History
Throughout the evolution of the definition of Barrett’s esophagus (BE) the one thing that has remained constant is the identification of some form of columnar epithelium upon histological analysis. The first reporting of such a tissue found in the esophagus is attributed to Schmidt in 18051. In the early part of the 20th Century, Stewart and Hartfall2 and Lyall3 noted that the presence of columnar epithelium in the esophagus—surrounding “ulcerations”—was an abnormality. Confusion and debate began to center on the origin of columnar epithelium in the esophagus and, in his famous treatise of 1950, Norman Rupert Barrett proposed that a condition existed in which congenitally short esophagus resulted in the stomach being drawn up into the chest cavity—a type of hiatal hernia4. However, even though the eponym of Barrett’s was to stick, his initial theory of the origin of columnar epithelium was soon to be found incorrect. It was Allison and Johnstone in 19535 who initially used the term ‘Barrett’s ulcer’ to refer to what was previously known as ‘peptic ulcer of the esophagus’, reasoning that this would distinguish it from ulceration of esophageal squamous epithelium, which had been given the name ‘reflux esophagitis’ by Barrett himself. Allison and Johnstone proposed that Barrett’s ulcer was not due to a congenitally short esophagus with herniation of the stomach, but instead to a congenital gastric-lined esophagus. From scrupulous examination of specimens, they noted that there was no peritoneal covering, the musculature was typically esophageal, islands of squamous epithelium existed within the columnar lining and that oxyntic (parietal) cells were absent. This led them to propose that:
“It appears better…to refer to that congenital abnormality which from the outside looks like oesophagus and from the inside looks like stomach as ‘oesophagus lined with gastric mucous membrane’.”
The association with reflux and hiatal hernia was spoken of, but they still considered BE to be wholly congenital, a view supported by embryological studies of fetal development. Barrett conceded his theory of congenitally short esophagus in 19576, now referring to this gastric-like lining as ‘lower oesophagus lined by columnar epithelium’, a term which in future publications was to be replaced by the eponym ‘Barrett’s esophagus’. He too still thought the condition to be of congenital origin, yet in 1957 he did acknowledge that an acquired pathogenesis may exist:-
“If the cardiac valve of a normal person were to become incompetent and if the lower oesophagus were, as a result, to be bathed for a long time by digestive gastric juice, the squamous epithelium could be eaten away and totally replaced by columnar cells.”
Thus, perhaps the eponym is merited. Two-years later, in 1959, Moersch, Ellis, and McDonald7 are credited with the first publication where the changes of the distal esophagus following reflux esophagitis are discussed without inferring a congenital origin, instead referring to ‘inflammatory metaplasia’. Their study of 36 esophageal resections was the first convincing evidence that persistent gastroesophageal reflux disease (GERD) was central to the etiology of columnar-lined esophagus. This perspective was strengthened in 1970 in a series of landmark canine experiments by Bremner et al.8 in which they showed normal esophageal squamous repair in the absence of GERD but re-epithelialization with a columnar lining when GERD was induced.
What we now consider the defining feature of BE—goblet cells—were first noted by Bosher and Taylor in 19519. Despite subsequent confirmatory observations5,10, the histology of BE continued to be debated for the next 20 years. In 1976, some clarity emerged from a study by Paull and colleagues11 with descriptions of three types of metaplasia, including specialized columnar epithelium—which we now call intestinal metaplasia, synonymous with BE. Paull only hinted at the potential carcinogenic importance of intestinal metaplasia, and it wasn’t until early 1990’s that intestinal metaplasia was generally accepted to be the most prevalent, distinctive epithelium, and highest in conferring risk of esophageal adenocarcinoma (EA)12,13.
Epidemiology
POPULATION PREVALENCE OF BARRETT’S ESOPHAGUS
The population prevalence of BE is a crucial statistic upon which all primary and secondary prevention strategies are based, yet it remains largely unknown primarily due to the fact that many individuals with BE are asymptomatic.14 The BE prevalence in selected populations—such as endoscopic or surgical series—is no substitute, and so there are few studies that provide an accurate estimate. The first reliable study, published in 1990, assessed the prevalence of long-segment BE in a large series of randomly selected subjects for autopsy and compared this to a prevalence from the endoscopy practice of the Mayo Clinic15. The finding of four BE cases was approximately 21 times of that which was expected (0.19), and this equated to an age- and sex-adjusted BE prevalence estimate of 376 per 100,000 population (0.376%). Other estimates of BE population prevalence come from randomly selected populations to undergo endoscopy. A Swedish study16 of 1,000 randomly selected volunteers detected a total of 16 cases of BE, five of which were classified as long-segment BE (>=2 cm), yielding population prevalences of 1.6% and 0.5%, respectively. An Italian study17 of 1,033 adults, reported a BE population prevalence of 1.3% (0.2% for long segment and 1.1% for short segment). A computer simulation disease model has also been used to estimate the population prevalence of BE.18 Aligning simulation models with EA rates from the US Surveillance Epidemiology and End Results (SEER) cancer registry data, the authors estimated a BE population prevalence of 5.6%. Thus, in predominantly European ancestral populations, estimates for BE population prevalence range from 0.4% to 5.6%. Perhaps surprisingly, Asian countries may also have BE population prevalences in this range, despite the lower incidence of EA. A recent meta-analysis of four Asian-based studies indicated a BE population prevalence of 0.7%19, and a Taiwan-based study of 3,385 subjects undergoing routine esophagogastroduodenoscopy examination as part of a health check-up provided a BE population prevalence of 2.6%.20 The Taiwan study provided details of segment length, showing that that the vast majority of diagnoses were short-segment BE, a characteristic that has previously been described in other selected endoscopic series from Asian countries, and which is in accordance with the lower incidence of EA in Asian populations.
INCIDENCE TRENDS OF BARRETT’S ESOPHAGUS
Assessing incidence trends of BE is a near impossibility due to the large pool of asymptomatic, undiagnosed subjects that we estimate to exist in most populations. As such, the best estimates are derived from clinical data with the hope that these may mirror relative change of all BE cases, including those never diagnosed. Typically, the denominator in such studies is the number of endoscopies, rather than the total population at risk.
In the US, the first report of BE incidence was from the Mayo Clinic which found a rate of 9.5 per 1,000 endoscopies per annum that was stable over the period 1965 to 1986; the crude rate did dramatically increase but this was wholly accounted for by a similar increase in the number of endoscopies15. Two other US studies also presented evidence for no change in BE incidence through the 1990s, once adjusted for number of endoscopies21,22. However, a fourth US study did provide evidence for an increase23. The authors found that endoscopy suspected BE increased from 32.2 to 82.8 per 1,000 endoscopies and histologically diagnosed BE from 6.7 to 27.6 per 1,000 endoscopies during 1991 to 2000. More recent studies covering the mid-90s through 2010, indicate that BE incidence has been stable and then may have declined24–26.
In contrast to the US, a majority of European studies have suggested an increase in BE incidence including Scotland27, UK28, Switzerland29, the Netherlands30, Spain31, and Northern Ireland32, as has a study from Australia33. The most recent European study from the UK and the Netherlands provides evidence for a decrease and then stabilization of BE incidence34.
Statistics of BE incidence need to be interpreted with several caveats in mind: increasing enthusiasm and education about BE, especially short-segment BE, and changes in referral patterns have not been measured and therefore remain unadjusted for. It has been suggested that the increasing epidemiological evidence of the strength of BE as a precursor to EA served to increase the awareness of this lesion27. It is unknown what effect this has had referral practices; obviously more patients are being referred for endoscopy but the relative influences of altered incidence of heartburn symptoms and increased awareness of sequelae complications, by both patient and provider, are unknown. Overall, it is likely the evidence supports increased BE incidence in Western Europe and Australia, whilst further evidence is needed to confirm or refute similar trends in the US.
INCIDENCE AND SURVIVAL OF ESOPHAGEAL ADENOCARCINOMA
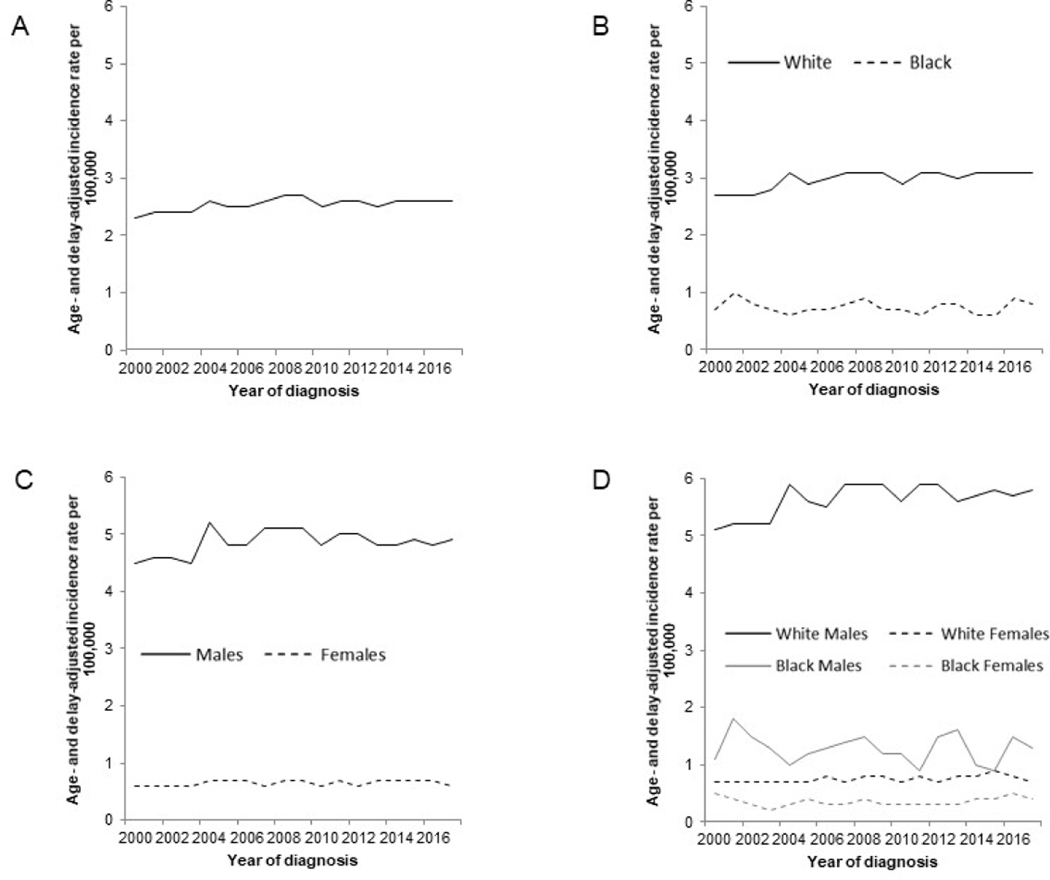
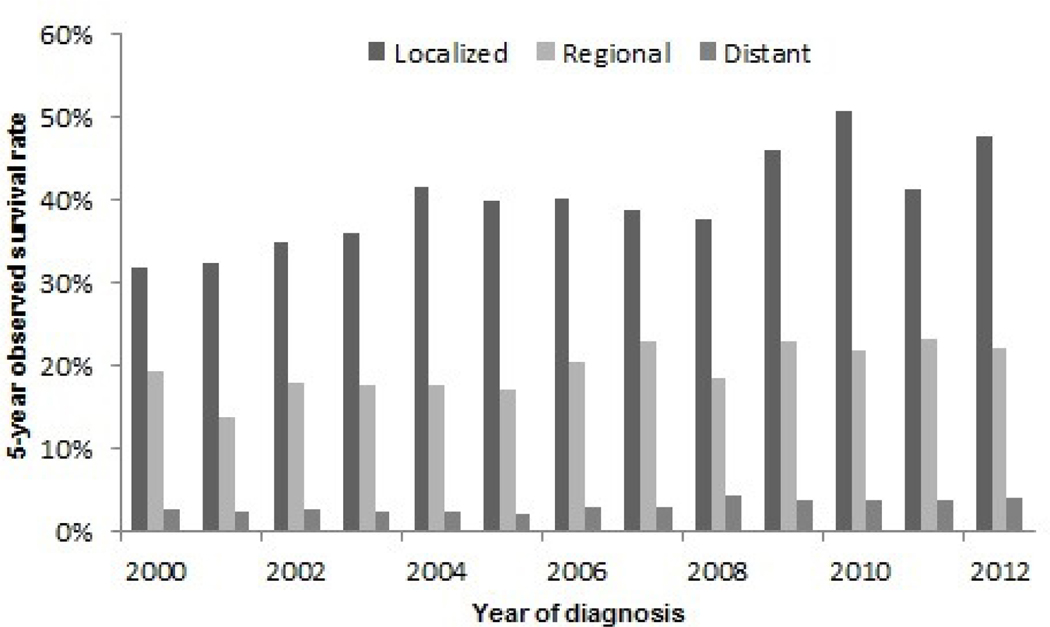
For the purpose of this review, the most recent data were analyzed from the SEER 9 registries35 (covering approximately 10% of the United States’ population) in which 21,358 cases of invasive EA (defined using International Classification of Diseases for Oncology, Third Edition (ICD-O-3) site codes, C15.0-C15.9; and histologic codes, M8140–8575) were diagnosed between 1975 and 2017. The overall age-adjusted incidence rate for EA during 1975–2017 was 2.0 per 100,000 person-years. EA incidence rates increased from 0.4 per 100,000 person-years in 1975 to 2.8 per 100,000 person-years in 2017 (Figure 1). EA incidence also varies by US state, with NAACCR data36 showing highest rates predominantly in northern and northeastern states (Figure 2). While EA rates increased dramatically between the mid-1970s through 2000,37 SEER 18 delay-adjusted data38 show that the rate of increase has slowed in subsequent years and EA incidence has stabilized in the U.S. through 2017 (Figure 3). Absolute rates of EA remain significantly higher among White males in the U.S. compared with females and non-Whites; however, similar rates of change and secular trends have been observed in all subgroups of the U.S. population. For EA cases in the SEER 18 registries39, median relative survival has increased from 10.5 months in 2000 to 13.1 months for persons diagnosed in 2016. Overall 5-year observed survival rates increased from 15.4% for patients diagnosed with EA in 2000 to 18.4% for patients diagnosed with EA in 2012. The greatest absolute improvement in survival trends occurred in EA patients diagnosed with localized disease, approximately 32% of patients diagnosed with localized EA in 2000 survived 5 years after their diagnosis, whereas the 5-year observed survival rate for patients diagnosed with localized EA in 2012 was 48% (Figure 4). Less striking improvements in 5-year survival rates were observed among patients diagnosed with regional (19% for patients diagnosed in 2000 vs. 22% for those diagnosed in 2012) or distant (2.5% for patients diagnosed in 2000 vs. 4.1% for those diagnosed in 2012) stage EA.
Figure 1.
Age-adjusted incidence rates of esophageal adenocarcinoma in the United States, 1975–2017. Rates are per 100,000 person-years. Data source: Surveillance, Epidemiology, and End Results (SEER) 9 Registries.
Figure 2 .
Age-adjusted incidence rates of esophageal adenocarcinoma in the United States by state, 2012–2016. Rates are per 100,000 person-years. Darker blue hues denote higher incidence rate categories of esophageal adenocarcinoma. There were no data for the time period assessed for the states filled white. Alaska, Hawaii, and Puerto Rico have been repositioned for maximal resolution. (Data from North American Association of Central Cancer Registries (NAACCR) Incidence Data - CiNA Analytic File, 1995–2016.
Figure 3.
Age- and delay-adjusted incidence rates of esophageal adenocarcinoma in the United States, 2000–2017. Rates are per 100,000 person-years. Age- and delay-adjusted incidence rates of esophageal adenocarcinoma are shown for: (A) All; (B) by race (White and Black); (C) by sex (Males and Females); and (D) by sex and race (White Males, Black Males, White Females and Black Females). (Data from Surveillance, Epidemiology, and End Results (SEER) 18 Registries.)
Figure 4.
Five-year survival rates for esophageal adenocarcinoma in the United States, 2000–2012, by stage at diagnosis (localized, regional and distant stage). (Data from Surveillance, Epidemiology, and End Results (SEER) 18 Registries.)
Nature of the Problem
The central problems in primary prevention (screening) and secondary prevention (surveillance) of EA is the large undiagnosed BE population and the suboptimal ability to triage risk in the diagnosed BE population, respectively. The large undiagnosed BE population results in a majority of EA cases presenting with late stage disease and a resultant poor prognosis. Below, we review the current epidemiologic evidence of risk factors, biomarkers, and algorithms that may be used to overcome this problem and identify a larger pool of subjects with BE. We then review the current evidence for triaging cancer risk in the diagnosed BE population, a difficulty that will be compounded should the primary prevention hurdle (population screening) be overcome.
Current Evidence
POPULATION SCREENING FOR BARRETT’S ESOPHAGUS
There is a major caveat to this section. A vast majority of the studies that are described have been conducted using selected BE populations; that is, patients who present with symptoms that merit endoscopic and histologic investigation. This should not be glossed over and it is an inherent limitation of studying a rare, largely asymptomatic condition that is expensive and difficult to diagnose.
Risk Factors
While clinical guidelines all recommend screening for BE, the screening population differs40–43. All guidelines, except those from the American Gastroenterological Association, condition screening for BE based on the presence of GERD symptoms (Table 1). Risk factors used to define high-risk for purposes of screening generally include age ≥50 years, male sex, Caucasian race, GERD symptoms, smoking, and obesity. Here, we review the literature supporting these high-risk determinants as well as other potential risk factors for BE and EA.
Table 1.
Barrett’s esophagus screening guidelines for select gastroenterological societies
| Society | Year | Screening Population |
|---|---|---|
| American College of Gastroenterology | 2016 | Men >5 years GERD, or with >weekly symptoms + ≥2 risk factors: >50 years, central obesity (waist circumference >102cm or WR >0.9), Caucasian, smoking, first-degree relative w/ BE or EA |
| British Society of Gastroenterology | 2014 | GERD with ≥3 risk factors: >50 years, Caucasian, male, obesity +/− (+) family history |
| American Society for Gastrointestinal Endoscopy | 2019 | Individuals with a family history of EA or BE (high risk) OR GERD with ≥ 1 risk factors (moderate risk): >50 years, male gender, Caucasian, smoking, obesity |
| American Gastroenterological Association | 2011 | Multiple risk factors: >50 years, Caucasian, male, chronic GERD, hiatal hernia, obesity |
Demographics
EA incidence increases with increasing age and is rare among persons aged <50 years. An intriguing and yet largely unexplained observation in EA is the striking sex disparity; across all countries, EA incidence rates in females remain significantly lower than those of males. The magnitude of the male predominance is greatest in the U.S., where the male:female incidence rate ratio approaches 9:144–46. EA is also more common in non-Hispanic whites than non-whites47. Likewise, BE is twice as common in men as in women and more common in non-Hispanic whites than other races/ethnicities48.
Environmental Risk Factors
Frequent GERD symptoms, cigarette smoking, and obesity are the main risk factors for EA and BE. Together, these three risk factors account for over 70% of all cases of EA in Western populations49,50, and are observed among the majority of patients with BE.
In a pooled analysis of individual-level data from 5 case-control studies participating in the Barrett’s and Esophageal Adenocarcinoma Consortium (BEACON; https://esocan.org/beacon/), there was a strong, dose-dependent relationship between frequency of GERD symptoms and EA risk. Compared to individuals with infrequent or no GERD symptoms, those with at least weekly and daily symptoms had five-fold (odds ratio [OR]=4.81, 95% confidence interval [CI], 3.39–6.82) and eight-fold (OR=7.96, 95%CI:4.51–14.04) higher risk of EA, respectively51. For BE, individuals with frequent GERD symptoms occurring in early adulthood have especially high-risk (first reported symptoms at age <30 years, OR=15.1, 95%CI:7.91–28.8)52.
Cook et al.53, using pooled individual-level data from 10 case-control and 2 cohort studies in BEACON, found that ever smoking was associated two-fold increased risk of EA (vs. never smoking; OR=1.96, 95%CI:1.64–2.34) and showed that EA risk increased with increasing pack-years smoking history. While EA risk among ever smokers appears to decline with increased years of smoking cessation, risk in formers smokers does not return to the level observed for never smokers53,54. The evidence is less clear for BE. In the largest study to date, risk of BE was 1.7-fold as high among ever smokers as it was among never smokers (OR=1.67, 95%CI:1.04–2.67)55. However, unlike for EA, BE risk does not increase with increasing cumulative exposure.
Compared to individuals with a normal body mass index (BMI <25.0 kg/m2), individuals with BMI of 30.0–34.9 kg/m2 and ≥40.0 kg/m2 have two-fold (OR=2.39, 95%CI:1.86–3.06) and five-fold (OR=4.76, 95%CI:2.96–7.66) higher risk of EA, respectively56. Obesity in childhood and adolescence may also confer increased risk of EA independent of adult BMI57,58. Increasing evidence suggests that abdominal obesity confers greater risk for EA and BE than overall obesity59,60. A meta-analysis found over two-fold increased risk of EA associated with abdominal obesity (OR=2.51, 95%CI:1.54–4.06)61. Likewise, abdominal obesity was associated with two-fold higher risk of BE (OR=1.98, 95%CI:1.52–2.57)61. These associations remained after controlling for BMI, while there were weak or no associations with BMI after controlling for abdominal obesity.
Alcohol consumption is not associated with increased risks of EA or BE62,63. The Continuous Update Project Report on diet, nutrition and physical activity by the World Cancer Research Fund/American Institute for Cancer Research, which considers results from only cohort studies, reported that no dietary factors were judged to have strong evidence of an association with risk of EA and that there were limited suggestive evidence for an inverse relationship between physical activity and risk of EA64.
Helicobacter pylori is a gram-negative bacterium that infects half the world’s human population and causes gastric cancer65. Conversely, H. pylori infection is associated with lower risks of EA and BE. Two meta-analyses both reported over 40% lower risk of EA for individuals infected with H. pylori (in particular, those with the CagA-positive H. pylori strain) compared with individuals uninfected with H. pylori66,67. There is also strong evidence that H. pylori infection is associated with lower risk of BE68,69.
Frequent users of aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) have lower risk of EA. A pooled analysis of individual-level participant data in BEACON found that any use of aspirin or NSAIDs was associated with 30% lower risk of EA (OR=0.68, 95%CI:0.56–0.83)70. Current users had especially lower risk for EA (OR=0.40, 95%CI:0.24–0.97), and risk was shown to decrease linearly with both increased frequency and duration of use70. In contrast, a pooled analysis among BE studies in BEACON found no association between any NSAIDs and BE (OR=1.00, 95%CI:0.76–1.32)71. Statins have also been shown to be associated with lower risks of EA and BE72,73.
Given the male predominance in BE and EA risk, studies have considered whether or not sex hormones might be involved. Case-control studies have found increased risk of EA associated with increased androgen:estrogen74, and increased risk of BE associated with higher levels of free testosterone and free dihydrotestosterone75,76. In a prospective study, pre-diagnostic concentrations of circulating dehydroepiandrosterone (highest quartile vs. lowest quartile: OR=0.28, 95%CI:0.13–0.64), estradiol (highest quartile vs. lowest quartile: OR=0.55, 95%CI:0.31–0.99), and free estradiol (highest quartile vs. lowest quartile: OR=0.56, 95%CI:0.30–1.03) were associated with lower risk of a combined outcome of EA and gastric cardia adenocarcinoma77. In a second prospective study of EA, contrary to long-standing hypotheses,78 higher circulating levels of testosterone were associated with lower risk for EA in males.79 A recently published Mendelian randomization study found an association between genetically predicted levels of follicle-stimulating and luteinizing hormones and risk of BE and EA but no associations with other sex hormones, including dehydroepiandrosterone sulfate, testosterone, and estradiol.80
Genetic Factors
Genome-wide association studies have identified and validated germline (inherited) loci associated with risk of EA and BE, including CRTC1, a transcription coactivator associated with increased cancerous activity, BARX1, a transcription factor that promotes esophageal differentiation, FOXF1, FOXP1, and TBX5, which encode transcription factors involved in esophageal development, and GDF7, which encodes a protein in the bone morphogenetic pathway which has been associated with BE.81–84
Algorithms
Early strategies to select patients for BE screening were based on only frequency and severity of GERD symptoms. However, as only around half of BE patients report symptoms of GERD16,17, symptoms alone discriminate poorly between persons with and without BE85–88. A number of risk stratification tools have since been developed that use demographic, lifestyle and clinical information to discriminate between individuals at high- and low-risk for BE, with varying degrees of success with respect to discriminatory accuracy85,89,90. They also require further examination in external populations and prospectively before clinical implementation can be recommended91,92. Three recent validation efforts have shown that the Michigan Barrett’s Esophagus pREdiction Tool (M-BERET) which incorporates GERD symptoms, age, waist-to-hip ratio, and pack-years of cigarette use to predict BE risk is robust and transportable to other populations88,93,94. However, the discriminatory ability of this tool (area under the receiver operating characteristic curve [AUC], ~0.70) is not at the level required for clinical application. To address this shortcoming, other factors including blood-based biomarkers86 and genetic information95,96 have been added to baseline models using demographic, lifestyle and clinical factors, with modest success.
PRECISION SURVEILLANCE OF BARRETT’S ESOPHAGUS
A similar caveat to that mentioned under population screening also applies to this section: all studies to have assessed preneoplastic (BE) tissues of EA cases have, by definition, been restricted to the BE subpopulation that is currently identified (symptomatology justifies endoscopic investigation). Rapid progressors, less symptomatic, and asymptomatic BE case populations—which comprise the majority as well as important, high-risk subsets of BE—are typically not identified and thus not studied. As such, evidence from studies of pre-neoplastic BE tissues may not be generalizable to the wider BE population, from which a majority of EA cases derive. It is here that case-control (cross-sectional) studies of EA compared with BE controls may offer additional insights for putative risk prediction markers by studying unselected and complete EA populations97.
Risk Factors
Risk factors for neoplastic progression in BE include age, sex, and cigarette smoking. Increasing age as a risk factor for neoplastic progression is inferred from cancer registry data which shows EA incidence of 1.0 per 100,000 person years aged 40–49 years, 3.9 for ages 50–59, 9.3 for ages 60–69, 13.7 for ages 70–79, and 13.6 for 80+ years38 and has empirical support as an independent predictor.98–103 Male BE subjects have been shown to have 2–3 times higher risk of developing EA compared with female BE subjects104 which is not attenuated in multivariable models.98–101,103,105 Cigarette smoking and pack-years have shown fairly consistent moderate associations with EA when compared with BE100,102,105–107, with a recent meta-analysis finding a 30–50% increased risk of ever-smoking compared with never-smoking 98. Other lifestyle factors and demographics, such as GERD, excess adiposity, alcohol consumption, and race do not have good evidence for being risk factors for neoplastic progression in BE. GERD has consistently been inversely associated with EA when compared with BE97,107,108. In a study that conducted separate analyses of EA by prior diagnosis of BE, GERD was positively associated with the 13% of EA cases that had a prior diagnosis of BE97, yet inversely associated with the remaining 87% of EA cases that did not have a prior diagnosis of BE, when each were compared with a BE control group. A plausible interpretation is that a majority of individuals diagnosed with EA do not have a recent history of severe GERD exposure109. With regards to excess adiposity, overweight at age 20 years (OR=2.6, 95%CI:1.2–5.5) and 10 years prior to questionnaire (OR=1.8, 95%CI:1.1–3.3) were associated with EA compared with BE controls in a hospital-based case-control study from The Netherlands107; however, this observation does not have support from a majority of other studies that have compared EA with BE98,100,102,106. In addition, abdominal obesity has been similarly null in relation to neoplastic progression in BE populations102,106. Studies of alcohol and neoplastic progression in BE have been null105,106,110 while race has been difficult to study due to the fact that most BE patients are of European ancestry.
Biomarkers
Endoscopic and Histologic Features
Various endoscopic features have been associated with neoplastic progression in BE, including metaplastic segment length with studies showing a 17–19% increased risk per cm after multivariable adjustment98,111,112. Moreover, a recent pooled analysis of 10 studies showing annual rates of progression from non-dysplastic BE to high-grade dysplasia or EA of 0.24% for short segment BE (<3 cm) compared with 0.76% for long-segment BE (≥3 cm)113. Other endoscopic features associated neoplastic progression in BE include esophageal contractility114, esophageal ulcer108,115, and nodularity 115,116. The evidence for whether hiatal hernia is associated with neoplastic progression in BE is mixed with many studies finding no association 99,108,111,117, although one of the largest case-control studies did report an OR of 1.2 per cm (95%CI:1.0–1.4)112. Esophageal stricture108 and esophagitis99,108 do not appear to be risk factors for neoplastic progression in BE.
The primary histologic feature associated with EA risk in BE is dysplasia. Diagnosis of high-grade dysplasia is often clinically treated as EA43 due to the high-risk of prevalent malignancy or subsequent progression. Low-grade dysplasia has a more contentious history as a marker of neoplastic risk due to low interobserver agreement and the possibility of true regression back to a non-dysplastic state118. Larger specimen size119 and simplified descriptive histologic criteria120 appear to improve interobserver agreement, while expert confirmation118 and persistence of low-grade dysplasia121–123 are associated with higher risks of neoplastic progression. However, cancer risk still varies markedly between studies of low-grade dysplasia populations98,113,118,124. In the US, this has resulted in low-grade dysplasia being used as a marker for either clinical intervention or increased surveillance, depending on patient-provider discussions118.
Molecular Biomarkers
Initial molecular biomarkers to stratify neoplastic risk in BE focused on using histochemistry to distinguish three subtypes of intestinal metaplasia125–127. Although subtype III was hypothesized to be a marker of disease progression128, further studies cast doubt upon the specificity129,130 and accuracy131 of this biomarker. Despite this initial disappointment, further histochemical studies have provided more promising results. For example, in a nested case-control study of 29 cancer cases and up to 5 matched controls per case, diffuse/intense TP53 staining in baseline BE biopsy was associated with an 11-fold increased risk of EA132. Prior smaller IHC studies of low-grade dysplastic BE cases had suggested this association133–135, and subsequent studies and meta-analyses offered corroborating evidence136,137. This body of evidence led the British Society of Gastroenterology to recommend considering TP53 immunohistochemistry as an adjunct diagnostic41,138.
A recent multiplexed immunofluorescence discovery and validation study of 14 markers implicated in BE progression or carcinogenesis more generally were tested in a multi-institutional case-control study comprised of 79 progressors matched with 287 nonprogressors139. A 3-tier (low-, intermediate-, and high-risk), 15-feature classifier based on ten biomarkers (TP53, HER2, K20, COX2, CD68, HIF1a, p16INK4A, AMACR, CD45RO, and nuclear morphology) estimated a hazard ratio of 9.4 (95%CI:4.6–19.2) when comparing high- with low-risk in the validation sample set, providing independent prognostic information. A subsequent external validation study estimated an OR of 4.7 (95%CI:2.5–8.8) when comparing high- and low-risk groups within 58 progressors and 210 matched nonprogressors140. A prevalence-adjusted positive predictive value of 23% at 5 years140 and evidence of costeffectiveness141 further emphasize the potential clinical value of this test. In a separate study, the authors also found that this risk classifier could detect prevalent high-grade dysplasia/EA as a field effect in BE biopsies without dysplasia, indefinite for dysplasia or low-grade dysplasia142, with an OR of 46 (95%CI:15–169) when comparing high- and low-risk groups. Therefore, this test may offer diagnostic as well as prognostic information which may be of particular value for BE patients in which intervention is not clearly indicated or desired.
Initial nucleic acid studies used flow cytometry to find that increased aneuploid and tetraploid cell fractions correlated with disease stage143,144. A cohort study12 showed that 13 of 62 patients had increased G2/tetraploid cell fractions in baseline BE biopsies, nine of which subsequently progressed to high-grade dysplasia/EA. A later study of the Seattle Barrett’s Esophagus Study cohort145 reported increased baseline tetraploidy and aneuploidy had five-year EA incidences of 56% and 43%, respectively, although a majority of progressors had high-grade dysplasia at baseline. Many additional studies have been conducted in support of aneuploidy and tetraploidy as biomarkers of neoplastic progression146, albeit with evidence of publication bias and significant heterogeneity, the latter of which likely stems from variable BE study populations, technologies and assays, and thresholds of exposure.
A recent retrospective case-control study has shown that TP53 mutations were more common (OR=13.8, 95%CI:3.2–61.0) in baseline BE biopsies of progressors (46%, 11/24) than nonprogressors (5%, 4/73) with significant associations also observed for ARID1B, APC, and ERBB2147. Importantly, Stachler and colleagues noted that these mutations are early biomarkers that appear to precede aneuploidy in esophageal adenocarcinogenesis, which is in agreement with their prior cross-sectional study148 and forms the backbone of the current molecular model. Building on this model, a study using a high-resolution SNP array has provided evidence that somatic copy number alterations of CDKN2A/B and FHIT were also predictive of neoplastic progression in nondysplastic baseline BE biopsy samples from 16 progressors and 42 nonprogressors149.
Other prospective studies have assessed multiple nucleic acid biomarkers in relation to neoplastic progression. One study based in the Seattle Barrett’s Esophagus Study cohort found that 17p loss of heterozygosity (LOH), tetraploidy, aneuploidy, and 9p LOH estimated a relative risk of 38.7 (95%CI:10.8–138.5) for EA diagnosis at 10-years of follow-up150, while another derived a 29 chromosomal feature model which was reported to have an AUC of 0.94 for predicting EA risk151. Timmer et al152 conducted a similar nucleic acid analysis study, but restricted to a BE population without dysplasia, finding that p16 loss, MYC gain, and aneusomy—when combined with age and BE circumferential segment length—identified a high-risk group with a hazard ratio of 8.7 (95%CI:2.6–29.8) for neoplastic progression when compared with the low-risk group.
Other biomarkers of neoplastic progression in BE to have recently been assessed include methylation153,154, mutational load155,156, and cellular apoptosis susceptibility gene (CAS/CSE1L)157. An in-depth discussion of these putative biomarkers is beyond the scope of this article but highlights the expansion of biomarkers being assessed in this field.
Finally, in addition to the prospective and retrospective studies described to have assessed preneoplastic (BE) tissues of EA cases, case-control (cross-sectional) studies comparing EA with BE are also of interest, as described at the outset of this section. Many of these discriminative markers are also in their infancy but include gene expression158, microRNA expression159–161, stromal lymphocytic phenotype162, neutrophil-lymphocyte ratio163, T-cell phenotype164,165, microbiome diversity166,167, and serum glycoproteins168, amongst others.
Algorithms
There are a limited number of algorithms for estimating risk of neoplastic progression in BE. One of the first was the Barrett’s Esophagus Assessment of Risk (BEAR) score by Brown et al in 2018169. This model estimated the risk of progressing from nondysplastic BE (N=2,591) to dysplasia (low-/high-grade) or EA (n=133). Using 10-fold cross validation, the model of age, sex, proton-pump inhibitor use, segment length, and history of esophageal candidiasis estimated an AUC of 0.76. Shortly thereafter, Parasa et al170 published their model—Progression in Barrett’s Esophagus (PIB) score using a BE cohort of 2,697 with 133 outcomes. This algorithm estimated risk of progressing from BE without dysplasia, indefinite for dysplasia, or with low-grade dysplasia to high-grade dysplasia or EA using 70% of the study population and included sex, smoking, segment length, and baseline-confirmed low-grade dysplasia resulting in an AUC of 0.76; the remaining 30% of the population demonstrated high model calibration. An external validation study, based in Northern Ireland and comprising 1,198 BE patients with 54 progressors, estimated the AUC of this model as 0.70171. This is the only external validation study to-date of any BE neoplastic progression model. A final model to be based on demographic/lifestyle/clinical factors was published by Holmberg et al99. This nested case-control study based in the Swedish National Patient Registry compared BE without dysplasia, indefinite for dysplasia, or with low-grade dysplasia (n=1,089) with high-grade dysplasia or EA previously diagnosed with BE (n=279). A final model of age, sex and maximal segment length estimated an AUC of 0.71.
Recently, Hoefnagel et al developed a prediction model that combined molecular markers with demographic and clinical variables172. This Dutch multicenter study included 334 nondysplastic BE patients, 32 of which progressed to high-grade dysplasia or EA. A model including age, BE circumferential length, and a clonicity score (based on fluorescence in-situ hybridization probes for 20q13.2, c-MYC [8q24.12], and centromeres of chromosomes 7 and 17) estimated an AUC of 0.88.
Finally, Vaughan et al109 brought together an array of demographic, lifestyle, clinical and molecular evidence to build an online risk calculator named IC-RISC (https://ic-risc.fredhutch.org). This calculator emphasizes the importance of simplicity of use and communication of risk. The latter is especially important for providers and patients if professional guidelines are to advocate for personalized decisions in individuals with low-grade dysplasia.
Controversies
A central controversy to the primary (screening) and secondary (surveillance) prevention strategies that underlie this review is whether BE is a necessary precursor of EA. Previous studies have reported that not all EA cases have concomitant BE.173 A study using a rigorous biopsy protocol found that only 62% of EA patients had detectable BE174, whilst previous EA series have detected a BE prevalence range of 23–100% 175–185. Sampling error as well as overgrowth and elimination by the expanding tumor are possible reasons for the variation of these estimates and the failure to observe BE in all EA subjects. The study of Chandrasoma and colleagues183 found the prevalence of intestinal metaplasia decreased with increasing tumor size and stage, supporting the overgrowth/elimination theory. Meanwhile, Smith et al186 conducted a comprehensive retrospective and prospective review of clinical records and pathology specimens of 21 EA patients who underwent esophageal mucosal resection, finding evidence for intestinal metaplasia in all cases. Despite this, the controversy continues with a recent study going so far as to suggest that presence/absence of adjacent BE defines two distinct EA phenotypes187; a concept previously contemplated182,188 but, overall, considered unlikely from the limited biological evidence that exists189–191.
Future Directions
Biomarkers for BE screening and risk triaging that have been discovered using biopsies will need to be validated in whole esophageal sampling specimens as well as total, unselected BE populations. A low-cost, single-timepoint specimen collection that can be used for sequential assessment of BE presence and neoplastic risk would be optimal. Algorithms that combine biomarkers and clinical parameters will need to be optimized and validated in external populations, and risk communication should be a central feature. If BE is determined to be an unnecessary pre-requisite for EA, then population BE screening programs should collect information on other putative biomarkers of neoplastic progression gleaned from prospective and case-control studies.
Future BE biomarker studies could be strengthened in the following ways: state the a priori plan for building statistical models; consider interactions, transformations, and splines; refrain from categorizing predictors; use and report betas for risk models; use cross-validation and aim for external validation; use informed and a priori stated criteria for desired sensitivity and specificity; and assess model performance by incorporating population disease risk.
Conclusions
Epidemiological studies of demographic, clinical, and molecular biomarkers for BE screening and surveillance provide optimism for accurate risk prediction and precision surveillance. Movement towards larger scale, collaborative studies—particularly focused on unselected BE populations without dysplasia, indefinite for dysplasia, or with low-grade dysplasia—is needed, as is further discovery and validation studies of biomarkers and algorithms. Cost-effective approaches for primary and secondary prevention of EA are within our grasp but it is imperative that we conduct larger studies with a stronger and more clinically-focused statistical framework.
KEY POINTS.
In the United States, the incidence of esophageal adenocarcinoma increased markedly during recent decades and has now stabilized.
The causes of the striking male predominance and racial difference in the incidence of esophageal adenocarcinoma remain unknown.
The main risk factors for esophageal adenocarcinoma and its precursor, Barrett’s esophagus, are gastroesophageal reflux disease, abdominal obesity, and cigarette smoking, yet these features occur in only a subset of cases and are largely prevalent in the general population, which weakens their discriminatory ability for screening and surveillance.
Esophageal adenocarcinoma patients that have a prior diagnosis of Barrett’s esophagus (less than 10% of all esophageal adenocarcinoma patients) have better outcomes compared with patients without a prior diagnosis of Barrett’s esophagus.
Biomarker discovery and validation studies in unselected Barrett’s esophagus populations using whole esophageal sampling are warranted, and all biomarker studies should strive to be larger and have a strengthened statistical framework.
Multiple prediction models have been derived for use in selecting high-risk patients for screening and surveillance; however, these models need further validation (temporal and geographic) and optimization before their clinical application can be recommended.
SYNOPSIS.
In the United States, the incidence of esophageal adenocarcinoma increased markedly since the 1970s with a recent stabilization. Despite evolving screening and surveillance strategies to diagnose, risk triage, and intervene in Barrett’s esophagus patients to prevent esophageal adenocarcinoma, most cases present with advanced disease and poor resultant survival. Epidemiological studies have identified the main risk factors for these conditions, including increasing age, male sex, white race, gastroesophageal reflux disease, abdominal obesity, cigarette smoking, and lack of infection with Helicobacter pylori. This review summarizes the current epidemiologic evidence with implications for screening and surveillance in Barrett’s esophagus and esophageal adenocarcinoma.
Clinics Care Points.
Screening for Barrett’s esophagus needs to go beyond patients reporting current symptoms of gastroesophageal reflux disease to include other established risk factors, such as smoking history and obesity.
To date, no screening or surveillance algorithm has sufficient discriminatory accuracy or external validation to support clinical use.
It is important to note that the vast majority of evidence for etiology and neoplastic progression is derived from selected Barrett’s esophagus populations.
Despite potential reverse-causation, case-control studies comparing esophageal adenocarcinoma with Barrett’s esophagus may derive additional neoplastic predictors given the ability to characterize all cancer patients with greater statistical power, as opposed to assessing a small subset of esophageal adenocarcinoma cases previously diagnosed with Barrett’s esophagus.
Biomarker studies must strive to use stronger statistical frameworks.
Larger, collaborative studies—particularly those focused on Barrett’s esophagus populations without dysplasia, indefinite for dysplasia, or with low-grade dysplasia—are needed to enhance biomarker discovery and validation efforts to increase the accuracy of predictive algorithms.
Footnotes
DISCLOSURE STATEMENT
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schmidt FA. De mammalium oesophage atque ventriculo. Halae, Batheana; 1805. [Google Scholar]
- 2.Stewart MJ, Hartfall SJ. Chronic peptic ulcer of the esophagus. The Journal of Pathology. 1929;32:9–14. [Google Scholar]
- 3.Lyall A Chronic peptic ulcer of the oesophagus: A report of eight cases. Br J Surg. 1937;24:534–547. [Google Scholar]
- 4.Barrett NR. Chronic peptic ulcer of the oesophagus and oesophagitis. Br J Surg. 1950;38:175–182. [DOI] [PubMed] [Google Scholar]
- 5.Allison PR, Johnstone AS. The oesophagus lined with gastric mucosa membrane. Thorax. 1953;8:87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrett NR. The lower esophagus lined by columnar epithelium. Surgery. 1957;41(6):881–894. [PubMed] [Google Scholar]
- 7.Moersch RN, Ellis FH, McDonald JR. Pathologic changes occurring in severe reflux esophagitis. Surgery, Gynecology and Obstetrics. 1959;108:476–484. [PubMed] [Google Scholar]
- 8.Bremner CG, Lynch VP, Ellis FH Jr., Barrett’s esophagus: congenital or acquired? An experimental study of esophageal mucosal regeneration in the dog. Surgery. 1970;68(1):209–216. [PubMed] [Google Scholar]
- 9.Bosher LH, Taylor FH. Heterotopic gastric mucosa in the esophagus with ulceration and stricture formation. The Journal of thoracic surgery. 1951;21:306–312. [PubMed] [Google Scholar]
- 10.Morson BC, Belcher JR. Adenocarcinoma of the esophagus and ectopic gastric mucosa. Br J Surg. 1952;6:127–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paull A, Trier JS, Dalton MD, Camp RC, Loeb P, Goyal RK. The histologic spectrum of Barrett’s esophagus. N Engl J Med. 1976;295(9):476–480. [DOI] [PubMed] [Google Scholar]
- 12.Reid BJ, Blount PL, Rubin CE, Levine DS, Haggitt RC, Rabinovitch PS. Flow-cytometric and histological progression to malignancy in Barrett’s esophagus: prospective endoscopic surveillance of a cohort. Gastroenterology. 1992;102(4; 1):1212–1219. [PubMed] [Google Scholar]
- 13.Paraf F, Flejou JF, Pignon JP, Fekete F, Potet F. Surgical pathology of adenocarcinoma arising in Barrett’s esophagus. Analysis of 67 cases. Am J Surg Pathol. 1995;19(2):183–191. [DOI] [PubMed] [Google Scholar]
- 14.Shaheen N Is there a “Barrett’s iceberg?”. Gastroenterology. 2002;123(2):636–639. [DOI] [PubMed] [Google Scholar]
- 15.Cameron AJ, Zinsmeister AR, Ballard DJ, Carney JA. Prevalence of columnar-lined (Barrett’s) esophagus. Comparison of population-based clinical and autopsy findings. Gastroenterology. 1990;99(4):918–922. [DOI] [PubMed] [Google Scholar]
- 16.Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology. 2005;129(6):1825–1831. [DOI] [PubMed] [Google Scholar]
- 17.Zagari RM, Fuccio L, Wallander MA, et al. Gastro-oesophageal reflux symptoms, oesophagitis and Barrett’s oesophagus in the general population: the Loiano-Monghidoro study. Gut. 2008;57(10):1354–1359. [DOI] [PubMed] [Google Scholar]
- 18.Hayeck TJ, Kong CY, Spechler SJ, Gazelle GS, Hur C. The prevalence of Barrett’s esophagus in the US: estimates from a simulation model confirmed by SEER data. Dis Esophagus. 2010;23(6):451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qumseya BJ, Bukannan A, Gendy S, et al. Systematic review and meta-analysis of prevalence and risk factors for Barrett’s esophagus. Gastrointest Endosc. 2019;90(5):707–717.e701. [DOI] [PubMed] [Google Scholar]
- 20.Chen YH, Yu HC, Lin KH, Lin HS, Hsu PI. Prevalence and risk factors for Barrett’s esophagus in Taiwan. World J Gastroenterol. 2019;25(25):3231–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macdonald CE, Wicks AC, Playford RJ. Ten years’ experience of screening patients with Barrett’s oesophagus in a university teaching hospital. Gut. 1997;41(3):303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conio M, Cameron AJ, Romero Y, et al. Secular trends in the epidemiology and outcome of Barrett’s oesophagus in Olmsted County, Minnesota. Gut. 2001;48(3):304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irani S, Parkman H, Krevsky B, Thomas R, Fisher R. A decade (1991–2000) of increasing incidence of endoscopic and histologic Barrett’s esophagus (BE) at a single academic medical center. Am J Gastroenterol. 2003;98:S16. [Google Scholar]
- 24.Musana AK, Resnick JM, Torbey CF, Mukesh BN, Greenlee RT. Barrett’s esophagus: incidence and prevalence estimates in a rural Mid-Western population. Am J Gastroenterol. 2008;103(3):516–524. [DOI] [PubMed] [Google Scholar]
- 25.Yachimski P, Lee RA, Tramontano A, Nishioka NS, Hur C. Secular trends in patients diagnosed with Barrett’s esophagus. Dig Dis Sci. 2010;55(4):960–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrick JL, Nguyen T, Cook MB. Temporal trends of esophageal disorders by age in the Cerner Health Facts database. Ann Epidemiol. 2016;26(2):151–154.e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prach AT, MacDonald TA, Hopwood DA, Johnston DA. Increasing incidence of Barrett’s oesophagus: education, enthusiasm, or epidemiology? Lancet. 1997;350(9082):933. [DOI] [PubMed] [Google Scholar]
- 28.Watson A, Reed PI, Caygill CPJ, Epstein O, Winslet MC, Pounder RE. Changing incidence of columnar-lined (Barrett’s) oesophagus (CLO) in the UK. Gut. 1999;44:W180. [Google Scholar]
- 29.Hurschler D, Borovicka J, Neuweiler J, et al. Increased detection rates of Barrett’s oesophagus without rise in incidence of oesophageal adenocarcinoma. Swiss Med Wkly. 2003;133(37–38):507–514. [DOI] [PubMed] [Google Scholar]
- 30.van Soest EM, Dieleman JP, Siersema PD, Sturkenboom MC, Kuipers EJ. Increasing incidence of Barrett’s oesophagus in the general population. Gut. 2005;54(8):1062–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alcedo J, Ferrandez A, Arenas J, et al. Trends in Barrett’s esophagus diagnosis in Southern Europe: implications for surveillance. Dis Esophagus. 2009;22(3):239–248. [DOI] [PubMed] [Google Scholar]
- 32.Coleman HG, Bhat S, Murray LJ, McManus D, Gavin AT, Johnston BT. Increasing incidence of Barrett’s oesophagus: a population-based study. Eur J Epidemiol. 2011. [DOI] [PubMed] [Google Scholar]
- 33.Kendall BJ, Whiteman DC. Temporal changes in the endoscopic frequency of new cases of Barrett’s esophagus in an Australian health region. Am J Gastroenterol. 2006;101(6):1178–1182. [DOI] [PubMed] [Google Scholar]
- 34.Masclee GM, Coloma PM, de Wilde M, Kuipers EJ, Sturkenboom MC. Letter: incidence rates of Barrett’s oesophagus and oesophageal adenocarcinoma in UK and the Netherlands - authors’ reply. Aliment Pharmacol Ther. 2014;40(4):404. [DOI] [PubMed] [Google Scholar]
- 35.Surveillance Epidemiology and End Results (SEER) Program. (www.seer.cancer.gov) SEER*Stat Database: Incidence – SEER Research Data, 9 Registries, Nov 2019 Sub (1975–2017) - Linked To County Attributes - Time Dependent (1990–2017) Income/Rurality, 1969–2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2020, based on the November 2019 submission. [Google Scholar]
- 36. SEER*Stat Database:. NAACCR Incidence Data - CiNA Analytic File, 1995–2016, Public Use (which includes data from CDC’s National Program of Cancer Registries (NPCR), CCCR’s Provincial and Territorial Registries, and the NCI’s Surveillance, Epidemiology and End Results (SEER) Registries), certified by the North American Association of Central Cancer Registries (NAACCR) as meeting high-quality incidence data standards for the specified time periods, submitted December 2018.
- 37.Thrift AP, Whiteman DC. The incidence of esophageal adenocarcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Ann Oncol. 2012;23(12):3155–3162. [DOI] [PubMed] [Google Scholar]
- 38.Surveillance Epidemiology and End Results (SEER) Program. (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases with Delay-Adjustment, Malignant Only, Nov 2019 Sub (2000–2017) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2018 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2020, based on the November 2019 submission. [Google Scholar]
- 39.Surveillance Epidemiology and End Results (SEER) Program. (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER Research Data, 18 Registries, Nov 2019 Sub (2000–2017) - Linked To County Attributes - Time Dependent (1990–2017) Income/Rurality, 1969–2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2020, based on the November 2019 submission. [Google Scholar]
- 40.Qumseya B, Sultan S, Bain P, et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest Endosc. 2019;90(3):335–359.e332. [DOI] [PubMed] [Google Scholar]
- 41.Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63(1):7–42. [DOI] [PubMed] [Google Scholar]
- 42.Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association Technical Review on the Management of Barrett’s Esophagus. Gastroenterology. 2011;140(3):e18–e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaheen NJ, Falk GW, Iyer PG, Gerson LB, American College of G. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. The American journal of gastroenterology. 2016;111(1):30–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie SH, Lagergren J. A global assessment of the male predominance in esophageal adenocarcinoma. Oncotarget. 2016;7(25):38876–38883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie SH, Lagergren J. The Male Predominance in Esophageal Adenocarcinoma. Clin Gastroenterol Hepatol. 2016;14(3):338–347.e331. [DOI] [PubMed] [Google Scholar]
- 46.Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64(3):381–387. [DOI] [PubMed] [Google Scholar]
- 47.Cook MB, Chow WH, Devesa SS. Oesophageal cancer incidence in the United States by race, sex, and histologic type, 1977–2005. Br J Cancer. 2009;101(5):855–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cook MB, Wild CP, Forman D. A systematic review and meta-analysis of the sex ratio for Barrett’s esophagus, erosive reflux disease, and nonerosive reflux disease. Am J Epidemiol. 2005;162(11):1050–1061. [DOI] [PubMed] [Google Scholar]
- 49.Engel LS, Chow WH, Vaughan TL, et al. Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst. 2003;95(18):1404–1413. [DOI] [PubMed] [Google Scholar]
- 50.Olsen CM, Pandeya N, Green AC, Webb PM, Whiteman DC, Study ftAC. Population Attributable Fractions of Adenocarcinoma of the Esophagus and Gastroesophageal Junction. Am J Epidemiol. 2011. [DOI] [PubMed] [Google Scholar]
- 51.Cook MB, Corley DA, Murray LJ, et al. Gastroesophageal Reflux in Relation to Adenocarcinomas of the Esophagus: A Pooled Analysis from the Barrett’s and Esophageal Adenocarcinoma Consortium (BEACON). PLoS ONE. 2014;9(7):e103508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thrift AP, Kramer JR, Qureshi Z, Richardson PA, El-Serag HB. Age at onset of GERD symptoms predicts risk of Barrett’s esophagus. Am J Gastroenterol. 2013;108(6):915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cook MB, Kamangar F, Whiteman DC, et al. Cigarette Smoking and Adenocarcinomas of the Esophagus and Esophagogastric Junction: A Pooled Analysis From the International BEACON Consortium. J Natl Cancer Inst. 2010;102(17):1344–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang QL, Xie SH, Li WT, Lagergren J. Smoking Cessation and Risk of Esophageal Cancer by Histological Type: Systematic Review and Meta-analysis. J Natl Cancer Inst. 2017;109(12). [DOI] [PubMed] [Google Scholar]
- 55.Cook MB, Shaheen NJ, Anderson LA, et al. Cigarette Smoking Increases Risk of Barrett’s Esophagus: An Analysis of the Barrett’s and Esophageal Adenocarcinoma Consortium. Gastroenterology. 2012;142(4):744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoyo C, Cook MB, Kamangar F, et al. Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: a pooled analysis from the International BEACON Consortium. Int J Epidemiol. 2012;41(6):1706–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cook MB, Freedman ND, Gamborg M, Sorensen TIA, Baker JL. Childhood body mass index in relation to future risk of oesophageal adenocarcinoma. Br J Cancer. 2015;112(3):601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levi Z, Kark JD, Shamiss A, et al. Body mass index and socioeconomic status measured in adolescence, country of origin, and the incidence of gastroesophageal adenocarcinoma in a cohort of 1 million men. Cancer. 2013;119(23):4086–4093. [DOI] [PubMed] [Google Scholar]
- 59.Steffen A, Huerta J-M, Weiderpass E, et al. General and abdominal obesity and risk of esophageal and gastric adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2015;137(3):646–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.El-Serag HB, Hashmi A, Garcia J, et al. Visceral abdominal obesity measured by CT scan is associated with an increased risk of Barrett’s oesophagus: a case-control study. Gut. 2014;63(2):220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh S, Sharma AN, Murad MH, et al. Central Adiposity is Associated with Increased Risk of Esophageal Inflammation, Metaplasia, and Adenocarcinoma: a Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Freedman ND, Murray LJ, Kamangar F, et al. Alcohol intake and risk of oesophageal adenocarcinoma: a pooled analysis from the BEACON Consortium. Gut. 2011;60(8):1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thrift AP, Cook MB, Vaughan TL, et al. Alcohol and the risk of Barrett’s esophagus: a pooled analysis from the International BEACON Consortium. Am J Gastroenterol. 2014;109(10):1586–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018Diet, Nutrition, Physical Activity, and Oesophageal Cancer. 2018. Available at dietandcancerreport.org.
- 65.Parsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325(16):1127–1131. [DOI] [PubMed] [Google Scholar]
- 66.Nie S, Chen T, Yang X, Huai P, Lu M. Association of Helicobacter pylori infection with esophageal adenocarcinoma and squamous cell carcinoma: a meta-analysis. Dis Esophagus. 2014;27(7):645–653. [DOI] [PubMed] [Google Scholar]
- 67.Xie FJ, Zhang YP, Zheng QQ, et al. Helicobacter pylori infection and esophageal cancer risk: an updated meta-analysis. World J Gastroenterol. 2013;19(36):6098–6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Z, Shaheen NJ, Whiteman DC, et al. Helicobacter pylori Infection Is Associated With Reduced Risk of Barrett’s Esophagus: An Analysis of the Barrett’s and Esophageal Adenocarcinoma Consortium. The American Journal of Gastroenterology. 2018;113(8):1148–1155. [DOI] [PubMed] [Google Scholar]
- 69.Eross B, Farkas N, Vincze A, et al. Helicobacter pylori infection reduces the risk of Barrett’s esophagus: A meta-analysis and systematic review. Helicobacter. 2018;23(4):e12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liao LM, Vaughan TL, Corley DA, et al. Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology. 2012;142(3):442–452 e445; quiz e422–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thrift AP, Anderson LA, Murray LJ, et al. Nonsteroidal Anti-Inflammatory Drug Use is Not Associated With Reduced Risk of Barrett’s Esophagus. Am J Gastroenterol. 2016;111(11):1528–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alexandre L, Clark AB, Bhutta HY, Holt S, Lewis MP, Hart AR. Statin use is associated with reduced risk of histologic subtypes of esophageal cancer: a nested case-control analysis. Gastroenterology. 2014;146(3):661–668. [DOI] [PubMed] [Google Scholar]
- 73.Nguyen T, Khalaf N, Ramsey D, El-Serag HB. Statin use is associated with a decreased risk of Barrett’s esophagus. Gastroenterology. 2014;147(2):314–323. [DOI] [PubMed] [Google Scholar]
- 74.Petrick JL, Falk RT, Hyland PL, et al. Association between circulating levels of sex steroid hormones and esophageal adenocarcinoma in the FINBAR Study. Plos One. 2018;13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cook MB, Wood S, Hyland PL, et al. Sex steroid hormones in relation to Barrett’s esophagus: an analysis of the FINBAR Study. Andrology. 2017;5(2):240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cook MB, Wood SN, Cash BD, et al. Association between circulating levels of sex steroid hormones and Barrett’s esophagus in men: a case-control analysis. Clin Gastroenterol Hepatol. 2015;13(4):673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Petrick JL, Hyland PL, Caron P, et al. Associations Between Prediagnostic Concentrations of Circulating Sex Steroid Hormones and Esophageal/Gastric Cardia Adenocarcinoma Among Men. J Natl Cancer Inst. 2019;111(1):34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Petrick JL, Cook MB. Do Sex Hormones Underlie Sex Differences in Cancer Incidence? Testing the Intuitive in Esophageal Adenocarcinoma. Am J Gastroenterol. 2020;115(2):211–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xie SH, Ness-Jensen E, Rabbani S, et al. Circulating Sex Hormone Levels and Risk of Esophageal Adenocarcinoma in a Prospective Study in Men. Am J Gastroenterol. 2020;115(2):216–223. [DOI] [PubMed] [Google Scholar]
- 80.Xie SH, Fang R, Huang M, et al. Association Between Levels of Hormones and Risk of Esophageal Adenocarcinoma and Barrett’s Esophagus. Clin Gastroenterol Hepatol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gharahkhani P, Fitzgerald RC, Vaughan TL, et al. Genome-wide association studies in oesophageal adenocarcinoma and Barrett’s oesophagus: a large-scale meta-analysis. Lancet Oncol. 2016;17(10):1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Becker J, May A, Gerges C, et al. Supportive evidence for FOXP1, BARX1, and FOXF1 as genetic risk loci for the development of esophageal adenocarcinoma. Cancer Med. 2015;4(11):1700–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Palles C, Chegwidden L, Li X, et al. Polymorphisms near TBX5 and GDF7 are associated with increased risk for Barrett’s esophagus. Gastroenterology. 2015;148(2):367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Su Z, Gay LJ, Strange A, et al. Common variants at the MHC locus and at chromosome 16q24.1 predispose to Barrett’s esophagus. Nat Genet. 2012;44(10):1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rubenstein JH, Morgenstern H, Appelman H, et al. Prediction of Barrett’s Esophagus Among Men. Am J Gastroenterol. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thrift AP, Garcia JM, El-Serag HB. A multibiomarker risk score helps predict risk for Barrett’s esophagus. Clin Gastroenterol Hepatol. 2014;12(8):1267–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dong J, Buas MF, Gharahkhani P, et al. Determining Risk of Barrett’s Esophagus and Esophageal Adenocarcinoma Based on Epidemiologic Factors and Genetic Variants. Gastroenterology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rubenstein JH, McConnell D, Waljee AK, et al. Validation and Comparison of Tools for Selecting Individuals to Screen for Barrett’s Esophagus and Early Neoplasia. Gastroenterology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Locke GR, Zinsmeister AR, Talley NJ. Can symptoms predict endoscopic findings in GERD? Gastrointest Endosc. 2003;58(5):661–670. [DOI] [PubMed] [Google Scholar]
- 90.Gerson LB, Edson R, Lavori PW, Triadafilopoulos G. Use of a simple symptom questionnaire to predict Barrett’s esophagus in patients with symptoms of gastroesophageal reflux. Am J Gastroenterol. 2001;96(7):2005–2012. [DOI] [PubMed] [Google Scholar]
- 91.Rubenstein JH, Thrift AP. Risk factors and populations at risk: selection of patients for screening for Barrett’s oesophagus. Best Pract Res Clin Gastroenterol. 2015;29(1):41–50. [DOI] [PubMed] [Google Scholar]
- 92.Thrift AP, Kanwal F, El-Serag HB. Prediction Models for Gastrointestinal and Liver Diseases: Too Many Developed, Too Few Validated. Clin Gastroenterol Hepatol. 2016;14(12):1678–1680. [DOI] [PubMed] [Google Scholar]
- 93.Thrift AP, Vaughan TL, Anderson LA, Whiteman DC, El-Serag HB. External Validation of the Michigan Barrett’s Esophagus Prediction Tool. Clin Gastroenterol Hepatol. 2017;15(7):1124–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ireland CJ, Thrift AP, Esterman A. Risk Prediction Models for Barrett’s Esophagus Discriminate Well and Are Generalizable in an External Validation Study. Dig Dis Sci. 2020. [DOI] [PubMed] [Google Scholar]
- 95.Kunzmann AT, Canadas Garre M, Thrift AP, et al. Information on Genetic Variants Does Not Increase Identification of Individuals at Risk of Esophageal Adenocarcinoma Compared to Clinical Risk Factors. Gastroenterology. 2019;156(1):43–45. [DOI] [PubMed] [Google Scholar]
- 96.Dong J, Buas MF, Gharahkhani P, et al. Determining Risk of Barrett’s Esophagus and Esophageal Adenocarcinoma Based on Epidemiologic Factors and Genetic Variants. Gastroenterology. 2018;154(5):1273–1281.e1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cook MB, Drahos J, Wood S, et al. Pathogenesis and progression of oesophageal adenocarcinoma varies by prior diagnosis of Barrett’s oesophagus. Br J Cancer. 2016;115(11):1383–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Krishnamoorthi R, Singh S, Ragunathan K, et al. Factors Associated with Progression of Barrett’s Esophagus: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2017. [DOI] [PubMed] [Google Scholar]
- 99.Holmberg D, Ness-Jensen E, Mattsson F, Lagergren J. Clinical prediction model for tumor progression in Barrett’s esophagus. Surg Endosc. 2019;33(9):2901–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cooper S, Menon S, Nightingale P, Trudgill N. Risk factors for the development of oesophageal adenocarcinoma in Barrett’s oesophagus: a UK primary care retrospective nested case-control study. United European Gastroenterol J. 2014;2(2):91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.de Jonge PJ, van Blankenstein M, Looman CW, Casparie MK, Meijer GA, Kuipers EJ. Risk of malignant progression in patients with Barrett’s oesophagus: a Dutch nationwide cohort study. Gut. 2010;59(8):1030–1036. [DOI] [PubMed] [Google Scholar]
- 102.Kambhampati S, Tieu AH, Luber B, Wang H, Meltzer SJ. Risk Factors for Progression of Barrett’s Esophagus to High Grade Dysplasia and Esophageal Adenocarcinoma. Sci Rep. 2020;10(1):4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bhat S, Coleman HG, Yousef F, et al. Risk of Malignant Progression in Barrett’s Esophagus Patients: Results from a Large Population-Based Study. J Natl Cancer Inst. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cook MB, Coburn SB, Lam JR, Taylor PR, Schneider JL, Corley DA. Cancer incidence and mortality risks in a large US Barrett’s oesophagus cohort. Gut. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Menke-Pluymers MB, Hop WC, Dees J, van Blankenstein M, Tilanus HW. Risk factors for the development of an adenocarcinoma in columnar-lined (Barrett) esophagus. The Rotterdam Esophageal Tumor Study Group. Cancer. 1993;72(4):1155–1158. [DOI] [PubMed] [Google Scholar]
- 106.Hardikar S, Onstad L, Blount PL, Odze RD, Reid BJ, Vaughan TL. The Role of Tobacco, Alcohol, and Obesity in Neoplastic Progression to Esophageal Adenocarcinoma: A Prospective Study of Barrett’s Esophagus. PLoS One. 2013;8(1):e52192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.de Jonge PJ, Steyerberg EW, Kuipers EJ, et al. Risk factors for the development of esophageal adenocarcinoma in Barrett’s esophagus. Am J Gastroenterol. 2006;101(7):1421–1429. [DOI] [PubMed] [Google Scholar]
- 108.Coleman HG, Bhat SK, Murray LJ, et al. Symptoms and Endoscopic Features at Barrett’s Esophagus Diagnosis: Implications for Neoplastic Progression Risk. Am J Gastroenterol. 2014;109(4):527–534. [DOI] [PubMed] [Google Scholar]
- 109.Vaughan TL, Onstad L, Dai JY. Interactive decision support for esophageal adenocarcinoma screening and surveillance. Bmc Gastroenterology. 2019;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lou Z, Xing H, Li D. Alcohol consumption and the neoplastic progression in Barrett’s esophagus: a systematic review and meta-analysis. PLoS One. 2014;9(10):e105612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pohl H, Wrobel K, Bojarski C, et al. Risk factors in the development of esophageal adenocarcinoma. Am J Gastroenterol. 2013;108(2):200–207. [DOI] [PubMed] [Google Scholar]
- 112.Avidan B, Sonnenberg A, Schnell TG, Chejfec G, Metz A, Sontag SJ. Hiatal hernia size, Barrett’s length, and severity of acid reflux are all risk factors for esophageal adenocarcinoma. Am J Gastroenterol. 2002;97(8):1930–1936. [DOI] [PubMed] [Google Scholar]
- 113.Chandrasekar VT, Hamade N, Desai M, et al. Significantly lower annual rates of neoplastic progression in short- compared to long-segment non-dysplastic Barrett’s esophagus: a systematic review and meta-analysis. Endoscopy. 2019;51(7):665–672. [DOI] [PubMed] [Google Scholar]
- 114.Yadlapati R, Triggs J, Quader F, et al. Reduced Esophageal Contractility Is Associated with Dysplasia Progression in Barrett’s Esophagus: A Multicenter Cohort Study. Dig Dis Sci. 2020. [DOI] [PubMed] [Google Scholar]
- 115.Alnasser S, Agnihotram R, Martel M, Mayrand S, Franco E, Ferri L. Predictors of dysplastic and neoplastic progression of Barrett’s esophagus. Can J Surg. 2019;62(2):93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Solanky D, Krishnamoorthi R, Crews N, et al. Barrett Esophagus Length, Nodularity, and Low-grade Dysplasia are Predictive of Progression to Esophageal Adenocarcinoma. J Clin Gastroenterol. 2019;53(5):361–365. [DOI] [PubMed] [Google Scholar]
- 117.Krishnamoorthi R, Borah B, Heien H, Das A, Chak A, Iyer PG. Rates and predictors of progression to esophageal carcinoma in a large population-based Barrett’s esophagus cohort. Gastrointest Endosc. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wani S, Rubenstein JH, Vieth M, Bergman J. Diagnosis and Management of Low-Grade Dysplasia in Barrett’s Esophagus: Expert Review From the Clinical Practice Updates Committee of the American Gastroenterological Association. Gastroenterology. 2016;151(5):822–835. [DOI] [PubMed] [Google Scholar]
- 119.Wani S, Mathur SC, Curvers WL, et al. Greater interobserver agreement by endoscopic mucosal resection than biopsy samples in barrett’s dysplasia. Clinical Gastroenterology and Hepatology. 2010;8(9):783–788.e782. [DOI] [PubMed] [Google Scholar]
- 120.Ten Kate FJC, Nieboer D, Ten Kate FJW, et al. Improved Progression Prediction in Barrett’s Esophagus With Low-grade Dysplasia Using Specific Histologic Criteria. Am J Surg Pathol. 2018;42(7):918–926. [DOI] [PubMed] [Google Scholar]
- 121.Duits LC, van der Wel MJ, Cotton CC, et al. Patients With Barrett’s Esophagus and Confirmed Persistent Low-Grade Dysplasia Are at Increased Risk for Progression to Neoplasia. Gastroenterology. 2017;152(5):993–1001.e1001. [DOI] [PubMed] [Google Scholar]
- 122.Kestens C, Offerhaus GJ, van Baal JW, Siersema PD. Patients With Barrett’s Esophagus and Persistent Low-grade Dysplasia Have an Increased Risk for High-grade Dysplasia and Cancer. Clin Gastroenterol Hepatol. 2016;14(7):956–962.e951. [DOI] [PubMed] [Google Scholar]
- 123.Song KY, Henn AJ, Gravely AA, et al. Persistent confirmed low-grade dysplasia in Barrett’s esophagus is a risk factor for progression to high-grade dysplasia and adenocarcinoma in a US Veterans cohort. Dis Esophagus. 2020;33(2). [DOI] [PubMed] [Google Scholar]
- 124.O’Byrne LM, Witherspoon J, Verhage RJJ, et al. Barrett’s Registry Collaboration of academic centers in Ireland reveals high progression rate of low-grade dysplasia and low risk from nondysplastic Barrett’s esophagus: report of the RIBBON network. Dis Esophagus. 2020. [DOI] [PubMed] [Google Scholar]
- 125.Jass JR, Filipe MI. The mucin profiles of normal gastric mucosa, intestinal metaplasia and its variants and gastric carcinoma. Histochem J. 1981;13(6):931–939. [DOI] [PubMed] [Google Scholar]
- 126.Jass JR, Filipe MI. A variant of intestinal metaplasia associated with gastric carcinoma: a histochemical study. Histopathology. 1979;3(3):191–199. [DOI] [PubMed] [Google Scholar]
- 127.Jass JR. Role of intestinal metaplasia in the histogenesis of gastric carcinoma. J Clin Pathol. 1980;33(9):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Peuchmaur M, Potet F, Goldfain D. Mucin histochemistry of the columnar epithelium of the oesophagus (Barrett’s oesophagus): a prospective biopsy study. J Clin Pathol. 1984;37(6):607–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Haggitt RC, Reid BJ, Rabinovitch PS, Rubin CE. Barrett’s esophagus. Correlation between mucin histochemistry, flow cytometry, and histologic diagnosis for predicting increased cancer risk. Am J Pathol. 1988;131(1):53–61. [PMC free article] [PubMed] [Google Scholar]
- 130.Endo T, Tamaki K, Arimura Y, et al. Expression of sulfated carbohydrate chain and core peptides of mucin detected by monoclonal antibodies in Barrett’s esophagus and esophageal adenocarcinoma. J Gastroenterol. 1998;33(6):811–815. [DOI] [PubMed] [Google Scholar]
- 131.Smith JL, Dixon MF. Is subtyping of intestinal metaplasia in the upper gastrointestinal tract a worthwhile exercise? An evaluation of current mucin histochemical stains. Br J Biomed Sci. 2003;60(4):180–186. [DOI] [PubMed] [Google Scholar]
- 132.Murray L, Sedo A, Scott M, et al. TP53 and progression from Barrett’s metaplasia to oesophageal adenocarcinoma in a UK population cohort. Gut. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Weston AP, Banerjee SK, Sharma P, Tran TM, Richards R, Cherian R. p53 protein overexpression in low grade dysplasia (LGD) in Barrett’s esophagus: immunohistochemical marker predictive of progression. Am J Gastroenterol. 2001;96(5):1355–1362. [DOI] [PubMed] [Google Scholar]
- 134.Younes M, Ertan A, Lechago LV, Somoano JR, Lechago J. p53 Protein accumulation is a specific marker of malignant potential in Barrett’s metaplasia. Dig Dis Sci. 1997;42(4):697–701. [DOI] [PubMed] [Google Scholar]
- 135.Skacel M, Petras RE, Rybicki LA, et al. p53 expression in low grade dysplasia in Barrett’s esophagus: correlation with interobserver agreement and disease progression. Am J Gastroenterol. 2002;97(10):2508–2513. [DOI] [PubMed] [Google Scholar]
- 136.Snyder P, Dunbar K, Cipher DJ, Souza RF, Spechler SJ, Konda VJA. Aberrant p53 Immunostaining in Barrett’s Esophagus Predicts Neoplastic Progression: Systematic Review and Meta-Analyses. Dig Dis Sci. 2019;64(5):1089–1097. [DOI] [PubMed] [Google Scholar]
- 137.Janmaat VT, van Olphen SH, Biermann KE, Looijenga LHJ, Bruno MB, Spaander MCW. Use of immunohistochemical biomarkers as independent predictor of neoplastic progression in Barrett’s oesophagus surveillance: A systematic review and meta-analysis. PLoS One. 2017;12(10):e0186305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.di Pietro M, Fitzgerald RC. Revised British Society of Gastroenterology recommendation on the diagnosis and management of Barrett’s oesophagus with low-grade dysplasia. Gut. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Critchley-Thorne RJ, Duits LC, Prichard JW, et al. A Tissue Systems Pathology Assay for High-Risk Barrett’s Esophagus. Cancer Epidemiol Biomarkers Prev. 2016;25(6):958–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Davison JM, Goldblum J, Grewal US, et al. Independent Blinded Validation of a Tissue Systems Pathology Test to Predict Progression in Patients With Barrett’s Esophagus. The American journal of gastroenterology. 2020: 10.14309/ajg.0000000000000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hao J, Critchley-Thorne R, Diehl DL, Snyder SR. A Cost-Effectiveness Analysis Of An Adenocarcinoma Risk Prediction MultiBiomarker Assay For Patients With Barrett’s Esophagus. Clinicoeconomics and Outcomes Research. 2019;11:623–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Critchley-Thorne RJ, Davison JM, Prichard JW, et al. A Tissue Systems Pathology Test Detects Abnormalities Associated with Prevalent High-Grade Dysplasia and Esophageal Cancer in Barrett’s Esophagus. Cancer Epidemiology Biomarkers & Prevention. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Reid BJ, Haggitt RC, Rubin CE, Rabinovitch PS. Barrett’s esophagus. Correlation between flow cytometry and histology in detection of patients at risk for adenocarcinoma. Gastroenterology. 1987;93(1):1–11. [PubMed] [Google Scholar]
- 144.Rabinovitch PS, Reid BJ, Haggitt RC, Norwood TH, Rubin CE. Progression to cancer in Barrett’s esophagus is associated with genomic instability. Lab Invest. 1989;60(1):65–71. [PubMed] [Google Scholar]
- 145.Reid BJ, Levine DS, Longton G, Blount PL, Rabinovitch PS. Predictors of progression to cancer in Barrett’s esophagus: baseline histology and flow cytometry identify low- and high-risk patient subsets. Am J Gastroenterol. 2000;95(7):1669–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Altaf K, Xiong JJ, la Iglesia D, Hickey L, Kaul A. Meta-analysis of biomarkers predicting risk of malignant progression in Barrett’s oesophagus. Br J Surg. 2017;104(5):493–502. [DOI] [PubMed] [Google Scholar]
- 147.Stachler MD, Camarda ND, Deitrick C, et al. Detection of Mutations in Barrett’s Esophagus Before Progression to High-Grade Dysplasia or Adenocarcinoma. Gastroenterology. 2018;155(1):156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stachler MD, Taylor-Weiner A, Peng S, et al. Paired exome analysis of Barrett’s esophagus and adenocarcinoma. Nat Genet. 2015;advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sepulveda JL, Komissarova EV, Kongkarnka S, et al. High-resolution genomic alterations in Barrett’s metaplasia of patients who progress to esophageal dysplasia and adenocarcinoma. Int J Cancer. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Galipeau PC, Li X, Blount PL, et al. NSAIDs modulate CDKN2A, TP53, and DNA content risk for progression to esophageal adenocarcinoma. PLoS Med. 2007;4(2):e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li X, Paulson TG, Galipeau PC, et al. Assessment of Esophageal Adenocarcinoma Risk Using Somatic Chromosome Alterations in Longitudinal Samples in Barrett’s Esophagus. Cancer Prev Res (Phila). 2015;8(9):845–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Timmer MR, Martinez P, Lau CT, et al. Derivation of genetic biomarkers for cancer risk stratification in Barrett’s oesophagus: a prospective cohort study. Gut. 2016;65(10):1602–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nieto T, Tomlinson CL, Dretzke J, et al. A systematic review of epigenetic biomarkers in progression from non-dysplastic Barrett’s oesophagus to oesophageal adenocarcinoma. BMJ Open. 2018;8(6):e020427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dilworth MP, Nieto T, Stockton JD, et al. Whole Genome Methylation Analysis of Nondysplastic Barrett Esophagus that Progresses to Invasive Cancer. Ann Surg. 2019;269(3):479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Eluri S, Brugge WR, Daglilar ES, et al. The Presence of Genetic Mutations at Key Loci Predicts Progression to Esophageal Adenocarcinoma in Barrett’s Esophagus. Am J Gastroenterol. 2015;110(6):828–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Eluri S, Klaver E, Duits LC, Jackson SA, Bergman JJ, Shaheen NJ. Validation of a biomarker panel in Barrett’s esophagus to predict progression to esophageal adenocarcinoma. Dis Esophagus. 2018;31(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jiang K, Neill K, Cowden D, et al. Expression of CAS/CSE1L, the Cellular Apoptosis Susceptibility Protein, Correlates With Neoplastic Progression in Barrett’s Esophagus. Appl Immunohistochem Mol Morphol. 2018;26(8):552–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Varghese S, Newton R, Ross-Innes CS, et al. Analysis of dysplasia in patients with Barrett’s esophagus based on expression pattern of 90 genes. Gastroenterology. 2015;149(6):1511–1518.e1515. [DOI] [PubMed] [Google Scholar]
- 159.Drahos J, Schwameis K, Orzolek LD, et al. MicroRNA Profiles of Barrett’s Esophagus and Esophageal Adenocarcinoma: Differences in Glandular Non-native Epithelium. Cancer Epidemiol Biomarkers Prev. 2016;25(3):429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Revilla-Nuin B, Parrilla P, Lozano JJ, et al. Predictive value of MicroRNAs in the progression of barrett esophagus to adenocarcinoma in a long-term follow-up study. Ann Surg. 2013;257(5):886–893. [DOI] [PubMed] [Google Scholar]
- 161.Wu X, Ajani JA, Gu J, et al. MicroRNA expression signatures during malignant progression from Barrett’s esophagus to esophageal adenocarcinoma. Cancer Prev Res (Phila). 2013;6(3):196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Porter RJ, Murray GI, Brice DP, Petty RD, McLean MH. Novel biomarkers for risk stratification of Barrett’s oesophagus associated neoplastic progression-epithelial HMGB1 expression and stromal lymphocytic phenotype. Br J Cancer. 2020;122(4):545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Campos VJ, Mazzini GS, Juchem JF, Gurski RR. Neutrophil-Lymphocyte Ratio as a Marker of Progression from Non-Dysplastic Barrett’s Esophagus to Esophageal Adenocarcinoma: a Cross-Sectional Retrospective Study. J Gastrointest Surg. 2020;24(1):8–18. [DOI] [PubMed] [Google Scholar]
- 164.Kavanagh ME, Conroy MJ, Clarke NE, et al. Impact of the inflammatory microenvironment on T-cell phenotype in the progression from reflux oesophagitis to Barrett oesophagus and oesophageal adenocarcinoma. Cancer Lett. 2016;370(1):117–124. [DOI] [PubMed] [Google Scholar]
- 165.Kavanagh ME, Conroy MJ, Clarke NE, et al. Altered T Cell Migratory Capacity in the Progression from Barrett Oesophagus to Oesophageal Adenocarcinoma. Cancer Microenviron. 2019;12(1):57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Snider EJ, Compres G, Freedberg DE, et al. Alterations to the Esophageal Microbiome Associated with Progression from Barrett’s Esophagus to Esophageal Adenocarcinoma. Cancer Epidemiology Biomarkers & Prevention. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Elliott DRF, Walker AW, O’Donovan M, Parkhill J, Fitzgerald RC. A non-endoscopic device to sample the oesophageal microbiota: a case-control study. Lancet Gastroenterol Hepatol. 2017;2(1):32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shah AK, Cao KA, Choi E, et al. Serum Glycoprotein Biomarker Discovery and Qualification Pipeline Reveals Novel Diagnostic Biomarker Candidates for Esophageal Adenocarcinoma. Mol Cell Proteomics. 2015;14(11):3023–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Brown CS, Lapin B, Goldstein JL, et al. Predicting Progression in Barrett’s Esophagus: Development and Validation of the Barrett’s Esophagus Assessment of Risk Score (BEAR Score). Ann Surg. 2018;267(4):716–720. [DOI] [PubMed] [Google Scholar]
- 170.Parasa S, Vennalaganti S, Gaddam S, et al. Development and Validation of a Model to Determine Risk of Progression of Barrett’s Esophagus to Neoplasia. Gastroenterology. 2018;154(5):1282–1289.e1282. [DOI] [PubMed] [Google Scholar]
- 171.Kunzmann AT, Thrift AP, Johnston BT, et al. External validation of a model to determine risk of progression of Barrett’s oesophagus to neoplasia. Aliment Pharmacol Ther. 2019;49(10):1274–1281. [DOI] [PubMed] [Google Scholar]
- 172.Hoefnagel SJM, Mostafavi N, Timmer MR, et al. A genomic biomarker-based model for cancer risk stratification of nondysplastic Barrett’s esophagus patients after extended follow up; results from Dutch surveillance cohorts. PLOS ONE. 2020;15(4):e0231419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tan MC, Mansour N, White DL, Sisson A, E-SH B, Thrift AP Systematic review with meta-analysis: prevalence of prior and concurrent Barrett’s oesophagus in oesophageal adenocarcinoma patients. Aliment Pharmacol Ther. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340(11):825–831. [DOI] [PubMed] [Google Scholar]
- 175.Duhaylongsod FG, Wolfe WG. Barrett’s esophagus and adenocarcinoma of the esophagus and gastroesophageal junction. J Thorac Cardiovasc Surg. 1991;102(1):36–41. [PubMed] [Google Scholar]
- 176.Haggitt RC, Tryzelaar J, Ellis FH, Colcher H. Adenocarcinoma complicating columnar epithelium-lined (Barrett’s) esophagus. Am J Clin Pathol. 1978;70(1):1–5. [DOI] [PubMed] [Google Scholar]
- 177.Hamilton SR, Smith RR, Cameron JL. Prevalence and characteristics of Barrett esophagus in patients with adenocarcinoma of the esophagus or esophagogastric junction. Hum Pathol. 1988;19(8):942–948. [DOI] [PubMed] [Google Scholar]
- 178.Streitz JM Jr., Ellis FH Jr., Gibb SP, Balogh K, Watkins E Jr. Adenocarcinoma in Barrett’s esophagus. A clinicopathologic study of 65 cases. Ann Surg. 1991;213(2):122–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cameron AJ, Lomboy CT, Pera M, Carpenter HA. Adenocarcinoma of the esophagogastric junction and Barrett’s esophagus. Gastroenterology. 1995;109(5):1541–1546. [DOI] [PubMed] [Google Scholar]
- 180.Clark GW, Smyrk TC, Burdiles P, et al. Is Barrett’s metaplasia the source of adenocarcinomas of the cardia? Arch Surg. 1994;129(6):609–614. [DOI] [PubMed] [Google Scholar]
- 181.Li H, Walsh TN, Hennessy TP. Carcinoma arising in Barrett’s esophagus. Surg Gynecol Obstet. 1992;175(2):167–172. [PubMed] [Google Scholar]
- 182.Sabel MS, Pastore K, Toon H, Smith JL. Adenocarcinoma of the esophagus with and without Barrett mucosa. Arch Surg. 2000;135(7):831–835. [DOI] [PubMed] [Google Scholar]
- 183.Chandrasoma P, Wickramasinghe K, Ma Y, DeMeester T. Is intestinal metaplasia a necessary precursor lesion for adenocarcinomas of the distal esophagus, gastroesophageal junction and gastric cardia? Dis Esophagus. 2007;20(1):36–41. [DOI] [PubMed] [Google Scholar]
- 184.Takubo K, Aida J, Naomoto Y, et al. Cardiac rather than intestinal-type background in endoscopic resection specimens of minute Barrett adenocarcinoma. Hum Pathol. 2009;40(1):65–74. [DOI] [PubMed] [Google Scholar]
- 185.Aida J, Vieth M, Shepherd NA, et al. Is carcinoma in columnar-lined esophagus always located adjacent to intestinal metaplasia?: a histopathologic assessment. Am J Surg Pathol. 2015;39(2):188–196. [DOI] [PubMed] [Google Scholar]
- 186.Smith J, Garcia A, Zhang R, DeMeester S, Vallone J, Chandrasoma P. Intestinal Metaplasia is Present in Most if Not All Patients Who Have Undergone Endoscopic Mucosal Resection for Esophageal Adenocarcinoma. Am J Surg Pathol. 2016;40(4):537–543. [DOI] [PubMed] [Google Scholar]
- 187.Sawas T, Killcoyne S, Iyer PG, et al. Identification of Prognostic Phenotypes of Esophageal Adenocarcinoma in 2 Independent Cohorts. Gastroenterology. 2018;155(6):1720–1728.e1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Johansson J, Johnsson F, Walther B, et al. Adenocarcinoma in the distal esophagus with and without Barrett esophagus: Differences in symptoms and survival rates. Arch Surg. 1996;131(7):708–713. [DOI] [PubMed] [Google Scholar]
- 189.von Rahden BHA, Kircher S, Lazariotou M, et al. LgR5 expression and cancer stem cell hypothesis: clue to define the true origin of esophageal adenocarcinomas with and without Barrett’s Esophagus? J Exp Clin Cancer Res. 2011;30:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mendes de Almeida JC, Chaves P, Pereira AD, Altorki NK. Is Barrett’s esophagus the precursor of most adenocarcinomas of the esophagus and cardia? A biochemical study. Ann Surg. 1997;226(6):725–733; discussion 733–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Engel U, McCombs R, Stranahan P, Pettijohn D, Hage E. Decrease in Le(x) expression in esophageal adenocarcinomas arising in Barrett’s epithelium. Cancer Epidemiol Biomarkers Prev. 1997;6(4):245–248. [PubMed] [Google Scholar]