Abstract
Background:
Hemophilia A (HA) inhibitor patients that fail traditional immune tolerance induction (ITI) have increased morbidity and mortality. Preclinical studies support factor VIII (FVIII) tolerance induction with a combined approach of anti-CD20 mediated transient B cell depletion and rapamycin mediated regulatory T cell (Treg) induction.
Methods:
Two refractory HA inhibitor patients were treated with rituximab, rapamycin, and FVIII ITI. Their clinical course, anti-FVIII immunoglobulins, cytokines, and select lymphocytes were followed.
Results:
One patient achieved complete and the other partial FVIII tolerance; both had marked annualized bleeding rate improvement. FVIII-specific immunoglobulins, but not total Treg counts, correlated with tolerance induction. IL-6 and IL-21 correlation with complete tolerance induction may support that down-regulation of T effectors and IgG4 production, respectively, contribute to the pathogenesis of tolerance induction.
Conclusions:
This regimen may be considered to induce FVIII tolerance in HA patients with refractory inhibitors. Further characterization of the FVIII-specific immune response is necessary to clarify the mechanism of immune tolerance.
Keywords: hemophilia A, immune tolerance, neutralizing antibody, rituximab, sirolimus
1 |. INTRODUCTION
Deficiency of coagulation factor VIII (FVIII), congenital hemophilia A (HA), results in spontaneous or trauma-induced bleeding incurring risk of life-threatening hemorrhage and recurrent hemarthroses. Standard care in severe HA (FVIII activity < 1%) is prophylactic exogenous FVIII infusion or, more recently, use of a subcutaneously administered FVIII-mimetic antibody1,2 aimed at preventing bleeding and hemophilic arthropathy.3 Approximately one third of patients with severe HA develop neutralizing alloantibodies against FVIII (inhibitors);4–6 they account for the bulk of hemophilia-related morbidity and mortality.5 Thus far, the only proven approach of establishing FVIII immunological tolerance is immune tolerance induction (ITI), accomplished via frequent infusions of high-dose FVIII administered over several months.6 Tolerance induction, defined as negative inhibitor titer and normal FVIII pharmacokinetics, with this approach occurs in ~70% of patients.7,8
In recalcitrant patients, a variety of immunosuppressive regimens have been tried with mixed results.9–11 In a prospective clinical trial of rituximab alone10 or in a retrospective review of rituximab with ITI in HA patients with refractory inhibitors, there was limited success at tolerance induction.11 Mechanistic studies support that antigen-presenting cells recognize FVIII and present cleaved peptides on human leukocyte antigen (HLA) class II molecules to CD4+ T cells, which under conditions of co-stimulation, initiate B cell activation and antibody formation.12 The anti-FVIII antibody response consists of both inhibitory and non-neutralizing antibodies, corresponding to high-affinity IgG4 and low-affinity IgG1 antibodies, respectively.13,14 In preclinical studies, rapamycin administered in conjunction with FVIII antigen induces antigen-specific T effector (Teff) cell deletion and induction of regulatory T cells (Treg), showing promise in preventing inhibitors.15 In a preclinical HA murine study, rapamycin combined with anti-CD20 therapy and conventional ITI was successful at eradicating or reducing inhibitor titer to <5 Bethesda units (BU) in the majority of HA mice with FVIII inhibitors.16
Here, we report the first successful use of combined rapamycin and anti-CD20 therapy with conventional ITI in refractory HA inhibitor patients. In an effort to better understand the mechanism of FVIII tolerance induction, longitudinal blood samples were queried for quantitative anti-FVIII IgG, total CD20+ and Treg populations, and plasma cytokines.
2 |. METHODS
Two pediatric patients with severe HA refractory to conventional ITI were treated with 100 units/kg/d of recombinant FVIII, rapamycin to target trough values of 5–15 ng/mL, and four weekly doses of 375 mg/m2 rituximab and monitored regularly as outlined in Figure 1A. Both patients were maintained on bypassing agents (either prothrombin complex concentrate or activated factor VII) for hemostatic prophylaxis while they had high titer inhibitors. Patients consented to a Children’s Hospital of Philadelphia Institutional Review Board (IRB) approved protocol solely for collection of plasma and peripheral blood mononuclear cells (PBMCs) in order to evaluate their anti-FVIII antibody response and cellular responses.
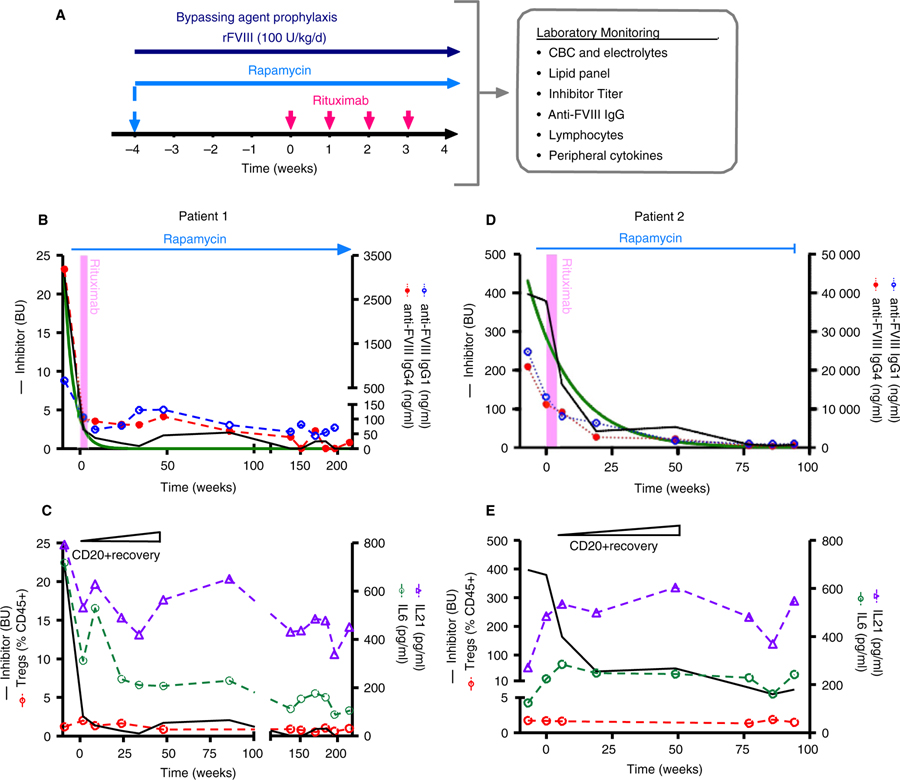
FIGURE 1.
Combination immune tolerance induction regimen and patient course. A, Regimen schematic. Patients were treated with a combination of rapamycin, rituximab, and FVIII and monitored routinely for adverse effects, factor VIII inhibitor titer, and immune responses. Hemostatic prophylaxis was achieved with bypassing agents (recombinant factor VIIa or anti-inhibitor coagulant complex, FEIBA) until low titer inhibitors were established after which patients were treated with FVIII prophylaxis. B-E, Patient course with combined immune tolerance induction regimen. Inhibitor titer (—) correlates with anti-FVIII IgG1 ( ) and IgG4 (
) and IgG4 ( ) for patient 1 (B) and 2 (D) and fit an exponential decay pattern (‒). Quantification of patients’ CD20+ B cell and regulatory T (Treg) cell responses for patient 1 (C) and 2 (E). CD20+ B cell reconstitution occurred at 48 weeks post-rituximab and no difference in Tregs (
) for patient 1 (B) and 2 (D) and fit an exponential decay pattern (‒). Quantification of patients’ CD20+ B cell and regulatory T (Treg) cell responses for patient 1 (C) and 2 (E). CD20+ B cell reconstitution occurred at 48 weeks post-rituximab and no difference in Tregs ( ) was noted after therapy. Peripheral interleukin (IL)-6 (
) was noted after therapy. Peripheral interleukin (IL)-6 ( ) and IL-21 (
) and IL-21 ( ) cytokine response correlated with inhibitor titer for patient 1 (C) but not patient 2 (E)
) cytokine response correlated with inhibitor titer for patient 1 (C) but not patient 2 (E)
Bethesda titers were measured via a heat-inactivated Nijmegen-modified Bethesda assay as previously described.17 Factor VIII specific IgG1 and IgG4 subclass enzyme-linked immunosorbent assays (ELISAs) were conducted as previously described,14,15 with FVIII-specific IgG1 and IgG4 standards generously provided by Jan Voorberg (Sanquin Blood Supply). Plasma cytokines were measured by a Luminex bead-based multiplex assay (Millipore Sigma) per manufacturer’s specifications18 including, (a) Th1 cytokines: IFNγ, TNFα, IL-2, IL-12p70; (b) Th2 cytokines: IL-4, IL-5, IL-6, IL-10; and (c) Th17: IL-17A, IL21. Flow cytometric analysis was conducted on a CytoFlex reader staining for CD3-Pacific Blue, CD4-APC eFluor 780, CD8-BV785, CD19-BV650, CD20-PE-Cy7, and intracellular FoxP3-PE (Thermo Fisher Scientific) to quantify percent B, T, and Treg cells and analyzed by FlowJo version 10. Cytokine and IgG subclass Pearson correlation was analyzed via GraphPad Prism version 7.0 with Bonferroni correction for multiple tests. Baseline annualized bleeding rates (ABR) were retrospectively determined by chart review of the 6 months prior to therapy and compared to prospectively collected data during ITI therapy.
3 |. RESULTS AND DISCUSSION
3.1 |. Patient characteristics
Patient characteristics are detailed in Table S1 in supporting information. Both patients had F8 mutations at high risk of inhibitor development and were non-Caucasian. Despite multiple prior ITI attempts (including rituximab in patient 1), both had long-standing high-titer inhibitors at regimen start (23 BU and 4.3 years for patient 1 and 397 BU and 12.8 years for patient 2). Patient 2 has a co-morbid congenital adenine diphosphate (ADP)-mediated platelet aggregation defect.
3.2 |. Regimen safety
There were no changes in lipid profile, renal function, peripheral blood cell counts, or infectious complications. Both patients had B cell reconstitution at ~48 weeks following rituximab. Shortly after achieving a low-titer inhibitor (~20 months into therapy), patient 2 was found to have a spontaneous small bowel hematoma and jejunal inflammation, concerning for a possible gastrointestinal vasculitis. Due to case reports of rapamycin inducing gastrointestinal vasculitis,19 rapamycin was discontinued. The patient’s jejunal inflammation resolved on follow-up imaging within 10 weeks.
3.3 |. Treatment response
Patient 1 had an abrupt decline to a low-titer inhibitor by week 2 and achieved complete tolerance 2.6 years into his modified ITI regimen (Figure 1B). After inhibitor resolution, he was maintained on prophylactic factor infusions. The patient was weaned off rapamycin approximately 4 years from start of therapy and continues to demonstrate a good pharmacokinetic response to FVIII with no detectable inhibitor off immunosuppression. Patient 1’s ABR declined from 6 to 0.2 with a single port-associated hematoma during ITI.
Patient 2 had a higher FVIII inhibitor titer (397 BU) and reached a low titer approximately 2 years into therapy (Figure 1D). At the time of rapamycin discontinuation 1.8 years into therapy due to gastrointestinal vasculitis, his inhibitor was 3 BU with approximately 30% of expected FVIII recovery. Of note, extrapolating from a fitted exponential decay curve of his inhibitor titer decline (fit: r2 = .921), his inhibitor titer was predicted to be <1 BU approximately 4 months after rapamycin was discontinued (Figure 1D). Despite only partial tolerance, he demonstrated clinically meaningful improvement including a reduction in ABR from 20 pre-therapy to 4.6 over the course of combined ITI therapy. After stopping rapamycin, he remained on FVIII ITI with a low titer inhibitor for approximately 1 year and then was transitioned to emicizumab prophylaxis. Eighteen months after he discontinued rapamycin and 6 months after stopping FVIII ITI, his chromogenic FVIII inhibitor titer was 44 BU supporting the importance of maintaining immunosuppressive therapy and/or FVIII exposure until tolerance is fully achieved. Consistent with prior publications, both patients’ anti-FVIII IgG1 and IgG4 values directly correlated with their Bethesda titer (patient 1: r2 values of .967 and .986; patient 2: r2 values of .878 and .902, respectively, P < .001).
As expected, B cell recovery occurred at ~48 weeks following rituximab in both patients (Figure 1C,E). There was no change in the total peripheral Treg population before or after rapamycin therapy wherein Treg cells accounted for 0.5%–2% of CD45+ lymphocytes in patient 1 and 1.3%–1.9% in patient 2 (Figure 1C,E). These values were similar to inhibitor patients on conventional ITI (n = 3, 0.5%–1.5%) and non-inhibitor controls (n = 2, 1%–3%). Longitudinal plasma cytokine analysis demonstrated a differential response between the two patients. In patient 1, there was a significant correlation between inhibitor titer and levels of IL-6 and IL-21 (r2 = .68 and .59, respectively, P ≤ .005) but not with the other cytokines measured (Figure 1C). In contrast, plasma cytokines, including IL-6 and IL-21, in patient 2 did not correlate with inhibitor titer (r2 = .15 and .22, respectively, P > .05; Figure 1E).
Rapamycin is known to preferentially target anergy in effector T (Teff) cells and thus favor differentiation of developing T cells into Tregs. A trial of anti-CD20 and rapamycin therapy in Pompe’s Disease20 was successful at preventing antibodies to enzyme replacement therapy, and the combination successfully eradicated inhibitors in HA mice.16 Based upon this data, we hypothesized that addition of rapamycin would induce a Treg response and thus help decrease the immune response to FVIII. This hypothesis is not well supported by the observed quantification of Tregs; however, we did not directly measure FVIII-specific Tregs and therefore cannot draw a definitive conclusion. However, the correlation of IL-6 and IL-21 with tolerance induction in patient 1 may support our hypothesis as IL-6 and IL-21 play complementary roles in immunity to stimulate Teffs, inhibit Tregs, and promote antibody (including IgG4) development. Decreasing IL-6 and IL-21 with continued rapamycin therapy may partially explain tolerance induction in patient 1 through negative regulation of the IL-6 mediated Treg suppression. The same pattern was not seen in Patient 2 who, unlike patient 1, did not achieve FVIII tolerance and experienced an amnestic response upon FVIII re-exposure while off immunomodulatory therapy.
This is the first report of combined rapamycin, rituximab, and FVIII ITI to induce FVIII tolerance in patients with previously refractory FVIII inhibitors. One patient successfully achieved tolerance, while the other, who had a partial but clinically meaningful response (397 to 3 BU), discontinued rapamycin due to potentially related vasculitis. This experience is consistent with that seen in preclinical studies in which low titer inhibitors (<50 BU) were eradicated in HA mice treated with combination immunomodulatory therapy.16 Both patients had clinically significant reductions in ABR and no infectious complications. The observed gastrointestinal vasculitis in one patient has been previously reported with rapamycin use raising a potential safety concern;19 however, the causality remains unclear. This regimen may provide an avenue for sustainable FVIII tolerance in patients with a history of refractory FVIII inhibitors and significant bleeding complications.
Supplementary Material
Essentials.
Refractory factor VIII (FVIII) inhibitors pose a significant challenge in the management of hemophilia A.
We report a novel combined T and B cell targeted regimen with FVIII for FVIII tolerance induction.
The regimen was well tolerated and led to significant clinical improvement in bleeding phenotype.
One patient achieved complete tolerance but the mechanism of tolerance needs further study.
ACKNOWLEDGMENTS
The authors would like to thank Dr Jan Voorberg for generously providing anti-FVIII IgG1 and IgG4 and Dr Valder Arruda for assistance with sample collection and processing.
Footnotes
CONFLICTS OF INTEREST
LAG has served as a consultant for Pfizer. BSD and LJR have no conflicts of interest to declare.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377(9):809–818. [DOI] [PubMed] [Google Scholar]
- 2.Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535–544. [DOI] [PubMed] [Google Scholar]
- 3.Council NHFMaSA. Recommendation concerning prophylaxis: regular administration of clotting factor concentrate to prevent bleeding 2012. www.hemophilia.org/NHFWebMainPgs/MainNHF.aspx?menuxml:id=57&contentxml:id=1007
- 4.Hay CR, Palmer B, Chalmers E, et al. Incidence of factor VIII inhibitors throughout life in severe hemophilia A in the United Kingdom. Blood. 2011;117(23):6367–6370. [DOI] [PubMed] [Google Scholar]
- 5.Walsh CE, Soucie JM, Miller CH, United States Hemophilia Treatment Center N. Impact of inhibitors on hemophilia A mortality in the United States. Am J Hematol. 2015;90(5):400–405. [DOI] [PubMed] [Google Scholar]
- 6.Kempton CL, Meeks SL. Toward optimal therapy for inhibitors in hemophilia. Blood. 2014;124(23):3365–3372. [DOI] [PubMed] [Google Scholar]
- 7.Hay CR, DiMichele DM, International Immune Tolerance S. The principal results of the International Immune Tolerance Study: a randomized dose comparison. Blood. 2012;119(6):1335–1344. [DOI] [PubMed] [Google Scholar]
- 8.Blanchette V, Key N, Ljung L, et al. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12(11):1935–1939. [DOI] [PubMed] [Google Scholar]
- 9.Valentino LA, Kempton CL, Kruse-Jarres R, et al. US Guidelines for immune tolerance induction in patients with haemophilia a and inhibitors. Haemophilia. 2015;21(5):559–567. [DOI] [PubMed] [Google Scholar]
- 10.Leissinger C, Josephson CD, Granger S, et al. Rituximab for treatment of inhibitors in haemophilia A. A Phase II study. Thromb Haemost. 2014;112(3):445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins PWMM, Hanley J, Keeling D, et al. Haemophilia Centre Doctors’ Organisation. Rituximab and immune tolerance in severe hemophilia A: a consecutive national cohort. J Thromb Haemost. 2009;7(5):787–794. [DOI] [PubMed] [Google Scholar]
- 12.Astermark J. FVIII inhibitors: pathogenesis and avoidance. Blood. 2015;125(13):2045–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whelan SF, Hofbauer CJ, Horling FM, et al. Distinct characteristics of antibody responses against factor VIII in healthy individuals and in different cohorts of hemophilia A patients. Blood. 2013;121(6):1039–1048. [DOI] [PubMed] [Google Scholar]
- 14.Lollar P. Pathogenic antibodies to coagulation factors. Part one: factor VIII and factor IX. JThrombHaemost. 2004;2(7):1082–1095. [DOI] [PubMed] [Google Scholar]
- 15.Liu CL, Ye P, Lin J, Djukovic D, Miao CH. Long-term tolerance to factor VIII is achieved by administration of interleukin-2/interleukin-2 monoclonal antibody complexes and low dosages of factor VIII. J Thromb Haemost. 2014;12(6):921–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biswas M, Rogers GL, Sherman A, et al. Combination therapy for inhibitor reversal in haemophilia A using monoclonal anti-CD20 and rapamycin. Thromb Haemost. 2017;117(1):33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller CH, Platt SJ, Rice AS, Kelly F, Soucie JM. Hemophilia Inhibitor Research Study I. Validation of Nijmegen-Bethesda assay modifications to allow inhibitor measurement during replacement therapy and facilitate inhibitor surveillance. J Thromb Haemost. 2012;10(6):1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millipore. Human Cytokine/Chemokine Magnetic Bead Panel: Millipore Sigma; 2019. http://www.emdmillipore.com/US/en/product/MILLIPLEX-MAP-Human-Cytokine-Chemokine-Magnetic-Bead-Panel-Immunology-Multiplex-Assay,MM_NF-HCYTOMAG-60K#anchor_PR [Google Scholar]
- 19.Hardinger KL, Cornelius LA, Trulock EP 3rd, Brennan DC. Sirolimus-induced leukocytoclastic vasculitis. Transplantation. 2002;74(5):739–743. [DOI] [PubMed] [Google Scholar]
- 20.Elder ME, Nayak S, Collins SW, et al. B-Cell depletion and immunomodulation before initiation of enzyme replacement therapy blocks the immune response to acid alpha-glucosidase in infantile-onset Pompe disease. J Pediatr. 2013;163(3):847–854.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.