Abstract
Hyperuricemia is associated with insulin resistance, pancreatic β-cell dysfunction and consequently with development of type 2 diabetes. Although a direct relationship between high levels of uric acid (UA) and the development of diabetes is still a controversial issue, there is some evidence that strongly points to pancreatic β-cells damage as a result of high serum UA levels. Here, the mechanisms underlying UA-induced β-cell damage are discussed. Available literature indicates that UA can decrease glucose-stimulated insulin secretion and cause β-cell death. The mechanisms underlying these effects are UA-induced oxidative stress and inflammation within the β-cells. UA also stimulates inducible nitric oxide (NO) synthase (iNOS) gene expression leading to NO-induced β-cell dysfunction. Thus hyperuricemia may potentially cause β-cell dysfunction, leading to diabetes. It may be hypothesized that in hyperuricemic subjects, UA-lowering drugs may be beneficial in preventing diabetes.
Keywords: Uric acid, Pancreatic β-cell, Type 2 diabetes, Nitric oxide
Background
Worldwide, the prevalence of diabetes is about 8–9 % [1, 2] and its incidence varies between 2.9 and 23.5 per 1000 population [3]. The worldwide prevalence of gout, defined as deposition of monosodium urate crystals mostly in the peripheral joints, ranges from 0.1 to 10 %, and its incidence varies 0.3 to 6 per 1000 person-years [4]. Both prevalence [5] and incidence [6] of diabetes are higher in patients with gout.
Uric acid (UA) is the end product of exogenous and endogenous purine (adenine and guanine) metabolism [7, 8]. The liver and the intestine are the major sites of endogenous UA production [9], which is about 300–400 mg/day [8]. Dietary contribution is approximately 300 mg/day with a total pool size of 1200 mg in men and 600 mg in women [10, 11]. UA homeostasis depends on a balance between production and catabolism [7], where 20–40 % of UA is excreted by the gastrointestinal tract and 60–80 % by the kidneys [7, 12]. Secreted UA by the intestine is further metabolized by the gut bacteria (intestinal uricolysis) [12]. UA is freely filtrated by the kidneys, of the filtrated load (plasma concentration of UA × glomerular filtration rate), 90 % is reabsorbed and therefore, fractional excretion of UA is about 10 % (7–12 %) [8, 12, 13]. Physiological functions of UA include but not limited to antioxidant property [9, 14], defense against neurological diseases [14], autoimmune diseases [9], and maintaining endothelial function [9].
High serum UA levels is a risk factor for type 2 diabetes mellitus (T2DM) as reported in different population-based studies [15–18]. According to meta-analyses of cohort studies, each 1 mg/dL (59.48 µmol/L) increase in serum levels of UA increases the risk of developing T2DM by about 6–17 % [19–21]. High UA concentrations is associated with both insulin resistance [16, 22] and β-cell dysfunction [23], two defects that are at the core of pathophysiology of T2DM [24]. In healthy subjects with normal serum UA concentrations, a positive correlation between serum UA levels and steady-state plasma glucose (SSPG) concentrations, an index of insulin resistance, has been reported [25]. In addition, renal clearance of UA is inversely associated with insulin resistance [25]. A direct relationship between changes in UA homeostasis and diabetes is still controversial [26, 27]. Using a multilocus Mendelian randomization approach, it has been shown that for each 1 mg/dL increase in circulating UA concentrations, there is an associated 20 % higher risk of diabetes, but the data does not support causality [28]. However, this approach to show potential causality has been criticized as it may dissociate the physiological serum-intracellular relationship [26]. In addition, acute euglycemic hyperinsulinemia decreased fractional UA excretion by 26 % (from 6.1 ± 0.8 % to 4.5 ± 0.6 %) in healthy subjects, indicating that insulin inhibits renal UA excretion [29] and that high UA levels causes insulin resistance by affecting the insulin signaling pathways [22]. Although a cause or effect relationship between hyperuricemia and diabetes is still a matter of debate, some experimental evidence indicates that high UA levels can damage pancreatic β-cells; this review aims to summarize the mechanisms underlying UA-induced β-cell damage.
Uptake of uric acid by pancreatic β-cells
Urate transporters include, (i) urate transporter 1/solute carrier family 22, member 12 (URAT1/SLC22A12), (ii) ATP-binding cassette subfamily G, member 2/breast cancer resistance protein (ABCG2/BCRP), and (iii) glucose transporter 9 (GLUT9/SLC2A9) [8]. Expression of URAT1 in endocrine pancreas is controversial; both low expression in pancreatic islets of rat [30] or no expressions in pancreatic β-cell lines (INS-1 cells and RIN-m5F cells) [31] have been reported. On the other hand, both variants of GLUT9 (GLUT9a and GLUT9b) are expressed in mouse insulinoma MIN6 cells, mouse islets, and human islets [32]. In addition, GLUT9 expression in pancreatic β-cells is specific [32]. Although human GLUT9 is a urate transporter [7], this carrier also participates in pancreatic β-cells function, as its knockdown resulted in reduced cellular ATP levels that correlated well with reductions in glucose-stimulated insulin secretion (GSIS) in MIN6 and INS cells [32].
Uric acid and β-cell dysfunction
In 1948, Griffiths reported that feeding rabbits with a diet that was deficient in methionine and cystin for 6–7 weeks decreased blood glutathione levels by about 40–53 % [33]. Intraperitoneal injection of UA (1 g/kg) to these rabbits increased blood glucose concentrations to hyperglycemic levels, and therefore, it has been suggested that UA exerts a diabetogenic action [33]. It has also been shown that inhibition of uricase (urate oxidase) in rats, along with UA feeding, increased serum glucose and decreased serum insulin, and therefore, decreased insulin:glucose ratio [34]. Uricase-knockout mice have glucose intolerance and are more susceptible to development of diabetes [23, 35]. In addition, in hyperuricemic subjects, β-cells fail to compensate variations of insulin sensitivity [36].
Inhibitory effect of uric acid on glucose‐stimulated insulin secretion
UA inhibits GSIS in isolated pancreatic rat islets [37, 38], pancreatic mouse islets [39], and pancreatic β-cell lines including Min6 cells [39, 40] and INS-1 cells [31, 38]. Inhibition varies between 30 and 80 % depending on the dose of UA, time of exposure, and different cell lines or different animal studied. High UA concentrations decreases GSIS by about 30–42 % in Min6 cells [39, 40], 44 % in isolated mouse islets [39], and 80 % in isolated rat islets [37]. Decreased GSIS in hyperuricemia may be due to decreases in MafA protein expression [39] as MafA is a key regulator of insulin secretion in β-cells [41].
The association between UA and insulin secretion is quite complex. It has been shown that UA increases GSIS in isolated perfused rat pancreas [42]. In addition, a positive correlation between serum UA and total insulin secretion has been reported using a hyperglycemic clamp technique in type 2 diabetic patients without hyperuricemia [43]. The effects of high UA levels on basal insulin secretion are not consistent. Inhibition in rat pancreatic islets [34, 37] and INS-1 cells [31] as well as no effects in INS-1 cells [38], Min6 cells [40], and isolated rat islets [38], have been reported.
Uric acid and β-cell death
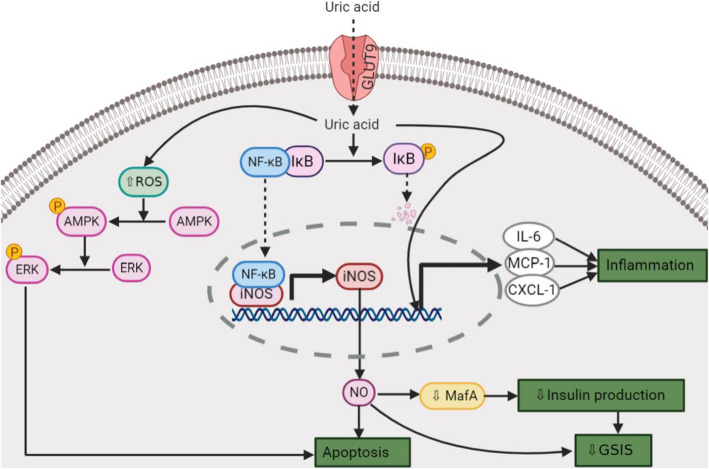
In addition to decreased GSIS, other mechanisms are involved in hyperuricemia-induced β-cell dysfunction, development of glucose intolerance, and T2DM. These include, increased inducible nitric oxide (NO) synthase (iNOS)-derived NO production [39, 40], increased inflammation [30, 39], increased oxidative stress [30, 31, 38], and increased apoptosis and β-cell death [39, 40]. These underlying mechanisms can be categorized under two major pathways that are activated by UA (Fig. 1): (1) The nuclear factor kappa B (NF-κB)-iNOS-NO signaling pathway, and (2) Reactive oxygen species (ROS)-AMP-activated protein kinase (AMPK)-extracellular signal-regulated kinase (ERK) signaling pathway.
Fig. 1.
Mechanisms underlying uric acid (UA)-induced β-cell dysfunction. UA probably enters the β-cells via glucose transporter 9 (GLUT9). Intracellular UA increases reactive oxygen species (ROS), which phosphorylates and activates AMP-activated protein kinase (AMPK) and then extracellular signal-regulated kinase (ERK). Phosphorylated ERK causes β-cell apoptosis. UA also phosphorylates and degrades inhibitor of kappa B (IκB) that permits the transcription factor nuclear factor kappa B (NF-κB) to enter the nucleus and increases expression of inducible nitric oxide synthase (iNOS). NO overproduction decreases glucose-stimulated insulin secretion (GSIS) and causes β-cell apoptosis. CXCL-1, chemokine (C-X-C motif) ligand 1; MCP-1, monocyte chemoattractant protein-1; IL-6, interleukin-6. Created with BioRender.com
In Min6 cells, UA activates the NF-κB signaling pathway by phosphorylation and degradation of inhibitor of κB (IκB) [39]; NF-κB increases iNOS expression and therefore NO production, which causes a decrease in GSIS and β-cell apoptosis [39]. In RIN-m5F cells, UA increases the mRNA expression of inflammatory mediators, including chemokine (C-X-C motif) ligand 1 (CXCL-1 or KC), monocyte chemoattractant protein-1 (MCP-1), and interleukin-6 (IL-6) [30].
High levels of UA inhibit the growth of the pancreatic β-cell lines (INS-1 and RIN-m5F) in a time- and dose-dependent manner via the ROS-AMPK-ERK signaling pathway [31]. High concentrations of UA also induce oxidative stress in these cell lines [31]. Elevated ROS increases phosphorylation of AMPK, which in turn increases ERK phosphorylation [31], thus inhibiting the cell growth [31]. Luteolin (a flavonoid), by suppressing UA-activated NF-κB-iNOS-NO signaling pathway [44], and resveratrol (a polyphenolic compound), by increasing miR-126 expression [40], protect the pancreatic β-cells from UA-induced dysfunction.
Uric acid and nitric oxide
It has been shown that the timing of serum UA peak (5:08) and serum NO nadir (5:32) coincide in healthy men, suggesting that their concentrations are physiologically related [45]. In addition, in male rats, serum UA levels are inversely correlated with serum NO metabolites, with hyperuricemia decreasing serum NO metabolite levels by about 40–50 % [46]. More details regarding circadian variations of NO metabolites can be found elsewhere [47].
UA increases iNOS expression in the β-cells, decreases GSIS, and causes apoptosis [39]. However, the potential role of NO in UA-induced β-cell dysfunction needs further investigations. NO produced by different NOS isoforms (i.e. endothelial NOS, neural NOS, and iNOS) exerts different effects on β-cell function [48], and in most cases, the eNOS/nNOS-derived NO has physiological relevance, whereas iNOS-derived NO in general has pathological effects. In endothelial cells, high UA levels decreases NO production [46, 49, 50], increases arginase activity [49], and suppresses insulin-stimulated phosphorylation of PKB (Akt) and eNOS [51]. In addition, in human umbilical vein endothelial (HUVEC) cells, a high concentration of UA causes mitochondrial calcium overload probably by switching the direction of mitochondrial sodium-calcium exchanger (NCXmito) function from efflux to influx. This calcium overload increases ROS production, which decreases eNOS expression and NO release, causing endothelial dysfunction [52]. Because NCXmito is involved in insulin secretion from β-cells [53], one can speculate that hyperuricemia can affect β-cell function via this pathway. However, further studies are needed to confirm these effects in the β-cells.
Uric acid‐lowering drugs in diabetes
Considering UA as a target for prevention/management of diabetes is still premature and needs to be evaluated in clinical trials. However, several lines of evidence indicate a potential favorable outcome of these drugs in diabetes. Zurapamic, an inhibitor of UA reabsorption in the kidneys, protects INS-1 cells and rat islets against UA-induced damage by decreasing URAT1 expression and oxidative stress [38]. Allopurinol, a competitive inhibitor of xanthine oxidase that decreases UA production, protects isolated islets from neonatal rats against the cytotoxic effects of styreptozotocin, probably via decreasing intracellular UA levels [54]. Benzbromarone, an uricosuric drug, inhibits fatty acid-binding protein 4 and improves glucose tolerance in type 2 diabetic db/db mice [55]. Allopurinol improves endothelial function in hypertensive type 2 diabetic patients [56]. In a retrospective cohort, it has been shown that compared with non-users, incidence of new-onset diabetes is lower in patients with gout being treated with benzbromarone [6].
Conclusions and perspectives
UA induces oxidative stress, the inflammatory response in the β-cells, and decreases GSIS, causing β-cell apoptosis. The threshold theory for the actions of UA on the β-cells hypothesizes that the detrimental effects of UA occurs above a given concentration. In support of this notion, it has been shown that the inhibitory effect of UA on GSIS in rat pancreatic islets has a sudden occurrence at a threshold of 6.7 mg/dL (0.4 mM) [37]. Other hypothesis of a potential association between UA and diabetes is that the effects of hyperuricemia, are potentiated in presence of other risk factors such as obesity or in genetically at risk subjects [34]. In support of this concept, a positive association has been found between serum UA levels and the body mass index [57]. Also, an association between serum UA levels and glucose homeostasis has been shown to be mediated by adiposity [58].
Regarding the association between UA and β-cell function, the effects of UA on the genes and proteins that are involved in insulin biosynthesis and secretion warrants further investigations. In addition, most mechanistic findings have been drawn from in vitro studies or from animal studies. As always, it should be noted that extending results from animal studies to humans needs abundance of caution, as UA metabolism is different between humans and rodents [59]. Unlike humans, rodents have uricase, and therefore, degrade UA more rapidly [59]. Thus, circulating UA concentrations in humans is about 5–20 fold higher than in most other mammals [12, 13].
All in all, hyperuricemia may potentially cause β-cell dysfunction and predispose subjects to metabolic disorders such as diabetes. If this holds true, then UA-lowering drugs may be helpful in prevention/management of diabetes, at least in subjects who are at risk for both hyperuricemia and diabetes.
Acknowledgements
This study was supported by Shahid Beheshti University of Medical Sciences (grant No. 24576). I would like to thank Prof. Khosrow Kashfi, an academic faculty member of City University of New York School of Medicine for editing of English grammar and syntax of the manuscript.
List of Abbreviations
- ABCG2
ATP-binding cassette subfamily G, member 2
- AMPK
AMP-activated protein kinase
- BCRP
Breast cancer resistance protein
- CXCL-1
Chemokine (C-X-C motif) ligand 1
- eNOS
Endothelial nitric oxide synthase
- ERK
Extracellular signal-regulated kinase
- GLUT9
Glucose transporter 9
- GSIS
Glucose-stimulated insulin secretion
- IL-6
Interleukin-6
- MCP-1
Monocyte chemoattractant protein-1
- NCXmito
mitochondrial sodium-calcium exchanger
- NF-κB
Nuclear factor kappa B
- nNOS
Neural nitric oxide synthase
- NO
Nitric oxide
- NOS
Nitric oxide synthase
- ROS
Reactive oxygen species
- SLC
Solute carrier family
- SSPG
Steady-state plasma glucose
- T2DM
Type 2 diabetes mellitus
- URAT1
Urate transporter 1
Authors’ contributions
A.GH designed and prepared the manuscript. The author(s) read and approved the final manuscript.
Funding
This work was not supported by any funding agency.
Availability of data and materials
Not Applicable.
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
A.GH is a member of the editorial board of BMC Endocrine Disorders.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Rutter GA, Pullen TJ, Hodson DJ, Martinez-Sanchez A. Pancreatic beta-cell identity, glucose sensing and the control of insulin secretion. Biochem J. 2015;466(2):203–18. doi: 10.1042/BJ20141384. [DOI] [PubMed] [Google Scholar]
- 3.Jaacks LM, Siegel KR, Gujral UP, Narayan KM. Type 2 diabetes: A 21st century epidemic. Best Pract Res Clin Endocrinol Metab. 2016;30(3):331–43. doi: 10.1016/j.beem.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. 2015;11(11):649–62. doi: 10.1038/nrrheum.2015.91. [DOI] [PubMed] [Google Scholar]
- 5.Suppiah R, Dissanayake A, Dalbeth N. High prevalence of gout in patients with Type 2 diabetes: male sex, renal impairment, and diuretic use are major risk factors. N Z Med J. 2008;121(1283):43–50. [PubMed] [Google Scholar]
- 6.Niu SW, Chang KT, Ta A, Chang YH, Kuo IC, Hung CC, et al. Decreased incidence of diabetes in patients with gout using benzbromarone. Rheumatology. 2018;57(9):1574–82. doi: 10.1093/rheumatology/key138. [DOI] [PubMed] [Google Scholar]
- 7.So A, Thorens B. Uric acid transport and disease. J Clin Invest. 2010;120(6):1791–9. doi: 10.1172/JCI42344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V. Regulation of uric acid metabolism and excretion. Int J Cardiol. 2016;213:8–14. doi: 10.1016/j.ijcard.2015.08.109. [DOI] [PubMed] [Google Scholar]
- 9.El Ridi R, Tallima H. Physiological functions and pathogenic potential of uric acid: A review. J Adv Res. 2017;8(5):487–93. doi: 10.1016/j.jare.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benn CL, Dua P, Gurrell R, Loudon P, Pike A, Storer RI, et al. Physiology of Hyperuricemia and Urate-Lowering Treatments. Front Med (Lausanne) 2018;5:160. doi: 10.3389/fmed.2018.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ndrepepa G. Uric acid and cardiovascular disease. Clin Chim Acta. 2018;484:150–63. doi: 10.1016/j.cca.2018.05.046. [DOI] [PubMed] [Google Scholar]
- 12.Bobulescu IA, Moe OW. Renal transport of uric acid: evolving concepts and uncertainties. Adv Chronic Kidney Dis. 2012;19(6):358–71. doi: 10.1053/j.ackd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandal AK, Mount DB. The molecular physiology of uric acid homeostasis. Annu Rev Physiol. 2015;77:323–45. doi: 10.1146/annurev-physiol-021113-170343. [DOI] [PubMed] [Google Scholar]
- 14.de Oliveira EP, Burini RC. High plasma uric acid concentration: causes and consequences. Diabetol Metab Syndr. 2012;4:12. doi: 10.1186/1758-5996-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dehghan A, van Hoek M, Sijbrands EJ, Hofman A, Witteman JC. High serum uric acid as a novel risk factor for type 2 diabetes. Diabetes Care. 2008;31(2):361–2. doi: 10.2337/dc07-1276. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan E, Pandya BJ, Chung L, Hariri A, Dabbous O. Hyperuricemia in young adults and risk of insulin resistance, prediabetes, and diabetes: a 15-year follow-up study. Am J Epidemiol. 2012;176(2):108–16. doi: 10.1093/aje/kws002. [DOI] [PubMed] [Google Scholar]
- 17.Bhole V, Choi JW, Kim SW, de Vera M, Choi H. Serum uric acid levels and the risk of type 2 diabetes: a prospective study. Am J Med. 2010;123(10):957–61. doi: 10.1016/j.amjmed.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juraschek SP, McAdams-Demarco M, Miller ER, Gelber AC, Maynard JW, Pankow JS, et al. Temporal relationship between uric acid concentration and risk of diabetes in a community-based study population. Am J Epidemiol. 2014;179(6):684–91. doi: 10.1093/aje/kwt320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kodama S, Saito K, Yachi Y, Asumi M, Sugawara A, Totsuka K, et al. Association between serum uric acid and development of type 2 diabetes. Diabetes Care. 2009;32(9):1737–42. doi: 10.2337/dc09-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lv Q, Meng XF, He FF, Chen S, Su H, Xiong J, et al. High serum uric acid and increased risk of type 2 diabetes: a systemic review and meta-analysis of prospective cohort studies. PLoS One. 2013;8(2):e56864. doi: 10.1371/journal.pone.0056864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu YL, Xu KF, Bai JL, Liu Y, Yu RB, Liu CL, et al. Elevation of serum uric acid and incidence of type 2 diabetes: A systematic review and meta-analysis. Chronic Dis Transl Med. 2016;2(2):81–91. doi: 10.1016/j.cdtm.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y, Hu Y, Huang T, Zhang Y, Li Z, Luo C, et al. High uric acid directly inhibits insulin signalling and induces insulin resistance. Biochem Biophys Res Commun. 2014;447(4):707–14. doi: 10.1016/j.bbrc.2014.04.080. [DOI] [PubMed] [Google Scholar]
- 23.Lu J, He Y, Cui L, Xing X, Liu Z, Li X, et al. Hyperuricemia Predisposes to the Onset of Diabetes via Promoting Pancreatic β-Cell Death in Uricase-Deficient Male Mice. 2020;69(6):1149–1163. [DOI] [PMC free article] [PubMed]
- 24.Ghasemi A, Norouzirad R. Type 2 diabetes: An updated overview. Critical Reviews™ in Oncogenesis. 2019;24(2):1–10. doi: 10.1615/CritRevOncog.2019030976. [DOI] [PubMed] [Google Scholar]
- 25.Facchini F, Chen YD, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. Jama. 1991;266(21):3008–11. [PubMed] [Google Scholar]
- 26.Johnson RJ, Merriman T, Lanaspa MA. Causal or Noncausal Relationship of Uric Acid With Diabetes. Diabetes. 2015;64(8):2720–2. doi: 10.2337/db15-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volpe A, Ye C, Hanley AJ, Connelly PW, Zinman B, Retnakaran R. Changes Over Time in Uric Acid in Relation to Changes in Insulin Sensitivity, Beta-Cell Function, and Glycemia. J Clin Endocrinol Metab. 2020;105(3):e651–9. doi: 10.1210/clinem/dgz199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sluijs I, Holmes MV, van der Schouw YT, Beulens JW, Asselbergs FW, Huerta JM, et al. A Mendelian Randomization Study of Circulating Uric Acid and Type 2 Diabetes. Diabetes. 2015;64(8):3028–36. doi: 10.2337/db14-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quiñones Galvan A, Natali A, Baldi S, Frascerra S, Sanna G, Ciociaro D, et al. Effect of insulin on uric acid excretion in humans. Am J Physiol. 1995;268(1 Pt 1):E1–5. doi: 10.1152/ajpendo.1995.268.1.E1. [DOI] [PubMed] [Google Scholar]
- 30.Roncal-Jimenez CA, Lanaspa MA, Rivard CJ, Nakagawa T, Sanchez-Lozada LG, Jalal D, et al. Sucrose induces fatty liver and pancreatic inflammation in male breeder rats independent of excess energy intake. Metabolism. 2011;60(9):1259–70. doi: 10.1016/j.metabol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Yamamoto T, Hisatome I, Li Y, Cheng W, Sun N, et al. Uric acid induces oxidative stress and growth inhibition by activating adenosine monophosphate-activated protein kinase and extracellular signal-regulated kinase signal pathways in pancreatic β cells. Mol Cell Endocrinol. 2013;375(1–2):89–96. doi: 10.1016/j.mce.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 32.Evans SA, Doblado M, Chi MM, Corbett JA, Moley KH. Facilitative glucose transporter 9 expression affects glucose sensing in pancreatic beta-cells. Endocrinology. 2009;150(12):5302–10. doi: 10.1210/en.2009-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griffiths M. Uric acid diabetes. J Biol Chem. 1948;172(2):853. [PubMed] [Google Scholar]
- 34.Scott FW, Trick KD, Stavric B, Braaten JT, Siddiqui Y. Uric acid-induced decrease in rat insulin secretion. Proc Soc Exp Biol Med. 1981;166(1):123–8. doi: 10.3181/00379727-166-41033. [DOI] [PubMed] [Google Scholar]
- 35.Lu J, Hou X, Yuan X, Cui L, Liu Z, Li X, et al. Knockout of the urate oxidase gene provides a stable mouse model of hyperuricemia associated with metabolic disorders. Kidney Int. 2018;93(1):69–80. doi: 10.1016/j.kint.2017.04.031. [DOI] [PubMed] [Google Scholar]
- 36.Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. Failure of beta-cell function to compensate lack of insulin action in hyperuricemic subjects. Diabetes Metab Res Rev. 2009;25(6):535–41. doi: 10.1002/dmrr.988. [DOI] [PubMed] [Google Scholar]
- 37.Rocić B, Vucić-Lovrencić M, Poje N, Poje M, Bertuzzi F. Uric acid may inhibit glucose-induced insulin secretion via binding to an essential arginine residue in rat pancreatic beta-cells. Bioorg Med Chem Lett. 2005;15(4):1181–4. doi: 10.1016/j.bmcl.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Xin Y, Wang K, Jia Z, Xu T, Xu Q, Zhang C, et al. Zurampic Protects Pancreatic β-Cells from High Uric Acid Induced-Damage by Inhibiting URAT1 and Inactivating the ROS/AMPK/ERK Pathways. Cell Physiol Biochem. 2018;47(3):1074–83. doi: 10.1159/000490184. [DOI] [PubMed] [Google Scholar]
- 39.Jia L, Xing J, Ding Y, Shen Y, Shi X, Ren W, et al. Hyperuricemia causes pancreatic β-cell death and dysfunction through NF-κB signaling pathway. PLoS One. 2013;8(10):e78284. doi: 10.1371/journal.pone.0078284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xin Y, Zhang H, Jia Z, Ding X, Sun Y, Wang Q, et al. Resveratrol improves uric acid-induced pancreatic β-cells injury and dysfunction through regulation of miR-126. Biomed Pharmacother. 2018;102:1120–6. doi: 10.1016/j.biopha.2018.03.172. [DOI] [PubMed] [Google Scholar]
- 41.Zhang C, Moriguchi T, Kajihara M, Esaki R, Harada A, Shimohata H, et al. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol. 2005;25(12):4969–76. doi: 10.1128/MCB.25.12.4969-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Worlicek H, Grabner W, Riemann JF. Effects of uric acid on the B cell in the isolated perfused rat pancreas [Abstract] Res Exp Med (Berl) 1981;178(2):165–75. doi: 10.1007/BF01851491. [DOI] [PubMed] [Google Scholar]
- 43.Robles-Cervantes JA, Ramos-Zavala MG, González-Ortiz M, Martínez-Abundis E, Valencia-Sandoval C, Torres-Chávez A, et al. Relationship between Serum Concentration of Uric Acid and Insulin Secretion among Adults with Type 2 Diabetes Mellitus. Int J Endocrinol. 2011;2011:107904. doi: 10.1155/2011/107904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding Y, Shi X, Shuai X, Xu Y, Liu Y, Liang X, et al. Luteolin prevents uric acid-induced pancreatic β-cell dysfunction. J Biomed Res. 2014;28(4):292–8. doi: 10.7555/JBR.28.20130170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanabrocki EL, Third JL, Ryan MD, Nemchausky BA, Shirazi P, Scheving LE, et al. Circadian relationship of serum uric acid and nitric oxide. Jama. 2000;283(17):2240–1. doi: 10.1001/jama.283.17.2240. [DOI] [PubMed] [Google Scholar]
- 46.Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67(5):1739–42. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 47.Bahadoran Z, Carlström M, Mirmiran P, Ghasemi A. Nitric oxide: To be or not to be an endocrine hormone? Acta Physiol. 2020;229(1):e13443. doi: 10.1111/apha.13443. [DOI] [PubMed] [Google Scholar]
- 48.Bahadoran Z, Mirmiran P, Ghasemi A. Role of Nitric Oxide in Insulin Secretion and Glucose Metabolism. Trends Endocrinol Metab. 2020;31(2):118–30. doi: 10.1016/j.tem.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 49.Zharikov S, Krotova K, Hu H, Baylis C, Johnson RJ, Block ER, et al. Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am J Physiol Cell Physiol. 2008;295(5):C1183–90. doi: 10.1152/ajpcell.00075.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16(12):3553–62. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 51.Choi YJ, Yoon Y, Lee KY, Hien TT, Kang KW, Kim KC, et al. Uric acid induces endothelial dysfunction by vascular insulin resistance associated with the impairment of nitric oxide synthesis. Faseb j. 2014;28(7):3197–204. doi: 10.1096/fj.13-247148. [DOI] [PubMed] [Google Scholar]
- 52.Hong Q, Qi K, Feng Z, Huang Z, Cui S, Wang L, et al. Hyperuricemia induces endothelial dysfunction via mitochondrial Na+/Ca2 + exchanger-mediated mitochondrial calcium overload. Cell Calcium. 2012;51(5):402–10. doi: 10.1016/j.ceca.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Nita II, Hershfinkel M, Fishman D, Ozeri E, Rutter GA, Sensi SL, et al. The mitochondrial Na+/Ca2 + exchanger upregulates glucose dependent Ca2 + signalling linked to insulin secretion. PLoS One. 2012;7(10):e46649. doi: 10.1371/journal.pone.0046649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nukatsuka M, Yoshimura Y, Nishida M, Kawada J. Allopurinol protects pancreatic beta cells from the cytotoxic effect of streptozotocin: in vitro study. J Pharmacobiodyn. 1990;13(4):259–62. doi: 10.1248/bpb1978.13.259. [DOI] [PubMed] [Google Scholar]
- 55.Cai HY, Wang T, Zhao JC, Sun P, Yan GR, Ding HP, et al. Benzbromarone, an old uricosuric drug, inhibits human fatty acid binding protein 4 in vitro and lowers the blood glucose level in db/db mice. Acta Pharmacol Sin. 2013;34(11):1397–402. doi: 10.1038/aps.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Butler R, Morris AD, Belch JJ, Hill A, Struthers AD. Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension. 2000;35(3):746–51. doi: 10.1161/01.hyp.35.3.746. [DOI] [PubMed] [Google Scholar]
- 57.Cook DG, Shaper AG, Thelle DS, Whitehead TP. Serum uric acid, serum glucose and diabetes: relationships in a population study. Postgrad Med J. 1986;62(733):1001–6. doi: 10.1136/pgmj.62.733.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mazidi M, Katsiki N, Mikhailidis DP, Banach M. The link between insulin resistance parameters and serum uric acid is mediated by adiposity. Atherosclerosis. 2018;270:180–6. doi: 10.1016/j.atherosclerosis.2017.12.033. [DOI] [PubMed] [Google Scholar]
- 59.Johnson RJ, Nakagawa T, Sanchez-Lozada LG, Shafiu M, Sundaram S, Le M, et al. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes. 2013;62(10):3307–15. doi: 10.2337/db12-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not Applicable.