Abstract
OBJECTIVE
The lack of randomized controlled trials comparing biologics for the treatment of juvenile idiopathic arthritis (JIA) has led to wide variation in treatment approaches. The objective of this study is to compare the efficacy and safety of abatacept, adalimumab, and etanercept in JIA patients treated at a tertiary pediatric institution.
METHODS
This was a single-center, retrospective chart review of patients initiated on abatacept, adalimumab, or etanercept from December 1, 2015, to August 31, 2018, at Monroe Carell Jr. Children's Hospital at Vanderbilt (VCH). The primary outcome was the change in the Physician Global Assessment (PGA) score after 4 to 6 months of biologic therapy. Secondary outcomes included change in laboratory markers of JIA disease activity, change in the number of joints with active disease or limitation of motion, reduction in corticosteroid dose, adverse effects, adherence among patients who have their medications filled at the institution's specialty pharmacy, and reason for discontinuation of therapy.
RESULTS
A total of 139 patients were included, with a median age of 13 years. Most patients, 80.6%, experienced a reduction in their PGA score after starting biologic therapy. There was not a statistically significant difference among the agents (p = 0.64). Adverse effects were reported in only 26.6% of patients, with the most frequent being injection site reactions or pain (n = 35). Ultimately, 32% of patients discontinued biologic therapy with a lack of efficacy being the most common reason.
CONCLUSIONS
Abatacept, adalimumab, and etanercept were not significantly different in efficacy and safety for the treatment of JIA at this single institution.
Keywords: abatacept, adalimumab, etanercept, juvenile idiopathic arthritis
Introduction
According to the American College of Rheumatology (ACR) recommendations for the treatment of juvenile idiopathic arthritis (JIA), the escalation of therapy depends on the active joint count, level of disease activity, and presence or absence of prognostic factors.1 When the active joint count is less than or equal to 4 in patients without poor prognostic features and low disease activity, monotherapy with non-steroidal anti-inflammatory drugs (NSAIDs) is recommended. Otherwise, intraarticular glucocorticoid injection is recommended as initial therapy. When the active joint count is greater than 4 in patients with moderate to high disease activity and poor prognostic features, methotrexate in addition to NSAIDs is preferred. Use of a biologic agent is not considered until there is continued disease activity after use of these initial treatments.2
There are no randomized controlled trials comparing biologics for the treatment of JIA. This has led to wide variation in treatment approaches among pediatric rheumatologists across the United States.3 Horneff et al4 performed an observational cohort study of German patients with polyarticular JIA receiving adalimumab, etanercept, or tocilizumab. These biologics showed comparable efficacy when looking at Juvenile Disease Activity Score and improvement in the Pediatric American College of Rheumatology Criteria (PedACR). Etanercept was used as the first-line biologic in 95.5% of patients, and there were no significant differences in efficacy between first-line and second-line biologics. A systematic review completed by Shepherd et al5 to assess the clinical effectiveness of etanercept, abatacept, adalimumab, and tocilizumab also found these biologics to be similar for the treatment of polyarticular JIA. The aim of this study is to compare the efficacy and safety of abatacept, adalimumab, and etanercept in JIA patients treated at Monroe Carell Jr. Children's Hospital (VCH) in the Pediatric Rheumatology Clinic.
Materials and Methods
Patients were identified by using prescription history and diagnosis code data from the electronic medical record. Data were collected through retrospective chart review of patients receiving care through the Pediatric Rheumatology Program at our institution. All JIA patients started on abatacept, adalimumab, or etanercept from December 1, 2015, to August 31, 2018, were eligible for inclusion. Patients were excluded if they had a primary diagnosis other than JIA for the indication of biologic therapy, age greater than 18 years at time of initiation, or no return clinic visit within 4 to 6 months after initiation of the biologic agent. Four to 6 months was chosen to give patients a comparable follow-up period while also taking into account the ACR recommendations that therapy may be escalated after this period. Only data from the first biologic agent during the study period were included for analysis; all subsequent changes in biologic therapy were excluded to preserve the independence of the data. Switching biologic agents is recommended by the ACR when escalation of therapy is warranted. Prior biologic therapy was not a part of the exclusion criteria in an effort to have a patient population most representative of that in current practice.
Baseline characteristics collected were age at initiation of biologic therapy, sex, race, weight, JIA type, duration of JIA, rheumatoid factor positivity, prior biologic use, and concomitant therapy at initiation of biologic. The primary outcome assessing the efficacy of abatacept, adalimumab, and etanercept was the change in the Physician Global Assessment (PGA) score after 4 to 6 months of biologic therapy. This measure was chosen because it is part of the PedACR, a widely accepted measure of response to treatment, and could be determined solely from chart review.6 A PGA scoring system was developed for this project with input from the Vanderbilt pediatric rheumatology providers (Table 1) and is based on a 5-point scale denoting the severity of patient symptoms for JIA, where higher scores represent worse status and a score of zero denotes no active joints. Symptoms were designated the following point totals: extremity pain (1 point); inflammation of extremities including extremity swelling, erythema, or tenderness (1 point); stiffness hindering daily activities (1 point); a decrease but not a complete suppression in the number of active joints (1 point). An increase or no change in the number of active joints counted as 2 points. Scores were calculated by using information provided in notes from clinic visits prior to initiation of biologic therapy (baseline) and at the 4- to 6-month follow-up visit. Symptoms were commonly charted by the provider; however, their omission was interpreted as an absence of symptoms.
Table 1.
Criteria for Physician Global Assessment Calculation in Patients With Juvenile Idiopathic Arthritis
| Points | Criteria |
|---|---|
| 0 |
|
| 1 |
|
| 2 |
|
Secondary outcomes included change in laboratory markers of JIA disease activity, change in the number of joints with active disease or limitation of motion, reduction in corticosteroid dose, adverse effects, adherence among patients who have their medications filled at the institution's specialty pharmacy, and reason for discontinuation of therapy. The specific laboratory markers assessed were erythrocyte sedimentation rate and C-reactive protein, which may indicate underlying inflammation not visible on physical examination.7 Adverse effects of interest were local injection site reactions or pain, infections, autoimmune disorders, and malignancy. Adherence was assessed by using a proportion-of-days-covered calculation with the date of the first fill used as the start of the observation window and the date of the follow-up visit, the end of the window. Prescribing trends were assessed by appropriateness of starting biologic dose, based on the package insert and escalation of current biologic therapy at follow-up visits.
To analyze the primary outcome of follow-up PGA score, the full model was fit by using proportional odds logistic regression with medication, age, race, weight, JIA type, time from baseline visit to follow-up, prior use of biologic, and baseline PGA score as covariates. Differences in adverse effects between the biologics were analyzed by using Pearson chi-squared test. For baseline characteristics, continuous variables were analyzed by using the Kruskal-Wallis test, while Pearson chi-squared test was used on categorical variables. A p value of 0.05 was considered significant. Descriptive statistics were used to describe the remainder of the results.
Results
During the study period, 233 patients were started on abatacept, adalimumab, or etanercept through the Pediatric Rheumatology Clinic. After patients were excluded for various reasons, a total of 139 patients were included in the final analysis (Figure 1). Fifteen patients received multiple biologic therapies during the study period; however, only the first instance of a biologic agent during this period was included for analysis. Refer to Table 2 for a summary of baseline characteristics by biologic agent. The p values listed in this table reflect a global analysis of the 3 groups and cannot be interpreted individually. The median age of patients was 13 years with a median weight of 47.1 kg. Most patients were female (72%) and white (76%). The median time on biologic therapy was 383 days, and the distribution of JIA type was 58.3% polyarticular, 40.3% oligoarticular, and 1.4% systemic. Of the 105 patients with available rheumatoid factor information, only 12% had positive findings. Most patients were not on scheduled NSAID or corticosteroid therapy when biologic therapy was started at 69% and 60%, respectively. For those patients receiving corticosteroid therapy, the median dose was 7.5 mg of prednisone equivalents/day and 76% of patients received the oral formulation. However, methotrexate use was common, with 76% of patients receiving this medication at initiation of biologic, and 86% of these patients using the injectable formulation. Most patients were started on a biologic less than 2 years following diagnosis of JIA (66%). Patients receiving adalimumab were more likely to have previous exposure to a different biologic (47%).
Figure 1.
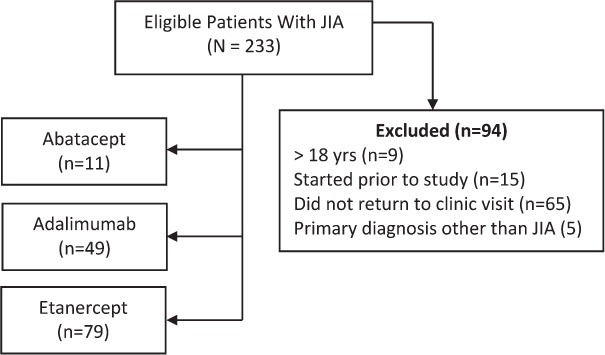
Participant flow diagram.
Table 2.
Summary of Baseline Characteristics by Biologic Agent
| Abatacept (n = 11) | Adalimumab (n = 49) | Etanercept (n = 79) | p value | |
|---|---|---|---|---|
| Age, median, yr | 13.7 | 13.9 | 12.1 | 0.18 |
| Female, n (%) | 8 (73) | 36 (73) | 56 (71) | 0.95 |
| Caucasian, n (%) | 7 (64) | 38 (78) | 60 (76) | 0.35 |
| JIA type, n (%) | ||||
| Oligoarticular | 5 (45) | 18 (37) | 33 (42) | 0.95 |
| Polyarticular | 6 (55) | 30 (61) | 45 (57) | |
| Systemic | 0 (0) | 1 (2) | 1 (1) | |
| Duration of JIA, n (%) | ||||
| <2 yr | 5 (46) | 25 (51) | 62 (78) | 0.002 |
| 2–5 yr | 1 (9) | 10 (20) | 6 (8) | |
| 5–10 yr | 2 (18) | 12 (25) | 6 (8) | |
| >10 yr | 3 (27) | 2 (4) | 5 (6) | |
| Prior biologic, n (%) | 2 (18) | 23 (47) | 5 (6) | <0.001 |
| Time on therapy, median, days | 322 | 343 | 419 | 0.35 |
JIA, juvenile idiopathic arthritis
For the primary outcome, 80.6% of patients experienced an improvement in their PGA score after 4 to 6 months of biologic therapy (Figure 2). The median change in PGA score was −1 for abatacept and adalimumab and −2 for etanercept, resulting in an overall change of −2. The multiple regression model found a higher age and baseline PGA score to be significant. For each 1-year increase in age, the odds of having a higher follow-up PGA score was 1.15 times higher (p < 0.036; 95% CI, 1.01–1.32). Each 1-unit increase in baseline PGA score was associated with a 1.67-fold increase in the odds of having a higher follow-up score (p = 0.007; 95% CI, 1.15–2.42). There was no statistically significant difference in follow-up PGA scores among the 3 agents (p = 0.640). No other covariates included in the full model were significantly associated with the follow-up PGA score.
Figure 2.
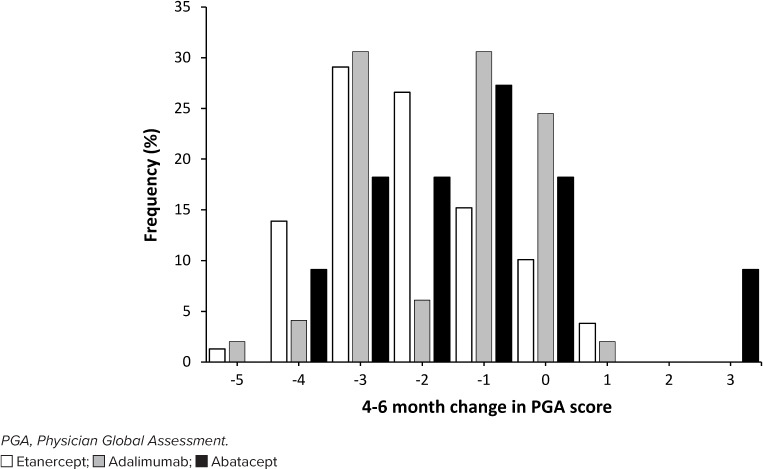
Change in PGA score at follow-up.
Most patients also experienced a reduction in the number of joints with active disease at follow-up (Figure 3). The median erythrocyte sedimentation rate and the C-reactive protein level were lower after initiation of biologic therapy, with a change from 23 mm/hr to 8.5 mm/hr and 11 mg/L to 0.7 mg/L, respectively. Of the 55 patients receiving any formulation of corticosteroid therapy at baseline, only 9 remained on therapy at follow-up. The starting dose of biologic therapy was appropriate in 89.2% of patients, and the dose or frequency was escalated in only 10% of patients at the 4- to 6-month follow-up, increasing to 20% at the last visit prior to the end of the study period or discontinuation of therapy. Adverse effects were uncommon, and there was no statistically significant difference among biologic agents (Table 3). Notably, there were no reported cases of autoimmune disorders or malignancy. Patients who had their medications filled at Vanderbilt Specialty Pharmacy (n = 74) demonstrated good adherence, with a median proportion of days covered of 0.96. There was no statistically significant difference between biologic agents (p = 0.54). Biologic therapy was discontinued in 32% of patients for reasons listed in Table 4, the most common being lack of efficacy.
Figure 3.
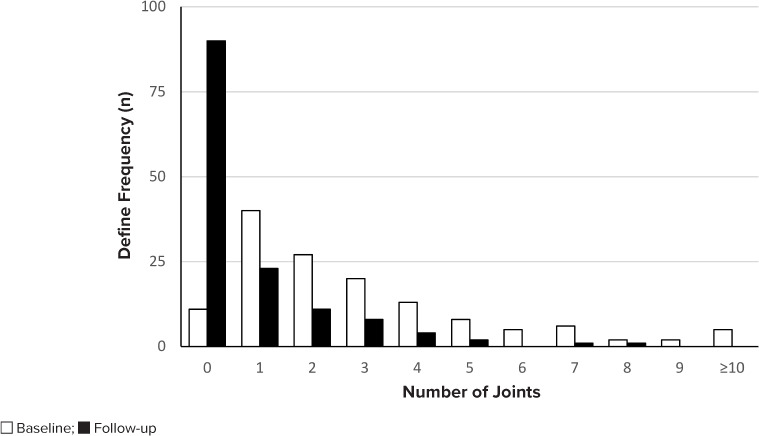
Joints with active disease.
Table 3.
Incidence of Adverse Effects
| Adverse Effect | Abatacept (n = 11), n (%) | Adalimumab (n = 49), n (%) | Etanercept (n = 79), n (%) | p value |
|---|---|---|---|---|
| Local injection site reactions/pain | 1 (9) | 15 (31) | 19 (24) | 0.31 |
| Infections | 0 (0) | 2 (4) | 1 (1) | — |
| None | 10 (91) | 33 (67) | 59 (75) | 0.26 |
Table 4.
Reasons for Discontinuation of Therapy
| Reason | Abatacept (n = 4), n | Adalimumab (n = 6), n | Etanercept (n = 35), n |
|---|---|---|---|
| Major side effect | 0 | 1 | 3 |
| Non-adherence | 0 | 1 | 6 |
| Inefficacy | 4 | 4 | 26 |
| Full course completed | 0 | 0 | 2 |
| Insurance change/mandate | 1 | 0 | 0 |
Discussion
Adalimumab, abatacept, and etanercept were not found to be different in regard to safety and efficacy for the treatment of JIA at this single institution. Not only did the PGA decline in the time frame of 4 to 6 months, but also the active joint count similarly decreased. Laboratory markers of continued disease activity were also reduced from biologic initiation to follow-up. The results of this study aligned with the well-documented efficacy of these agents for this rheumatologic disease and previous indirect comparisons.2,4,5
For the treatment of JIA at this institution, etanercept was the most commonly prescribed biologic with adalimumab being more likely to be used second line. Most patients received an adequate trial of biologic therapy at approximately 1 year despite 32% of patients discontinuing the biologic for various reasons. Most patients were appropriately on methotrexate as an additional disease-modifying antirheumatic drug. The addition of biologic therapy allowed for weaning or discontinuation of corticosteroid therapy, likely due to improvement in disease activity. This is desired given the known adverse effects of corticosteroids and allowed for reservation for acute flares of disease. Most patients were not receiving corticosteroids at the time of biologic initiation, which reflects the department's steroid-sparing practice. NSAID use may be underreported because only scheduled use was included since the frequency of as-needed NSAIDs was not well documented. The low incidence of rheumatoid factor positivity was expected because it excludes the diagnosis of oligoarticular JIA and most children with polyarticular JIA are rheumatoid factor negative.8,9 Lack of efficacy was the most common reason for biologic discontinuation in this study, which was also observed in the study performed by Horneff et al.4 Similarly, very few patients discontinued their biologic owing to adverse effects. The lack of cases of autoimmune disease or malignancy was reassuring and provides additional data regarding serious adverse effects associated with these agents.
One of the strengths of this study was that it included patients from a large academic medical center. The Vanderbilt Pediatric Rheumatology Department averages approximately 100 patient visits per week on the main campus. The study period of almost 3 years allowed for patients to be followed up for long-term adverse effects. In addition, fill data through Vanderbilt Specialty Pharmacy enabled us to assess adherence for a subset of patients. This indirect comparison of 3 biologic therapies for the treatment of JIA adds to the preexisting evidence supporting their use. Providers may continue to prescribe biologics, based on patient-specific factors, given their comparable efficacy.
This study also had multiple limitations, the first being it was a retrospective chart review. The recent approval of subcutaneous abatacept limited the number of patients who were eligible for inclusion and thus comparison. It is unknown if the frequent use of adalimumab as a second-line biologic resulted in an underestimation of its efficacy. The lower discontinuation rate may speak to its utility in treating refractory cases of JIA. This study was not powered to detect a difference in discontinuation rates between the 3 biologic agents. It is important to note inefficacy as a discontinuation reason was up to the provider's or patient's interpretation. The absence of a statistically significant difference in the primary outcome argues against a difference in the discontinuation rates. The most common reason for exclusion was the time to follow-up visit not occurring within the 4- to 6-month time frame. Standardization of the first follow-up visit may allow for a larger comparison to be performed. For this study, the PGA score was calculated on chart review and not by the provider during the office visit. Furthermore, the PGA score itself is not a validated measure of JIA disease activity. With the documentation of the PGA score becoming more common practice at VCH, a study using this measure for efficacy in the future may be easier to complete. Adding the PGA score to the note template used in the Pediatric Rheumatology Clinic may help promote documentation of this measure at all visits. The ultimate goal would be documentation of all components of the PedACR to assess response to treatment.
The results of this study provide directions for future JIA research. Future comparisons of efficacy will be warranted once there is a larger proportion of pediatric patients receiving subcutaneous abatacept. Including only patients with no prior biologic exposure may provide more information as to which agent should be used first line. The reformulation of adalimumab as citrate-free has increased patient satisfaction owing to decreased pain with injection. Providers may now use adalimumab preferentially over etanercept for this reason along with the potential for increased compliance owing to its every 2-week dosing frequency.
Conclusions
Most patients included in this study experienced an improvement in their PGA score after starting abatacept, adalimumab, and etanercept regardless of which biologic they received. These 3 biologics were largely well tolerated with most patients experiencing no adverse effects. Unfortunately, lack of efficacy was the most common reason for biologic discontinuation. From these results, prescribers should continue to prescribe a particular biologic, based on patient-specific factors, until future data suggest otherwise.
Acknowledgments
Preliminary results were presented at PPAG Annual Meeting Resident Project Presentations in Oklahoma City, OK, on April 13, 2019.
ABBREVIATIONS
- ACR
American College of Rheumatology;
- JIA
juvenile idiopathic arthritis;
- NSAIDs
nonsteroidal anti-inflammatory drugs;
- PedACR
Pediatric American College of Rheumatology Criteria;
- PGA
Physician Global Assessment;
- VCH
Monroe Carell Jr. Children's Hospital at Vanderbilt
Footnotes
Disclosure. The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Ethical Approval and Informed Consent. The research protocol was approved by the Vanderbilt Institutional Review Board and written informed consent was not required.
References
- 1.Beukelman T, Patkar NM, Saag KG et al. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res. 2011;63(4):465–482. doi: 10.1002/acr.20460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ringold S, Angeles-Han ST, Beukelman T et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Treatment of Juvenile Idiopathic Arthritis: Therapeutic Approaches for Non-Systemic Polyarthritis, Sacroiliitis, and Enthesitis. Arthritis Care Res. 2019;71(6):717–734. doi: 10.1002/acr.23870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Mater H, Balevic SJ, Freed GL, Clark SJ. Prescribing for children with rheumatic disease: perceived treatment approaches between pediatric and adult rheumatologists. Arthritis Care Res. 2018;70(2):268–274. doi: 10.1002/acr.23273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horneff G, Klein A, Klotsche J et al. Comparison of treatment response, remission rate and drug adherence in polyarticular juvenile idiopathic arthritis patients treated with etanercept, adalimumab or tocilizumab. Arthritis Res Ther. 2016;18(1):272. doi: 10.1186/s13075-016-1170-3. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shepherd J, Cooper K, Harris P et al. The clinical effectiveness and cost-effectiveness of abatacept, adalimumab, etanercept and tocilizumab for treating juvenile idiopathic arthritis: a systematic review and economic evaluation. Health Technol Assess. 2016;20(34):1–222. doi: 10.3310/hta20340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Consolaro A, Giancane G, Schiappapietra B et al. Clinical outcome measures in juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2016;14(1):23. doi: 10.1186/s12969-016-0085-5. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swart JF, de Roock S, Prakken BJ. Understanding inflammation in juvenile idiopathic arthritis: how immune biomarkers guide clinical strategies in the systemic onset subtype. Eur J Immunol. 2016;46(9):2068–2077. doi: 10.1002/eji.201546092. [DOI] [PubMed] [Google Scholar]
- 8.Petty RE, Southwood TR, Manners P et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–392. [PubMed] [Google Scholar]
- 9.Hinks A, Marion MC, Cobb J et al. Brief report: the genetic profile of rheumatoid factor-positive polyarticular juvenile idiopathic arthritis resembles that of adult rheumatoid arthritis. Arthritis Rheumatol. 2018;70(6):957–962. doi: 10.1002/art.40443. [DOI] [PMC free article] [PubMed] [Google Scholar]


