Abstract
To determine the effect of COVID-19 convalescent plasma on mortality, we aggregated patient outcome data from 10 randomized clinical trials, 20 matched control studies, 2 dose-response studies, and 96 case reports or case series. Studies published between January 1, 2020, and January 16, 2021, were identified through a systematic search of online PubMed and MEDLINE databases. Random effects analyses of randomized clinical trials and matched control data demonstrated that patients with COVID-19 transfused with convalescent plasma exhibited a lower mortality rate compared with patients receiving standard treatments. Additional analyses showed that early transfusion (within 3 days of hospital admission) of higher titer plasma is associated with lower patient mortality. These data provide evidence favoring the efficacy of human convalescent plasma as a therapeutic agent in hospitalized patients with COVID-19.
Abbreviations and Acronyms: COVID-19, coronavirus disease 2019; OR, odds ratio; RCT, randomized clinical trial; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
Article Highlights.
-
•
There remains a lack of consensus on convalescent plasma use in hospitalized patients with COVID-19.
-
•
Meta-analyses of randomized clinical trials and matched control data demonstrated that patients with COVID-19 transfused with convalescent plasma exhibited a lower mortality rate compared with patients receiving standard treatments.
-
•
Additional analyses showed that early transfusion (within 3 days of hospital admission) of high-titer plasma is associated with lower mortality.
-
•
These data provide evidence favoring the efficacy of human convalescent plasma as a therapeutic agent in hospitalized patients with COVID-19.
Convalescent plasma is a century-old passive antibody therapy that has been used to treat outbreaks of novel infectious diseases, including those affecting the respiratory system.1 , 2 At the onset of the pandemic, human convalescent plasma was used worldwide as it represented the only antibody-based therapy for coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).2, 3, 4, 5 Despite the emerging availability of monoclonal antibody therapies and vaccines for use in nonhospitalized patients through federal emergency authorization routes, convalescent plasma use has persisted (~100,000 units per month in the United States in early 2021) during subsequent waves of the COVID-19 pandemic because of surging hospitalizations and mortality rates.6, 7, 8, 9 However, evidence for the efficacy of therapeutic COVID-19 convalescent plasma still requires definitive support from large randomized clinical trials (RCTs). As a result, there remains a lack of consensus on convalescent plasma use in hospitalized patients with COVID-19.10 , 11 Smaller RCTs, matched control studies, and case series studies investigating convalescent plasma therapy for COVID-19 have emerged and provided a positive efficacy signal.12, 13, 14, 15, 16, 17, 18 Most of these studies, however, lacked appropriate statistical power or were terminated early. Also, many studies have transfused patients only after clinical progression to severe COVID-19 respiratory distress, which opposes historical data highlighting the efficacy of early convalescent plasma transfusion and overlooks viral neutralization as the fundamental mechanism for convalescent plasma therapy.1 , 2
There is an urgent need to determine the efficacy of potential treatments amid the ongoing COVID-19 pandemic. Although a “living” systematic review has summarized a broad-ranging clinical experience with convalescent plasma,10 , 11 this approach may be limited because it employed stringent inclusion criteria for aggregating patient outcomes, which prevented a preliminary assessment of convalescent plasma efficacy. Given the insufficient patient outcome data available from RCTs, we used a pragmatic approach for study selection to aggregate COVID-19 clinical outcomes, focusing solely on mortality data from RCTs, matched control studies, dose-response investigations, and case series or case reports in real time. Our primary objective was to derive an aggregate estimate of the mortality rates from transfused and nontransfused cohorts of contemporaneous COVID-19 studies. As an exploratory objective, we assessed whether the time from hospital admission to convalescent plasma transfusion was associated with mortality of patients.
Methods
Eligibility
We included RCTs, matched control trials, dose-response studies, and case series or case reports published on preprint servers or peer-reviewed journals that investigated the impact of human convalescent plasma therapy on mortality of patients with COVID-19.
Literature Search and Data Extraction
We performed a systematic search of the online PubMed and MEDLINE databases from January 1, 2020, through January 16, 2021. Keywords used in the search included ((convalescent plasma) OR (convalescent serum)) AND COVID-19 (and medical subject headings) using the following limits: Humans. No language restrictions were imposed. The references of all eligible studies were reviewed to identify other potentially eligible studies. To be considered eligible for inclusion, studies must have included patients with confirmed diagnosis of COVID-19, used convalescent plasma treatment, and reported mortality. Randomized clinical trials, matched control studies, dose-response studies, case series, and case reports were included. Two reviewers (S.A.K. and J.W.S.) independently screened the titles and abstracts of all studies identified by the search to determine eligibility. Studies that were deemed potentially eligible had their full text reviewed (S.A.K. and J.W.S.) to determine whether they met the criteria for inclusion in the review. Disagreement was resolved by consensus. Two reviewers (S.A.K. and J.W.S.) extracted study and patient characteristics as well as clinical information (additional information for each study is available in in Supplemental Tables 1-6, available online at http://www.mayoclinicproceedings.org).
Two reviewers (S.A.K. and J.W.S.) independently assessed the risk of bias for mortality data of each included study using the Cochrane risk of bias criteria (for RCTs; Supplemental Table 1, available online at http://www.mayoclinicproceedings.org) and the Newcastle-Ottawa Scale (for matched control studies; Supplemental Table 2, available online at http://www.mayoclinicproceedings.org).19, 20, 21 Dose-response studies were evaluated with the Newcastle-Ottawa Scale. The criteria developed by the Mayo Clinic Evidence-Based Practice Research Program informed our assessment of bias in the mortality data reported by case series and case reports.22
Data Synthesis
For RCTs and matched control studies, we recorded the number of survivors and nonsurvivors in transfused and nontransfused cohorts to calculate odds ratios (ORs) with 95% CIs. For dose-response studies, we recorded the number of survivors and nonsurvivors among patients who were transfused with higher titer and lower titer convalescent plasma units to calculate ORs with 95% CIs. Aggregate mortality rates were calculated for transfused and, if applicable, nontransfused patients at the longest reported vital status for each study.
Using the DerSimonian-Laird random effects method,23 we computed aggregate ORs with 95% CIs separately for RCTs and matched control studies. We also computed aggregate ORs with 95% CIs for RCTs and matched control studies combined. Simple random effects meta-regression analyses evaluated the moderator variables (ie, cohort age, proportion of cohort receiving mechanical ventilation, and duration of study follow-up) on mortality for all clinical studies. The I 2 statistic was used to quantify heterogeneity. On the basis of historical data,1 we performed an exploratory subgroup analysis to assess the impact of early transfusion (within 3 days of hospital admission) compared with late transfusion (>3 days after hospital admission) on mortality of patients with COVID-19. All analyses were performed with Comprehensive Meta-analysis software (Biostat, version 3.3.070). Tests were 2 tailed, and α was .05. Figures were made with R software (R Foundation for Statistical Computing). The number needed to treat was calculated using aggregate data from controlled studies.24 Dose-response studies, case series, and case reports were not included in the meta-analysis but were described in a narrative.
Certainty of Evidence Assessment
We used the Grading of Recommendations Assessment, Development, and Evaluation approach to assess the certainty of evidence regarding the impact of convalescent plasma on mortality of patients with COVID-19.25 The risk of bias assessments for RCT and matched control data informed our certainty of evidence assessment.
Results
Search Results
The literature search yielded 780 studies, of which 128 studies met the eligibility criteria and were included in the systematic review (Supplemental Figure 1, available online at http://www.mayoclinicproceedings.org). The analyses included a total of 10 RCTs,13 , 17 , 18 , 26, 27, 28, 29, 30, 31 20 matched control studies,14 , 16 , 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50 2 dose-response studies,51 , 52 and 96 case series or case reports.3 , 14 , 15 , 43 , 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143 Overall, these studies reported outcomes from 35,055 patients with COVID-19 in 31 countries (Tables 1 and 2 ; Supplemental Table 3, available online at http://www.mayoclinicproceedings.org). The age of patients enrolled in these studies ranged from 4 to 100 years, with a greater proportion of men than of women in most studies (proportion of women, 0%-100%; Supplemental Tables 4-6). All studies included patients with diagnosed COVID-19, with most studies including hospitalized patients with severe or life-threatening COVID-19. At the time of plasma transfusion, the proportion of patients on mechanical ventilation varied by study from 0% to 100%. The duration of follow-up ranged from 2 to 118 days (Supplemental Tables 4-6). In most studies, patients were eligible to receive concomitant and experimental therapies, such as antivirals, steroids, and chloroquine or hydroxychloroquine.
Table 1.
Mortality Rates Among COVID-19 Patients: Randomized Clinical Trials and Matched Control Studiesa
| Study | Location | Convalescent plasma |
Control |
Statistics |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Survivor | Nonsurvivor | Mortality (%) | Survivor | Nonsurvivor | Mortality (%) | OR | P | 95% CI | ||
| Randomized clinical trials | ||||||||||
| Avendano-Sola et al18 | Spain | 38 | 0 | 0 | 39 | 4 | 9 | 0.11 | .15 | 0.01-2.19 |
| Rasheed et al17 | Iraq | 20 | 1 | 5 | 20 | 8 | 29 | 0.13 | .06 | 0.01-1.09 |
| Gharbharan et al13 | The Netherlands | 37 | 6 | 14 | 32 | 11 | 26 | 0.47 | .18 | 0.16-1.42 |
| AlQahtani et al26 | Bahrain | 19 | 1 | 5 | 18 | 2 | 10 | 0.47 | .56 | 0.04-5.69 |
| Libster et al27 | Argentina | 78 | 2 | 3 | 76 | 4 | 5 | 0.49 | .41 | 0.09-2.74 |
| Li et al12 | China | 43 | 8 | 16 | 38 | 12 | 24 | 0.59 | .30 | 0.22-1.59 |
| Ray et al28 | India | 30 | 10 | 25 | 26 | 14 | 35 | 0.62 | .33 | 0.24-1.63 |
| Simonovich et al29 | Argentina | 197 | 25 | 11 | 93 | 12 | 11 | 0.98 | .96 | 0.47-2.04 |
| Agarwal et al30 | India | 201 | 34 | 14 | 198 | 31 | 14 | 1.08 | .77 | 0.64-1.83 |
| Bajpai et al31 | India | 11 | 3 | 21 | 14 | 1 | 7 | 3.82 | .27 | 0.35-41.96 |
| Random effects model | 674 | 90 | 12 | 554 | 99 | 15 | 0.76 | .14 | 0.54-1.09 | |
| Random effects model excluding Agarwal et al | 473 | 56 | 11 | 356 | 68 | 16 | 0.65 | .04 | 0.43-0.98 | |
| Matched control studies | ||||||||||
| Duan et al32 | China | 10 | 0 | 0 | 7 | 3 | 30 | 0.10 | .15 | 0.01-2.28 |
| Perotti et al42 | Italy | 43 | 3 | 7 | 16 | 7 | 30 | 0.16 | .01 | 0.04-0.69 |
| Omrani et al44 | Qatar | 39 | 1 | 3 | 35 | 5 | 13 | 0.18 | .13 | 0.02-1.61 |
| Hegerova et al45 | Washington | 18 | 2 | 10 | 14 | 6 | 30 | 0.26 | .13 | 0.05-1.49 |
| Salazar et al,33 | Texas | 146 | 6 | 4 | 235 | 34 | 13 | 0.28 | .01 | 0.12-0.69 |
| Alsharidah et al46 | Kuwait | 111 | 24 | 18 | 143 | 90 | 39 | 0.34 | <.001 | 0.21-0.57 |
| Zeng et al47 | China | 1 | 5 | 83 | 1 | 14 | 93 | 0.36 | .50 | 0.02-6.85 |
| Donato et al48 | New York | 36 | 11 | 23 | 775 | 565 | 42 | 0.42 | .01 | 0.21-0.83 |
| Salazar et al49 | Argentina | 647 | 221 | 25 | 1288 | 1010 | 44 | 0.44 | <.001 | 0.37-0.52 |
| Liu et al50 | New York | 34 | 5 | 13 | 118 | 38 | 24 | 0.46 | .13 | 0.17-1.25 |
| Xia et al34 | China | 135 | 3 | 2 | 1371 | 59 | 4 | 0.52 | .27 | 0.16-1.67 |
| Abolghasemi et al16 | Iran | 98 | 17 | 15 | 56 | 18 | 24 | 0.54 | .10 | 0.26-1.13 |
| AlShehry et al35 | Saudi Arabia | 30 | 10 | 25 | 78 | 46 | 37 | 0.57 | .16 | 0.25-1.26 |
| Budhiraja et al36 | India | 248 | 85 | 26 | 241 | 120 | 33 | 0.69 | .03 | 0.50-0.96 |
| ah Yoon et al37 | New York | 50 | 23 | 32 | 45 | 28 | 38 | 0.74 | .39 | 0.37-1.46 |
| Rogers et al38 | Rhode Island | 56 | 8 | 13 | 149 | 28 | 16 | 0.76 | .52 | 0.33-1.77 |
| Altuntas et al39 | Turkey | 669 | 219 | 25 | 642 | 246 | 28 | 0.85 | .15 | 0.69-1.06 |
| Klapholz et al40 | New Jersey | 37 | 10 | 21 | 38 | 9 | 19 | 1.14 | .80 | 0.42-3.13 |
| Klein et al41 | Maryland | 25 | 9 | 26 | 26 | 8 | 24 | 1.17 | .78 | 0.39-3.51 |
| Moniuszko-Malinowska et al43 | Poland | 49 | 6 | 11 | 672 | 43 | 6 | 1.91 | .16 | 0.78-4.72 |
| Random effects model | 2482 | 668 | 21 | 5950 | 2377 | 29 | 0.57 | <.001 | 0.45-0.72 | |
| Overall random effects modelb | 2955 | 724 | 20 | 6306 | 2445 | 28 | 0.58 | <.001 | 0.47-0.71 | |
OR, odds ratio.
Random effects model excludes trial by Agarwal et al.
Table 2.
Mortality Rates Among COVID-19 Patients: Dose-Response Studies
Meta-analysis
Randomized Clinical Trials
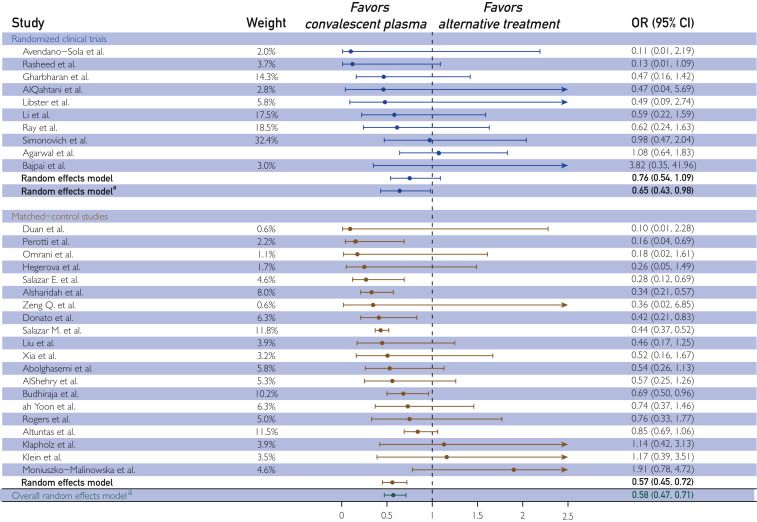
When data from the 10 RCTs were aggregated, there was no association between convalescent plasma therapy and mortality (OR, 0.76; 95% CI, 0.54 to 1.09; P=.14; I 2 =7%; Table 1; Figure ). Although the heterogeneity was low, 1 RCT (Agarwal et al30) demonstrated a directionally different effect, had a large statistical weight (34.2), and represented the primary source of heterogeneity (ΔI 2=7%). In addition, in the context of COVID-19, neutralizing antibodies are hypothesized to represent the primary active agent in convalescent plasma and the marker of plasma potency.144 , 145 In this regard, as mentioned later, 2 studies reported a dose-response relationship between convalescent plasma antibody level and mortality, suggesting the need for a sufficient amount of antibody for therapeutic success.144 , 145 The trial of Agarwal et al30 included a large proportion of patients (~70%) in the convalescent plasma arm who received plasma with low levels of SARS-CoV-2 antibodies less than 1:80, with approximately 30% receiving plasma with no detectable antibodies.30 Thus, there were strong analytical and biologic rationales to exclude this study from statistical models.
Figure.
The effect of human convalescent plasma therapy on mortality of patients with COVID-19. Forest plot illustrating odds ratios (ORs) and 95% CIs computed for each study and aggregated for each study type (DerSimonian-Laird random effects model). Data are separated by study type; randomized clinical trials are presented in blue, and matched control studies are presented in orange. The overall OR pooled across all controlled studies is presented in green. Relative study weights are provided. The I2 values were 0 (randomized clinical trial model), 61 (matched control study model), and 53 (overall model combining randomized clinical trial and matched control studies). aRandom effects model excludes trial by Agarwal et al.
When analyses were performed on data from 9 RCTs excluding the study of Agarwal et al,30 patients transfused with convalescent plasma exhibited a lower mortality rate compared with nontransfused patients with COVID-19 (11% vs 16% mortality; OR, 0.65; 95% CI, 0.43 to 0.98; P=.04; I 2=0%; Table 1; Figure). The aggregate OR (0.65) indicates that convalescent plasma was associated with a 35% reduction in the odds of mortality among patients with COVID-19.
Matched Control Studies
When we aggregated mortality data from the 20 matched control studies, patients transfused with convalescent plasma exhibited a lower mortality rate compared with nontransfused patients (21% vs 29% mortality; OR, 0.57; 95% CI, 0.45 to 0.72; P<.001; I 2=61%; Table 1; Figure).
Randomized Clinical Trials and Matched Control Studies
Aggregation of mortality data from all controlled studies including RCTs and matched control studies indicated that patients transfused with convalescent plasma exhibited a 42% reduction in mortality rate compared with patients receiving standard treatments (20% vs 28% mortality; OR, 0.58; 95% CI, 0.47 to 0.71; P<.001; I 2=53%; Table 1; Figure). Simple random effects meta-regression analyses indicated that cohort age (P=.23), proportion of cohort receiving mechanical ventilation (P=.51), and duration of study follow-up (P=.29) did not affect the aggregate OR computed for all controlled studies.
Subgroup Analysis: Effect of Days Between Hospital Admission and Plasma Transfusion
Sixteen studies (n=6 RCTs, n=10 matched control studies) reported the number of days between hospital admission and convalescent plasma transfusion (Supplemental Table 4). Exploratory analysis revealed that the mortality reduction associated with convalescent plasma transfusion was greater in studies that transfused patients within 3 days of hospital admission (OR, 0.44; 95% CI, 0.32-0.61) compared with studies that transfused patients more than 3 days after hospital admission (OR, 0.79; 95% CI, 0.62 to 0.98; random effects test of heterogeneity between subgroups, P=.005). However, this analysis was strongly influenced by the study by Altuntas et al,39 which transfused patients more than 3 days after admission (relative weight, 73%). On removal of the study by Altuntas et al,39 the number of days from hospital admission to transfusion no longer affected the mortality reduction associated with convalescent plasma transfusion (transfusion within 3 days of hospitalization, 0.44 [0.32-0.60]; transfusion >3 days after hospitalization, 0.61 [0.36-0.68]; random effects test of heterogeneity between subgroups, P=.23).
Additional Evidence
Dose-Response Studies
Two studies investigated the association between convalescent plasma antibody levels and the risk of mortality from COVID-19.3 , 52 Although different criteria were used to categorize convalescent plasma units as higher and lower antibody level, both studies found a dose-response association between antibody level and COVID-19 mortality, such that patient mortality was lower in the subgroups transfused with higher titer plasma. The aggregate mortality rate of patients with COVID-19 transfused with higher titer convalescent plasma was less than that of patients transfused with lower titer plasma (22% vs 29% mortality; Table 2).
Case Series and Case Reports
The aggregate mortality rate among patients with COVID-19 transfused with convalescent plasma reported in uncontrolled studies was 13% (range, 0%-100%), which is comparable to the mortality rates exhibited by transfused cohorts from clinical trials and matched control studies (Supplemental Table 3). Case series and case report data included diverse cohorts of patients with varying inherent risk for COVID-19 complications. Several studies explored immunosuppressed patients with suppressed antibody production due to hematologic malignant neoplasms, cancer-directed therapy, or X-linked agammaglobulinemia and provided an important “experiment of nature” to evaluate convalescent plasma efficacy for COVID-19.84 , 92 , 124 , 146 For example, Jin et al92 highlighted a series of 3 patients with X-linked agammaglobulinemia with severe COVID-19 who failed to respond to other supportive treatments but demonstrated strong improvements in oxygen requirements and viral clearance within days of receiving convalescent plasma transfusions.
Risk of Bias
Overall, we deemed the risk of bias for mortality data to be low to moderate for RCTs and low to moderate for matched control studies. We present the full judgment for each study in Supplemental Tables 1 and 2. The risk of bias for uncontrolled studies is inherently high. Visual inspection of the funnel plot to assess publication bias shows that 1 study falls below the 95% CI and 2 studies fall above the 95% CI (Supplemental Figure 2, available online at http://www.mayoclinicproceedings.org). The funnel plot shows symmetry in the effect sizes among studies with low standard error and asymmetry among studies with greater standard error, suggesting that smaller studies with larger standard error may be more likely to report an effect of convalescent plasma. However, the Egger regression test suggests that there is no significant asymmetry of the plot (intercept, −0.17; P=.67).
Certainty of Evidence
The certainty in the estimate of the effect of convalescent plasma on mortality is moderate to high.147 This judgment was based on the consistency of the results between RCTs and matched control studies and the corroborating evidence from dose-response studies and other uncontrolled case data. In aggregating data from all controlled studies, the meta-analyses provided precise estimates, did not demonstrate substantial heterogeneity, and demonstrated no strong evidence of publication bias. The inherent limitations of the included studies rendered the certainty of evidence judgment to be moderate to high.
Number Needed to Treat
Based on the aggregate OR (0.58; 95% CI, 0.47 to 0.71) computed for all controlled studies and the aggregate mortality rate (28%) expressed by nontransfused cohorts among the controlled studies, to avoid 1 death, the number needed to be transfused with convalescent plasma rather than only to receive the standard of care is 11 (range, 8-16).
Discussion
This analysis represents the most current aggregation of mortality data from contemporaneous COVID-19 convalescent plasma studies. The aggregate mortality rate of transfused patients with COVID-19 was lower than that of nontransfused patients with COVID-19. Additional analyses demonstrated that early transfusion of high-titer plasma reduces mortality among patients with COVID-19. These results favor the efficacy of convalescent plasma as a COVID-19 therapeutic agent. The primary biologic hypothesis for the efficacy of convalescent plasma is antibody-mediated SARS-CoV-2 viral neutralization and interference with viral replication, although other biologic mechanisms may also contribute to the mitigation of symptoms.2 The mortality reduction associated with convalescent plasma aligns with similar analyses of historical data from convalescent plasma trials for viral diseases, such as the 1918 influenza epidemic,1 severe acute respiratory syndrome,148 and H1N1 influenza.149 Our findings are discordant with those of a previous living systematic review,10 , 11 which concluded that there is insufficient evidence to determine the impact of convalescent plasma on all-cause mortality based on only 2 RCTs, including 1 prematurely terminated RCT (Li et al12). This discordance reflects differences in the studies included in the analysis. Our approach was pragmatic and used less stringent study inclusion criteria, allowing the inclusion of 30 controlled studies, of which a majority found a directionally similar effect of convalescent plasma, and our analyses stratified by study design (eg, RCTs and matched control studies) revealed similar aggregate ORs.
Mechanistic and clinical data support the reduction in mortality associated with convalescent plasma administration. Importantly, convalescent plasma contains SARS-CoV-2–neutralizing antibodies.150 , 151 Convalescent plasma administration increases SARS-CoV-2 clearance in patients with COVID-19,12 , 32 including immunocompromised individuals,84 , 92 , 107 , 118 indicating an antiviral effect. Viral neutralization is then posited to reduce the inflammatory response and thus to lessen the likelihood that an overexuberant immune response progresses to lung damage, interference with gas exchange, and death. Additional evidence arising from animal studies shows that administration of human convalescent plasma is protective against SARS-CoV-2 infection.152 , 153 Antibody-mediated interference with viral replication may increase tissue repair and eventually be manifested as reduced mortality. In addition, convalescent plasma transfusion is associated with reductions in inflammatory markers, such as chemokines, cytokines, and C-reactive protein.124 , 154 Concomitant reductions in inflammation and improved gas exchange may underlie the reductions in oxygen requirements associated with convalescent plasma, even in critically ill patients. These findings provide mechanistic evidence for the reduction in mortality observed in patients receiving convalescent plasma.
There are several limitations to this analysis, including the aggregation of mortality data across study populations that varied by the nation of data origin, the timing relative to worldwide progression of the pandemic, the clinical diagnostic and treatment algorithms, the plasma antibody titer and administration volume, the latency between COVID-19 diagnosis and transfusion, and the duration of follow-up after transfusion. Also, we did not consult a librarian when constructing our search terms. However, high-quality evidence from large RCTs remains unavailable, and the continuing global health emergency related to COVID-19 necessitated a practical real-time aggregation of existing mortality data. We note that the reports cited herein include positive results from different countries, suggesting that efficacy is robust across different health care systems. Given the safety of convalescent plasma administered to patients with COVID-19,3 , 4 the results of this real-time systematic review and meta-analysis provide encouragement for its continued use as a therapy and may have broad implications for the treatment of COVID-19 and design of RCTs. Importantly, many of the patients enrolled in the studies included in the analyses received convalescent plasma transfusions later in their disease course. In this context, before antibiotics and effective vaccinations, convalescent plasma therapy was widely understood to be most efficacious very early in the course of hospitalizations.2 , 155 As a result, our analysis may underestimate the mortality reduction achievable through early administration of high-titer convalescent plasma for COVID-19.
Conclusion
This real-time systematic review and meta-analysis of contemporaneous studies highlights that the mortality rate of transfused patients with COVID-19 was lower than that of nontransfused patients with COVID-19 and suggests that early transfusion of high-titer plasma represents the optimal use scenario to reduce the risk of mortality among patients with COVID-19. These results favor the efficacy of convalescent plasma as a COVID-19 therapeutic agent.
Acknowledgments
The authors express their gratitude to the convalescent plasma donors.
Drs Klassen, Senefeld, Casadevall, and Joyner contributed equally to this article.
Footnotes
Grant Support: This work was supported by grants from the National Heart, Lung, and Blood Institute (5R35HL139854 to M.J.J.), the National Institute of Diabetes and Digestive and Kidney Diseases (5T32DK07352 to J.W.S. and C.C.W.), the Natural Sciences and Engineering Research Council of Canada (PDF-532926-2019 to S.A.K.), the National Institutes of Health (1-F32-HL154320-01 to J.W.S.), the National Institute of Allergy and Infectious Diseases (R21 AI145356 and R21 AI152318 to D.F.; R01 AI1520789 to A.C.), and the National Heart, Lung, and Blood Institute (R01 HL059842 to A.C.).
Potential Competing Interests: The authors report no competing interests.
Supplemental material can be found online at http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Luke T.C., Kilbane E.M., Jackson J.L., Hoffman S.L. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145(8):599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- 2.Casadevall A., Pirofski L. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130(4):1545–1548. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joyner M.J., Bruno K.A., Klassen S.A., et al. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin Proc. 2020;95(9):1888–1897. doi: 10.1016/j.mayocp.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joyner M., Wright R.S., Fairweather D., et al. Early safety indicators of COVID-19 convalescent plasma in 5,000 patients. J Clin Invest. 2020;130(9):4791–4797. doi: 10.1172/JCI140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloch E.M., Shoham S., Casadevall A., et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020;130(6):2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Association of Blood Banks America’s Blood Centers, American Red Cross. Joint statement: blood community encourages individuals to donate blood, convalescent plasma during national blood donor month and beyond. https://mk0americasbloogf2jd.kinstacdn.com/wp-content/uploads/2021/01/NBDM_Joint-Statement_Jan-2021-1.15.21_final.pdf Accessed January 15, 2021.
- 7.Center for Systems and Engineering at Johns Hopkins University COVID-19 dashboard. https://coronavirus.jhu.edu/map.html Accessed January 30, 2021.
- 8.US Food & Drug Administration Emergency Use Authorization (EUA) for emergency use of bamlanivimab for the treatment of mild to moderate COVID-19. https://www.fda.gov/media/143602/download Accessed January 30, 2021.
- 9.US Food & Drug Administration Emergency Use Authorization (EUA) for emergency use of Moderna COVID-19 vaccine. https://www.fda.gov/media/144636/download Accessed January 30, 2021.
- 10.Piechotta V., Chai K.L., Valk S.J., et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev. 2020;7(7):CD013600. doi: 10.1002/14651858.CD013600.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chai K.L., Valk S.J., Piechotta V., et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev. 2020;10:CD013600. doi: 10.1002/14651858.CD013600.pub3. [DOI] [PubMed] [Google Scholar]
- 12.Li L., Zhang W., Hu Y, et al. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients with Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA. 2020;324(5):460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gharbharan A., Jordans C.C.E., GeurtsvanKessel C., et al. Convalescent plasma for COVID-19. A randomized clinical trial. medRxiv. July 30, 2020. https://www.medrxiv.org/content/10.1101/2020.07.01.20139857v1 Accessed Jan 15, 2021.
- 14.Salazar E., Christensen P.A., Graviss E.A., et al. Treatment of coronavirus disease 2019 patients with convalescent plasma reveals a signal of significantly decreased mortality. Am J Pathol. 2020;190(11):2290–2303. doi: 10.1016/j.ajpath.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartman W., Hess A.S., Connor J.P. Hospitalized COVID-19 patients treated with convalescent plasma in a mid-size city in the midwest. Transl Med Commun. 2020;5(1):17. doi: 10.1186/s41231-020-00068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abolghasemi H., Eshghi P., Cheraghali A.M., et al. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: results of a multicenter clinical study. Transfus Apher Sci. 2020;59(5):102875. doi: 10.1016/j.transci.2020.102875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasheed A.M., Fatak D.F., Hashim H.A., et al. The therapeutic effectiveness of convalescent plasma therapy on treating critically-ill COVID-19 patients residing in respiratory care units in hospitals in Baghdad, Iraq. Infez Med. 2020;28(3):357–366. [PubMed] [Google Scholar]
- 18.Avendano-Sola C., Ramos-Martinez A., Munez-Rubio E., et al. Convalescent plasma for COVID-19: a multicenter, randomized clinical trial. medRxiv. September 29, 2020. https://www.medrxiv.org/content/10.1101/2020.08.26.20182444v3 Accessed January 15, 2021.
- 19.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 20.Higgins J.P.T., Savović J., Page M., Elbers R., Sterne J. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.1. Cochrane. Higgins J.P.T., Thomas J., Chandler J., et al., editors. 2020. Chapter 8: Assessing risk of bias in a randomized trial.www.training.cochrane.org/handbook Accessed January 20, 2021. [Google Scholar]
- 21.Wells G., Shea B., O’Connell D., et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed January 20, 2021.
- 22.Murad M.H., Sultan S., Haffar S., Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 24.Schunemann H., Vist G., Higgins J.P.T., et al. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.1. Higgins J.P.T., Thomas J., Chandler J., et al., editors. Cochrane; 2020. Chapter 15: Interpretting results and drawing conclusions.https://training.cochrane.org/handbook/current/chapter-15#section-15-4 Accessed January 20, 2021. [Google Scholar]
- 25.Schunemann H., Higgins J., Vist G., et al. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.1. Cochrane. Higgins J.P.T., Thomas J., Chandler J., et al., editors. 2020. Chapter 14: Completing ‘summary of findings’ tables and grading the certainty of the evidence.www.training.cochrane.org/handbook Accessed January 20, 2021. [Google Scholar]
- 26.AlQahtani M., Abdulrahman A., AlMadani A., et al. Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID-19 disease. medRxiv. November 4, 2020. https://www.medrxiv.org/content/10.1101/2020.11.02.20224303v1 Accessed January 15, 2021. [DOI] [PMC free article] [PubMed]
- 27.Libster R., Pérez Marc G., Wappner D., et al. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med. 2021;384(7):610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ray Y., Paul S.R., Bandopadhyay P., et al. Clinical and immunological benefits of convalescent plasma therapy in severe COVID-19: insights from a single center open label randomised control trial. medRxiv. November 29, 2020. https://www.medrxiv.org/content/10.1101/2020.11.25.20237883v1.full Accessed January 15, 2021.
- 29.Simonovich V.A., Burgos Pratx L.D., Scibona P., et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021;384(7):619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agarwal A., Mukherjee A., Kumar G., Chatterjee P., Bhatnagar T., Malhotra P. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) [erratum appears in BMJ. 2020;371:m4232] BMJ. 2020;371:m3939. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bajpai M., Maheshwari A., Chhabra K., et al. Efficacy of convalescent plasma therapy compared to fresh frozen plasma in severely ill COVID-19 patients: a pilot randomized controlled trial. medRxiv. October 27, 2020. https://www.medrxiv.org/content/10.1101/2020.10.25.20219337v1 Accessed January 15, 2021.
- 32.Duan K., Liu B., Li C., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salazar E., Christensen P.A., Graviss E.A., et al. Significantly decreased mortality in a large cohort of COVID-19 patients transfused early with convalescent plasma containing high-titer anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein IgG. Am J Pathol. 2021;191(1):90–107. doi: 10.1016/j.ajpath.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia X., Li K., Wu L., et al. Improved clinical symptoms and mortality on severe/critical COVID-19 patients utilizing convalescent plasma transfusion. Blood. 2020;136(6):755–759. doi: 10.1182/blood.2020007079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.AlShehry N., Zaidi S.Z.A., AlAskar A., et al. Safety and efficacy of convalescent plasma for severe COVID-19: interim report of a multicenter phase II study from Saudi Arabia. Saudi J Med Med Sci. 2021;9(1):16–23. doi: 10.4103/sjmms.sjmms_731_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Budhiraja S., Dewan A., Aggarwal R., et al. Effectiveness of convalescent plasma therapy in Indian patients with COVID-19. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3726179 Accessed January 15, 2021. [DOI] [PMC free article] [PubMed]
- 37.ah Yoon H., Bartash R., Gendlina I., et al. Treatment of severe COVID-19 with convalescent plasma in the Bronx, NYC. JCI Insight. 2021;6(4):e142270. doi: 10.1172/jci.insight.142270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rogers R., Shehadeh F., Mylona E., et al. Convalescent plasma for patients with severe COVID-19: a matched cohort study. medRxiv. August 21, 2020. https://www.medrxiv.org/content/10.1101/2020.08.18.20177402v1 Accessed January 15, 2021. [DOI] [PMC free article] [PubMed]
- 39.Altuntas F., Ata N., Yigenoglu T.N., et al. Convalescent plasma therapy in patients with COVID-19. Transfus Apher Sci. 2020 Sep 19:102955. doi: 10.1016/j.transci.2020.102955. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klapholz M., Pentakota S.R., Zertuche J.-P., et al. Matched cohort study of convalescent COVID-19 plasma (CCP) treatment in severely or life threateningly ill COVID-19 patients. Open Forum Infect Dis. 2021;8(2):ofab001. doi: 10.1093/ofid/ofab001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klein M.N., Wang E.W., Zimand P., et al. Kinetics of SARS-CoV-2 antibody responses pre-and post–COVID-19 convalescent plasma transfusion in patients with severe respiratory failure: an observational case-control study. medRxiv. December 11, 2020. https://www.medrxiv.org/content/10.1101/2020.12.10.20247007v1 Accessed January 20, 2021. [DOI] [PMC free article] [PubMed]
- 42.Perotti C., Baldanti F., Bruno R., et al. Covid-19 Plasma Task Force. Mortality reduction in 46 severe Covid-19 patients treated with hyperimmune plasma. A proof of concept single arm multicenter trial. Haematologica. 2020;105(12):2834–2840. doi: 10.3324/haematol.2020.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moniuszko-Malinowska A., Czupryna P., Zarębska-Michaluk D., et al. Convalescent plasma transfusion for the treatment of COVID-19—experience from Poland: a multicenter study. J Clin Med. 2021;10(1):28. doi: 10.3390/jcm10010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Omrani A.S., Zaqout A., Baiou A., et al. Convalescent plasma for the treatment of patients with severe coronavirus disease 2019: a preliminary report. J Med Virol. 2021;93(3):1678–1686. doi: 10.1002/jmv.26537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hegerova L., Gooley T.A., Sweerus K.A., et al. Use of convalescent plasma in hospitalized patients with COVID-19: case series. Blood. 2020;136(6):759–762. doi: 10.1182/blood.2020006964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alsharidah S., Ayed M., Ameen R.M., et al. COVID-19 convalescent plasma treatment of moderate and severe cases of SARS-CoV-2 infection: a multicenter interventional study. Int J Infect Dis. 2021;103:439–446. doi: 10.1016/j.ijid.2020.11.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng Q.L., Yu Z.J., Gou J.J., et al. Effect of convalescent plasma therapy on viral shedding and survival in patients with coronavirus disease 2019. J Infect Dis. 2020;222(1):38–43. doi: 10.1093/infdis/jiaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donato M., Park S., Baker M., et al. Clinical and laboratory evaluation of patients with SARS-CoV-2 pneumonia treated with high-titer convalescent plasma: a prospective study. medRxiv. August 4, 2020. https://www.medrxiv.org/content/10.1101/2020.07.20.20156398v3 Accessed Janary 15, 2021. [DOI] [PMC free article] [PubMed]
- 49.Salazar M.R., Gonzalez S.E., Regairaz L., et al. Effect of convalescent plasma on mortality in patients with COVID-19 pneumonia. medRxiv. October 9, 2020. https://www.medrxiv.org/content/10.1101/2020.10.08.20202606v1 Accessed January 15, 2021.
- 50.Liu S.T.H., Lin H.M., Baine I., et al. Convalescent plasma treatment of severe COVID-19: a propensity score–matched control study. Nat Med. 2020;26(11):1708–1713. doi: 10.1038/s41591-020-1088-9. [DOI] [PubMed] [Google Scholar]
- 51.Joyner M.J., Carter R.E., Senefeld J.W., et al. Convalescent plasma antibody levels and the risk of death from Covid-19. N Engl J Med. 2021 Jan 13 doi: 10.1056/NEJMoa2031893. NEJMoa2031893. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maor Y., Cohen D., Paran N., et al. Compassionate use of convalescent plasma for treatment of moderate and severe pneumonia in COVID-19 patients and association with IgG antibody levels in donated plasma. EClinicalMedicine. 2020;26:100525. doi: 10.1016/j.eclinm.2020.100525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baang J.H., Smith C., Mirabelli C., et al. Prolonged severe acute respiratory syndrome coronavirus 2 replication in an immunocompromised patient. J Infect Dis. 2021;223(1):23–27. doi: 10.1093/infdis/jiaa666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balashov D., Trakhtman P., Livshits A., et al. SARS-CoV-2 convalescent plasma therapy in pediatric patient after hematopoietic stem cell transplantation. Transfus Apher Sci. 2020 Nov 1:102983. doi: 10.1016/j.transci.2020.102983. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bao Y., Lin S.Y., Cheng Z.H., et al. Clinical features of COVID-19 in a young man with massive cerebral hemorrhage—case report. SN Compr Clin Med. 2020 May 23:1–7. doi: 10.1007/s42399-020-00315-y. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Betrains A., Godinas L., Woei-A-Jin F.J.S.H., et al. Convalescent plasma treatment of persistent severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection in patients with lymphoma with impaired humoral immunity and lack of neutralising antibodies. Br J Haematol. 2020 Dec 13 doi: 10.1111/bjh.17266. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 57.Bhumbra S., Malin S., Kirkpatrick L., et al. Clinical features of critical coronavirus disease 2019 in children. Pediatr Crit Care Med. 2020;21(10):e948–e953. doi: 10.1097/PCC.0000000000002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ilona B., László G., Marienn R., et al. Successful administration of convalescent plasma in critically ill COVID-19 patients in Hungary: the first two cases. Orv Hetil. 2020;161(27):1111–1121. doi: 10.1556/650.2020.31901. [DOI] [PubMed] [Google Scholar]
- 59.Bradfute S.B., Hurwitz I., Yingling A.V., et al. Severe acute respiratory syndrome coronavirus 2 neutralizing antibody titers in convalescent plasma and recipients in New Mexico: an open treatment study in patients with coronavirus disease 2019. J Infect Dis. 2020;222(10):1620–1628. doi: 10.1093/infdis/jiaa505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choudhury A., Reddy G.S., Venishetty S., et al. COVID-19 in liver transplant recipients—a series with successful recovery. J Clin Transl Hepatol. 2020;8(4):467–473. doi: 10.14218/JCTH.2020.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Christensen J., Kumar D., Moinuddin I., et al. Coronavirus disease 2019 viremia, serologies, and clinical course in a case series of transplant recipients. Transplant Proc. 2020;52(9):2637–2641. doi: 10.1016/j.transproceed.2020.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Çınar O.E., Sayınalp B., Karakulak E.A., et al. Convalescent (immune) plasma treatment in a myelodysplastic COVID-19 patient with disseminated tuberculosis. Transfus Apher Sci. 2020;59(5):102821. doi: 10.1016/j.transci.2020.102821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clark E., Guilpain P., Filip I.L., et al. Convalescent plasma for persisting COVID-19 following therapeutic lymphocyte depletion: a report of rapid recovery. Br J Haematol. 2020;190(3):e154–e156. doi: 10.1111/bjh.16981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diorio C., Anderson E.M., McNerney K.O., et al. Convalescent plasma for pediatric patients with SARS-CoV-2–associated acute respiratory distress syndrome. Pediatr Blood Cancer. 2020;67(11):e28693. doi: 10.1002/pbc.28693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Donzelli M., Ippolito M., Catalisano G., et al. Prone positioning and convalescent plasma therapy in a critically ill pregnant woman with COVID-19. Clin Case Rep. 2020;8(12):3352–3358. doi: 10.1002/ccr3.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dulipsingh L., Ibrahim D., Schaefer E.J., et al. SARS-CoV-2 serology and virology trends in donors and recipients of convalescent plasma. Transfus Apher Sci. 2020;59(6):102922. doi: 10.1016/j.transci.2020.102922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Easterlin M.C., De Beritto T., Yeh A.M., Wertheimer F.B., Ramanathan R. Extremely preterm infant born to a mother with severe COVID-19 pneumonia. J Investig Med High Impact Case Rep. 2020;8 doi: 10.1177/2324709620946621. 2324709620946621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Einollahi B., Cegolon L., Abolghasemi H., et al. A patient affected by critical COVID-19 pneumonia, successfully treated with convalescent plasma. Transfus Apher Sci. 2020;59(6):102995. doi: 10.1016/j.transci.2020.102995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Erkurt M.A., Sarici A., Berber İ., Kuku İ., Kaya E., Özgül M. Life-saving effect of convalescent plasma treatment in Covid-19 disease: clinical trial from eastern Anatolia. Transfus Apher Sci. 2020;59(5):102867. doi: 10.1016/j.transci.2020.102867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferrari S., Caprioli C., Weber A., Rambaldi A., Lussana F. Convalescent hyperimmune plasma for chemo-immunotherapy induced immunodeficiency in COVID-19 patients with hematological malignancies. Leuk Lymphoma. 2021 Jan 18:1–9. doi: 10.1080/10428194.2021.1872070. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Figlerowicz M., Mania A., Lubarski K., et al. First case of convalescent plasma transfusion in a child with COVID-19–associated severe aplastic anemia. Transfus Apher Sci. 2020;59(5):102866. doi: 10.1016/j.transci.2020.102866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fisher D.L., Pavel A., Malnick S. Rapid recovery of taste and smell in a patient with SARS-CoV-2 following convalescent plasma therapy. QJM. 2021 Jan 8:hcaa341. doi: 10.1093/qjmed/hcaa341. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fung M., Nambiar A., Pandey S., et al. Treatment of immunocompromised COVID-19 patients with convalescent plasma. Transpl Infect Dis. 2020 Sep 29:e13477. doi: 10.1111/tid.13477. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gazitúa R., Briones J.L., Selman C., et al. Convalescent plasma in COVID-19. Mortality-safety first results of the prospective multicenter FALP 001-2020 trial. medRxiv. December 2, 2020. https://www.medrxiv.org/content/10.1101/2020.11.30.20218560v1.full Accessed January 30, 2021.
- 75.Gemici A., Bilgen H., Erdoğan C., et al. A single center cohort of 40 severe COVID-19 patients who were treated with convalescent plasma. Turk J Med Sci. 2020;50(8):1781–1785. doi: 10.3906/sag-2009-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.González S.E., Regairaz L., Ferrando N.S., González Martínez V.V., Salazar M.R., Estenssoro E. [Convalescent plasma therapy in COVID-19 patients, in the Province of Buenos Aires] Medicina (B Aires) 2020;80(5):417–424. [PubMed] [Google Scholar]
- 77.Hahn M., Condori M.E.H., Totland A., Kristoffersen E.K., Hervig T.A. [Patient with severe covid-19 treated with convalescent plasma] Tidsskr Nor Laegeforen. 2020;140(12) doi: 10.4045/tidsskr.20.0501. [DOI] [PubMed] [Google Scholar]
- 78.Hartman W.R., Hess A.S., Connor J. Use of COVID-19 convalescent plasma as prophylaxis in a patient with new onset ALL. Clin Oncol Case Rep. 2020;4(1) [Google Scholar]
- 79.Hatzl S., Eisner F., Schilcher G., et al. Response to “COVID-19 in persons with haematological cancers. Leukemia. 2020;34(8):2265–2270. doi: 10.1038/s41375-020-0914-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hovey J.G., Tolbert D., Howell D. Burton’s agammaglobulinemia and COVID-19. Cureus. 2020;12(11):e11701. doi: 10.7759/cureus.11701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hu X., Hu C., Jiang D., et al. Effectiveness of convalescent plasma therapy for COVID-19 patients in Hunan, China. Dose-Response. 2020;18(4) doi: 10.1177/1559325820979921. 1559325820979921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang S., Shen C., Xia C., Huang X., Fu Y., Tian L. A retrospective study on the effects of convalescent plasma therapy in 24 patients diagnosed with COVID-19 pneumonia in February and March 2020 at 2 centers in Wuhan, China. Med Sci Monit. 2020;26:e928755. doi: 10.12659/MSM.928755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abid M.B., Chhabra S., Buchan B., et al. Bronchoalveolar lavage–based COVID-19 testing in patients with cancer. Hematol Oncol Stem Cell Ther. 2020 Oct 8;S1658-3876(20) doi: 10.1016/j.hemonc.2020.09.002. 30149-7. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hueso T., Pouderoux C., Péré H., et al. Convalescent plasma therapy for B-cell depleted patients with protracted COVID-19 disease. Blood. 2020;136(20):2290–2295. doi: 10.1182/blood.2020008423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Im J.H., Nahm C.H., Baek J.H., Kwon H.Y., Lee J.S. Convalescent plasma therapy in coronavirus disease 2019: a case report and suggestions to overcome obstacles. J Korean Med Sci. 2020;35(26):e239. doi: 10.3346/jkms.2020.35.e239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jaiswal V., Nasa P., Raouf M., et al. Therapeutic plasma exchange followed by convalescent plasma transfusion in critical COVID-19—an exploratory study. Int J Infect Dis. 2020;102:332–334. doi: 10.1016/j.ijid.2020.10.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jamir I., Lohia P., Pande R.K., Setia R., Singhal A.K., Chaudhary A. Convalescent plasma therapy and remdesivir duo successfully salvaged an early liver transplant recipient with severe COVID-19 pneumonia. Ann Hepatobiliary Pancreat Surg. 2020;24(4):526–532. doi: 10.14701/ahbps.2020.24.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jamous F., Meyer N., Buus D., et al. Critical illness due to Covid-19: a description of the surge in a single center in Sioux Falls. S D Med. 2020;73(7):312–317. [PubMed] [Google Scholar]
- 89.Ji F., Liu W., Hao D., et al. Use of convalescent plasma therapy in eight mild COVID-19 patients. New Microbes New Infect. 2021;39:100814. doi: 10.1016/j.nmni.2020.100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jiang J., Miao Y., Zhao Y., et al. Convalescent plasma therapy: helpful treatment of COVID-19 in a kidney transplant recipient presenting with severe clinical manifestations and complex complications. Clin Transplant. 2020;34(9):e14025. doi: 10.1111/ctr.14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jin C., Gu J., Yuan Y., et al. Treatment of 6 COVID-19 patients with convalescent plasma. medRxiv. May 26, 2020. https://www.medrxiv.org/content/10.1101/2020.05.21.20109512v1.full Accessed January 15, 2021.
- 92.Jin H., Reed J.C., Liu S.T.H., et al. Three patients with X-linked agammaglobulinemia hospitalized for COVID-19 improved with convalescent plasma. J Allergy Clin Immunol Pract. 2020;8(10):3594–3596.e3. doi: 10.1016/j.jaip.2020.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Karataş A., İnkaya A.Ç., Demiroğlu H., et al. Prolonged viral shedding in a lymphoma patient with COVID-19 infection receiving convalescent plasma. Transfus Apher Sci. 2020;59(5):102871. doi: 10.1016/j.transci.2020.102871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ahn J.Y., Sohn Y., Lee S.H., et al. Use of convalescent plasma therapy in two covid-19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci. 2020;35(14):e149. doi: 10.3346/jkms.2020.35.e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Katz-Greenberg G., Yadav A., Gupta M., et al. Outcomes of COVID-19–positive kidney transplant recipients: a single-center experience. Clin Nephrol. 2020;94(6):318–321. doi: 10.5414/CN110311. [DOI] [PubMed] [Google Scholar]
- 96.Kong Y., Cai C., Ling L., et al. Successful treatment of a centenarian with coronavirus disease 2019 (COVID-19) using convalescent plasma. Transfus Apher Sci. 2020;59(5):102820. doi: 10.1016/j.transci.2020.102820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lancman G., Mascarenhas J., Bar-Natan M. Severe COVID-19 virus reactivation following treatment for B cell acute lymphoblastic leukemia. J Hematol Oncol. 2020;13(1):131. doi: 10.1186/s13045-020-00968-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lima B., Gibson G.T., Vullaganti S., et al. COVID-19 in recent heart transplant recipients: clinicopathologic features and early outcomes. Transpl Infect Dis. 2020;22(5):e13382. doi: 10.1111/tid.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Luetkens T., Metcalf R., Planelles V., et al. Successful transfer of anti–SARS-CoV-2 immunity using convalescent plasma in an MM patient with hypogammaglobulinemia and COVID-19. Blood Adv. 2020;4(19):4864–4868. doi: 10.1182/bloodadvances.2020002595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.London J., Boutboul D., Lacombe K., et al. Severe COVID-19 in patients with B cell alymphocytosis and response to convalescent plasma therapy. J Clin Immunol. 2021;41(2):356–361. doi: 10.1007/s10875-020-00904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lubnow M., Schmidt B., Fleck M., et al. Secondary hemophagocytic lymphohistiocytosis and severe liver injury induced by hepatic SARS-CoV-2 infection unmasking Wilson’s disease: balancing immunosuppression. Int J Infect Dis. 2021;103:624–627. doi: 10.1016/j.ijid.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Malsy J., Veletzky L., Heide J., et al. Sustained response after remdesivir and convalescent plasma therapy in a B-cell depleted patient with protracted COVID-19. Clin Infect Dis. 2020 Oct 26:ciaa1637. doi: 10.1093/cid/ciaa1637. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martinez-Resendez M.F., Castilleja-Leal F., Torres-Quintanilla A., et al. Initial experience in Mexico with convalescent plasma in COVID-19 patients with severe respiratory failure, a retrospective case series. medRxiv. July 20, 2020. https://www.medrxiv.org/content/10.1101/2020.07.14.20144469v1 Accessed January 18, 2021.
- 104.Mehta S.A., Rana M.M., Motter J.D., et al. Incidence and outcomes of COVID-19 in kidney and liver transplant recipients with HIV: report from the National HOPE in Action Consortium. Transplantation. 2021;105(1):216–224. doi: 10.1097/TP.0000000000003527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Anderson J., Schauer J., Bryant S., Graves C.R. The use of convalescent plasma therapy and remdesivir in the successful management of a critically ill obstetric patient with novel coronavirus 2019 infection: a case report. Case Rep Womens Health. 2020;27:e00221. doi: 10.1016/j.crwh.2020.e00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Milošević I., Jovanović J., Stevanovic O. Atypical course of COVID-19 in patient with Bruton agammaglobulinemia. J Infect Dev Ctries. 2020;14(11):1248–1251. doi: 10.3855/jidc.13840. [DOI] [PubMed] [Google Scholar]
- 107.Mira E., Yarce O.A., Ortega C., et al. Rapid recovery of a SARS-CoV-2–infected X-linked agammaglobulinemia patient after infusion of COVID-19 convalescent plasma. J Allergy Clin Immunol Pract. 2020;8(8):2793–2795. doi: 10.1016/j.jaip.2020.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moore J.L., Ganapathiraju P.V., Kurtz C.P., Wainscoat B. A 63-year-old woman with a history of non-Hodgkin lymphoma with persistent SARS-CoV-2 infection who was seronegative and treated with convalescent plasma. Am J Case Rep. 2020;21:e927812. doi: 10.12659/AJCR.927812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Naeem S., Gohh R., Bayliss G., et al. Successful recovery from COVID-19 in three kidney transplant recipients who received convalescent plasma therapy. Transpl Infect Dis. 2020 Aug 19:e13451. doi: 10.1111/tid.13451. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Niu A., McDougal A., Ning B., et al. COVID-19 in allogeneic stem cell transplant: high false-negative probability and role of CRISPR and convalescent plasma. Bone Marrow Transplant. 2020;55(12):2354–2356. doi: 10.1038/s41409-020-0972-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Olivares-Gazca J.C., Priesca-Marín J.M., Ojeda-Laguna M., et al. Infusion of convalescent plasma is associated with clinical improvement in critically ill patients with COVID-19: a pilot study. Rev Invest Clin. 2020;72(3):159–164. doi: 10.24875/RIC.20000237. [DOI] [PubMed] [Google Scholar]
- 112.Pal P., Ibrahim M., Niu A., et al. Safety and efficacy of COVID-19 convalescent plasma in severe pulmonary disease: a report of 17 patients. Transfus Med. 2020 Oct 19 doi: 10.1111/tme.12729. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 113.Peng F., Tu L., Yang Y., et al. Management and treatment of COVID-19: the Chinese experience. Can J Cardiol. 2020;36(6):915–930. doi: 10.1016/j.cjca.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ragab D., Salah-Eldin H., Afify M., Soliman W., Badr M.H. A case of COVID-19, with cytokine storm, treated by consecutive use of therapeutic plasma exchange followed by convalescent plasma transfusion: a case report. J Med Virol. 2021;93(4):1854–1856. doi: 10.1002/jmv.26630. [DOI] [PubMed] [Google Scholar]
- 115.Rahman M.H., Akter R., Behl T., et al. COVID-19 outbreak and emerging management through pharmaceutical therapeutic strategy. Curr Pharm Des. 2020;26(41):5224–5240. doi: 10.2174/1381612826666200713174140. [DOI] [PubMed] [Google Scholar]
- 116.Antony S.J., Singh J., de Jesus M., Lance J. Early use of tocilizumab in respiratory failure associated with acute COVID-19 pneumonia in recipients with solid organ transplantation. IDCases. 2020;21:e00888. doi: 10.1016/j.idcr.2020.e00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rizvi S., Danic M., Silver M., LaBond V. Cytosorb filter: an adjunct for survival in the COVID-19 patient in cytokine storm? A case report. Heart Lung. 2021;50(1):44–50. doi: 10.1016/j.hrtlng.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rodriguez Z., Shane A.L., Verkerke H., et al. COVID-19 convalescent plasma clears SARS-CoV-2 refractory to remdesivir in an infant with congenital heart disease. Blood Adv. 2020;4(18):4278–4281. doi: 10.1182/bloodadvances.2020002507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schwartz S.P., Walker T.C., Kihlstrom M., et al. Extracorporeal membrane oxygenation for COVID-19–associated multisystem inflammatory syndrome in a 5-year-old. Am Surg. 2020 Dec 29 doi: 10.1177/0003134820983198. 0003134820983198. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shankar R., Radhakrishnan N., Dua S., et al. Convalescent plasma to aid in recovery of COVID-19 pneumonia in a child with acute lymphoblastic leukemia. Transfus Apher Sci. Published online. 2020 Sep 24:102956. doi: 10.1016/j.transci.2020.102956. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shen C., Wang Z., Zhao F., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Szwebel T.-A., Veyer D., Robillard N., et al. Usefulness of plasma SARS-CoV-2 RNA quantification by droplet-based digital PCR to monitor treatment against COVID-19 in a B-cell lymphoma patient. Stem Cell Rev Rep. 2021;17(1):296–299. doi: 10.1007/s12015-020-10107-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tan L., Kang X., Zhang B., et al. A special case of COVID-19 with long duration of viral shedding for 49 days. medRxiv. March. 2020;27 doi: 10.1002/mco2.2. https://www.medrxiv.org/content/10.1101/2020.03.22.20040071v1 Accessed January 15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tremblay D., Seah C., Schneider T., et al. Convalescent plasma for the treatment of severe COVID-19 infection in cancer patients. Cancer Med. 2020;9(22):8571–8578. doi: 10.1002/cam4.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Trimarchi H., Gianserra R., Lampo M., Monkowski M., Lodolo J. Eculizumab, SARS-CoV-2 and atypical hemolytic uremic syndrome. Clin Kidney J. 2020;13(5):739–774. doi: 10.1093/ckj/sfaa166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Van Damme K.F.A., Tavernier S., Van Roy N., et al. Case report: convalescent plasma, a targeted therapy for patients with CVID and severe COVID-19. Front Immunol. 2020;11:596761. doi: 10.3389/fimmu.2020.596761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.van Oers N.S.C., Hanners N.W., Sue P., et al. SARS-CoV-2 infection associated with hepatitis in an infant with X-linked severe combined immunodeficiency. Clin Immunol. 2021;224:108662. doi: 10.1016/j.clim.2020.108662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vlachogianni G., Hassapopoulou-Matamis H., Politis C., Fylaki E., Mentis A. A case of COVID-19 convalescent plasma donation in Greece: directed donation for compassionate use in the donor’s critically ill father. Transfus Clin Biol. 2020;27(4):269–270. doi: 10.1016/j.tracli.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang M., Yang X., Yang F., et al. Convalescent plasma therapy in critically ill coronavirus disease 2019 patients with persistently positive nucleic acid test, case series report. Medicine (Baltimore) 2020;99(36):e21596. doi: 10.1097/MD.0000000000021596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang B., Van Oekelen O., Mouhieddine T., et al. A tertiary center experience of multiple myeloma patients with COVID-19: lessons learned and the path forward. J Hematol Oncol. 2020;13(1):94. doi: 10.1186/s13045-020-00934-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wei B., Hang X., Xie Y., et al. Long-term positive severe acute respiratory syndrome coronavirus 2 ribonucleic acid and therapeutic effect of antivirals in patients with coronavirus disease: case reports. Rev Soc Bras Med Trop. 2020;53:e20200372. doi: 10.1590/0037-8682-0372-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wright Z., Bersabe A., Eden R., Cap A. Successful use of COVID-19 convalescent plasma in a patient recently treated for follicular lymphoma. Clin Lymphoma Myeloma Leuk. 2021;21(1):66–68. doi: 10.1016/j.clml.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xu X., Ong Y.K., Wang D.Y. Role of adjunctive treatment strategies in COVID-19 and a review of international and national clinical guidelines. Mil Med Res. 2020;7(1):22. doi: 10.1186/s40779-020-00251-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang B., Yang J., Zhou L., et al. Inflammatory cytokine depletion in severe coronavirus disease 2019 infectious pneumonia: a case report. Medicine (Baltimore) 2020;99(49):e23449. doi: 10.1097/MD.0000000000023449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ye M., Fu D., Ren Y., et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol. 2020;92(10):1890–1901. doi: 10.1002/jmv.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yi S.G., Rogers A.W., Saharia A., et al. Early experience with COVID-19 and solid organ transplantation at a US high-volume transplant center. Transplantation. 2020;104(11):2208–2214. doi: 10.1097/TP.0000000000003339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Avanzato V.A., Matson M.J., Seifert S.N., et al. Case study: prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell. 2020;183(7):1901–1912.e9. doi: 10.1016/j.cell.2020.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yokoyama A.P.H., Wendel S., Bonet-Bub C., et al. Impact of convalescent plasma transfusion (CCP) in patients with previous circulating neutralizing antibodies (nAb) to COVID-19. medRxiv. December 11, 2020. https://www.medrxiv.org/content/10.1101/2020.12.08.20246173v1 Accessed January 30, 2021.
- 139.Zeng H., Wang D., Nie J., et al. The efficacy assessment of convalescent plasma therapy for COVID-19 patients: a multi-center case series. Signal Transduct Target Ther. 2020;5(1):219. doi: 10.1038/s41392-020-00329-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang B., Liu S., Tan T., et al. Treatment with convalescent plasma for critically ill patients with severe acute respiratory syndrome coronavirus 2 infection. Chest. 2020;158(1):e9–e13. doi: 10.1016/j.chest.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang L.L., Liu Y., Guo Y.G., et al. Convalescent plasma rescued a severe COVID-19 patient with chronic myeloid leukemia blast crisis and myelofibrosis. Turk J Haematol. 2020 Sep 29 doi: 10.4274/tjh.galenos.2020.2020.0400. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang L.B., Pang R.R., Qiao Q.H., et al. Successful recovery of COVID-19–associated recurrent diarrhea and gastrointestinal hemorrhage using convalescent plasma. Mil Med Res. 2020;7(1):45. doi: 10.1186/s40779-020-00273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang L., Pang R., Xue X., et al. Anti–SARS-CoV-2 virus antibody levels in convalescent plasma of six donors who have recovered from COVID-19. Aging (Albany NY) 2020;12(8):6536–6542. doi: 10.18632/aging.103102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.US Food & Drug Administration Updated evidence to support the emergency use of COVID-19 convalescent plasma—as of 9/23/2020. https://www.fda.gov/media/142386/download Accessed January 30, 2021.
- 145.Joyner M.J., Senefeld J.W., Klassen S.A., et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience. medRxiv. August 12, 2020. https://www.medrxiv.org/content/10.1101/2020.08.12.20169359v1 Accessed January 15, 2021.
- 146.Senefeld J., Klassen S.A., Ford S.K., et al. Therapeutic use of convalescent plasma in COVID-19 patients with immunodeficiency: a systematic review. medRxiv. November 10, 2020. https://www.medrxiv.org/content/10.1101/2020.11.08.20224790v1 Accessed Jan 15, 2021.
- 147.Murad M.H. Clinical practice guidelines: a primer on development and dissemination. Mayo Clin Proc. 2017;92(3):423–433. doi: 10.1016/j.mayocp.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 148.Cheng Y., Wong R., Soo Y.O.Y., et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24(1):44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hung I.F.N., To K.K.W., Lee C.-K., et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447–456. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Robbiani D.F., Gaebler C., Muecksch F., Lorenzi J.C.C., Wang Z., Cho A. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584(7821):437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Klein S.L., Pekosz A., Park H.S., et al. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J Clin Invest. 2020;130(11):6141–6150. doi: 10.1172/JCI142004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Imai M., Iwatsuki-Horimoto K., Hatta M., et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc Natl Acad Sci U S A. 2020;117(28):16587–16595. doi: 10.1073/pnas.2009799117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sun J., Zhuang Z., Zheng J., et al. Generation of a broadly useful model for COVID-19 pathogenesis, vaccination, and treatment. Cell. 2020;182(3):734–743.e5. doi: 10.1016/j.cell.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bandopadhyay P., Rozario R.D., Lahiri A., et al. Nature and dimensions of the systemic hyper-inflammation and its attenuation by convalescent plasma in severe COVID-19. J Infect Dis. 2021 Jan 12:jiab010. doi: 10.1093/infdis/jiab137. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Casadevall A., Scharff M.D. Return to the past: the case for antibody-based therapies in infectious diseases. Clin Infect Dis. 1995;21(1):150–161. doi: 10.1093/clinids/21.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.