Abstract
This commentary suggests specific strategies and language that clinicians can use to address mistrust of COVID-19 vaccines among racial and ethnic minorities.
“The health care system has failed me and my family many times before. Why should I believe this vaccine will be any different?” Clinicians may feel ill-equipped to address concerns about the coronavirus disease 2019 (COVID-19) vaccine that are rooted in the sociopolitical mistrust of communities that experience health disparities, discrimination, and structural injustice in their everyday lives. Current recommendations for talking to patients about COVID-19 vaccines do not provide specific guidance on how to discuss mistrust (1). We suggest specific strategies and language that clinicians can use to address mistrust of COVID-19 vaccines among racial and ethnic minorities.
Reducing COVID-19 vaccine mistrust is a national priority. Mistrust of COVID-19 vaccines is widespread, particularly among people of color. Only 18% of Black Americans and 40% of Latinx Americans trust that a COVID-19 vaccine will be effective (2). Even fewer trust that it will be safe. The impact of this mistrust is alarming: Fewer than half of Black Americans intend to get vaccinated against COVID-19 (3).
Mistrust in COVID-19 vaccines must be addressed to reduce the disproportionate burden of COVID-19 morbidity and mortality among people of color. This mistrust is multifactorial and is not restricted to concerns about COVID-19 vaccine safety and efficacy. It is rooted in a history of unethical medical and public health experimentation involving communities of color, as well as structural inequities in government institutions (for example, police, criminal justice, child welfare, and public schools). As a result, a primary strategy to decrease mistrust has been to leverage trusted community leaders to engage communities of color in public health campaigns.
Clinicians can play an important role in mitigating mistrust of COVID-19 vaccines. The personal clinicians of Black and Latinx patients, regardless of clinician race, remain trustworthy sources of COVID-19 vaccine information (4). Although race-concordant patient–clinician relationships may nurture trust among patients of color and may even facilitate information-seeking behaviors about COVID-19 (5), most patients of color do not have a race-concordant clinician. Addressing COVID-19 vaccine mistrust can be a powerful way for any clinician to convey an openness to discussing patient concerns about COVID-19 vaccination and also an interest in patients' lived experiences with structural injustice.
These discussions with patients about COVID-19 vaccines can occur now as an important part of health promotion and counseling. Initiating these discussions early can start the process of building trust in COVID-19 vaccines among patients of color. Anticipate the possibility of a multivisit process, rather than a single discussion.
Four specific communication strategies may help promote trust among patients of color about COVID-19 vaccines. First, lead with listening. Patients, particularly those from marginalized communities, desire health care interactions in which their experiences are heard and validated. Consider beginning the COVID-19 vaccine conversation with an open-ended invitation for the patient to share their perspective, such as “You may be hearing a lot about COVID vaccines. Tell me what you think about them.” Doing so can also help avoid erroneous assumptions about a patient's self-identity or experience.
This approach differs from standard vaccine communication practices for well-established and routinely recommended vaccines. For these vaccines, starting the conversation with the implicit assumption that vaccination will happen, such as “You're due for your tetanus booster today,” has been associated with increased vaccine uptake. For COVID-19 vaccines, however, which have, to date, been made available only through an emergency use authorization, a more appropriate approach is to facilitate shared decision making.
Second, tailor responses to patient concerns. If patients respond to an invitation to share their perspective about COVID-19 vaccines either equivocally (such as “I'm not sure.”) or negatively (such as “Those vaccines aren't for me.”), do not provide reassurance prematurely. Doing so may be ineffective if it is done before listening to and exploring the patient's concerns in detail. Rather, engage with the patient nonjudgmentally and collaboratively. Motivational interviewing (MI) techniques can facilitate this engagement (6) (Table, top) and have been found to be effective in improving vaccine uptake among those who voice reluctance (7). These techniques can also be used to explore and address COVID-19 vaccine concerns rooted in structural injustice (Table, bottom), complementing other suggested COVID-19 communication approaches (8). Of importance, a clinician's vaccine recommendation, a factor associated with increased uptake (9), can be integrated with MI. For instance, consider asking permission to make a recommendation and, if granted, stating, “In my view, the benefits of COVID-19 vaccination outweigh the risks. I am strongly recommending these vaccines to my patients.”
Table. COVID-19 Vaccine Communication Strategies for the Clinical Encounter to Help Address Mistrust.
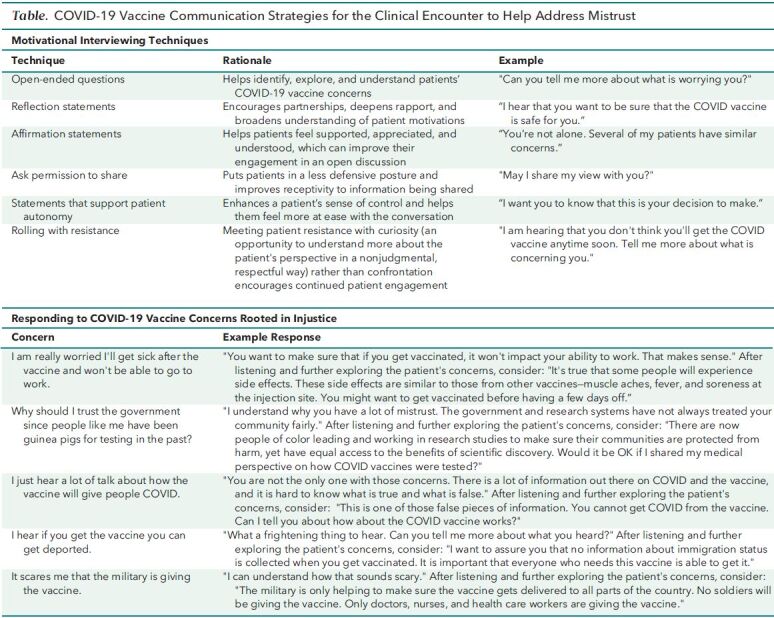
Third, briefly describe the regulatory and development processes surrounding COVID-19 vaccines using accessible language. There may be confusion about, and negative connotations with, terms used to describe the vaccine regulatory process (10). The term “emergency use authorization,” for instance, may lead to misunderstanding or exacerbate mistrust. Patients may believe that a new vaccine, for instance, has been authorized “emergently” before any data could be reviewed, harkening back to days of experimentation on enslaved people, indigenous people, prisoners, immigrants, and unknowing Black Americans. Consider simple factual statements that avoid confusing terminology, such as “The FDA has authorized these vaccines now after reviewing a lot of evidence carefully” (see also bottom part of the Table).
Lastly, acknowledge uncertainty. The shortage of COVID-19 vaccines is unprecedented, and delivery schedules are changeable. There is much not yet known about COVID-19 vaccines, particularly regarding their long-term safety, their effect on transmission of severe acute respiratory syndrome coronavirus 2, and their efficacy against new strains. Acknowledging uncertainty creates transparency, and transparency is key to facilitating trust during public health emergencies.
COVID-19 vaccines are not routine vaccines, nor are these routine circumstances. This past year has laid bare harsh health disparities and structural injustice in the United States, activating and exacerbating mistrust among people of color. Clinicians need to address this mistrust to help patients at highest risk for COVID-19 gain the benefits of COVID-19 vaccines. Prioritizing these discussions now in routine clinic visits, even over other health maintenance or stable chronic disease management issues, may help increase the acceptance of COVID-19 vaccinations and improve health outcomes among persons of color.
Footnotes
This article was published at Annals.org on 9 February 2021
References
- 1. Centers for Disease Control and Prevention. Talking to recipients about COVID-19 vaccines. 2 November 2020. Accessed at www.cdc.gov/vaccines/covid-19/hcp/talking-to-patients.html on 7 December 2020.
- 2. COVID Collaborative; UnidosUS; NAACP. Coronavirus Vaccine Hesitancyin Black and Latinx Communities. 2020. Accessed at https://static1.squarespace.com/static/5f85f5a156091e113f96e4d3/t/5fb72481b1eb2e6cf845457f/1605837977495/VaccineHesitancy_BlackLatinx_Final_11.19.pdf on 7 December 2020.
- 3. Funk C, Tyson A.Intent to get a COVID-19 vaccine rises to 60% as confidence in research and development process increases. Pew Research Center; 3 December 2020. Accessed at www.pewresearch.org/science/2020/12/03/intent-to-get-a-covid-19-vaccine-rises-to-60-as-confidence-in-research-and-development-process-increases/ on 14 December 2020.
- 4. Hamel L, Kirzinger A, Muñana C, et al. KFF COVID-19 vaccine monitor: December 2020. Kaiser Family Foundation; 15 December 2020. Accessed at www.kff.org/coronavirus-covid-19/report/kff-covid-19-vaccine-monitor-december-2020/ on 15 December 2020.
- 5. Alsan M , Stanford FC , Banerjee A , et al. Comparison of knowledge and information-seeking behavior after general COVID-19 public health messages and messages tailored for black and latinx communities: a randomized controlled trial. Ann Intern Med. 2020. doi: 10.7326/M20-6141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. Guilford Pr; 1991.
- 7. Dempsey AF , Pyrznawoski J , Lockhart S , et al. Effect of a health care professional communication training intervention on adolescent human papillomavirus vaccination: A cluster randomized clinical trial. JAMA Pediatr. 2018;172:e180016. [PMID: ] doi: 10.1001/jamapediatrics.2018.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. VitalTalk. COVID collaborative resources. 2021. Accessed at www.vitaltalk.org/topics/covid-collaborative-resources/ on 22 January 2021.
- 9. National Vaccine Advisory Committee.. Recommendations from the National Vaccine Advisory committee: standards for adult immunization practice. Public Health Rep. 2014 Mar-Apr;129:115-23. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu BF , Quinn SC , Egnoto M , et al. Public understanding of medical countermeasures. Health Secur. 2017 Mar/Apr;15:194-206. [PMID: ] doi: 10.1089/hs.2016.0074 [DOI] [PMC free article] [PubMed] [Google Scholar]


