Abstract
How does the time of day of a practice session affect learning of a new motor sequence in the elderly? Participants practiced a given finger tapping sequence either during morning or evening hours. All participants robustly improved performance speed within the session concurrent with a reorganization of the tapping pattern of the sequence. However, evening-trained participants showed additional gains overnight and at 1 wk posttraining; moreover, evening training led to a further reorganization of the tapping pattern offline. A learning experience preceding nocturnal sleep can lead to a task-specific movement routine as an expression of novel “how to” knowledge in the elderly.
The ability to acquire new procedural (“how to”) knowledge, including new motor skills, is often reduced in older adults. The long-term performance enhancement and task mastery are the product of a good lesson (online learning) that has been successfully consolidated (offline learning) (Korman et al. 2003). Previous findings suggest that the online gains attained during practice and the capacity to express additional offline gains afterward may be unevenly affected by aging.
The ability to improve performance online during the training session is generally well preserved in older adults (Durkina et al. 1995; Howard et al. 2004; Yan et al. 2010; Ehsani et al. 2015; Korman et al. 2015). However, their ability to retain, and moreover to further improve offline between sessions is often hampered (Tucker et al. 2011; Wilson et al. 2012). This is of impact on the ability to acquire new lasting skills, as the offline gains are considered a product of successfully completed memory consolidation processes (Spencer et al. 2007; Terpening et al. 2013; Backhaus et al. 2016). Offline processes are subject to the effect of different states during the postlearning interval (e.g., wake and sleep) (Walker and Stickgold 2010) and subsequent activities that may exert mnemonic interference effects (Korman et al. 2005, 2007, 2015). Altogether, the decline in offline learning may be related to several factors: (1) age-dependent changes in neuroplasticity (King et al. 2013, 2017), (2) a higher selectivity (“gating”) in the elderly for what is to be retained in long-term memory (Korman et al. 2015), and (3) blunted circadian rhythms and changes in sleep quality and architecture (Hood and Amir 2017).
In a recent study, following a multisession training on a finger tapping sequence task, we found that elderly participants who trained in the evening hours had significantly lower forgetting rates over a 6-mo period, than participants receiving morning training (Gal et al. 2019). That implies that evening training in the elderly may better engage (and sustain) memory consolidation processes and thus result in a more robust skill representation. Here, we tested this hypothesis, in the same participants as in Gal et al. (2019), by analyzing changes in performance that occur overnight and 1 wk after the first session of evening or morning practice.
Detailed description of the methods has been published elsewhere (Gal et al. 2019). In short, differences in online (within session) and offline (posttraining) learning were assessed following practice on a given five-element sequence (4–1–3–2–4) in two groups of healthy morning-oriented participants (60–75 yr old, N = 14/group). The sequence was tapped repeatedly using the keys of an ergonomic response box with the left, nondominant, hand, “as fast and accurately as possible.” The practice session consisted of 14 blocks, separated by 30-sec breaks, with 60 key presses in each block (equivalent to 12 repetitions of the sequence). Performance was retested overnight (24 h) and a week later (1 wk) to evaluate offline gains and their persistence. All sessions were conducted at the same time of day for the same participant according to the group assignment: morning (Morn, 8–10:30 a.m.) or evening (Eve, 6–9 p.m.). Performance was assessed in terms of mean block duration, within-sequence duration (that is, mean time to complete four sequence transitions [4–1, 1–3, 3–2, 2–4]), and between-sequence transition (4–4) time. These parameters were averaged across four consecutive blocks at the beginning (start) and end (end) of the training session, as well as at 24-h and a 1-wk retests. As the accuracy was very high, all analyses were conducted on the correctly performed complete sequences. Learning gains were assessed using a repeated measure approach with time point (online learning: start and end; offline learning: end, 24 h and 1 wk) as a within-subject factor and group as a between-subject factor. Two-tailed t-tests were used to directly compare between the gains attained by each group. Detailed statistics are provided in Supplemental Material 1.
Time of day effects in online learning
Overall, no differences were observed between the Morn and the Eve groups in the online learning phase. The training session was very effective in both groups, with block duration decreasing from an average of 44.48 ± 3.02 to 33.16 ± 2.46 sec (start and end, respectively), with a significant effect of time points (F(1,26) = 45.30, P < 0.001, ɳ2 = 0.64) (Supplemental Material Fig. 1). There was, however, no significant effect of group (F(1,26) = 0.52, P = 0.479) or group × time point interaction (F(1,26) = 1.20, P = 0.284) (Fig. 2A, online gains). Given the differences between individual participants’ absolute performance, we also compared the individually normalized gains. On average, the gains in block duration at end-training relative to start-training (percentage) were somewhat larger in the Morn group (22.39% ± 3.11%, 27.06% ± 3.57%; Eve and Morn groups, respectively) but the difference was not significant (t(26) = −0.99, P = 0.334).
Figure 2.
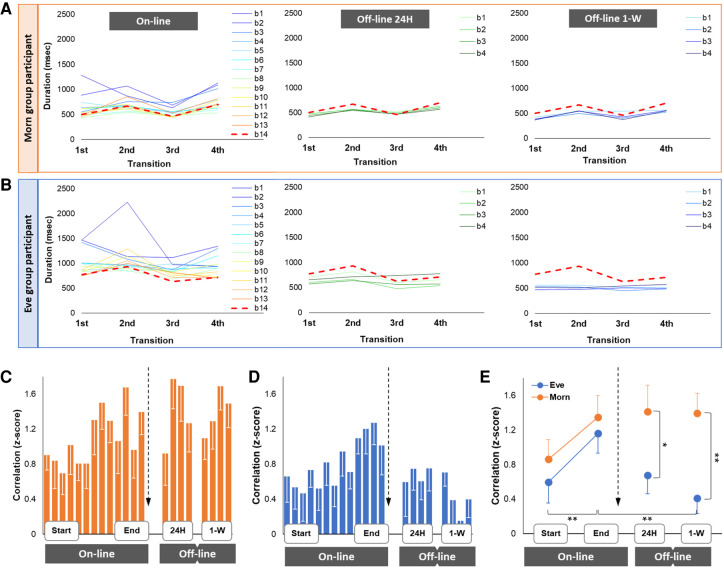
Changes in the tapping pattern (vector of four within-sequence transitions). Changes in the tapping pattern for two representative participants from Morn (A) and Eve (B) groups. Mean interkey transitions of the sequence (connected with thin colored lines) for each block during the online (training) and following the offline phases (24-h and 1-wk retests) are shown. The shape of each line illustrates a tapping pattern for a single block. The last training block is represented by thick dashed red line (the “seed” reference block) in all panels. Group averages of individual normalized Pearson correlation coefficients (bars) between tapping patterns at the end of practice (14-th block—black arrow) and each block during the practice (blocks 1–13) and 24-h and 1-wk retest are shown for Morn (C) and Eve (D) groups. (E) Differences in the changes of the tapping pattern between the groups. Mean correlation coefficients across the initial and final four blocks of the training session and each retest. Bars indicate standard error of the mean (S.E.M.). (*) 0.1 > P > 0.05, (**) P < 0.05.
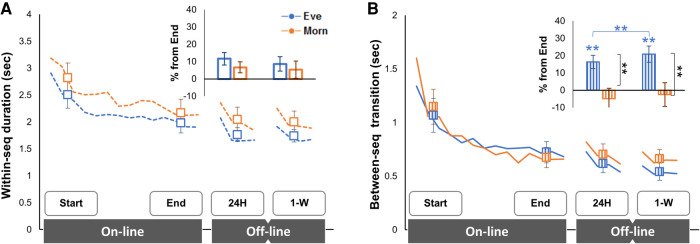
The online gains in block duration were the product of shortening in both the within-sequence duration and the between-sequence transition time (F(1,26) = 42.22, P < 0.001, ɳ2 = 0.62; F(1,26) = 41.26, P < 0.001, ɳ2 = 0.61, respectively) (Fig. 1A,B). There was neither a significant effect of Group nor significant group × time points interaction on either interval type (see the full statistical report in Supplemental Material 1; Supplemental Tables; Supplemental SM-Fig. 1).
Figure 1.
The time course and the learning gains in the Eve (blue) and Morn (orange) groups for within-sequence duration (A) and between-sequence transition (B). Group averages of all performance blocks (lines) and time points (markers) representing the mean of four performance blocks during the online (start to end) and offline (end to 24 h and 1 wk) learning phases are shown. (Insets) Gains in performance at 24-h and 1-wk retests relative to the end of training (percentage). Bars indicate standard error of the mean (S.E.M.). (**) P < 0.05.
Time of day effects in offline changes in performance
There were additional, offline, improvements in block duration by the 24-h and 1-wk retests compared with the end-training performance (F(2,52) = 6.93, P = 0.002, ɳ2 = 0.21), with no significant effect of group (F(1,26) = 0.78, P = 0.385) or group × time point interaction (F(2,52) = 1.54, P = 0.224). Nevertheless, the normalized offline improvements (gains relative to the end-training performance, in percentage) at the 24-h (9.13% ± 2.99%) and 1-wk (13.12% ± 3.51%) retests were significant in the Eve group (t(13) = 3.05, P = 0.009; t(13) = 3.73, P = 0.003, respectively) but not in the Morn group (4.24% ± 3.57%, t(13) = 1.19, P = 0.257; 3.07% ± 5.11%, t(13) = 0.60, P = 0.558 at 24 h and 1 wk, respectively) (Fig. 1A, right bars, inset). Because these results suggest a dichotomy in the offline improvement, a theoretically important observation, we further explored the likelihood of gains across the consolidation interval using Bayesian one-way repeated ANOVA with three time points (end, 24 h and 1 wk). The Bayes factor in the Morn group suggested anecdotal evidence for H0 (BF = 0.16), while the evidence for H1 in the Eve group was extremely strong (BF = 157.65).
Both the within-sequence duration and the between-sequence transition time improved offline (F(2,52) = 5.81, P = 0.005, ɳ2 = 0.18; F(2,52) = 4.49, P = 0.016, ɳ2 = 0.15; respectively). For the between-sequence transition, but not the within-sequence duration, there was a significant interaction of group × time point (F(2,52) = 3.72, P = 0.031) (Fig. 1, offline). Indeed, the effect of time point was significant in the Eve group only (F(2,26) = 13.61, P < 0.001, ɳ2 = 0.51). Moreover, at both 24 h and 1 wk postlearning, the groups differed in their offline improvements at the 24 h and 1 wk (t(26) = 2.23, P = 0.034; t(26) = 2.70, P = 0.012, respectively) (Fig. 1B, offline).
Tapping patterns
Mean durations between each two consecutive sequence key presses of the sequence were computed for each performance block. These mean values compose the individual within-sequence tapping pattern at a given block (see representative individual examples, Fig. 2A,B). Changes in strategy of sequence execution, which presumably reflect changes in the representation of the task (Povel and Collard 1982), were estimated by analyzing changes in the tapping pattern of the sequence in reference to the pattern attained at the end of practice (block 14) (Gabitov et al. 2017, 2019a,b). The end of the practice session was chosen as a reference because this allowed direct assessments of the changes in the representation of the trained sequence during both online and offline learning phases. In each group, the Fisher's transformed Pearson's correlation coefficients with the mean tapping pattern generated during block 14 were calculated for all other, preceding or succeeding, performance blocks.
During training, the degree of similarity to the tapping pattern formed by the end of training progressively increased (F(1,26) = 21.53, P < 0.001, ɳ2 = 0.45). There was no significant effect of group (F(1,26) = 0.51, P = 0.483) or a significant time point × group interaction (F(1,26) = 0.13, P = 0.719), suggesting that time of training did not affect the course and the magnitude of the online changes in the tapping pattern (Fig. 2C).
Across the two offline intervals, there was a marginally significant trend for a time point effect (F(2,52) = 2.96, P = 0.061, ɳ2 = 0.10) but a significant effect of group (F(1,26) = 4.80, P = 0.038, ɳ2 = 0.16). Moreover, there was also a significant time point × group interaction (F(2,52) = 3.96, P = 0.025, ɳ2 = 0.13), suggesting that time of day in which training was afforded affected the course and the magnitude of the offline changes in the tapping pattern in favor of the Eve group (Fig. 2C).
Post-hoc analyses of the tapping pattern for each group, separately, showed a significant effect of time point across the offline intervals for the Eve group (F(2,26) = 8.46, P = 0.001, ɳ2 = 0.39), but not the Morn group (F(2,26) = 0.05, P = 0.952) (Fig. 2E). The difference between the groups in the correlation indices vis à vis the end of training was marginally significant at 24 h (t(26) = −2.00, P = 0.056); however, at 1-wk retest the difference was highly significant (t(26) = −3.37, P = 0.002). Note that the significant decrease in the correlation in the Eve group does not imply that the tapping pattern of the sequence at 24-h and 1-wk retests became more similar to the initial pattern before any training was afforded. In fact, the tapping patterns at the 24-h and 1-wk retests, in both groups, differed significantly from the initial pattern (Supplemental Material 2; Supplemental SM-Fig. 2).
Finally, we tested the relationship between changes in tapping patterns and gains in performance in the general sample, using bivariate Spearman correlations. There was a significant correlation between gains in between-sequence transition and z-scores of the tapping pattern at 1 wk (r = −0.516, P = 0.005), indicating that larger performance gains in the between-sequence transition were associated with greater change in the tapping patterns (Supplemental SM-Fig. 3). There were no significant correlations between gains in block duration or within-sequence duration and changes in the tapping pattern.
Note that it is unlikely that differences in offline changes between groups are related to differences in post-training sleep quality or quantity, or chronotype (see Supplemental Material 3).
The results of the current study indicate that while in terms of speed (fluency) the two groups showed comparable levels of performance, significant offline gains were expressed only by the Eve group. Moreover, following consolidation period, there was a qualitative difference between the groups in the way the movement sequence was generated. These findings suggest that time of day, wherein a novel sequence of finger movements is practiced, can constitute a significant factor in the ability of morning-type elderly to benefit from offline learning.
The gains in within-sequence duration were independent of the time of day of training. The small advantage in speed after Eve training was found to be related to a shortening of the between-sequence key press intervals—a measure considered to represent sequence planning time (Friedman and Korman 2012, 2016). Moreover, significant qualitative changes occurred in the tapping pattern (vis à vis the pattern attained at the end of the session but also compared with the initial sequence tapping pattern) overnight in the Eve, but not in the Morn group; these qualitative changes were well maintained (and even enhanced) a week later. We conjecture that these differences in the offline reorganization of the movement routine may account for slower forgetting rates that were observed in the same Eve group participants at the 7-mo retest after the completion of the multisession training protocol (Gal et al. 2019). In order to gain insight into the nature of underlying processes and their effect on memories, changes in the tapping pattern should be considered together with changes in performance speed (Gabitov et al. 2017; Gabitov et al. 2019a). The significant correlation between the offline gains in between-sequence transition time and changes in the tapping pattern at 1-wk retest suggests a faster selection of the required action (next sequence) due to the newly formed/reorganized representation of the task (Diedrichsen and Kornysheva 2015).
The current results support the notion that shifts in representations of trained movement sequences occur offline when consolidation processes triggered by the training experience take place (Korman et al. 2003; Gabitov et al. 2019a). Moreover, these consolidation phase processes in older adults are more effective following evening practice, perhaps because of its proximity to sleep (Tucker et al. 2011; Korman et al. 2015). Circadian effects may also be at work although it has been suggested that motor performance may be less prone to circadian effects (Schmidt et al. 2015); implicit memory retrieval may, in fact, be better at off-peak than at peak alertness hours in both the young and elderly (May et al. 2005).
The current results suggest that distinct changes in the temporal organization of individual sequence elements and a general reorganization of the tapping patterns, as it has been proposed by previous studies in younger adults (Korman et al. 2003; Friedman and Korman 2016; Gabitov et al. 2019a), can also occur in older individuals. In this population, the potential for change in temporal organization of sequence elements may be constrained by the time of day of the learning experience or/and its proximity to sleep (Spencer et al. 2007; Korman et al. 2015; Backhaus et al. 2016). Altogether, scheduling a single motor practice session to evening hours should be considered in planning interventions to enhance motor abilities in the healthy elderly and even in the context of rehabilitation protocols to promote better long-term skill consolidation.
Supplementary Material
Acknowledgments
The E.J. Safra Brain Research Center for the Study of Learning Disabilities at the University of Haifa is gratefully acknowledged for partially funding this project.
Footnotes
[Supplemental material is available for this article.]
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.052522.120.
References
- Backhaus W, Braass H, Renne T, Gerloff C, Hummel FC. 2016. Motor performance is not enhanced by daytime naps in older adults. Front Aging Neurosci 8: 125 10.3389/fnagi.2016.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Kornysheva K. 2015. Motor skill learning between selection and execution. Trends Cogn Sci 19: 227–233. 10.1016/j.tics.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkina M, Prescott L, Furchtgott E, Cantor J, Powell DA. 1995. Performance but not acquisition of skill learning is severely impaired in the elderly. Arch Gerontol Geriatr 20: 167–183. 10.1016/0167-4943(94)00594-W [DOI] [PubMed] [Google Scholar]
- Ehsani F, Abdollahi I, Mohseni Bandpei MA, Zahiri N, Jaberzadeh S. 2015. Motor learning and movement performance: older versus younger adults. Basic Clin Neurosci 6: 231–238. [PMC free article] [PubMed] [Google Scholar]
- Friedman J, Korman M. 2012. Kinematic strategies underlying improvement in the acquisition of a sequential finger task with self-generated vs. Cued repetition training. PLoS One 7: e52063 10.1371/journal.pone.0052063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J, Korman M. 2016. Offline optimization of the relative timing of movements in a sequence is blocked by retroactive behavioral interference. Front Hum Neurosci 10: 623–623. 10.3389/fnhum.2016.00623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabitov E, Boutin A, Pinsard B, Censor N, Fogel SM, Albouy G, King BR, Benali H, Carrier J, Cohen LG, et al. 2017. Re-stepping into the same river: competition problem rather than a reconsolidation failure in an established motor skill. Sci Rep 7: 9406 10.1038/s41598-017-09677-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabitov E, Boutin A, Pinsard B, Censor N, Fogel SM, Albouy G, King BR, Carrier J, Cohen LG, Karni A, et al. 2019a. Susceptibility of consolidated procedural memory to interference is independent of its active task-based retrieval. PLoS One 14: e0210876 10.1371/journal.pone.0210876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabitov E, Lungu O, Albouy G, Doyon J. 2019b. Weaker inter-hemispheric and local functional connectivity of the somatomotor cortex during a motor skill acquisition is associated with better learning. Front Neurol 10: 1242 10.3389/fneur.2019.01242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal C, Gabitov E, Maaravi-Hesseg R, Karni A, Korman M. 2019. A delayed advantage: multi-session training at evening hours leads to better long-term retention of motor skill in the elderly. Front Aging Neurosci 11: 321 10.3389/fnagi.2019.00321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood S, Amir S. 2017. The aging clock: circadian rhythms and later life. J Clin Invest 127: 437–446. 10.1172/JCI90328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard DV, Howard JH Jr, Japikse K, DiYanni C, Thompson A, Somberg R. 2004. Implicit sequence learning: effects of level of structure, adult age, and extended practice. Psychol Aging 19: 79–92. 10.1037/0882-7974.19.1.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BR, Fogel SM, Albouy G, Doyon J. 2013. Neural correlates of the age-related changes in motor sequence learning and motor adaptation in older adults. Front Hum Neurosci 7: 142 10.3389/fnhum.2013.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BR, van Ruitenbeek P, Leunissen I, Cuypers K, Heise K-F, Santos Monteiro T, Hermans L, Levin O, Albouy G, Mantini D, et al. 2017. Age-related declines in motor performance are associated with decreased segregation of large-scale resting state brain networks. Cereb Cortex 28: 4390–4402. 10.1093/cercor/bhx297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korman M, Raz N, Flash T, Karni A. 2003. Multiple shifts in the representation of a motor sequence during the acquisition of skilled performance. Proc Natl Acad Sci 100: 12492–12497. 10.1073/pnas.2035019100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korman M, Flash T, Karni A. 2005. Resistance to interference and the emergence of delayed gains in newly acquired procedural memories: synaptic and system consolidation? Behav Brain Sci 28: 74–75. 10.1017/S0140525X05320024 [DOI] [Google Scholar]
- Korman M, Doyon J, Doljansky J, Carrier J, Dagan Y, Karni A. 2007. Daytime sleep condenses the time course of motor memory consolidation. Nat Neurosci 10: 1206–1213. 10.1038/nn1959 [DOI] [PubMed] [Google Scholar]
- Korman M, Dagan Y, Karni A. 2015. Nap it or leave it in the elderly: a nap after practice relaxes age-related limitations in procedural memory consolidation. Neurosci Lett 606: 173–176. 10.1016/j.neulet.2015.08.051 [DOI] [PubMed] [Google Scholar]
- May CP, Hasher L, Foong N. 2005. Implicit memory, age, and time of day: paradoxical priming effects. Psychol Sci 16: 96–100. 10.1111/j.0956-7976.2005.00788.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povel DJ, Collard R. 1982. Structural factors in patterned finger tapping. Acta Psychol 52: 107–123. 10.1016/0001-6918(82)90029-4 [DOI] [PubMed] [Google Scholar]
- Schmidt C, Collette F, Reichert CF, Maire M, Vandewalle G, Peigneux P, Cajochen C. 2015. Pushing the limits: chronotype and time of day modulate working memory-dependent cerebral activity. Front Neurol 6: 199 10.3389/fneur.2015.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RM, Gouw AM, Ivry RB. 2007. Age-related decline of sleep-dependent consolidation. Learn Mem 14: 480–484. 10.1101/lm.569407 [DOI] [PubMed] [Google Scholar]
- Terpening Z, Naismith S, Melehan K, Gittins C, Bolitho S, Lewis SJ. 2013. The contribution of nocturnal sleep to the consolidation of motor skill learning in healthy ageing and Parkinson's disease. J Sleep Res 22: 398–405. 10.1111/jsr.12028 [DOI] [PubMed] [Google Scholar]
- Tucker M, McKinley S, Stickgold R. 2011. Sleep optimizes motor skill in older adults. J Am Geriatr Soc 59: 603–609. 10.1111/j.1532-5415.2011.03324.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. 2010. Overnight alchemy: sleep-dependent memory evolution. Nat Rev Neurosci 11: 218 10.1038/nrn2762-c1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JK, Baran B, Pace-Schott EF, Ivry RB, Spencer RM. 2012. Sleep modulates word-pair learning but not motor sequence learning in healthy older adults. Neurobiol Aging 33: 991–1000. 10.1016/j.neurobiolaging.2011.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan JH, Abernethy B, Li X. 2010. The effects of ageing and cognitive impairment on on-line and off-line motor learning. Appl Cogn Psychol 24: 200–212. 10.1002/acp.1551 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.