Zarrinkar et al., 2009; Galanis et al., 2015).
Quizartinib and sorafenib are among a sizable number of FLT3 inhibitors that have been developed that are either in late-stage clinical trials, or that have been FDA approved. Other FLT3 inhibitors include midostaurin (PKC412; Rydapt; Novartis Pharma AG (Basel, Switzerland)), which is a first generation FLT3 inhibitor approved for mutant FLT3-positive AML (Weisberg et al., 2002; Levis et al., 2017), crenolanib besylate (CP-868596; AROG Pharmaceuticals, LLC (Dallas, TX, USA)) (Smith et al., 2014), which is a second generation FLT3 inhibitor, and gilteritinib (ASP2215, XOSPATA; Astellas Pharma US, Inc. (Northbrook, IL, USA) (Thom, 2015; Lee et al., 2017) which is another second generation FLT3 inhibitor FDA-approved for patients with relapsed or refractory AML with an identified FLT3 mutation, based on results of the ADMIRAL trial (NCT02421939).
Hyperactivated FLT3 can also result from mutations in the E3 ubiquitin ligase, CBL, which catalyzes ubiquitylation of both non-mutated FLT3 and FLT3-ITD (Sargin et al., 2007). Loss-of-function CBL mutations occur in a small percentage (1.1%) of AML/MDS patients but are particularly common (16%) in patients with inv(16)/t(16;16) chromosomal abnormalities (Reindl et al., 2009; Haferlach et al., 2010).
Transforming mutations of KIT in AML are detected in about 5% of patients, and are typically located in exon 17, encoding the kinase domain activation loop, with D816V being the primary mutation. Interestingly, this same mutation is typical of systemic mastocytosis (SM), a rare disorder characterized by overproduction of mast cells that accumulate in the skin and organs) (Nagata et al., 1995; Hirota et al., 1998; Willmore-Payne et al., 2005; Kemmer et al., 2004). Prognosis is generally poor for advanced SM patients, such as SM with an associated hematologic neoplasm (SM-AHN), mast cell leukemia (MCL), and aggressive SM (ASM), and treatment options are limited. Over 80% of SM patients have mutations in KIT, with KIT-D816V the most common. This mutation is characterized by an active state conformation (Mol et al., 2004).
The KIT-N822K mutation is expressed in Kasumi-1 and SKNO-1 cell lines, which also express the t(8;21)(q22;q22) translocation that generates the RUNX1-RUNX1T1 fusion gene (Larizza et al., 2005; Becker et al., 2008). The KIT-N822K mutation has been characterized as being less transforming than KIT-D816V, namely in its ability to induce cell proliferation or suppress apoptosis (Omori et al., 2017). However, activating KIT mutations are common in RUNX1-RUNX1T1-positive AML, with KIT mutations identified in nearly half of RUNX1-RUNX1T1-positive AML patients studied (Wang et al., 2005; Jiao et al., 2009). The importance of KIT-N822K in AML pathogenesis is also exemplified by its demonstrated cooperation with RUNX1-RUNX1T1 in inducing AML in mice, supporting the two-hit model dictating that signaling molecules and transcription factors are both required for AML to fully develop (Wang et al., 2011).
Adding to the complexity of kinase inhibitors and at the same time providing a structural basis for their activity are their classification as either “type 1” and “type 2”, based on the distinct ways in which they physically interact with the growth factor receptor(s) they target. Briefly, while all FLT3 inhibitors competitively block ATP binding by physically associating with the ATP-binding site of the TKD, type 1 inhibitors only interact with the ATP-binding site of an active receptor whereas type 2 inhibitors only interact with a region proximal to the ATP-binding site of an inactive receptor (Larrosa and Baer, 2017). Certain TKD mutations, such as FLT3-D835Y, favor the active conformation of the FLT3 receptor, and so are more sensitive to type 1 inhibitors than type 2 inhibitors (Larrosa and Baer, 2017).
The first “type 1” TKI demonstrated to exhibit substantial clinical activity against KIT exon 17 mutations was midostaurin (Growney et al., 2005; Gleixner et al., 2006; Gotlib et al., 2016), which, in addition to being approved for mutant FLT3-positive AML was also approved by the FDA for SM in 2017 (Levis et al., 2017). Recently, the more selective KIT inhibitor BLU-285 (avapritinib; Blueprint Medicines Corporation (Cambridge, MA, USA)) has been reported to have impressive clinical activity in early phase clinical trials, with a 72% overall response rate in ASM patients (Evans et al., 2017).
In this report, we compared the mutant FLT3 and mutant KIT inhibitory activity of a panel of inhibitors that included wide spectrum first generation inhibitors and highly selective second generation inhibitors, characterized as either type 1 or type 2 ATP-competitive inhibitors. Our findings support the clinical development of inhibitors capable of potently targeting FLT3-ITD as well as FLT3-TKD, and KIT mutations such as KIT-D816V, as therapeutics for a broader AML population characterized by either mutant FLT3 or mutant KIT. In contrast, other inhibitors with a more narrow range of targets due to type 2 classification, such as quizartinib, or a type 1 inhibitor like BLU-285 that preferentially inhibits mutant KIT over mutant FLT3, are ideally suited for a more focused subset of AML.
Materials and Methods
Chemical compounds and biologic reagents
For in vitro studies, midostaurin, crenolanib and quizartinib, were purchased from Haoyuan chemexpress (Shanghai, China). Sorafenib was purchased from LC Laboratories (Woburn, MA). Gilteritinib (Hydrochloride) was purchased from Chemietek (Indianapolis, IN). BLU-285 was purchased from Cayman Chemical (Ann Arbor, MI). All drugs were dissolved in DMSO to obtain a 10 mM stock solution. Serial dilutions were then made, to obtain final dilutions for cellular assays with a final concentration of DMSO not exceeding 0.1%.
Cell lines
Ba/F3 (interleukin [IL]-3-dependent murine pro-B) cells were transduced with KRAS G12D containing murine stem cell virus (MSCV) retroviruses harboring IRES-GFP and rendered growth factor-independent via IL-3 withdrawal from culture media (to develop Ba/F3-KRAS-G12D cells) (Weisberg et al., 2015). FLT3-ITD- and FLT3-D835Y-containing murine stem cell virus (MSCV) retroviruses were transfected into Ba/F3 cells as previously described (Kelly et al., 2002). A Ba/F3 cell line expressing FLT3-ITD with a mutation in the ATP-binding pocket (F691L) was developed as previously described (Cools et al., 2004). Ba/F3-BCR-ABL1 cells were obtained by transfecting the IL-3-dependent murine hematopoietic Ba/F3 cell line with a pGD vector containing p210BCR-ABL1 cDNA (Sattler et al., 1996). Ba/F3 cells engineered to express wt FLT3+CBL.Ins(SK366) and wt FLT3+CBL.Y371H were developed as previously described (Fernandes et al., 2010). Ba/F3 and Ba/F3-KIT-D816V cells were developed as previously described (Zermati et al., 2003).
The human AML-derived, FLT3-ITD-expressing line, MOLM14 (Matsuo et al., 1997), was provided to us by Dr. Scott Armstrong (DFCI). Human AML-derived, FLT3-ITD-expressing MV4,11 cells were obtained from Dr. Anthony Letai (Dana-Farber Cancer Institute). Kasumi-1-luc+ and SKNO-1-luc+ cells (both of which express KIT-N822K and express the t(8;21)(q22;q22) translocation that generates the RUNX1-RUNX1T1 fusion gene) were a gift from Dr. Andrew Kung (Memorial Sloan Kettering Cancer Center). TF-1 and TF-1-KIT-D816V cells were developed as previously described (Yang et al., 2010).
These cell lines were cultured with 5% CO2 at 37°C, at a concentration of 2×105 to 5×105 cells/mL in Gibco RPMI 1640 media with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. SKNO-1-luc+ cells and TF-1 cells were cultured in RPMI 1640 media with 10% FBS and 1% penicillin/streptomycin and supplemented with GM-CSF (2 ng/mL). Parental Ba/F3 cells were cultured in RPMI 1640 media with 10% FBS and 1% penicillin/streptomycin and supplemented with 20% WEHI-conditioned media (as a source of IL-3).
The MV4-11, MOLM14, SKNO-1-luc, and Kasumi-1-luc cell lines were submitted for cell line authentication and were authenticated within 6 months of manuscript preparation through cell line short tandem repeat (STR) profiling (Molecular Diagnostics Core, Dana-Farber Cancer Institute). All cell lines tested matched ≥80% with lines listed in the DSMZ Cell Line Bank STR database (https://www.dsmz.de/catalogues/catalogue-human-and-animal-cell-lines/quality-assurance/identity-control/authentication-of-cell-lines.html). All cell lines were confirmed to be virus- and mycoplasma-free.
Cell proliferation studies
The Trypan blue exclusion assay, previously described (Weisberg et al., 2002), was utilized for cell counting prior to seeding for CellTiter-Glo experiments. CellTiter-Glo (Promega, Madison, WI) was used for proliferation studies according to manufacturer instructions. Cell viability is shown in graphs as the percentage of control (untreated) cells; error bars represent the standard deviation for each data point.
Immunoblotting
Protein lysate preparation, immunoblotting, and immunoprecipitation were carried out as has been previously described (Weisberg et al., 2002).
Antibodies
Anti-GAPDH (14C10) (rabbit mAb, #2118) (Cell Signaling Technology (Danvers, MA)) was used at a dilution of 1:3000. Also purchased from Cell Signaling Technology were phospho-KIT (Tyr719) (rabbit Ab, #3391), phospho-KIT (Tyr703) (rabbit Ab, #3073), KIT (D13A2) XP (rabbit mAb, #3074), and phospho-FLT3 (Tyr591) (54H1) (mouse mAb, #3466); each was used at a dilution of 1:1000. FLT3/Flk-2 (C-20) (sc-479) (Santa Cruz Biotechnology, Inc. (Dallas, TX)) was used at a dilution of 1:1000, and p-TYR (4G10), #05-321 (MilliporeSigma (Burlington, MA)) was used at a dilution of 1:1000.
Non-invasive in vivo bioluminescence study
All animal studies were performed according to protocols approved by the Dana-Farber Cancer Institute's Institutional Animal Care and Use Committee.
Bioluminescence imaging was carried out as previously described (Weisberg et al., 2005). Briefly, Ba/F3.FLT3.CBL.Y371H-luc+ cells suspended in 1X PBS were implanted intravenously (1.5 × 106 cells/mouse) in the female NCr-nude mice (7 weeks of age; Taconic Laboratories, NY). Animals were randomized 3 days post implantation using total flux values (sum of prone and supine bioluminescence values) into vehicle control and midostaurin, 100 mg/kg once daily by oral gavage for 21 days (n=10/group). Bioluminescence imaging was performed once weekly after treatment initiation, and body weights were measured twice weekly.
Midostaurin was formulated as a pre-concentrate/microemulsion with 5% drug powder, 34% Vit E TPGS, 42.5% PEG400, 8.5% Corn oil, and 10% Ethanol. The pre-concentrate was then dissolved in purified water at a 24:76 ratio the day of treatment. Stocks of midostaurin were purchased from LC Laboratories (Woburn, MA) and Medchemexpress (Monmouth Junction, NJ).
For this study, P < 0.05 was considered to be statistically significant. The data had similar variance, and met the assumptions of the tests carried out. The Mann-Whitney test (two-tailed) was carried out to assess differences in leukemia burden between vehicle and drug-treated mice and the Gehan-Breslow-Wilcoxon test was carried out for survival curve comparisons.
Results
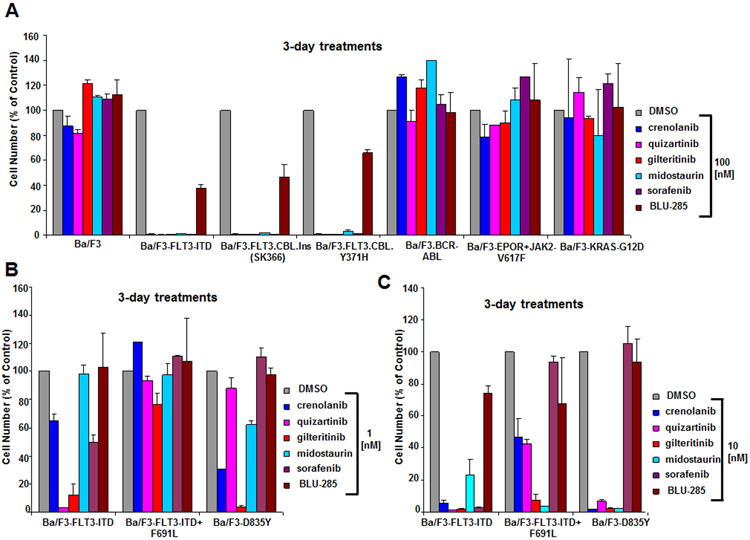
As mutated FLT3 is expressed in a large percentage of AML patients and has been identified as a promising therapeutic target, we first investigated and compared the potencies of a panel of FLT3 and KIT inhibitors against isogenic Ba/F3 cells expressing human oncogenic FLT3 (Ba/F3-FLT3-ITD). The KIT-targeting drug, BLU-285, was considerably less potent (IC50 near 100 nM) against Ba/F3-FLT3-ITD cells than a panel of targeted FLT3 inhibitors, including crenolanib, quizartinib, gilteritinib, midostaurin, and sorafenib (nearly all cells were killed at 1 nM by quizartinib and at 10 nM for the other FLT3 inhibitors) (Fig 1A and Supplementary Fig 1A). The selective nature of the FLT3 inhibitors was validated by their lack of anti-proliferative activity at 100 nM against parental Ba/F3 cells cultured in the presence of WEHI cell-conditioned media (used as a source of IL-3) or Ba/F3 cells driven by oncogenes other than FLT3, including BCR-ABL1, JAK2-V617F, and KRAS-G12D (Fig 1A).
Figure 1. Anti-proliferative effects of BLU-285 and FLT3 inhibitors on growth of oncogene-driven Ba/F3 cell lines.
(A) Anti-proliferative effects of BLU-285 and FLT3 inhibitors (100 nM) on growth of Ba/F3 cells driven by oncogenic FLT3 (Ba/F3-FLT3-ITD) or hyperactivated FLT3 (Ba/F3.FLT3.CBL.Ins (SK366) and Ba/F3.FLT3.CBL.Y371H) versus Ba/F3 cells driven by other oncogenes (Ba/F3.p210, Ba/F3-EPOR+JAK2-V617F, Ba/F3-KRAS-G12D), following 3 days of treatment. (B-C) Comparison of effects of BLU-285 and FLT3 inhibitors against Ba/F3-FLT3-ITD versus Ba/F3-FLT3-ITD+F691L cells and Ba/F3-D835Y cells at 1 nM (B) and 10 nM (C).
Point mutations in the tyrosine kinase domain of FLT3, such as the FLT3-D835Y mutation and the “gatekeeper” FLT3-F691L mutation, have been reported to diminish the potency of some FLT3 inhibitors, with FLT3-F691L conferring particularly strong resistance to sorafenib when co-expressed with FLT3-ITD (Baker et al., 2013). Briefly, sorafenib was reported to have an IC50 of 210 nM against Ba/F3-D835Y, compared with an IC50 of 11 nM for quizartinib and an IC50 of 1.5 nM for midostaurin (Baker et al., 2013). In this report, sorafenib also exhibited an IC50 of 1300 nM against Ba/F3-FLT3-ITD+F691L, compared with an IC50 of 210 nM for quizartinib and an IC50 of 19 nM for midostaurin (Baker et al., 2013). We observed a similar trend in terms of potency for these inhibitors as reported by Baker et al. (Fig 1B,C). At concentrations of 1-10 nM, gilteritinib, crenolanib, quizartinib, and sorafenib exhibited higher potency against Ba/F3-FLT3-ITD than Ba/F3-FLT3-ITD+F691L, consistent with what has been previously reported for quizartinib (Albers et al., 2013) (Fig 3B,C). The potency of midostaurin, in contrast, was similar against both Ba/F3-FLT3-ITD+F691L and Ba/F3-FLT3-ITD, as has been previously shown (Albers et al., 2013) (Fig 1B,C). BLU-285 at 1-10 nM showed comparatively little activity against Ba/F3-FLT3-ITD+F691L and Ba/F3-FLT3-ITD (Fig 1B,C). Consistent with published findings (Smith et al., 2015), quizartinib and sorafenib displayed higher potency against Ba/F3-FLT3-ITD than Ba/F3-D835Y (Fig 1B,C). Gilteritinib, midostaurin, and crenolanib potently killed Ba/F3 cells expressing either D835Y or FLT3-ITD at 1-10 nM, whereas BLU-285 had comparatively little effect on the growth of both cell lines at the same concentrations (Fig 1B,C). At 100 nM, we generally observed strong efficacy of the FLT3 inhibitors, with the exception of sorafenib (Supplementary Fig 1B). BLU-285 showed efficacy at 100 nM against Ba/F3 cells expressing FLT3-D835Y or co-expressing FLT3-ITD+FLT3-F691L, however it was less potent than the other inhibitors (Supplementary Fig 1B).
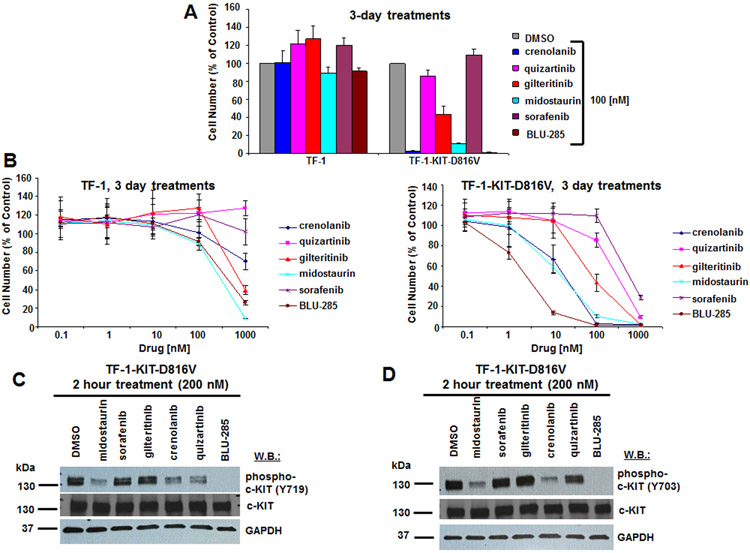
Figure 3. Anti-proliferative effects of BLU-285 and FLT3 inhibitors on growth of TF-1-KIT-D816V cells, and effects of FLT3 inhibitors versus BLU-285 on phosphorylation of KIT.
(A) Anti-proliferative effects of BLU-285 and FLT3 inhibitors (100 nM) on growth of TF-1 and TF-1-KIT-D816V cells following 3 days of treatment. (B) Proliferation studies: Growth curves showing drug treatment effects on TF-1 or TF-1-KIT-D816V cells following 3 days of treatment. (C,D) Immunoblots: Measurement of drug effects on phosphorylation of KIT in TF-1-KIT-D816V cells following two hour treatments with inhibitors at a concentration of 200 nM. Phospho-KIT (Y719) antibody was used for (C) and phospho-KIT (Y703) antibody was used for (D).
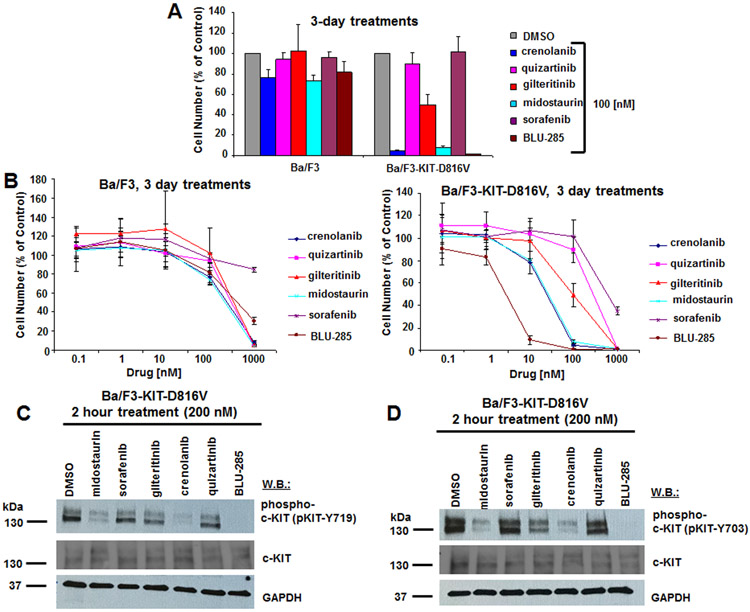
Considering the prevalence of KIT-D816V in AML and the ability of targeted FLT3 inhibitors to also target mutant KIT, we were interested in comparing the potencies of our selected panel of FLT3 and KIT inhibitors in clinical development. We first investigated the potency of these inhibitors against isogenic Ba/F3 cells expressing KIT-D816V. At 100 nM, crenolanib and midostaurin showed similar potency to BLU-285 with the majority of Ba/F3-KIT-D816V cells killed at that concentration (Fig 2A). However, the potency of BLU-285 was around 10-fold higher than that of crenolanib and midostaurin, with an IC50 for BLU-285 between 1 and 10 nM and an IC50 for crenolanib and midostaurin between 10 and 100 nM (Fig 2B). Similar results were observed with TF-1-D816V cells treated with the inhibitors (Fig 3A, B). A sizable therapeutic window was observed for crenolanib, midostaurin, and BLU-285, with growth factor-dependent parental Ba/F3 cells and TF-1 cells showing very little response to the inhibitors in terms of growth inhibition, as compared to their KIT-D816V-transformed counterparts (Fig 2A, B and Fig 3A, B). At 200 nM, midostaurin and crenolanib, like BLU-285, led to strong inhibition of phosphorylation of KIT at tyrosine residues 703 and 719 in Ba/F3-KIT-D816V cells (Fig 2C,D) and TF-1-KIT-D816V cells (Fig 3C,D). Inhibition of phospho-KIT by midostaurin and BLU-285 was apparent at a concentration of 100 nM as well in TF-1-KIT-D816V cells (Supplementary Fig 2). Quizartinib inhibited phosphorylation of KIT at tyrosine residue 719, however not at tyrosine residue 703, in TF-1-KIT-D816V cells, however this did not correlate with the lack of growth suppression of TF-1-KIT-D816V cells and thus its significance is unclear.
Figure 2. Anti-proliferative effects of BLU-285 and FLT3 inhibitors on growth of Ba/F3-KIT-D816V cells, and effects of FLT3 inhibitors versus BLU-285 on phosphorylation of KIT.
(A) Anti-proliferative effects of BLU-285 and FLT3 inhibitors (100 nM) on growth of Ba/F3 and Ba/F3-KIT-D816V cells following 3 days of treatment. (B) Proliferation studies: Growth curves showing drug treatment effects on Ba/F3 or Ba/F3-KIT-D816V cells following 3 days of treatment. (C,D) Immunoblots: Measurement of drug effects on phosphorylation of KIT in Ba/F3-KIT-D816V cells following 2 hour treatments with inhibitors at a concentration of 200 nM. Phospho-KIT (Y719) antibody was used for (C) and phospho-KIT (Y703) antibody was used for (D).
In addition to mutational activation, the wt forms of FLT3 and KIT can also be activated by mutant forms of the E3 ubiquitin ligase c-CBL (Fernandes et al., 2010). CBL is altered in 1-3% of patients with de novo AML (Reindl et al., 2009). Loss-of-function mutations in CBL lead to a transformed phenotype characterized by hyperactivation or hypersensitivity of FLT3 or KIT (Fernandes et al., 2010). We were interested in comparing the efficacy of BLU-285 and midostaurin against mutant CBL-dependent cells. In patients, it would be expected that both FLT3 and KIT are activated by CBL mutations and dual FLT3/KIT inhibitors would be therefore predicted to be necessary for efficacy. We therefore focused primarily on experimental models containing wt FLT3 in combination with mutant CBL. We observed potent and selective growth suppression by our panel of FLT3 inhibitors at a concentration of 100 nM, including crenolanib, quizartinib, gilteritinib, midostaurin, and sorafenib, which were used to treat Ba/F3 cells characterized by hyperactivation of wt human FLT3 due to impaired c-CBL E3 ligase activity (Ba/F3-CBL.Ins (SK366) and Ba/F3-CBL.Y371H) (Fig 1A). BLU-285 was less potent against the mutant CBL-expressing lines, similar to that observed with this agent against Ba/F3-FLT3-ITD cells (Fig 1A).
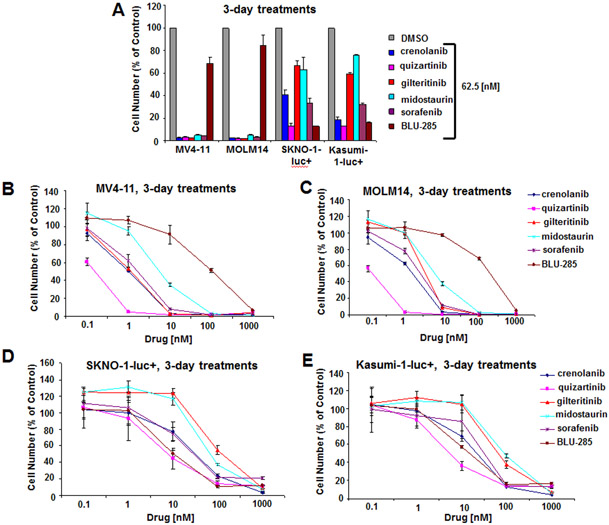
We next investigated the activity of BLU-285 and our panel of FLT3 inhibitors against FLT3-ITD-positive human AML cell lines (MV4-11 and MOLM14) and against mutant KIT (Asn822Lys)-expressing human AML cells (SKNO-1-luc+ and Kasumi-1-luc+ ). At a concentration of 62.5 nM, the potency of the FLT3 inhibitors was considerably higher as compared to BLU-285 against the two mutant FLT3-expressing lines, with the majority of cells growth suppressed by the FLT3 inhibitors and very little inhibition by BLU-285 (Fig 4A). In contrast, the potency of several of the FLT3 inhibitors (sorafenib, crenolanib, quizartinib) and BLU-285 against the mutant KIT-expressing lines at 62.5 nM was similarly strong with the majority of cells growth inhibited (Fig 4A). Full dose-response curves reflect the lower sensitivity of MV4-11 and MOLM14 cells to BLU-285 as compared to the panel of FLT3 inhibitors, as well as the similar potency of BLU-285 and several of the FLT3 inhibitors against mutant KIT-expressing Kasumi-1-luc+ and SKNO-1-luc+ cells (Fig 4B-E and Supplementary Fig 3).
Figure 4. Anti-proliferative effects of BLU-285 and FLT3 inhibitors on growth of FLT3-ITD- and KIT (N822K mutation)-expressing human AML cell lines.
(A) Anti-proliferative effects of BLU-285 and FLT3 inhibitors (62.5 nM) on growth of N822K-expressing and FLT3-ITD-expressing human AML cell lines following 3 days of treatment. (B,C) Proliferation studies: Growth curves showing drug treatment effects on FLT3-ITD+ MV4-11 and MOLM14 cells following 3 days of treatment. (D,E) Proliferation studies: Growth curves showing drug treatment effects on N822K+ SKNO-1-luc+ and Kasumi-1-luc+ cells following 3 days of treatment.
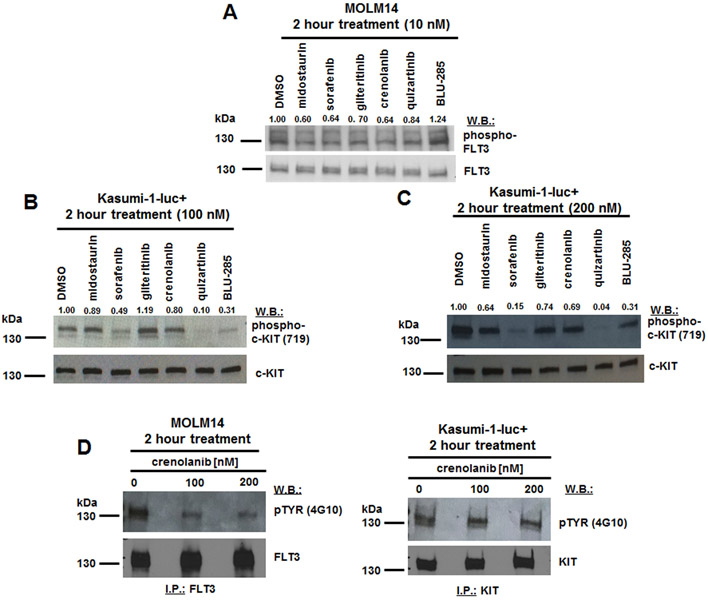
Signaling studies were performed and show inhibition of phosphorylation of FLT3 in MOLM14 cells by FLT3 inhibitors at 10 nM but not by BLU-285 (Fig 5A). Strongest inhibition of phosphorylation of KIT in Kasumi-1-luc+ cells was observed for sorafenib and quizartinib, as well as BLU-285, at 100-200 nM (Fig 5B, C). Despite strong inhibition of Kasumi-1 cell growth by crenolanib, we observed no significant inhibition of KIT phosphorylation at tyrosine residue 719 by crenolanib in these cells (Fig 5B,C). A KIT immunoprecipitation followed by pTYR immunoblot was performed using DMSO- or crenolanib (100, 200 nM)-treated Kasumi-1-luc+ cells to ensure that all KIT phosphorylation sites were investigated as potential targets for inhibition by crenolanib; a FLT3 immunoprecipitation followed by pTYR immunoblot was also performed in parallel using crenolanib-treated MOLM14 cells as a positive control (Fig 5D and Supplementary Fig 4). As expected, crenolanib at 100-200 nM inhibited FLT3 phosphorylation in MOLM14 cells, however we did not observe inhibition of KIT phosphorylation in crenolanib (100, 200 nM)-treated Kasumi-1-luc+ cells (Fig 5D and Supplementary Fig 4). It is thus possible that the inhibitory effect of crenolanib on Kasumi-1-luc+ cell growth is independent of KIT, and may be an off-target effect.
Figure 5. Effects of FLT3 inhibitors versus BLU-285 on phosphorylation of FLT3 in FLT3-ITD-expressing MOLM14 cells and on phosphorylation of KIT in N822K-expressing Kasumi-1-luc+ cells.
(A-C) Immunoblots: Measurement of drug effects on phosphorylation of FLT3 in MOLM14 cells (A) and on phosphorylation of KIT in Kasumi-1-luc+ cells (B, C) following 2 hour treatments with inhibitors at the indicated concentrations. Phospho-KIT (Y719) antibody was used for B and C. (D) KIT immunoprecipitation/pTYR immunoblot for crenolanib (100-200 nM) -treated Kasumi-1; as positive control, FLT3 immunoprecipitation/pTYR immunoblot for crenolanib (100-200 nM)-treated MOLM14.
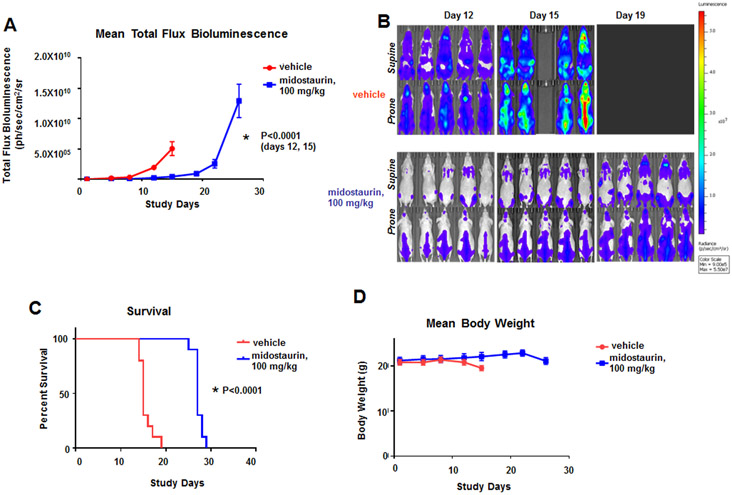
We next asked whether midostaurin could disrupt the mutant CBL-mediated proliferative activity in an in vivo model of mutant CBL characterized by hypersensitization of TK receptors such as FLT3 and KIT (Fernandes et al., 2010) (Fig 6). Midostaurin significantly lowered leukemia burden as a function of bioluminescence in mice (Fig 6A, B and Supplementary Fig 5), and significantly increased median survival, 15 days for vehicle control vs. 26 days for midostaurin (Fig 6C). Midostaurin was well tolerated at the tested dose (Fig 6D). Taken together, these data suggest that mutant CBL-driven growth can be efficiently targeted in vivo by midostaurin and this model may also show efficacy with other dual FLT3/KIT inhibitors. Poor in vitro efficacy of BLU-285 in this model would imply that it has inferior clinical efficacy in AML with mutant CBL compared to midostaurin.
Figure 6. Effects of midostaurin in vivo against mice harboring Ba/F3.FLT3.CBL.Y371H-luc+ cells.
(A) Measure of leukemia burden in vehicle- versus midostaurin-treated mice for CBL.Y371H murine model. Vehicle-treated, female Ncr nude mice were administered vehicle by oral gavage once daily x 21 days. Midostaurin-treated mice were administered 100 mg/kg midostaurin by oral gavage once daily x 21 days. For total flux bioluminescence, the Mann-Whitney test (two-tailed) was carried out for CBL.Y371H BLI for Day 12: Vehicle versus midostaurin, p=0.0001. The Mann-Whitney test (two-tailed) was carried out for CBL.Y371H BLI for Day 15: Vehicle versus midostaurin. P<0.0001. (B) Effects of midostaurin in vivo against mice harboring Ba/F3.FLT3.CBL.Y371H-luc+ cells. Supine and Prone (High Scale), Day 12 – 19 Representative Images (n=5). (C) Measure of survival of vehicle- versus midostaurin-treated mice for CBL.Y371H murine model. Gehan-Breslow-Wilcoxon test was carried out for survival curve comparisons. P<0.0001. (D) Mean weights of mice from the CBL.Y371H murine model.
A comparison of IC50s generated for the inhibitors tested against human AML cell lines expressing mutant FLT3 or mutant KIT is shown in Supplementary Table 1, and a summary of key findings is shown in Supplementary Table 2. Taken together, our data suggest that FLT3 inhibitors can kill AML cells expressing mutant KIT with similar potency to BLU-285. However, BLU-285 is less potent than the FLT3 inhibitors against AML cells expressing oncogenic FLT3.
Discussion
Activating mutations in type-3 RTKs are prevalent in AML and lead to deregulation of major signaling pathways. Common in AML are mutations of the type-3 RTK, FLT3, which have been detected in around one-third of AML patients (Stirewalt and Radich, 2003). Another commonly mutated type-3 RTK is KIT, which is expressed in mast cells and hematopoietic stem cells, among other tissues (Miettinen et al., 2005). Gain-of-function mutations in KIT have been detected in hematologic malignancies, including AML and SM, as well as non-hematologic malignancies, such as germ cell tumor and GIST (Miettinen et al., 2005).
FLT3 inhibitors are divided into type 1 or type 2 classifications due to the manner with which they interact with kinase targets: namely, type 1 inhibitors target either FLT3-ITD or FLT3-TKD point mutations and type 2 inhibitors target FLT3-ITD, however generally not FLT3 TKD point mutations although there are exceptions (Smith et al., 2015; Larrosa-Garcia and Baer, 2017). “Type 1” tyrosine kinase inhibitors (TKIs) bind to the catalytically active “DFG-in” conformation of a kinase and directly compete with ATP in the ATP binding site; they are distinguishable from “type 2” or “DFG-out” ATP-competitive inhibitors, which contrarily engage a hydrophobic binding site adjacent to the ATP binding site and bind exclusively to the inactive conformation of the kinase (Liu and Gray, 2006). For instance, while imatinib (classified as a type II TKI) (Roskoski, 2016), is a potent inhibitor of wt KIT, it does not effectively inhibit the KIT-D816V mutant (Frost et al., 2002). Interestingly, dasatinib (classified as a type I/I½A TKI) (Roskoski, 2016), inhibits KIT D816V potently in preclinical models (Shah et al., 2006), but clinical results were modest.
Examples of type 1 FLT3 inhibitors that potently inhibit both FLT3-ITD and FLT3-TKD mutants such as FLT3-D835Y, which favors the active conformation, include the following first generation inhibitors (lacking specificity for FLT3): sunitinib (SU11248), midostaurin, and lestaurtinib (CEP-701), and the following second generation inhibitors (that have higher potency and selectivity for FLT3): KW-2449, crenolanib, and gilteritinib (Larrosa-Garcia and Baer, 2017). Examples of type 2 FLT3 inhibitors that potently inhibit FLT3-ITD but not FLT3-D835Y include the following first generation inhibitors: sorafenib, ponatinib (AP24534) and tandutinib (MLN518), and the following second generation inhibitor: quizartinib (Larrosa-Garcia and Baer, 2017).
Exon 17 KIT mutations like KIT-D816V shift the KIT kinase conformation to the active state, and are thus insensitive to “type 2” TKIs, such as imatinib, which only bind to the inactive kinase conformation (Mol et al., 2004). Midostaurin belongs to the ATP-competitive “type-I” class of inhibitors, which bind to the catalytically active conformation of KIT, and was among the first “type 1” TKIs to demonstrate activity against KIT-D816V, with an IC50 of 30-40 nM reported in preclinical studies and clinical efficacy in advanced SM patients that led to its FDA approval (Growney et al., 2005; Gleixner et al., 2006; Gotlib et al., 2016).
BLU-285 is another “type 1” inhibitor demonstrated to interact with the active conformation of KIT and PDGFRA and potently inhibit KIT-D816V and PDGFRA-D842V (Evans et al., 2017). BLU-285 has shown impressive clinical activity in a phase 1 study in ASM and GIST patients with KIT mutations as well as GIST patients with PDGFRA mutations (Evans et al., 2017).
In our studies shown here, we rationally selected a panel of agents ranging from two first generation, broad spectrum FLT3 inhibitors (midostaurin and sorafenib) to several more highly targeted, second generation FLT3 inhibitors (crenolanib, quizartinib, and gilteritnib), with inhibitors categorized as either type 1 or type 2 ATP-competitive inhibitors, to compare their effectiveness as therapeutics for AML characterized by mutant FLT3 or mutant KIT. Included in our panel of inhibitors, for the purpose of direct comparison with the more FLT3-selective inhibitors tested, such as quizartinib and gilteritinib, was the highly KIT-selective BLU-285. As predicted and previously reported (Heinrich et al., 2012a; Kampa-Schittenhelm et al., 2013), the “type 2” FLT3 inhibitors quizartinib and sorafenib (Zhang et al., 2008; Zarrinkar et al., 2009; Larrosa-Garcia and Baer, 2017) were inactive against KIT-D816V-expressing Ba/F3 and TF-1 cells. In contrast, despite the “type 1” inhibitor crenolanib showing modest inhibition of non-mutated KIT (Heinrich et al., 2012b; Larrosa-Garcia and Baer, 2017), crenolanib- like the “type 1” inhibitor midostaurin- was highly effective in suppressing growth of KIT-D816V-expressing cells via inhibition of KIT phosphorylation. Our results support previous reports showing potent induction of apoptosis of KIT-D816V-expressing cells by crenolanib (Shittenhelm et al., 2014). Finally, the “type 1” classified ATP-competitive inhibitor, gilteritinib (Thom, 2015; Lee et al., 2017; Larrosa-Garcia and Baer, 2017), which is under clinical investigation due to its efficacious and selective pan-FLT3 mutant inhibitory activity, showed growth inhibition of KIT-D816V-expressing cells that was intermediate between that of the “type 2” inhibitors and the other “type 1” inhibitors, midostaurin, crenolanib, and BLU-285, against KIT-D816V-expressing cells. However, the inhibitory effect of gilteritinib on KIT phosphorylation was modest in Ba/F3-KIT-D816V cells and undetectable in TF-1-KIT-D816V cells.
Our results strongly suggest that classification of FLT3/KIT inhibitors as type 1 or type 2 is an important consideration, especially if genotyping information is available for a patient. An AML patient characterized by KIT-D816V or FLT3-D835Y is likely to benefit more from a type 1 ATP-competitive inhibitor, such as midostaurin, BLU-285, or crenolanib, than a type 2 ATP-competitive inhibitor.
The KIT-N822K mutation, which is expressed in Kasumi-1 and SKNO-1 cell lines, is common in RUNX1-RUNX1T1-positive AML and plays an important role in AML pathogenesis through its association with RUNX1-RUNX1T1 (Larizza et al., 2005; Becker et al., 2008; Wang et al., 2011). Among the most efficacious of the FLT3 inhibitors of KIT-N822K-expressing AML cells and exhibiting a potency similar to that of BLU-285 was quizartinib, which was followed by sorafenib and crenolanib. Results for BLU-285, sorafenib, and quizartinib are consistent with the previously demonstrated high potency of these inhibitors against the KIT-N822K mutant (Hu et al., 2008; Kampa-Schittenhelm et al., 2013; Evans et al., 2017). Although Kasumi-1 and SKNO-1 were potently growth inhibited by crenolanib, there was little observed inhibition of phosphorylation of KIT by crenolanib in Kasumi-1 cells; these results suggest that the crenolanib-induced growth suppression may be KIT-independent.
The potencies of midostaurin against both mutant FLT3-positive AML and mutant KIT-positive AML are such that one would expect concentrations determined in vitro in our assays to be achievable in human patients. Plasma concentrations of midostaurin reported in a clinical trial for AML were a few μM (Fischer et al., 2010). As the IC50s for midostaurin against the panel of oncogene-driven mouse lines and human AML lines were no higher than 100 nM, achievable plasma concentrations in patients would be expected to be more than adequate for midostaurin to achieve efficacy in AML patients. In addition, as has been previously observed (Stone et al., 2017), treatment of AML patients harboring mutant FLT3 with midostaurin as a single agent is not likely to lead to a complete remission and thus combination with other agents could potentially translate into additional clinical benefit. Overall response rates of first generation inhibitors such as midostaurin have been substantially improved when these inhibitors are combined with standard chemotherapy (Fischer et al., 2010; Ravandi et al., 2013; Strati et al., 2015).
In general, for mutant FLT3-positive MOLM14 and MV4-11, IC50s for the FLT3 inhibitors tested were 10 nM or lower, and 100-1000 nM for BLU-285. For mutant KIT-positive SKNO-1-luc+ and Kasumi-1-luc+, IC50s for all inhibitors were 100 nM or lower. For TF-1-D816V cells, IC50s for most inhibitors were less than or equal to 100 nM. Exceptions were quizartinib and sorafenib, which showed IC50s in the 100-1000 nM range. The different half-lives of the second generation FLT3 inhibitors would need to be taken into consideration when predicting efficacy in patients and optimal dosing. For instance, crenolanib has a short half-life (6-8 hours) without any accumulation after chronic dosing, and this requires a thrice-daily dosing regimen. In contrast, quizartinib and gilteritinib need to only be administered once daily.
In conclusion, we present here a comparison of the anti-proliferative activities of midostaurin, sorafenib, crenolanib, quizartinib, gilteritinib, and BLU-285 against AML cells driven by or expressing various oncogenic FLT3 or KIT alleles. Specifically, our studies reveal a broad spectrum of activity against FLT3-ITD (quizartinib>gilteritinib/ crenolanib>sorafenib/midostaurin>>BLU-285), FLT3-D835Y (gilteritinib>crenolanib>midostaurin>quizartinib>>sorafenib/BLU-285), FLT3-ITD+F691L (midostaurin>gilteritinib>quizartinib>crenolanib>BLU-285>sorafenib), KIT-D816V (BLU-285>crenolanib/midostaurin>gilteritinib>quizartinib/sorafenib), KIT-N822K (quizartinib>BLU-285>sorafenib/crenolanib>midostaurin>gilteritinib), and CBL.Ins (SK366) and Ba/F3.FLT3CBL.Y371H (quizartinib/sorafenib/gilteritinib/crenolanib>midostaurin>>BLU-285).
Taken together, our results highlight the potential for FLT3 inhibitors to be developed clinically for an AML population characterized by mutant FLT3 or mutant KIT expression, with a highly KIT-targeted agent like BLU-285 optimal for a more specific AML subpopulation characterized by mutant KIT expression. In addition, our findings support the consideration of type 1 inhibitors able to target both FLT3-ITD and FLT3-TKD mutants or KIT-D816V as promising treatments for a more general AML patient population characterized by mutations in either FLT3 or KIT. Contrary to this, type 2 inhibitors, such as quizartinib or sorafenib, may be considered optimal treatments for more restricted AML subtypes.
Supplementary Material
Acknowledgements
Ellen Weisberg: Wrote paper and designed the research study
Chengcheng Meng: Performed the research
Abigail Case: Performed the research
Martin Sattler: Designed the research study
Hong L. Tiv: Designed and performed mouse study
Prafulla C. Gokhale: Designed and performed mouse study
Sara Buhrlage: Wrote the paper
Xiaoxi Liu: Assessed purity of midostaurin stock for in vivo testing
Jing Yang: Performed the research
Jinhua Wang: Provided essential reagents
Nathanael Gray: Provided essential reagents
Richard Stone: Designed the research study
Sophia Adamia: Provided essential reagents
Patrice Dubreuil: Provided essential reagents
Sebastien Letard: Provided essential reagents
James D. Griffin: Wrote the paper and designed the research study
This work was funded by Project Program Grant, NIH PO1CA66996.
COI statement:
James D. Griffin receives funding and has received a royalty payment from Novartis Pharmaceuticals and receives funding from Eli Lilly and Company.
Nathanael Gray is a founder, science advisory board member (SAB) and equity holder in Gatekeeper, Syros, Petra, C4, B2S and Soltego. The Gray lab receives or has received research funding from Novartis, Takeda, Astellas, Taiho, Janssen, Kinogen, Voronoi, Her2llc, Deerfield and Sanofi.
Ellen Weisberg has received a royalty payment from Novartis Pharmaceuticals.
Richard Stone does Ad Hoc consulting for and receives clinical research support to Dana-Farber Cancer Institute from the following companies: Abbvie, Agios, Arog, and Novartis. He does Ad Hoc consulting for the following companies: Astrazeneca, Cornerstone, Jazz, Daiichi-Sankyo, Otsuka/Astex, Pfizer, and Stemline. He is on the Advisory Board of the following companies: Actinium, Amgen, Astellas, and Macrogenics. He is on the Data Safety and Monitoring Board for the following companies: Argenx, Celgene, and Takeda. He is an Ad Hoc Consultant and on the Steering Committee and Data Safety and Monitoring Board for Celgene.
References
- Albers C, Leischner H, Verbeek M, Yu C, Illert AL, Peschel C, von Bubnoff N & Duyster J (2013) The secondary FLT3-ITD F691L mutation induces resistance to AC220 in FLT3-ITD+ AML but retains in vitro sensitivity to PKC412 and Sunitinib. Leukemia 27, 1416–1418. [DOI] [PubMed] [Google Scholar]
- Bacher U, Haferlach C, Kern W, Haferlach T & Schnittger S (2008) Prognostic relevance of FLT3–TKD mutations in AML: the combination matters-an analysis of 3082 patients. Blood 111, 2527–2537. [DOI] [PubMed] [Google Scholar]
- Baker SD, Zimmerman EI, Wang YD, Orwick S, Zatechka DS, Buaboonnam J, Neale GA, Olsen SR, Enemark EJ, Shurtleff S, Rubnitz JE, Mullighan CG & Inaba H (2013) Emergence of polyclonal FlT3 tyrosine kinase domain mutations during sequential therapy with sorafenib and sunitinib in FLT3-ITD-positive acute myeloid leukemia. Clinical Cancer Research 19, 5758–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker H, Pfeifer D, Afonso JD, Nimer SD, Veelken H, Schwabe M & Lubbert M (2008) Two cell lines of t(8;21) acute myeloid leukemia with activating KIT exon 17 mutation: models for the ‘second hit’ hypothesis. Leukemia 22, 1792–1794. [DOI] [PubMed] [Google Scholar]
- Cools J, Mentens N, Furet P, Fabbro D, Clark JJ, Griffin JD, Marynen P & Gilliland DG (2004) Prediction of resistance to small molecule FLT3 inhibitors: implications for molecularly targeted therapy of acute leukemia. Cancer Research 64, 6385–6389. [DOI] [PubMed] [Google Scholar]
- Evans EK, Gardino AK, Kim JL, Hodous BL, Shutes A, Davis A, Zhu XJ, Schmidt-Kittler O, Wilson D, Wilson K, DiPietro L, Zhang Y, Brooijmans N, LaBranche TP, Wozniak A, Gebreyohannes YK, Schoffski P, Heinrich MC, DeAngelo DJ, Miller S, Wolf B, Kohl N, Guzi T, Lydon N, Boral A & Lengauer C (2017) A precision therapy against cancers driven by KIT/PDGFRA mutations. Science Translational Medicine 9 (414). [DOI] [PubMed] [Google Scholar]
- Fernandes MS, Reddy MM, Croteau NJ, Walz C, Weisbach H, Podar K, Band H, Carroll M, Reiter A, Larson RA, Salgia R, Griffin JD & Sattler M (2010) Novel oncogenic mutations of CBL in human acute myeloid leukemia that activate growth and survival pathways depend on increased metabolism. Journal of Biological Chemistry 285, 32596–32605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer T, Stone RM, Deangelo DJ, Galinsky I, Estey E, Lanza C, Fox E, Ehninger G, Feldman EJ, Schiller GJ, Klimek VM, Nimer SD, Gilliland DG, Dutreix C, Huntsman-Labed A, Virkus J & Giles FJ (2010) Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. Journal of Clinical Oncology 28, 4339–4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohling S, Schlenk RF, Breitruck J, Benner A, Kreitmeier S, Tobis K, Dohner H, Dohner K; AML Study Group Ulm. Acute myeloid leukemia. (2002) Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood 100, 4372–4380. [DOI] [PubMed] [Google Scholar]
- Frost MJ, Ferrao PT, Hughes TP & Ashman LK (2002) Juxtamembrane mutant V560Gkit is more sensitive to imatinib (STI571) compared with wild-type c-kit whereas the kinase domain mutant D816Vkit is resistant. Molecular Cancer Therapeutics 1, 1115–1124. [PubMed] [Google Scholar]
- Galanis A & Levis M (2015) Inhibition of c-Kit by tyrosine kinase inhibitors. Haematologica 100, e77–e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleixner KV, Mayerhofer M, Aichberger KJ, Derdak S, Sonneck K, Bohm A, Gruze A Samorapoompichit P, Manley PW, Fabbro D, Pickl WF, Sillaber C & Valent P (2006) PKC412 inhibits in vitro growth of neo-plastic human mast cells expressing the D816V-mutated variant of KIT: comparison with AMN107, imatinib, and cladribine (2CdA) and evaluation of cooperative drug effects. Blood 107, 752–759. [DOI] [PubMed] [Google Scholar]
- Gotlib J, Kluin-Nelemans HC, George TI, Akin C, Sotlar K, Hermine O, Awan FT, Hexner E, Mauro MJ, Sternberg DW, Villeneuve M, Huntsman Labed A, Stanek EJ, Hartmann K, Horny HP, Valent P & Reiter A (2016) Efficacy and safety of midostaurin in advanced systemic mastocytosis. New England Journal of Medicine 374, 2530–2541. [DOI] [PubMed] [Google Scholar]
- Growney JD, Clark JJ, Adelsperger J, Stone R, Fabbro D, Griffin JD & Gilliland DG (2005) Activation mutations of human c-KIT resistant to imatinib mesylate are sensitive to the tyrosine kinase inhibitor PKC412. Blood 106, 721–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haferlach C, Dicker F, Kohlmann A, Schindela S, Weiss T, Kern W, Schnittger S & Haferlach T (2010) AML with CBFB-MYH11 rearrangement demonstrate RAS pathway alterations in 92% of all cases including a high frequency of NF1 deletions. Leukemia 24, 1065–1069. [DOI] [PubMed] [Google Scholar]
- Heinrich MC, Griffith D, McKinley A, Patterson J, Presnell A, Ramachandran A & Debiec-Rychter M (2012b) Crenolanib inhibits the drug-resistant PDGFRA D842V mutation associated with imatinib-resistant gastrointestinal stromal tumors. Clinical Cancer Research 18, 4375–4384. [DOI] [PubMed] [Google Scholar]
- Heinrich MC, Marino-Enriquez A, Presnell A, Donsky RS, Griffith DJ, McKinley A, Patterson J, Taguchi T, Liang C-W & Fletcher JA (2012a) Sorafenib inhibits many kinase mutations associated with drug-resistant gastrointestinal stromal tumors. Molecular Cancer Therapeutics 11, 1770–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y & Kitamura Y (1998) Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279, 577–580. [DOI] [PubMed] [Google Scholar]
- Horiike S, Yokota S, Nakao M, Iwai T, Sasai Y, Kaneko H, Taniwaki M, Kashima K, Fujii H, Abe T & Misawa S (1997) Tandem duplications of the FLT3 receptor gene are associated with leukemic transformation of myelodysplasia. Leukemia 11, 1442–1446. [DOI] [PubMed] [Google Scholar]
- Hu S, Niu H, Minkin P, Orwick S, Shimada A, Inaba H, Dahl GV, Rubnitz J & Baker SD (2008) Comparison of antitumor effects of multitargeted tyrosine kinase inhibitors in acute myelogenous leukemia. Molecular Cancer Therapeutics 7, 1110–1120. [DOI] [PubMed] [Google Scholar]
- Jiao B, Wu CF, Liang Y, Chen HM, Xiong SM, Chen B, Shi JY, Wang YY, Wang JH, Chen Y, Li JM, Gu LJ Tang JY, Shen ZX, Gu BW, Zhao WL, Chen Z & Chen SJ (2009) AML1-ETO9a is correlated with C-KIT overexpression/mutations and indicates poor disease outcome in t(8;21) acute myeloid leukemia M2. Leukemia 23, 1598–1604. [DOI] [PubMed] [Google Scholar]
- Kampa-Schittenhelm KM, Heinrich MC, Akmut F, Dohner H, Dohner K, & Schittenhelm MM (2013) Quizartinib (AC220) is a potent second generation class III tyrosine kinase inhibitor that displays a distinct inhibition profile against mutant-FLT3, -PDGFRA and –KIT isoforms. Molecular Cancer 12, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly LM, Liu Q, Kutok JL, Williams IR, Boulton CL, & Gilliland DG (2002) FLT3 internal tandem duplication mutations associated with human acute myeloid leukemias induce myeloproliferative disease in a murine bone marrow transplant model. Blood 99, 310–318. [DOI] [PubMed] [Google Scholar]
- Kemmer K, Corless CL, Fletcher JA, McGreevey L, Haley A, Griffith D, Cummings OW, Wait C, Town A & Heinrich MC (2004) KIT mutations are common in testicular seminomas. American Journal of Pathology 164, 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larizza L, Magnani I & Beghini A (2005) The Kasumi-1 cell line: a t(8;21)-kit mutant model for acute myeloid leukemia. Leukemia & Lymphoma 46, 247–255. [DOI] [PubMed] [Google Scholar]
- Larrosa-Garcia M & Baer MR (2017) FLT3 inhibitors in acute myeloid leukemia: current status and future directions. Molecular Cancer Therapeutics 16, 991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LY, Hernandez D, Rajkhowa T, Smith SC, Raman JR, Nguyen B, Small D & Levis M (2017) Preclinical studies of gilteritinib, a next-generation FLT3 inhibitor. Blood 129, 257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis M (2017) Midostaurin approved for FLT3-mutated AML. Blood 129, 3403–3406. [DOI] [PubMed] [Google Scholar]
- Liu Y & Gray NS (2006) Rational design of inhibitors that bind to inactive kinase conformations. Nature Chemical Biology 2, 358–364. [DOI] [PubMed] [Google Scholar]
- Matsuo Y, MacLeod RA, Uphoff CC, Drexler HG, Nishizaki C, Katayama Y, Kimura G, Fujii N, Omoto E, Harada M & Orita K (1997) Two acute monocytic leukemia (AML-M5a) cell lines (MOLM13 and MOLM14) with interclonal phenotypic heterogeneity showing MLL-AF9 fusion resulting from an occult chromosome insertion, ins(11;9)(q23;p22p23). Leukemia 11, 1469–1477. [DOI] [PubMed] [Google Scholar]
- Miettinen M & Lasota J (2005) KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Applied Immunohistochemistry & Molecular Morphology 13, 205–220. [DOI] [PubMed] [Google Scholar]
- Mol CD, Dougan DR, Schneider TR, Skene RJ, Kraus ML, Scheibe DN, Snell GP, Zou H, Sang BC & Wilson KP (2004) Structural basis for autoinhibition and STI-571 inhibition of c-Kit tyrosine kinase. Journal of Biological Chemistry 279, 31655–31663. [DOI] [PubMed] [Google Scholar]
- Nagata H, Worobec AS, Oh CK, Chowdhury BA, Tannenbaum S, Suzuki Y & Metcalfe DD (1995) Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proceedings of the National Academy of Sciences USA 92, 10560–10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K, Sonoda Y, Fujimoto T & Misawa S (1996) Internal tandem duplication of the FLT3 gene found in acute myeloid leukemia. Leukemia 10, 1911–1918. [PubMed] [Google Scholar]
- Omori I, Yamaguchi H, Miyake K, Miyak e N., Kitano T & Inokuchi K (2017) D816V mutation in the KIT gene activation loop has greater cell-proliferative and anti-apoptotic ability than N822K mutation in core-binding factor acute myeloid leukemia. Experimental Hematology 52, 56–64. [DOI] [PubMed] [Google Scholar]
- Ravandi F, Alattar ML, Grunwald MR, Rudek MA, Rajkhowa T, Richie MA, Pierce S, Daver N, Garcia-Manero G, Faderl S, Nazha A, Konopleva M, Borthakur G, Burger J, Kadia T, Dellasala S, Andreeff M, Cortes J, Kantarjian H, & Levis M (2013) Phase 2 study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation. Blood 121, 4655–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reindl C, Quentmeier H, Petropoulos K, Greif PA, Benthaus T, Argiropoulos B, Mellert G, Vampati S, Duyster J, Buske C, Bohlander SK, Humphries KR, Hiddemann W & Spiekermann K (2009) CBL exon 8/9 mutants activate the FLT3 pathway and cluster in core binding factor/11q deletion acute myeloid leukemia/myelodysplastic syndrome subtypes. Clinical Cancer Research 15, 2238–2247. [DOI] [PubMed] [Google Scholar]
- Roskoski R Jr. (2016) Classification of small molecule protein kinase inhibitors based upon the structures of their drug-enzyme complexes. Pharmacological Research 103, 26–48. [DOI] [PubMed] [Google Scholar]
- Sargin B, Choudhary C, Crosetto N, Schmidt MHH, Grundler R, Rensinghoff M, Thiessen C, Tickenbrock L, Schwable J, Brandts C, August B, Koschmieder S, Bandi SR, Duyster J, Berdel WE, Muller-Tidow C, Dikic I & Serve H (2007) Flt3-dependent transformation by inactivating c-Cbl mutations in AML. Blood 110, 1004–1012. [DOI] [PubMed] [Google Scholar]
- Sattler M, Salgia R, Okuda K, Uemura N, Durstin MA, Pisick E, Xu G, Li JL, Prasad KV & Griffin JD (1996) The proto-oncogene product p120CBL and the adaptor proteins CRKL and c-CRK link c-ABL, p190BCR-ABL and p210BCR-ABL to the phosphatidylinositol-3' kinase pathway. Oncogene 12, 839–846. [PubMed] [Google Scholar]
- Schittenhelm M, Akmut F, Illing B, Frey J, Schuste r K., Chandran AR, Kanz L & Kampa-Schittenhelm KM (2014) Gain-of-function KIT mutations sensitize the mutant isoform to the type I tyrosine kinase inhibitor crenolanib: a rationale for the therapeutic use in systemic mastocytosis (SM) and core binding factor leukemias (CBFL). Blood 124, 2230. [Google Scholar]
- Shah NP, Lee FY, Luo R, Jiang Y, Donker M & Akin C (2006) Dasatinib (BMS-354825) inhibits kITD816V, an imatinib-resistant activating mutation that triggers neoplastic growth in most patients with systemic mastocytosis. Blood 108, 286–291. [DOI] [PubMed] [Google Scholar]
- Smith CC, Lasater EA, Lin KC, Wang Q, McCreery MQ, Stewart WK, Damon LE, Perl AE, Jeschke GR, Sugita M, Carroll M, Kogan SC, Kuriyan J & Shah NP (2014) Crenolanib is a selective type I pan-FLT3 inhibitor. Proceedings of the National Academy of Sciences USA 111, 5319–5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, Lin K, Stecula A, Sali A & Shah NP (2015) FLT3 D835 mutations confer differential resistance to type II FLT3 inhibitors. Leukemia 29, 2390–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, Wang Q, Chin CS, Salerno S, Damon LE, Levis MJ, Perl AE, Travers KJ, Wang S, Hunt JP, Zarrinkar PP, Schadt EE, Kasarskis A, Kuriyan J, Shah NP (2012) Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature 485, 260–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirewalt DL & Radich JP (2003) The role of FLT3 in haematopoeitic malignancies. Nature Reviews Cancer 3, 650–665. [DOI] [PubMed] [Google Scholar]
- Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, Thiede C, Prior TW, Dohner K, Marcucci G, Lo-Coco F, Klisovic RB, Wei A, Sierra J, Sanz MA, Brandwein JM, de Witte T, Niederwieser D, Appelbaum FR, Medeiros BC, Tallman MS, Krauter J, Schlenk RF, Ganser A, Serve H, Ehninger G, Amadori S, Larson RA & Dohner H (2017) Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. New England Journal of Medicine 377, 454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strati P, Kantarjian H, Ravandi F, Nazha A, Borthakur G, Daver N, Kadia T, Estrov Z, Garcia-Manero G, Konopleva M, Rajkhowa T, Durand M, Andreeff M, Levis M, & Cortes J (2015) Phase I/II trial of the combination of midostaurin (PKC412) and 5-azacytidine for patients with acute myeloid leukemia and myelodysplastic syndrome. American Journal of Hematology 90, 276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U, Wermke M, Bornhauser M, Ritter M, Neubauer A, Ehninger G & Illmer T (2002) Analysis of FLT3- activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood 99, 4326–4335. [DOI] [PubMed] [Google Scholar]
- Thom C (2015) Preliminary data on ASP2215: tolerability and efficacy in acute myeloid leukemia patients. Future Oncology 11, 2499–2501. [DOI] [PubMed] [Google Scholar]
- Wang YY, Zhao LJ, Wu CF, Liu P, Shi L, Liang Y, Xiong SM, Mi JQ, Chen Z, Ren R & Chen SJ (2011) C-KIT mutation cooperates with full-length AML1-ETO to induce acute myeloid leukemia in mice. Proceedings of the National Academy of Sciences USA 108, 2450–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-Y, Zhou G-B, Yin T, Chen B, Shi J-Y, Liang W-X, Jin X-L, You J-H, Yang G, Shen Z-X, Chen J, Xiong S-M, Chen G-Q, Xu F, Liu Y-W, Chen Z & Chen S-J. (2005) AML1-ETO and C-KIT mutations/overexpression in t(8;21) leukemia: Implication in stepwise leukemogenesis and response to Gleevec. Proceedings of the National Academy of Sciences USA 102, 1104–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg E, Boulton C, Kelly LM, Manley P, Fabbro D, Meyer T, Gilliland DG & Griffin JD (2002) Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell 1, 433–443. [DOI] [PubMed] [Google Scholar]
- Weisberg E, Manley PW, Breitenstein W, Bruggen J, Cowan-Jacob SW, Ray A, Huntly B, Fabbro D, Fendrich G, Hall-Meyers E, Kung AL, Mestan J, Daly GQ, Callahan L, Catley L, Cavazza C, Azam M, Neuberg D, Wright RD, Gilliland DG & Griffin JD (2005) Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell 7, 129–141. [DOI] [PubMed] [Google Scholar]
- Weisberg E, Nonami A, Chen Z, Liu F, Zhang J, Sattler M, Nelson E, Cowens K, Christie AL, Mitsiades C, Wong KK, Liu Q, Gray N & Griffin JD (2015) Identification of Wee1 as a novel therapeutic target for mutant RAS-driven acute leukemia and other malignancies. Leukemia 29, 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmore-Payne C, Holden JA, Tripp S & Layfield LJ (2005) Human malignant melanoma: detection of BRAF- and c-kit-activating mutations by high-resolution amplicon melting analysis. Human Pathology 35, 486–493. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Kiyoi H, Nakano Y, Suzuki R, Kodera Y, Miyawaki S, Asou N, Kuriyama K, Yagasaki F, Shimazaki C, Akiyama H, Saito K, Nishimura M, Motoji T, Shinagawa K, Takeshita A, Saito H, Ueda R, Ohno R & Naoe T (2001) Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood 97, 2434–2439. [DOI] [PubMed] [Google Scholar]
- Yang Y, Letard S, Borge L, Chaix A, Hanssens K, Lopez S, Vita M, Finetti P, Birnbaum D, Bertucci F, Gomez S, de Sepulveda P & Dubreuil P(2010) Pediatric mastocytosis-associated KIT extracellular domain mutations exhibit different functional and signaling properties compared with KIT-phosphotransferase domain mutations. Blood 116, 1114–1123. [DOI] [PubMed] [Google Scholar]
- Zarrinkar PP, Gunawardane RN, Cramer MD, Gardner MF, Brigham D, Belli B, Karaman MW, Pratz KW, Pallares G, Chao Q, Sprankle KG, Patel HK, Levis M, Armstrong RC, James J & Bhagwat SS (2009) AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML). Blood 114, 2984–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zermati Y, De Sepulveda P, Feger F, Letard S, Kersual J, Casteran N, Gorochov G, Dy M, Ribadeau Dumas A, Dorgham K, Parizot C, Bieche Y, Vidaud M, Lortholary O, Arock M, Hermine O & Dubreuil P (2003) Effect of tyrosine kinase inhibitor STI571 on the kinase activity of wild-type and various mutated c-kit receptors found in mast cell neoplasm. Oncogene 22, 660–664. [DOI] [PubMed] [Google Scholar]
- Zhang W, Konopleva M, Shi YX, McQueen T, Harris D, Ling X, Estrov Z, Quintas-Cardama A, Small D, Cortes J & Andreeff M (2008) Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. Journal of the National Cancer Institute 100, 184–198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.