Abstract
OBJECTIVES
Neonatal seizures are common complications. Phenobarbital is the agent of choice but leads to adverse neurologic outcomes. There has been increased use of newer agents like levetiracetam. The objective of this study was determining the rate of seizure resolution in neonates treated with phenobarbital or levetiracetam.
METHODS
This was a retrospective, single-center, cohort study from June 1, 2012–June 1, 2018 evaluating seizure resolution in neonates following first-line treatment with phenobarbital versus levetiracetam. Data were collected via review of the patient's charts in the electronic medical record. The primary outcome was seizure resolution without addition of a second antiepileptic agent. Logistic regression was used to assess the impact of pertinent variables.
RESULTS
Each group included 73 patients. The mean gestational age was 36.01 and 37.91 weeks for the phenobarbital and levetiracetam groups, respectively (p = 0.011). The phenobarbital group had higher rates of intraventricular hemorrhage at baseline. The median birth weight was 2750 and 3002 grams in the phenobarbital and levetiracetam groups, respectively (p = 0.10). Forty-five neonates (61.6%) achieved seizure resolution with phenobarbital compared with 30 neonates (41.1%) with levetiracetam (p = 0.01). In neonates who did not receive a benzodiazepine, seizure resolution was similar between groups (51–52%). In neonates who received a benzodiazepine, seizure resolution rate was 94.1% (16/17 neonates) for phenobarbital and 18.2% (4/22 neonates) for levetiracetam.
CONCLUSIONS
These findings suggest seizure resolution with levetiracetam, and phenobarbital may be impacted by benzodiazepine administration. If no benzodiazepine is used, these agents demonstrated similar efficacy. Further research into the pharmacodynamic interaction with benzodiazepines is necessary.
Keywords: benzodiazepines, efficacy, epilepsy, levetiracetam, newborn, phenobarbital, seizures
Introduction
Seizures are one of the most frequent neurologic complications occurring in neonates. They can vary in severity from a self-limited event to a life-threatening disorder.1 The most common etiology of neonatal seizures is hypoxic-ischemic encephalopathy (HIE). Other causes include infection, metabolic disorders, genetic disorders, congenital malformations, and cerebral infarction or intracerebral hemorrhage.2,3
Two consensus guidelines1,4 recommend using phenobarbital as the first-line agent in seizures without a treatable etiology. This recommendation is based on evidence that phenobarbital and phenytoin are equally efficacious, although each of these agents as monotherapy has shown to resolve seizures in less than half of the cases.5 Phenobarbital is recommended preferentially compared with phenytoin due to its more predictable pharmacokinetics in the neonatal population.6
Phenobarbital is a sedative hypnotic that has anticonvulsant properties through hyperpolarization of the neuronal membrane via action on γ-aminobutyric acid (GABA)-A receptor subunits.7 Concerns about the safety of phenobarbital exist due to commonly known respiratory, cardiovascular, and central nervous system effects. Additionally, phenobarbital induces widespread neuronal apoptosis in the developing rat brain.8,9 Long-term follow-up studies in children have also identified an association between receiving phenobarbital in infancy and poor neurologic outcomes at the ages of 1 to 2 years.10,11
Due to poor outcomes with phenobarbital, practitioners have started using newer antiepileptic drugs (AEDs) such as levetiracetam.12–14 Levetiracetam inhibits voltage-gated N-type calcium channels and modulates glutamate release through binding of the synaptic vesicle 2A (SV2A).15,16 Levetiracetam has a lower rate of neurologic adverse effects compared with pheno-barbital. In a study assessing neurologic outcomes in children formerly treated with phenobarbital or levetiracetam, a higher cumulative exposure to phenobarbital was associated with poorer neurologic outcomes compared with levetiracetam including a higher incidence of cerebral palsy and lower scores on the Bayley Scales of Infant Development.10 In stark contrast to the data with phenobarbital, levetiracetam has demonstrated neuroprotective effects against neuronal apoptosis following a hypoxic-ischemic event.17 However, the data assessing levetiracetam in this population are limited and there have been no randomized phase III clinical trials in the neonatal population assessing comparative efficacy of levetiracetam.
Despite a lack of strong evidence to support levetiracetam use, but based on the safety concerns associated with phenobarbital, in 2015 University of Kentucky HealthCare's Kentucky Children's Hospital changed its clinical practice guideline for neonatal seizures from using phenobarbital to using levetiracetam first line. Initial data have shown comparable seizure resolution rates to historical phenobarbital data in neonates treated with levetiracetam first line at Kentucky Children's Hospital.18 The purpose of this study is to retrospectively assess the rate of seizure resolution in neonates after initial treatment with phenobarbital or levetiracetam and assess other variables that affect this outcome.
Materials and Methods
Study Design. This was an observational retrospective cohort study from June 1, 2012–June 1, 2018 of hospitalized neonates, 0 to 28 days of age, who received either levetiracetam or phenobarbital first line for clinically or electrographically confirmed seizures. The institutional protocol allowed for the emergent administration of a benzodiazepine (BZD), rapid attainment of video electroencephalogram (vEEG), and loading with the recommended first-line AED: phenobarbital prior to June 2015 and levetiracetam after July 2015. AED administration was not delayed if a vEEG could not be obtained rapidly. Per the protocols, the recommended dosing was as follows: phenobarbital 20 mg/kg followed by 2.5 mg/kg twice daily and levetiracetam 50 mg/kg followed by 20 mg/kg twice daily all given intravenously. Phenobarbital was re-loaded, if clinically indicated, to achieve goal serum concentrations, defined by neurology, typically 15 to 40 mg/L. Dosing could not be completely standardized due to the retrospective nature of this study. Pharmacy billing records were used to identify all patients who received at least 1 dose of levetiracetam or phenobarbital during the study period. Patients were excluded if they received an AED for an indication other than treatment of a neonatal seizure or if they deviated from the institution's clinical protocol and received a medication other than levetiracetam or phenobarbital first line. In addition, patients were excluded if they received any AED at an outside hospital prior to transfer to the institution. If patients did not have seizure activity confirmed by vEEG or neurologist's clinical assessment, they were excluded. If there was not adequate baseline demographic information in the electronic medical record, patients were also excluded. Patients were not excluded based on the receipt of a BZD because the protocol allowed for administration of abortive doses of a BZD prior to loading with the initial AED.
Data Sources. Data were collected via review of medical records in the Sunrise electronic health record (Allscripts, Chicago, IL). Patient demographic information including sex, ethnicity, gestational age, type of delivery, birth weight, and age at seizure onset was collected. Medication information collected includes whether a BZD was administered prior to initial AED load, loading and maintenance dose of initial AED, noted adverse effects, additional AEDs given, AEDs continued on discharge, and phenobarbital serum concentrations. Seizure characteristics, such as etiology of seizure, clinical or electroencephalogram confirmation of seizure, and seizure control, were based on documentation from treating physicians within the electronic health record.
Outcome. The primary outcome was seizure resolution after first-line AED. Seizure resolution was defined as resolution of clinical features associated with seizures (e.g., abnormal gaze or eye movement, tongue thrusting, apnea, clonic, tonic, or jerking movements) or normalization of electroencephalogram, documented by the treating neurologist, with no recurrence of seizures prior to discharge. Seizure resolution after first-line AED was defined as seizure resolution as above with use of a single AED with or without administration of an abortive BZD prior. A posthoc analysis was performed to assess the influence various factors on seizure resolution after first-line AED. Variables assessed included the following: low birth weight, congenital abnormalities, birth trauma, premature gestational age, postconceptional age HIE, renal dysfunction, intraventricular hemorrhage (IVH), first-line medication used (levetiracetam or phenobarbital), BZD received prior, and an interaction term between first-line medication used and BZD received prior. Low birth weight was defined as < 2500 grams, and premature gestational age was defined as < 37 weeks. Congenital abnormalities, birth trauma, HIE, renal dysfunction, and IVH were captured if they were documented problems in the neonatology history and physical note.
Statistics. Statistical analysis was performed using SPSS Statistics version 23 (IBM, Armonk, NY), and modeling was performed using SAS version 9.4 (SAS Institute, Cary, NC). The predetermined level of significance was set at α = 0.05. Pearson χ2 tests were used for categorical data, independent samples T tests were used for continuous data and non-parametric comparisons of medians were used for comparison of baseline characteristics that were not normally distributed. Multivariate logistic regression was used to model the impact of select variables on seizure resolution in neonates.
Results
Enrollment. There were 510 patients identified who were aged 0 to 28 days upon admission and had received a dose of levetiracetam or phenobarbital during hospitalization. Based on predefined exclusion criteria, 364 were excluded from analysis. The most common reasons for exclusion were receiving phenobarbital for an alternative indication or the patient was found to be age >28 days at receipt of first AED. This left 146 patients included for analysis: 73 patients in the levetiracetam group and 73 patients in the phenobarbital group (Figure 1).
Figure 1.
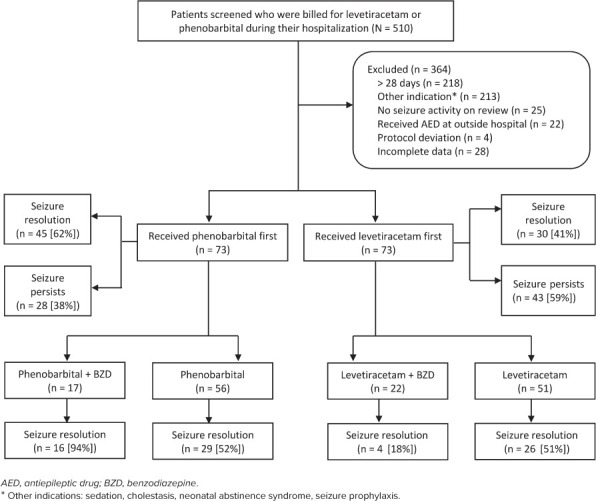
Identification and enrollment of study population.
Baseline Characteristics. Patients were similar in terms of baseline characteristics, with 2 exceptions. Patients started on phenobarbital had a higher rate of IVH (20.8% versus 8.2%, p = 0.031) and were approximately 2 weeks younger in terms of gestational age (36.01 weeks versus 37.91 weeks, p = 0.011) (Table 1). The population was majority white and the rate of HIE was approximately 30% in each group, which is consistent with the reported rate of this seizure etiology.1
Table 1.
Baseline Demographic Data of Neonates Started on Levetiracetam or Phenobarbital
| Demographic | Phenobarbital (n = 73) | Levetiracetam (n = 73) | p value |
|---|---|---|---|
| Gestational age, mean ± SD, wk | 36.01 ± 5.1 | 37.91 ± 2.33 | 0.011 |
| Postconceptional age, mean ± SD, wk | 36.73 ± 5.03 | 38.61 ± 2.38 | 0.011 |
| Age at seizure onset, mean ± SD, days | 4.93 ± 5.96 | 4.90 ± 6.35 | 0.507 |
| Birth weight, median (IQR), grams | 2750 (2046–3298) | 3002.5 (2535–3492.8) | 0.100 |
| Race, n (%) | |||
| Caucasian | 60 (82.2) | 63 (86.3) | 0.439 |
| Other | 13 (17.8) | 10 (13.7) | |
| Type of birth, n (%) | |||
| Vaginal non-assisted | 20 (27.4) | 26 (36.1) | 0.551 |
| Vaginal assisted | 7 (9.6) | 5 (6.9) | |
| Emergent C-section | 32 (43.8) | 25 (34.7) | |
| Non-emergent C-section | 14 (19.2) | 16 (22.2) | |
| Apgar scores, mean ± SD | |||
| At 1 min | 3.94 ± 2.96 | 4.68 ± 3.26 | 0.181 |
| At 5 min | 5.90 ± 2.73 | 6.21 ± 2.82 | 0.543 |
| Seizure Etiology | |||
| Intraventricular hemorrhage, n (%) | 15 (20.8) | 6 (8.2) | 0.031 |
| Hypoxic-ischemic encephalopathy, n (%) | 17 (23.3) | 22 (30.1) | 0.35 |
| Patient cooled*, n (%) | 14 (87.5) | 20 (90.9) | 1.00 |
| Congenital malformations, n (%) | 16 (21.9) | 13 (18.3) | 0.589 |
| Received benzodiazepine prior, n (%) | 17 (23.3) | 22 (30.1) | 0.35 |
* All patients received whole body cooling for 72 hours. Patients were placed on video electroencephalogram monitoring during cooling, but prophylactic anticonvulsants were not used. There were no dose adjustments in medications made for cooling.
Treatment. For the 73 neonates started on levetiracetam, the average loading dose was 48.2 ± 12.74 mg/kg, which is within 5% of the 50 mg/kg loading dose recommended per the institutional protocol. For the 73 neonates started on phenobarbital, the average loading dose was 19.67 ± 3.96mg/kg, which is also within 5% of the 20 mg/kg loading dose recommended per the institutional protocol. Doses of each medication were adjusted to clinical effect. The average maximum maintenance dose was 29.9 ± 16.04 mg/kg/dose for levetiracetam and 4.48 mg/kg/dose ± 2.18 for phenobarbital.
Thirty-nine neonates received a BZD prior to loading of the initial AED; 17 in the phenobarbital group (23.3% [17/73]) and 22 in the levetiracetam group (30.1% [22/73]). When a BZD was used, lorazepam was the most commonly used agent at 92.3% (36/39 neonates). All of those given a BZD in the phenobarbital group received lorazepam (17/17) compared with 86% of those in the levetiracetam group (19/22 neonates). Other BZDs included midazolam and diazepam. When seizures persisted, fosphenytoin was the most commonly used second-line AED in both groups.
Efficacy Outcomes. Of those neonates started on phenobarbital, 61.6% (n = 45) achieved seizure resolution compared with 41.1% (n = 30) of those started on levetiracetam (p = 0.01) (Figure 2). Overall seizure resolution increased to 76.7% (56/73 neonates) in both groups following administration of second-line AED, most commonly, fosphenytoin.
Figure 2.
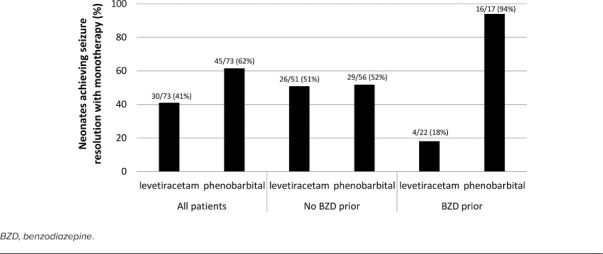
Percent of neonates achieving seizure resolution after receiving phenobarbital or levetiracetam first line in the whole cohort, and in those who did or did not receive a benzodiazepine prior.
Multivariate logistic regression was used to model the impact of select variables on seizure resolution after first-line AED. The only characteristics found to significantly impact seizure resolution following first-line AED were the AED administered and the use of a BZD. When a subgroup analysis accounting for BZD administration was performed, there was a significant interaction with the AED used. If no BZD administration, the rates of seizure resolution with first-line AED were approximately 51% regardless of which AED administered, levetiracetam or phenobarbital. Importantly, if the patient had received a BZD prior to first-line AED, the probability of seizure resolution was 94.1% (16/17 neonates) in the phenobarbital group and 18.2% (4/22 neonates) in the levetiracetam group (Figure 2). Thus, patients who were administered an abortive BZD and given phenobarbital were 98.3% more likely to obtain seizure resolution compared with patients receiving levetiracetam after a BZD (odds ratio = 0.017; 95% confidence interval: 0.002–0.167) (Figure 2).
Discussion
This is the largest cohort study to date assessing both levetiracetam and phenobarbital for neonatal seizures. The most interesting finding from this study is this effect of BZD administration. For those neonates who did not receive a BZD, seizure resolution rate was the same irrespective of first-line AED. Each agent achieved seizure resolution in half of the cases, which is consistent with original literature, which suggests about 50% seizure resolution with first-line agents.5 What appears to be driving the statistical and clinical difference in seizure resolution rates between neonates started on these 2 agents is administration of a BZD prior to loading of the AED. Those neonates who received a BZD and then levetiracetam were much less likely to achieve seizure resolution, whereas those who received a BZD and then phenobarbital achieved seizure resolution in all but 1 case.
Potential Mechanisms of Action. A potential mechanistic explanation for these results derives from the activity of these medications at the GABAA and SV2A receptors. Benzodiazepines function through binding the GABA receptor and enhancing endogenous GABA activity. This opens the chloride channel allowing an influx of chloride and causing membrane hyperpolarization. It has been demonstrated that BZDs increase the frequency of GABA receptor currents. Phenobarbital is thought to bind to a different regulatory site near the chloride channel and increase the duration of GABA receptor currents without affecting the frequency.19 Therefore, these medications could work synergistically at the GABA receptor increasing both the frequency and duration of GABA receptor currents. In contrast, the action of SV2A is to facilitate exocytosis of glutamate containing vesicles. It has been found to play a role in action potential-dependent neurotransmission, but not in action potential-independent neurotransmission.20 Perhaps, because this vesicle is action potential-dependent, the hyperpolarization of the membrane via BZD modulation of the GABA receptor could lead to decreased efficacy of levetiracetam due to reduced occurrence of action potentials and BZD suppression of neurotransmission. This potential interaction supports the results in this study but merit further validation in animal and human models.
Another potential mechanistic rationale for decreased efficacy with BZD use is the reversed chloride gradient in the neonatal brain. Due to overexpression of the sodium-potassium-chloride import channel, and underexpression of the potassium-chloride exporter, there are higher intracellular chloride concentrations and lower extracellular chloride concentrations in the neonatal brain.15 Activation of GABA by an agonist, such as BZDs, may lead to an efflux of chloride, instead of an influx as it does in the mature brain, which may cause depolarization of the membrane.
Context in the Literature. The role of BZDs in the child and adult population for aborting seizures is more recognized than it is in the neonatal population. Neonatal seizure guidelines do not comment on the use of a BZD prior to AED administration and approximately one-quarter of this population received a BZD prior to levetiracetam or phenobarbital. The high use of BZDs at this institution is likely because BZDs are available in the automated dispensing cabinets on the floor, whereas phenobarbital and levetiracetam are mixed in the satellite pharmacy. In an emergent situation, BZDs are more readily available for administration. The rate of use of BZDs at this institution will be further investigated internally. Institutions that do not use BZDs for neonatal seizures could likely consider levetiracetam and phenobarbital to have equivalent efficacy. However, these results indicate that using a BZD followed by phenobarbital results in significantly more seizure resolution than with levetiracetam. This would mitigate the risk of neuronal apoptosis by preventing cumulative exposure of phenobarbital.
This report adds to the limited evidence regarding levetiracetam in the neonatal population. The data that exists for levetiracetam in this population includes mostly surveys, case reports, and cohort studies. Surveys of pediatric neurologist practices have demonstrated a wide variability of prescribing for neonatal seizures with the majority of institutions still using phenobarbital as the first-line agent but with increasing recommendations for newer agents, including levetiracetam.12–14,21 A pharmacokinetic evaluation of levetiracetam in neonates demonstrated a larger volume of distribution and lower clearance compared with adult patients, but no adverse effects.22 Several institutions have reported on the use of levetiracetam as a first- or second-line agent in observational, descriptive studies23–26 and only 2 cohort studies to date have compared levetiracetam with phenobarbital.27,28 One of these demonstrated improved seizure resolution rates after first-line levetiracetam compared with phenobarbital; however, the study assessed infants aged >28 days.28 Another study compared levetiracetam with phenobarbital for neonatal seizures caused by HIE but did not include neonates with seizures from other etiologies.27 These 2 cohorts and a meta-analysis comparing levetiracetam data to historic phenobarbital data in neonates29 have suggested that levetiracetam could be equivalent or superior to phenobarbital for seizure control. A recent phase II prospective randomized trial of phenobarbital and levetiracetam contradicts the results from these cohort studies and demonstrated superior seizure resolution with phenobarbital.30 However, the initial loading dose of levetiracetam was 40 mg/kg in the phase II study, and the authors concluded that larger doses of levetiracetam should be explored in this population. Our results are consistent with previous cohort studies in that the levetiracetam cohort that did not receive BZDs had similar results to the phenobarbital cohort that did not receive BZDs.
Limitations. Most of the limitations of this study are due to the retrospective design. Seizure resolution needed to be documented in the electronic medical record, which means that cases that achieved seizure resolution could have been missed due to incomplete documentation. Due to timing and emergent treatment, not all seizures could be verified by electroencephalogram. Although initial loading and maintenance dosing of AEDs is recommended by institutional protocol, the dose could not be standardized, nor could the timing or indication of adding an additional AED. Timing of addition of a second agent would directly affect the primary outcome of seizure resolution after first-line AED particularly if clinicians did not wait for response prior to adding a second agent. Due to documentation, it was not possible to assess the timing of AED administration and timing of seizure resolution. Serum concentrations of levetiracetam were also not available because they are not yet routinely collected in clinical practice but could elucidate important information in levetiracetam non-responders. Additionally, there was limited information regarding adverse effects from the medications. This study also did not assess long-term outcomes of these patients, particularly poor neurologic outcomes, which is an important factor in use of levetiracetam or phenobarbital.
Research Implications. A prospective, randomized phase III trial is needed to assess the efficacy of a levetiracetam compared with phenobarbital. Preferably all studies in this population should include long-term follow-up for neurologic adverse consequences. Further research into the interaction between BZDs and levetiracetam is also needed to elucidate this pharmacodynamic interaction because this was a surprising new finding. Perhaps an effective multimodal approach likely includes the administration of a BZD followed by phenobarbital for acute severe seizure resolution and levetiracetam for chronic management; this approach should be investigated further.
Conclusion
These data suggest that if a BZD is not used, seizure resolution rates are similar between neonates who received phenobarbital or levetiracetam. However, if a BZD is used emergently, loading with phenobarbital may be preferred to use of levetiracetam due to the much lower rates of seizure resolution when a BZD is used prior to levetiracetam.
ABBREVIATIONS
- AED
antiepileptic drug;
- BZD
benzodiazepine;
- GABA
γ-aminobutyric acid;
- HIE
hypoxicischemic encephalopathy;
- IVH
intraventricular hemorrhage;
- SV2A
synaptic vesicle 2A;
- vEEG
video electroencephalogram
Footnotes
Disclosure. The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all data and take responsibility for the integrity and accuracy of the data analysis.
Ethical Approval and Informed Consent. Given the nature of this study, the project was exempt from institution board/ethics committee review and informed consent was not obtained.
References
- 1.WHO/ILAE/IRCCS Guidelines on neonatal seizures. 2011. Accessed December 19, 2020. https://apps.who.int/iris/bitstream/handle/10665/77756/9789241548304_eng.pdf;jsessionid=BE19939E2012E08B18F92FF3DA3CB540?sequence=1.
- 2.Glass HC, Shellhaas RA, Wusthoff CJ et al. Contemporary profile of seizures in neonates: a prospective cohort study. J Pediatr. 2016;174:98–103. doi: 10.1016/j.jpeds.2016.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tekgul H, Gauvreau K, Soul J et al. The current etiologic profile and neurodevelopmental outcome of seizures in term newborn infants. Pediatrics. 2006;117(4):1270–1280. doi: 10.1542/peds.2005-1178. [DOI] [PubMed] [Google Scholar]
- 4.Queensland Health Neonatal seizures. Maternity and neonatal clinical guideline 2017. Accessed December 19, 2020. https://www.health.qld.gov.au/__data/assets/pdf_file/0030/143697/g-seizures.pdf.
- 5.Painter MJ, Scher MS, Stein AD et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med. 1999;341(7):485–489. doi: 10.1056/NEJM199908123410704. [DOI] [PubMed] [Google Scholar]
- 6.Hennig S, Norris R, Tu Q et al. Population pharmacokinetics of phenytoin in critically ill children. J Clin Pharmacol. 2015;55(3):355–364. doi: 10.1002/jcph.417. [DOI] [PubMed] [Google Scholar]
- 7.Lewis CB, Adams N. Phenobarbital. Accessed December 19, 2020. https://www.ncbi.nlm.nih.gov/books/NBK532277/
- 8.Bittigau P, Sifringer M, Ikonomidou C. Antiepileptic drugs and apoptosis in the developing brain. Ann N Y Acad Sci. 2003;993(1):103–114. doi: 10.1111/j.1749-6632.2003.tb07517.x. discussion 123–104. [DOI] [PubMed] [Google Scholar]
- 9.Kaushal S, Tamer Z, Opoku F, Forcelli PA. Anticonvulsant drug-induced cell death in the developing white matter of the rodent brain. Epilepsia. 2016;57(5):727–734. doi: 10.1111/epi.13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maitre NL, Smolinsky C, Slaughter JC, Stark AR. Adverse neurodevelopmental outcomes after exposure to phenobarbital and levetiracetam for the treatment of neonatal seizures. J Perinatol. 2013;33(11):841–846. doi: 10.1038/jp.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farwell JR, Lee YJ, Hirtz DG et al. Phenobarbital for febrile seizures—effects on intelligence and on seizure recurrence. N Engl J Med. 1990;322(6):364–369. doi: 10.1056/NEJM199002083220604. [DOI] [PubMed] [Google Scholar]
- 12.Blume HK, Garrison MM, Christakis DA. Neonatal seizures: treatment and treatment variability in 31 United States pediatric hospitals. J Child Neurol. 2009;24(2):148–154. doi: 10.1177/0883073808321056. [DOI] [PubMed] [Google Scholar]
- 13.Bartha AI, Shen J, Katz KH et al. Neonatal seizures: multicenter variability in current treatment practices. Pediatr Neurol. 2007;37(2):85–90. doi: 10.1016/j.pediatrneurol.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Glass HC, Kan J, Bonifacio SL, Ferriero DM. Neonatal seizures: treatment practices among term and preterm infants. Pediatr Neurol. 2012;46(2):111–115. doi: 10.1016/j.pediatrneurol.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mruk AL, Garlitz KL, Leung NR. Levetiracetam in neonatal seizures: a review. J Pediatr Pharmacol Ther. 2015;20(2):76–89. doi: 10.5863/1551-6776-20.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loscher W, Gillard M, Sands ZA et al. Synaptic vesicle glycoprotein 2A ligands in the treatment of epilepsy and beyond. CNS Drugs. 2016;30(11):1055–1077. doi: 10.1007/s40263-016-0384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilicdag H, Daglioglu K, Erdogan S et al. The effect of levetiracetam on neuronal apoptosis in neonatal rat model of hypoxic ischemic brain injury. Early Hum Dev. 2013;89(5):355–360. doi: 10.1016/j.earlhumdev.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Kreimer AM, Littrell RA, Gibson JB, Leung NR. Effectiveness of levetiracetam as a first-line anticonvulsant for neonatal seizures. J Pediatr Pharmacol Ther. 2019;24(4):320–326. doi: 10.5863/1551-6776-24.4.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Twyman RE, Rogers CJ, Macdonald RL. Differential regulation of gamma-aminobutyric acid receptor channels by diazepam and phenobarbital. Ann Neurol. 1989;25(3):213–220. doi: 10.1002/ana.410250302. [DOI] [PubMed] [Google Scholar]
- 20.Kaminski RM, Gillard M, Klitgaard H. Targeting SV2A for discovery of antiepileptic drugs. In: Noebels JL, Avoli M, Rogawski MA, et al., editors. Jasper's Basic Mechanisms of the Epilepsies. Bethesda, MD: National Center for Biotechnology Information (US); 2012. [PubMed] [Google Scholar]
- 21.Silverstein FS, Ferriero DM. Off-label use of antiepileptic drugs for the treatment of neonatal seizures. Pediatr Neurol. 2008;39(2):77–79. doi: 10.1016/j.pediatrneurol.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Merhar SL, Schibler KR, Sherwin CM et al. Pharmacokinetics of levetiracetam in neonates with seizures. J Pediatr. 2011;159(1):152–154.e153. doi: 10.1016/j.jpeds.2011.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan O, Chang E, Cipriani C et al. Use of intravenous levetiracetam for management of acute seizures in neonates. Pediatr Neurol. 2011;44(4):265–269. doi: 10.1016/j.pediatrneurol.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Ramantani G, Ikonomidou C, Walter B et al. Levetiracetam: safety and efficacy in neonatal seizures. Eur J Paediatr Neurol. 2011;15(1):1–7. doi: 10.1016/j.ejpn.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Abend NS, Gutierrez-Colina AM, Monk HM et al. Levetiracetam for treatment of neonatal seizures. J Child Neurol. 2011;26(4):465–470. doi: 10.1177/0883073810384263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falsaperla R, Vitaliti G, Mauceri L et al. Levetiracetam in neonatal seizures as first-line treatment: a prospective study. J Pediatr Neurosci. 2017;12(1):24–28. doi: 10.4103/jpn.JPN_172_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao LM, Hussain SA, Zaki T et al. A comparison of levetiracetam and phenobarbital for the treatment of neonatal seizures associated with hypoxic-ischemic encephalopathy. Epilepsy Behav. 2018;88:212–217. doi: 10.1016/j.yebeh.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Grinspan ZM, Shellhaas RA, Coryell J et al. Comparative effectiveness of levetiracetam vs phenobarbital for infantile epilepsy. JAMA Pediatr. 2018;172(4):352–360. doi: 10.1001/jamapediatrics.2017.5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McHugh DC, Lancaster S, Manganas LN. A systematic review of the efficacy of levetiracetam in neonatal seizures. Neuropediatrics. 2018;49(1):12–17. doi: 10.1055/s-0037-1608653. [DOI] [PubMed] [Google Scholar]
- 30.Sharpe C, Reiner GE, Davis SL et al. Levetiracetam versus phenobarbital for neonatal seizures: a randomized controlled trial. Pediatrics. 2020;145(6):e20193182. doi: 10.1542/peds.2020-036806. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]


