Summary
Oxytocin was once understood solely as a neuropeptide with a central role in social bonding, reproduction, parturition, lactation and appetite regulation. Recent evidence indicates that oxytocin enhances glucose uptake and lipid utilization in adipose tissue and skeletal muscle, suggesting that dysfunction of the oxytocin system could underlie the pathogenesis of insulin resistance and dyslipidaemia. Murine studies revealed that deficiencies in oxytocin signalling and oxytocin receptor expression lead to obesity despite normal food intake, motor activity and increased leptin levels. In addition, plasma oxytocin concentration is notably lower in obese individuals with diabetes, which may suggest an involvement of the oxytocin system in the pathogenesis of cardiometabolic disease. More recently, small scale studies demonstrated that intranasal administration of oxytocin was associated with significant weight loss as well as improvements in insulin sensitivity and pancreatic β‐cell responsivity in human subjects. The multi‐pronged effects of oxytocin signalling on improving peripheral insulin sensitivity, pancreatic function and lipid homeostasis strongly suggest a role for this system as a therapeutic target in obesity and diabetes management. The complexity of obesity aetiology and the pathogenesis of obesity‐related metabolic complications underscore the need for a systems approach to better understand the role of oxytocin in metabolic function.
Keywords: beta cell function, glucose metabolism, insulin sensitivity, lipid metabolism
Introduction
Oxytocin was first characterised by Sir Henry H. Dale in 1906 as an extract made from the human posterior pituitary gland causing uterine contractions in a pregnant cat 1, 2. It was not long after when physicians began using the pituitary extract to stimulate uterine muscle contractions during labour. The extract was described then as having ‘oxytocic’ activity, derived from the Greek words ‘οξύς’, meaning sharp, and ‘τόκος’, meaning birth – in reference to its uterotonic action. Kamm et al. then successfully isolated the pressor and oxytocic components of the posterior pituitary lobe extracts and named the oxytocic fraction ‘oxytocin’ 3, formally, in 1928. The function of oxytocin was studied comprehensively in animals in the following decades, and the use of oxytocin in clinical obstetrics enabled considerable advances in the understanding of its physiological action in women. Physicians found that higher dosages of oxytocin could be used to induce abortions, prevent post‐partum haemorrhaging and treat heterotopic cardiac arrhythmias 4. However, knowledge gaps remained: studies of its physiological action in human males were lacking, and the metabolic effects of oxytocin in its natural or synthesised form were still largely unknown.
It was not until 1963 that accounts of the metabolic effects of oxytocin emerged 5: administration of oxytocin (Syntocinon) to 20 puerperal women caused prompt, sustained elevations in non‐esterified fatty acids (NEFAs), followed by reductions in blood glucose 30 min to 1 h later. Similar elevations in NEFA were observed in non‐pregnant women as well as women in labour (natural and oxytocin‐induced) 5, 6, except that glucose levels did not fall significantly in the women in labour 6, 7. By then, scientists had already noted a multitude of interspecies and sex differences in the expression, receptor sensitivity and metabolic effects of oxytocin 5, 8, which made inference of its equivalent functions in humans difficult. Regardless, research into oxytocin continued to flourish with the discovery of its effects in lactation, social bonding, cognition and appetite regulation 9.
Today, synthetic oxytocin (Pitocin/Syntocinon) is regularly prescribed to induce as well as augment labour. Unpublished data from the 2010 World Health Organisation Global Survey on Maternal and Perinatal Health showed that nearly 10% of deliveries globally are induced 10. In the intensified effort to remediate the obesity epidemic, oxytocin has been cast into the spotlight for its potential use in obesity therapeutics, following results from human and animal studies showing it could reduce food and calorie intake 11, 12, 13, 14. While oxytocin has been shown in murine studies to increase fat oxidation, improve insulin sensitivity and glucose transport in peripheral tissues, marked species differences in the localization and expression patterns of oxytocin receptors (OTRs) suggest that some of its observed effects may be species‐specific. Animal studies also suggest that the effects of oxytocin differ in the obese and diabetic state. The central effects of oxytocin on appetite regulation and metabolic homeostasis have been reviewed previously 15, 16, 17, 18, 19. The peripheral actions of oxytocin have not been assessed extensively in literature, particularly in regard to its effects in humans and translational implications. In this review, we aim to expand current knowledge concerning the role of the oxytocin system by reviewing studies on the peripheral effects of oxytocin and its role in human appetite regulation, metabolic homeostasis and other areas pertinent to obesity and diabetes management.
Biochemistry and physiology of the human oxytocin system
Oxytocin in humans
Oxytocin belongs to the category of neurohypophysial hormones that are nonapeptides (oligopeptides comprising nine amino acids) characterised by a disulphide bridge between cysteine residues 1 and 6. This results in a peptide with a cyclic hexapeptide ring structure and a highly flexible C‐terminal α‐amidated three‐residue tail. The isoleucine at position 3 and leucine at position 8 differentiates oxytocin from vasopressin, another neurohypophysial hormone with a similar primary structure, and it is generally believed that differences in polarity of the amino acid residues at these positions enable the specific binding of oxytocin and vasopressin to their respective receptors 20. Vasopressin and oxytocin are produced by specific neurons in the hypothalamo‐neurohypophysial system 21. Oxytocin is co‐synthesised with neurophysin I, a 93–95 residue disulphide‐rich protein that ensures proper targeting, packaging and storage of oxytocin within the granula before release into the bloodstream. The two peptides are complexed in a 1:1 ratio and moved along the axon where they are eventually stored in the axon terminals until neural inputs elicit their release. The protonated α‐amino group (Cys‐1) in oxytocin is the principal binding residue for neurophysin; the complex is held together by electrostatic and hydrogen bonds. Due to the binding dependence on amino group protonation (pKa ~6.4), oxytocin and neurophysin are kept tightly bound in the neurosecretory granula that has an acidic environment (pH ~5.5). The dissociation of the complex is facilitated by the change in pH as neurosecretory granules are released into the plasma (pH ~7.4).
External to the hypothalamus, oxytocin gene expression has been detected in a wide range of cells and tissues (Table 1). In humans, estimates of median plasma half‐life of oxytocin range from 3.2 min after a single 2 U bolus injection to 4.8 min during a 500 mU min−1 infusion 22. Oxytocin is rapidly metabolised in the liver and kidneys, and also in the plasma during pregnancy by secreted oxytocinase (leucyl/cystinyl aminopeptidase), with some degree of metabolism also taking place in the mammary glands 23, 24. In humans, leucyl/cystinyl aminopeptidase activity has also been found to be abundantly expressed in placenta, heart, kidney and small intestine. It has also been detected at lower levels in neuronal cells in the brain, as well as in skeletal muscle, spleen, liver, testes and colon 25, 26, 27. The hydrophobic region near the N‐terminus in the deduced sequence indicates that oxytocinase is a type II integral membrane protein, with northern blots analyses demonstrating that two forms of mRNAs are expressed, likely to be in relation to its multiple functions including peptide degradation (vasopressin, oxytocin, angiotensins), glucose transporter type 4 (GLUT4) receptor translocation and release of its secreted form into the circulation under specific physiological conditions (i.e. second and third trimester and after parturition) 25, 26, 27.
Table 1.
Expression of oxytocin and oxytocin receptors in human cells and tissues
| Cell or tissue | Oxytocin mRNA/co‐localization with neurophysin | Oxytocin receptor mRNA/protein expression | Reference |
|---|---|---|---|
| Reproductive system | |||
| Amnion | + | + | 144, 145 |
| Chorion | + | + | 144, 145 |
| Decidua | + | + | 144, 145 |
| Endometrium | + | 146 | |
| Epididymis | + | + | 147 |
| Foreskin fibroblasts | + | 148 | |
| Mammary tissue | + | 149, 150 | |
| Myometrium | + | + | 145, 146, 150, 151, 152 |
| Ovary (corpus luteum) | + | + | 28, 153, 154, 155, 156 |
| Placenta | + | + | 152, 157 |
| Prostate | + | + | 38, 147, 158 |
| Testis | + | + | 38, 147, 154, 159, 160 |
| Brain regions | |||
| Central structures | + | + | 161 |
| Cerebellum | + | + | 161 |
| Frontal lobe | + | + | 161 |
| Insula and CC | + | + | 161 |
| Occipital lobe | + | + | 38, 161 |
| Parietal lobe | + | + | 38, 161 |
| Temporal lobe | + | + | 161 |
| Other peripheral tissue and organs | |||
| Adrenal gland | + | 159 | |
| Caval vein | + | 38 | |
| Gastrointestinal tract | + | + | 38, 162 |
| Heart | + | 38 | |
| Kidney | + | 38 | |
| Larynx | + | 38 | |
| Lungs | + | 38 | |
| Pancreas | + | 38 | |
| Skeletal muscle | + | 38 | |
| Skin epidermis | + | + | 163 |
| Spleen | + | 38 | |
| Thymus | + | 164, 165 | |
| Thyroid | + | 38 | |
| Trachea | + | 38 | |
| Vascular endothelium | + | 166 | |
| Primary cells | |||
| Aortic endothelial cells | + | 68 | |
| Aortic smooth muscle cells | + | 68 | |
| Dermal fibroblasts | + | + | 163 |
| Human airway smooth muscle cells | + | 38 | |
| Human lymphocytes | + | 167 | |
| Human myoblasts | + | + | 57 |
| Human peripheral blood mononuclear cells | + | 168 | |
| Human primary fibroblastic cells (HFF) | + | 148 | |
| Human primary trophoblasts | + | 152 | |
| Keratinocytes | + | + | 163 |
| Osteoblasts | + | + | 88, 169, 170 |
| Osteoclasts | + | 171 | |
| Cell lines | |||
| Breast cancer (MCF7) | + | 146, 149 | |
| Human 1.1B4 clonal β‐cells | + | 83 | |
| Human breast BT20 cells | + | 149 | |
| Human breast Hs578T cells | + | 149, 172 | |
| Human endometriosis cell line (12Z) | + | 146 | |
| Human granulosa‐lutein cell line (HGL5) | + | 173 | |
| Human osteosarcoma cell lines (U2OS, MG63, OS15 and SaOS2) | + | 148 | |
| Human THP‐1 monocytes/macrophages | + | 68 | |
| Human umbilical vein endothelial cells (HUVEC) | + | 167 | |
The human oxytocin receptor
Molecular cloning experiments have confirmed that the human OTR is a member of the rhodopsin‐type class I G‐protein‐coupled receptor superfamily, characterised by seven hydrophobic transmembrane α‐helices joined by alternating intracellular and extracellular loops, with an extracellular N‐terminal domain and a cytoplasmic C‐terminal domain 9, 20, 28. The human OTR is 388 amino acids long with a relative molecular mass (Mr) of 42,716. Oxytocin binding involves the extracellular N‐terminus E1 and the extracellular loops E2 and E3 of the OTR. Specifically, Arg 34 within the distal segment of the N‐terminal domain has been shown in mutagenesis studies to be important for high‐affinity oxytocin binding. The human OTR also contains a number of sites for possible post‐translational modifications: three consensus N‐glycosylation sites in the N‐terminal region and possible phosphorylation sites in the third cytoplasmic loop and in the C‐terminal region of the receptor 28. Site‐directed mutagenesis studies suggest that post‐translational modifications at the N‐glycosylation sites do not influence ligand affinity or receptor trafficking 29. In the human myometrium, binding of oxytocin to its receptor leads to the activation of heterotrimeric G‐proteins 30 resulting in the activation of phospholipase C, which hydrolyses phosphatidylinositol 4,5‐bisphosphate (PIP2) to diacylglycerol and inositol triphosphate (IP3). IP3 triggers the increase in cytosolic Ca2+, which binds to calmodulin and activates the myosin light‐chain kinase for myometrial muscle contraction. Additionally, Ca2+‐mediated diacylglycerol activation of protein kinase C (PKC) forms an integral part of the intracellular Ca2+ oscillator mechanisms, generating phasic contractions in pregnant myometrial tissue, as well as inflammatory mediators associated with the onset of labour 31, 32. Both oxytocin and vasopressin are OTR agonists, with the former having approximately 10‐fold higher affinity for the human OTR 9. Further, a 100‐fold higher concentration of vasopressin in relation to oxytocin is needed to elicit the same electrophysiological responses in ligand specificity studies 28. Mounting evidence suggests that the human OTR binding site for antagonists is different from that of agonists: studies with chimeric receptors showed that the binding site for OTR antagonists was formed principally at transmembrane domains 1, 2, and conditionally at domain 7, with no binding activity at the N‐terminus 33, 34. The human OTR is also known to exist as homodimers or heterodimers with vasopressin receptor 1a (V1a) and vasopressin receptor 2 (V2), and oligomerization of the human OTR has also been demonstrated 35, 36. The specific functions of dimerization or oligomerization of the human OTR have not been clearly elucidated, although it has been suggested that targeting of dimeric human OTR may induce greater signalling potency and that oligomerization of human OTR may mediate possible crosstalk between the protomers leading to negative and positive cooperative ligand binding 35, 37.
The OTR is expressed ubiquitously in human cells and tissues (Table 1). Evidence suggests that the human OTR gene is differentially expressed in various tissues 38 and may be subject to further variation by external regulatory agents such as hormones and mediators of inflammation. In uterine myometrial cells, OTR expression is increased by oxytocin and oestradiol, and this expression rises significantly more with both hormones combined 39. Data from transfection studies also suggest progesterone may inhibit binding of oxytocin to human OTR 40. OTR expression is also reduced by proinflammatory cytokines tumour necrosis factor‐alpha and interleukin‐13 in airway smooth muscle cells, while interferon‐alpha, interleukin‐6 and interleukin‐1β have been shown to down‐regulate OTR in human myometrial cells 38, 41, 42. Lysophospholipids have also been linked to increased translation of OTRs, supporting a possible role for high lysophospholipase expression in the human amnion during OTR‐mediated initiation of labour 43, 44.
Cellular mechanisms of oxytocin in glucose metabolism
Glucose uptake in skeletal muscle
Oxytocin was shown to increase glucose uptake in murine C2C12 myoblasts, through the stimulation of intracellular Ca2+ release and activation of AMP‐activated protein kinase in a time‐dependent and dose‐dependent manner 45, although it could not be concluded whether these effects were associated with specific binding of oxytocin to the OTR. Down‐regulation of fatty acid binding protein 4 (Fabp4, also known as aP2) mRNA expression was also observed with oxytocin treatment of murine C2C12 myotubes 46. Although no direct links have been established between Fabp4 and glucose metabolism in muscle cells, increases of Fabp4 mRNA expression in skeletal muscle cells (bovine and murine) have been linked to the inhibition of myoblast differentiation as well as transdifferentiation of myotubes and muscle‐derived stem cells into adipocyte‐like cells 47, 48. Ectopic Fabp4 expression in muscle‐derived stem cells also caused an up‐regulation of stanniocalcin‐2 expression that is linked to reduced skeletal muscle growth 47, 49. Oxytocin‐mediated down‐regulation of Fabp4 gene expression may therefore have important implications for the maintenance of optimal muscle‐adipose ratios for adequate glucose disposal and muscle glycogen storage, as well as preventing insulin resistance resulting from lipid infiltration of skeletal muscle. Macrophage recruitment in the obese state is thought to result in impaired triglyceride (TG) deposition and increased lipolysis in adipose tissue 50, leading to excess circulating TG and NEFA levels that may disrupt mitochondrial oxidative phosphorylation and insulin‐stimulated glucose transport in muscle 51. Reduction of macrophage infiltration in adipose tissue was observed in obese mice treated with oxytocin, and this suggests a role for oxytocin in reducing NEFA‐mediated insulin resistance in obesity 51, 52, 53.
Oxytocin may also directly influence glucose metabolism through promotion of muscle cell differentiation. Increased oxytocin production has been associated with the anabolic effects of steroids in bovine and ovine skeletal muscle 54, 55. In murine studies, subcutaneous injections of oxytocin improved muscle regeneration in old mice subjected to cardiotoxin muscle injury, to a level comparable with that of young mice 56. Further intracellular signalling studies in human and mice myoblasts linked the observations to oxytocin‐mediated promotion of myogenic cell proliferation through extracellular signal‐regulated kinase 1/2 phosphorylation in the mitogen‐activated protein kinase/extracellular signal‐related kinase pathway 2, 56. Binding of oxytocin and the OTR‐selective agonist [Thr 4, Gly 7] OT to the OTRs in human myoblast cultures was also shown to promote myoblast fusion 57, substantiating prior observations of oxytocin‐mediated myogenic differentiation in murine L6‐C5 skeletal muscle cells 58. An oxytocin‐induced augmentation of muscle mass would, in turn, directly affect glucose uptake and insulin sensitivity.
Glucose uptake in adipose tissue
Oxytocin receptors have been detected in both human and murine adipocytes 9, 59, 60. Oxytocin was shown to elicit transient increases in intracellular Ca2+ and stimulate PKC activity 61, 62, which increases glucose uptake in murine adipocytes, and has been reported to be similar to phorbol esters in its ability to initiate glucose transporter translocation (presumably GLUT1 and GLUT4), without the glucose transport activity of insulin 62, 63, 64. Again, it is unclear if these effects were the result of specific binding of oxytocin to the OTR. In further evidence of its insulin‐like activity in adipose tissue, oxytocin stimulated glucose oxidation via enhancement of pyruvate dehydrogenase activity in murine epipidymal adipocytes 64. To add, this process was found to be independent of insulin‐mediated glucose transport. Glucose oxidation was augmented with oxytocin to a lesser extent compared with insulin stimulation 64.
Nevertheless, no evidence for an oxytocin–PKC interaction or an associated effect on glucose uptake in human adipocytes was found. Cortright et al. found that inhibition of PKC activity enhanced glucose transport in human adipocytes above that of controls incubated with insulin alone 65. However, 2 μM of the bisindolylmaleimide GF109203X was used to inhibit PKC in adipocytes, which is above the established IC50 values for most PKC isoforms, but well below the IC50 value for the PKCζ isoform (~6 μM) 65, 66. Therefore, it is likely that not all PKC isoforms were sufficiently inhibited in this study. As PKCζ has been shown to be required for insulin‐mediated glucose transport in cultured human adipocytes 67, the question of whether oxytocin‐mediated PKC activation augments glucose uptake in human adipocytes remains to be clarified. Furthermore, macrophage infiltration was reduced in the epididymal white adipose tissue (WAT) of obese mice treated with oxytocin over 2 weeks 52. To add, oxytocin decreased both superoxide production and release of proinflammatory cytokines from human endothelial cells and THP‐1 monocytes and macrophages 68. Oxytocin‐mediated reductions in macrophage localization and cytokine production may alleviate their inhibitory effects on GLUT4‐mediated glucose transport and prevent amplification of proinflammatory response within adipose tissue 50, 51. It is not currently known if oxytocin or OTR signalling increases brown adipose tissue (BAT) glucose uptake – central administration of oxytocin was recently associated with increased BAT thermogenesis but did not alter plasma glucose levels in murine studies 69. In addition, antagonism of the adenosine receptor A1R in oxytocin neurons in the paraventricular nucleus by central administration of caffeine was recently associated with increased Ucp1 and PGC1β expression in mouse BAT, as well as increased energy expenditure 70. More investigation is needed to determine the role of oxytocin in cell differentiation and substrate metabolism in BAT.
Hepatic, pancreatic and whole‐body glucose metabolism
In lean control mice, chronic oxytocin treatment induced a concomitant increase in mRNA expression for gluconeogenesis (Glucose‐6‐phosphatase catalytic subunit – G6pc, Fructose‐1,6‐bisphosphatase 1 – Fbp1 and Phosphoenolpyruvate carboxykinase – Pck1) and glycolysis (glucokinase – Gck, ATP‐dependent 6‐phosphofructokinase – Pfkl and Pyruvate kinase – Pklr) in the liver, suggesting increased futile cycling (Table 2). A trend for lowered glycaemia was observed in pyruvate tolerance tests in the same mice that had undergone oxytocin treatment; the concomitant increase in the mRNA expression of hepatic glycogen phosphorylase (Pygl) with no change in glycogen synthase expression corroborated the decrease in liver glycogen content 52. Taken together, the data suggest oxytocin treatment enhanced net hepatic glucose oxidation. In vitro, reduced glycogen synthase activity and increased glycogen phosphorylase activity in oxytocin‐treated rat hepatocytes was reported 71.
Table 2.
Changes in hepatic glucose metabolism gene or protein expression in response to oxytocin administration
| Glucose metabolism gene/protein | Hepatocytes/perfused liver | Murine lean | Murine obese |
|---|---|---|---|
| Glycolysis | |||
| ATP‐dependent 6‐phosphofructokinase (Pfkl) | Up 52 | No change 52 | |
| Glucokinase (Gck) | Up 52 | No change 52 | |
| Pyruvate kinase (Pklr) | Up 52 | No change 52 | |
| Glycogenesis | |||
| Glycogen phosphorylase (Pygl) | Up 71, † | Up 52 | No change 52 |
| Glycogen synthase (Gys2) | Activity inhibition 71, † | No change 52 | No change 52 |
| Gluconeogenesis | |||
| Fructose‐1,6‐bisphosphatase 1 (Fbp1) | Up 52 | No change 52 | |
| Glucose‐6‐phosphatase (G6pc) | Up 52 | Up 52 | |
| Phosphoenolpyruvate carboxykinase (Pck1) | Up 52 | No change 52 | |
Except for Ariño et al. where gender was unspecified, all murine models represented are male.
Increased adipose tissue inflammation in obesity also enhances fatty acid delivery to the liver and hepatic gluconeogenesis, thereby promoting fasting and postprandial hyperglycemia that leads to disruption of glucose homeostasis 51. Although no direct links have been established, the reduction of macrophage infiltration in adipose tissue observed in obese mice treated with oxytocin suggests a role for oxytocin in reducing NEFA‐mediated up‐regulation of gluconeogenesis in Type 2 diabetes mellitus (T2DM) and obesity 52.
Oxytocin appears to directly modulate pancreatic function. Oxytocin was shown to stimulate insulin secretion in isolated mouse pancreatic islets independent of glucose concentration 72. The mechanisms by which oxytocin modulates murine insulin secretion was shown to be two‐pronged: centrally via vagal cholinergic neurons innervating β‐cells 73 and peripherally by stimulation of phosphoinositide turnover and activation of PKC in pancreatic β‐cells 72. Oxytocin was also shown to increase both insulin and glucagon secretion in vivo as well as in situ and appears to have a greater effect on glucagon secretion than on insulin secretion (and to a much greater extent in insulin‐deficient diabetic rats), which is in line with the observed increase in hepatic glucose output in canine studies as well as in oxytocin‐infusion studies in men 74, 75, 76. The extent to which the increase in insulin secretion is attributed to a direct effect of oxytocin or to indirect stimulation by glucagon (which can be an insulin secretagogue itself) remains unclear. Interestingly, perfusion studies in the rat pancreas also showed oxytocin mediates insulin release from β‐cells through binding with vasopressin receptor 1b (V1b), but not by V1a or OTR 77.
The observed effects of oxytocin on peripheral systems suggest that the oxytocin system may have a significant role in whole body glucose metabolism. In support of this hypothesis, decreased insulin sensitivity and impaired glucose tolerance were found in oxytocin‐deficient (Oxt −/−) and high fat diet‐fed OTR‐deficient (Oxtr −/−) mice 78, 79. In addition, peripheral oxytocin administration was shown to improve insulin sensitivity as well as glucose tolerance in mice maintained on standard and high‐fat diets, as well as in obese diabetic (db/db) mice 80, 81, 82, 83, 84. However, in a study conducted in obese (ob/ob) mice, 2 weeks of chronic treatment with oxytocin was associated with deteriorations in glucose and insulin tolerance. This was attributed to the oxytocin‐induced increase in corticosterone levels that enhanced hepatic gluconeogenesis without changing hepatic glycogenolysis and glycolysis in the leptin‐deficient mouse model – an extreme model of obesity 52. The data, however, are inconclusive in humans. Improvements in glucose tolerance, lowering of postprandial plasma glucose and insulin concentrations have been reported in subjects with normal weight and obesity who were given oxytocin 11, 13, 14, 85, 86. In contrast, increases in plasma glucose and hepatic glycogenolytic activity concurrent with an absence of effects on peripheral insulin sensitivity have also been reported 76 (see: VI. Therapeutic role of oxytocin in human clinical trials).
Oxytocin and lipid metabolism
Adipogenesis
Oxytocin receptor mRNA is highly expressed in mouse adipocytes and increased during differentiation 87. The data on oxytocin effects on adipocyte differentiation appear divided. In one study, both oxytocin and carbotocin (a stable oxytocin analogue) dose‐dependently inhibited adipocyte differentiation in human multipotent adipose‐derived stem cells 88. It also reduced Fabp4 mRNA expression and adipocyte number in the humerus of ovariectomised mice 88.
However, human fat cells were shown to increase intracellular H2O2 production in response to oxytocin, and although direct associations have not been established, multiple lines of evidence suggest increased H2O2 initiates and drive adipocyte differentiation 64, 89, 90. In support of oxytocin's pro‐adipogenic effects, rises in the mRNA levels of Fabp4, Pparg (peroxisome proliferator‐activated receptor gamma), Glut4, Adipoq (adiponectin) and Lep (Leptin) in the epididymal WAT of oxytocin‐treated (2 weeks) rats were reported 91. Expression of endothelial marker Pecam1 also increased, indicating enhanced angiogenesis 91. To add, the average diameter of epididymal adipocytes decreased and this coincided with increased protein content in epididymal WAT without alterations in WAT mass. Similar observations in adipocyte size were made in mesenteric and epididymal fat pads from oxytocin‐treated (2 weeks) diet‐induced obese (DIO) mice 80. The discrepancy in results could be attributed to the longer treatment duration in the ovariectomised mice, in addition to the gender of rodents used (female mice in Elabd et al.; male rats in Eckertova et al.; male mice in Maejima et al.) and differences in fat depots studied, which could affect adipocyte turnover rate. Furthermore, increases in intracellular Ca2+ appear to inhibit early WAT differentiation and drive differentiation at later stages and could therefore confound direct comparison of data 53, 92. Further studies should be undertaken to confirm the chronic effects of oxytocin on adipogenesis.
Lipogenesis, lipolysis and β‐oxidation
Oxytocin increases fatty acid synthesis in isolated rat adipocytes 93 and, like insulin, is linked to the activation of pyruvate dehydrogenase, a rate‐limiting step in fatty acid synthesis from endogenous substrates and glucose 64. However, the rate at which fatty acid synthesis occurs with oxytocin is reduced compared with insulin 93. Oxytocin treatment of 3T3‐L1 adipocytes was also associated with an increase in lipoprotein lipase (Lpl) mRNA expression 82. Taken together, the data suggest a role for oxytocin in the regulation of adipocyte fatty acid availability from endogenous synthesis as well as extracellular uptake. In the same in vitro study of 3T3‐L1 adipocytes, oxytocin treatment also increased mRNA expression of hormone sensitive lipase (Hsl; or Lipe), patatin‐like phospholipase domain containing 2 (Pnpla2), acyl‐CoA oxidase 1 (Acox1), peroxisomal bifunctional enzyme (Ehhadh), medium chain specific acyl‐CoA dehydrogenase (Acadm), uncoupling protein 3 (Ucp3) and peroxisome proliferator‐activated receptor alpha (Ppara), suggesting a role for oxytocin in augmenting lipolysis and β‐oxidation in adipose tissue (Table 3) 82.
Table 3.
Changes in lipid metabolism gene expression in WAT in response to oxytocin administration
| Lipid metabolism gene | Murine adipocyte (in vitro) | Murine lean | Murine obese |
|---|---|---|---|
| Lipid synthesis in WAT | |||
| Acetyl‐coenzyme A carboxylase α (Acaca) | No change 82 | ||
| Acyl‐CoA desaturase 1 (Scd1) | Up 82 | Up 82 | Up 82 |
| Diacylglycerol O‐acyltransferase homologue 1 (Dgat1) | No change 82 | ||
| Fatty acid transporter (Cd36) | Up 82 | ||
| Fatty acid synthase (Fasn) | No change 52 | Down 52No change 82 | |
| Glucose transporter type 4 (Glut4) | Up 91 | ||
| Lipoprotein lipase (Lpl) | Up 82 | No change 52 | Down 52Up 82 |
| Peroxisome proliferator‐activated receptor gamma (Pparg) | Up 91 | ||
| Phosphoenolpyruvate carboxykinase (Pck1) | No change 52 | Up 52 | |
| Pyruvate dehydrogenase | Up 64 | ||
| Lipolysis in WAT | |||
| Fatty acid‐binding protein (Fabp4) | Up 91 | ||
| Hormone sensitive lipase (Lipe) | Up 82 | No change 52 | Up† 52Up 82 |
| Patatin‐like phospholipase domain containing 2 (Pnpla2) | Up† 82 | Up 82 | |
| β‐oxidation in WAT | |||
| Acyl‐CoA oxidase 1 (Acox1) | Up 82 | Up 82 | Up 82 |
| Leptin (Lep) | Up 91 | ||
| Medium chain acyl‐CoA dehydrogenase (Acadm) | Up 82 | Up 82 | Up 82 |
| Peroxisomal bifunctional enzyme (Ehhadh) | Up 82 | Up 82 | Up 82 |
| Peroxisome proliferator‐activated receptor alpha (Ppara) | Up 82 | No change 82 | |
| Uncoupling protein 3 (Ucp3) | Up† 82 | Up 82 | Up 82 |
Denotes trend; not statistically significant. All murine models represented in Table 3 are male. WAT, white adipose tissue.
In studies of lean mice, Altirriba et al. showed that oxytocin did not change Lpl mRNA expression in epididymal WAT 52. Oxytocin also did not modify the mRNA expression of fatty acid synthase (Fasn) and phosphoenolpyruvate carboxykinase (Pck1) in epididymal WAT. However, Eckertova et al. observed an up‐regulation of Fabp4, Lep, Pparg and Glut4 mRNA expression in the epididymal WAT of normal Wistar rats, suggesting oxytocin may enhance adipocyte maintenance and glucose uptake in adipose tissue 91. To add, Hsl mRNA expression was also unaffected in the epididymal WAT of lean mice, suggesting no effect on lipolysis. Oxytocin did not affect fat mass in either study. Oxytocin treatment also had no effect on macrophage localization in the WAT of lean mice 52. One may speculate that WAT receptor sensitivity to oxytocin may be enhanced in obesity or that oxytocin may have discernable effects only on macrophages polarised towards the classically activated, proinflammatory (M1) phenotype. Further studies will be needed to ascertain the mechanisms.
Oxytocin regulation of lipid metabolism in the obese/diabetic state appears to be different. In DIO mice, Lpl and Cd36 mRNA expression in epididymal WAT were up‐regulated by oxytocin treatment 82, substantiating the lowered plasma TG concentrations. Oxytocin did not modify mRNA expression of acetyl‐coenzyme A carboxylase α (Acaca), Fasn and diacylglycerol O‐acyltransferase homologue 1 (Dgat1) in epididymal WAT, enzymes which are involved in lipogenesis and TG storage, in the DIO mice 82. Oxytocin increased expression of lipolysis enzymes, such as Hsl and Pnpla2, and β‐oxidation genes Acox1, Ehhadh, Acadm and Ucp3 82 in the epididymal WAT of DIO mice. Accordingly, oxytocin treatment of DIO mice led to decreased TG and glycerol content in epididymal WAT and did not change NEFA content. In ob/ob mice, Lpl and Fasn mRNA expression in epididymal WAT were down‐regulated 52. Pck1 mRNA was also increased in the epididymal WAT of the oxytocin‐treated ob/ob mice, which may possibly be attributed to an increase in expression of Cd36, suggesting increased fatty acid re‐esterification and increased futile cycling of TG 52.
Overall, the increase in lipolysis enzymes together with TG and fatty acid uptake, with no concurring change in lipogenesis (and TG storage) and no net change in NEFA release in murine adipocytes 93, mice 52, 82 as well as in humans 85 (slight decrease) suggests a possible role for oxytocin in futile metabolic cycling that could help ameliorate obesity by promoting the use of fat as energy substrate (thermogenesis) in WAT and preventing NEFA‐mediated lipotoxic damage 82, 94. NEFAs may also be channelled to β‐oxidation, which could underlie the decreased respiratory quotient (RQ) observed in oxytocin‐treated DIO rodents and healthy men 13, 80, 82.
Interestingly, central oxytocin infusion did not modify lipid metabolism genes in murine skeletal muscle and liver 82, suggesting that the principal action of oxytocin is on adipose tissue. To corroborate, the expression of OTRs in adipose tissue was documented to be ninefold greater than that of skeletal muscle in lean rats 60 and comparable with classical oxytocin target tissues 52. Oxytocin‐mediated reduction of WAT macrophage infiltration 52 may also remediate obesity‐associated lipolysis in WAT and increase hepatic TG synthesis from enhanced fatty acid esterification 51. With respect to adipose tissue depots, continuous oxytocin administration for 2 weeks did not lead to any reductions in retroperitoneal or epididymal WAT mass in normal rats 91. In DIO mice, however, continuous oxytocin infusion for 10–13 days reduced subcutaneous and visceral fat mass as well as adipocyte size 80, 95, adding to evidence that oxytocin may have greater effects in the obese state compared with the lean state.
Alterations in the oxytocin system in obesity and diabetes
Obesity, diabetes and metabolic syndrome
Data regarding the oxytocin system in rodent models of obesity and human subjects with obesity appear divided. Plasma oxytocin levels were reported to be decreased in high fat diet‐fed mice (mice developed glucose intolerance, profound insulin resistance and leptin resistance), and oxytocin release in the paraventricular nucleus of these mice was shown to be blunted 96. Plasma oxytocin concentration was also lower in obese Zucker (fa/fa) rats, although hypothalamic oxytocin gene expression was not changed. Further, gene expression of synaptotagmin 4 (Syt4), a negative regulator of oxytocin exocytosis, and Fto, a proposed transcription cofactor for oxytocin expression, was also not altered in the fa/fa rats, suggesting that lowered plasma oxytocin concentration was not attributed to reduced oxytocin release 60. Both OTR mRNA and protein were significantly up‐regulated in the epididymal WAT of the fa/fa rats, although this could be attributed to OTR expression with higher macrophage localization. However, despite a several‐fold increase in OTR mRNA, there was substantially reduced OTR protein expression in skeletal muscle compared with lean controls. It was concluded that increased oxytocinase activity may have been responsible for the lowering of plasma oxytocin concentrations, as hepatic oxytocinase activity was higher in fa/fa rats than in the lean controls. Oxytocinase activity was also increased in adipose tissue and decreased in skeletal muscle of fa/fa rats, while plasma oxytocinase activity was not different when compared with controls 60.
In human studies, mean overnight serum oxytocin levels were reported to be higher in overweight women compared with healthy normal‐weight controls by Schorr et al. Similar findings were reported by Stock et al. in men and women with obesity 97. Mean oxytocin concentration was positively associated with body mass index (BMI), fat mass and lean mass in premenopausal women across a wide body weight range 98. In contrast, metabolic syndrome – the clustering of cardiometabolic risk factors including central obesity, insulin resistance, dyslipidaemia and hypertension – has instead been associated with reduced fasting serum oxytocin in larger scale mixed gender studies by Yuan et al. 99. To add, fasting serum oxytocin concentration has been negatively associated with BMI, waist circumference, glycosylated haemoglobin (HbA1c), fasting glucose and postprandial glucose, fasting and postprandial insulin, TG, the Homeostasis Model Assessment of Insulin Resistance (HOMA‐IR) score and C‐reactive protein in a study of subjects with normal weight and subjects with overweight/obesity 100. In another study of subjects with and without the metabolic syndrome (normal weight and overweight), fasting serum oxytocin was also negatively associated with BMI, waist circumference, HbA1c, fasting glucose and postprandial glucose, postprandial insulin, TG, HOMA‐IR score, C‐reactive protein and positively associated with high molecular weight adiponectin 99. The discrepancy in these observations could be attributed to differences in subject characteristics (healthy lean vs. overweight/obese, with or without metabolic syndrome and diabetes), as well as differences in assay method used for measuring oxytocin. Serum oxytocin concentrations in the study by Schorr et al. were reported to be two orders of magnitude higher than plasma oxytocin values reported from studies using radioimmunoassay (RIA) with extraction 101, while Yuan et al. and Qian et al. combined serum extraction with enzyme immunoassay and reported serum values that were more similar to values obtained from RIA with extraction. The measurement of oxytocin in human plasma or serum is still a controversial subject – an optimal method for measuring oxytocin has not yet been established, and there appears to be a large discordance in the reporting of plasma or serum oxytocin concentrations. While absolute levels of oxytocin cannot be compared between studies, there is no reason to believe that data derived from any of these methods are not valid, insofar as detecting between‐group differences, correlations with relevant endpoints and relative changes with interventions.
The apparent discrepancy in the results also suggests that abnormal glycaemia may be a modifying factor in the oxytocin–obesity relationship. The fasting oxytocin concentrations of subjects with metabolic syndrome who also had drug‐naive prediabetes or diabetes were approximately half that of control subjects with metabolic syndrome but had normal glycaemia and HbA1c 102. In African–American men with overweight and obesity, subjects in the highest tertile for fasting urinary oxytocin – a proxy for peripheral oxytocin levels – had lower weight, HbA1c, oral glucose tolerance test area‐under‐the‐curve insulin and C‐peptide, and liver function enzymes compared with subjects in the lowest tertile. The highest tertile for fasting urinary oxytocin was also associated with fewer diagnosed T2DMs and lower metformin use 103. However, the validity of urinary oxytocin as a proxy for peripheral oxytocin concentrations is still a matter of dispute 104, 105. In Qian et al., fasting oxytocin concentration in overweight subjects (BMI ≥25 kg m−2) with normal glucose tolerance was not different compared with normal‐weight subjects with normal glucose tolerance 100, again suggesting that glucose homeostasis is an important factor modifying the obesity–oxytocin relationship. Fasting oxytocin levels in T2DM subjects (both normal weight and overweight) were also significantly decreased when compared with subjects with normal glucose tolerance 100.
In older men (50–85 years of age), however, Szulc et al. found an association between increased fasting serum oxytocin levels and higher odds of metabolic syndrome after adjusting for confounders 106. This alone would suggest that the oxytocin system may be altered with age. However, the storage period of the samples analysed by Szulc et al. (18 years) was a significant study limitation. On the other end of the age spectrum, fasting oxytocin concentration was lower in children and adolescents with obesity compared with normal‐weight controls, although the obese group also had less favourable parameters for glucose homeostasis (e.g. HOMA‐IR in the obese group was ~threefold greater than in the control group). Therefore, it was not clear if the observed difference in oxytocin was due to obesity per se. In fact, subgroup analyses in the same study revealed that the children and adolescents with obesity and metabolic syndrome had significantly lower fasting oxytocin compared with those that had obesity without metabolic syndrome 107, supporting the notion that dysglycaemia may be a key factor in the lowering of plasma oxytocin concentrations in obesity.
Regarding ageing, studies indicate that production of oxytocin in response to challenging stimuli is not different in elderly men compared with younger men 108. A post‐mortem study on oxytocin neurons in the human paraventricular nucleus showed that oxytocin neuron size (an indicator of neuron activity) does not change with age 109. It is likely, therefore, that the increase in oxytocin in the plasma of older subjects with metabolic syndrome functions as a compensatory mechanism for the maintenance of metabolic homeostasis where ageing‐related deficits may have developed in classical metabolic regulatory systems. While human data are not available, reduced central oxytocin binding has been reported in aged rat brains 110, while reductions in OTR expression has been observed in muscle satellite cells of older mice 56. The oxytocin response to stress in rats is also blunted with age 111. One can speculate that changes in cholesterol trafficking and compartmentalization may have a part to play. Briefly, cholesterol is required for OTR stabilization and high affinity binding on plasma membranes, thus activation of the OTR may depend on intracellular cholesterol distribution in cells. In adipocyte hypertrophy, up‐regulation of sterol regulatory element‐binding protein 2 produces ‘cholesterol‐depleted’ plasma membranes 112. Because OTR activity is cholesterol‐dependent, increases in the number of hypertrophic adipocytes and lower adipocyte turnover associated with ageing suggest that the OTRs in adipose tissue of older subjects with obesity may have lower binding activity and may be more prone to thermal or proteolytic degradation 9.
Therapeutic role of oxytocin in human clinical trials
Oxytocin system in energy balance
The anorexigenic effect of oxytocin has been extensively documented in animals 12, 52, 80, 82, 83. However, definitive studies on the anorexigenic effects of oxytocin in humans are still lacking in light of discrepant results. In a study consisting of healthy men across the weight spectrum, 24 international units (IU) of intranasal oxytocin acutely reduced overall caloric intake with a preferential reduction in fat intake. Fasting levels of the anorexigenic hormone cholecystokinin was also increased without affecting appetite or other appetite‐regulating hormones (leptin, active ghrelin and peptide YY) 13. In contrast, 40 mU min−1 intravenous oxytocin infusion in 10 healthy men and women reduced satiety levels without affecting the volume of a liquid meal consumed 113. In addition, Ott et al. found no acute effects on total calorie intake in 20 healthy, normal‐weight men administered intranasal oxytocin (24 IU) and provided a buffet in the fasted state 11. The contrasting outcomes could be explained by the different routes of oxytocin administration studied (intravenous vs. intranasal). The distinct central and peripheral effects associated with both routes of administration have not been clearly elucidated. Specifically, it has been suggested that intravenous oxytocin does not cross the blood–brain barrier in sufficient quantities to mediate the central effects of oxytocin 114, and the existence of a feed‐forward mechanism that increases endogenous oxytocin secretion has been disputed 115. Endogenous peripheral and central levels of oxytocin were also shown to be largely dissociated, suggesting that plasma oxytocin concentrations may be a poor proxy for cerebrospinal fluid (CSF) oxytocin concentrations 114. However, when labelled oxytocin was administered exogenously (IV and intranasal) to rhesus macaques, labelled oxytocin concentrations increased in both CSF and plasma and were correlated with each other, suggesting similar mechanisms might be operating in humans 115. Of interest, Sabatier et al. also previously showed that when α‐Melanocyte‐stimulating hormone (α‐MSH) melanocortin‐4 receptors (which mediate the role of α‐MSH in feeding behaviour) on oxytocin neurons are stimulated, dendritic oxytocin release is activated in hypothalamic neurons while oxytocin secretion into the circulation via the posterior pituitary is simultaneously inhibited 116, suggesting other factors should be considered when evaluating the relationships between oxytocin (be it central or peripheral) and feeding behaviour.
Another likely reason could be that oxytocin may have a more discernible effect in persons with obesity compared with normal‐weight persons, because the inhibitory effect of oxytocin on food intake was shown to be greater in subjects with obesity compared with normal‐weight controls 14. Other differences, including the control of caloric intake prior to the study visits and method of ad libitum feeding used in the study designs, may also explain the discrepant data.
Although oxytocin did not affect ad libitum food intake in the study by Ott et al., it reduced the consumption of chocolate cookies (palatable food item) provided as a snack, suggesting that oxytocin inhibits reward‐driven but not hunger‐driven feeding. In a replication of the study by Thienel et al., oxytocin markedly reduced both hunger‐driven and reward‐driven food intake in men with obesity in contrast to normal‐weight men, with a greater effect size in snack intake reduction. There was however no effect on preferential macronutrient intake 14. The inhibition of palatable snack intake and concomitant reduction of hormones related to basal corticotropic function and hypothalamic–pituitary–adrenal axis activity strongly suggest a role for oxytocin in suppressing the activity of endocrine stress axes (activation of which increases the salience of pleasurable or compulsive activities, such as the consumption of ‘comfort foods’) 117. Recently, intranasal oxytocin (24 IU) administration in 10 male subjects with overweight and obesity led to bilateral hypoactivation in the ventral tegmental area (associated with hedonic regulation of human appetite) in response to viewing high‐calorie food stimuli 118. Whole‐brain analysis by functional magnetic resonance imaging also showed reduced activation in reward‐related food motivation brain regions in subjects treated with oxytocin. In addition, oxytocin administration was associated with reduced activity in the hypothalamus, a region central to homeostatic control of feeding, in male subjects across the weight spectrum 118, 119. Oxytocin also increased functional magnetic resonance imaging activation in brain regions involved in cognitive control, a process that enables individuals to suppress behavioural impulses to achieve a desired outcome, e.g. dieting. Taken together, the data suggest that the processes leading to hyperactivation of hedonic food motivation pathways, reduced cognitive control and increased hypothalamic activation in obesity could be blunted by oxytocin treatment.
Acute oxytocin administration has been observed to have no effect on basal and postprandial energy expenditure in humans 11, 13, 14, 85. The resting RQ in the fasted state in oxytocin‐treated men across the body weight spectrum was shown to be decreased in a crossover study, concomitant with a decrease in carbohydrate utilization rate and increase in fat oxidation rate 13. Oxytocin was also shown in individuals with obesity to similarly reduce RQ with decreased carbohydrate utilization and increased fat utilization in the fasted state; however, the data did not achieve statistical significance after correcting for reductions in calorie and macronutrient intake 14. The effects of longer term or chronic oxytocin on energy expenditure are currently unknown.
Oxytocin and pancreatic function
The effect of oxytocin on human pancreatic function has been rarely studied. In an early study of young normal‐weight men, oxytocin infusion (0.06 U min−1) did not change basal insulin, glucagon and plasma glucose in the first 10 min of infusion. In response to an intravenous glucose bolus, the oxytocin infusion group showed an increase in insulin production within the first 10 min that returned to control levels in 30 min. Plasma glucagon and glucose response to glucose load were unchanged compared with controls. A lower dose (0.03 U min−1) was not associated with any changes in glucose homeostasis 120. In another study in men of a similar profile, peripheral oxytocin infusion (0.2 U min−1) was associated with an increase of plasma glucagon in the first 30 min and increased above‐basal insulin secretion after 40 min of infusion. Plasma glucose rose in the first 30 min too, which was presumed to be an effect of increased glucagon production. However, hepatic glucose rose transiently independent of changes in glucagon concentration in pancreatic clamp studies, suggesting a direct effect of oxytocin on hepatic glucose production. It was not clear if it was an effect of transient enhancement of glycogenolysis or gluconeogenesis, or induction of hepatic insulin resistance 76.
In contrast, intranasal oxytocin (24 IU) in men of a similar profile did not have any effect on basal insulin or glucagon concentrations within the first hour of administration. The oxytocin group did however show a more rapid rise in serum insulin and blunted peak insulin and C‐peptide responses after a glucose load was given. In the oral minimal model analyses, β‐cell function was significantly enhanced by oxytocin when compared with placebo, reflected by a more than twofold increase in the disposition index of participants (the disposition index evaluates the appropriateness of pancreatic insulin secretion in relation to peripheral insulin sensitivity). Specifically, oxytocin administration was associated with the enhancement of the dynamic response to glucose, which suggested that β‐cells were better able to secrete insulin in response to a rise in circulating glucose concentrations 85.
The discrepant results may be associated with the different routes of administration used. Cerebrospinal fluid and plasma concentrations of exogenous labelled oxytocin delivered intravenously were observed to be higher compared with intranasal administration in primate studies 115. Much higher plasma oxytocin concentrations may be attained by the intravenous route, which may allow oxytocin to interact more quickly and directly with peripheral organs. The observed incongruities may also be attributed to the different oxytocin doses used, although notably, the doses used in the cited studies led to supraphysiological concentrations of oxytocin in the systemic circulation that are typically far above what is needed to exert physiological effects 114. Oxytocin has been shown to bind with low affinity to vasopressin receptors V1a, V1b and V2 77, 121. Therefore, supraphysiological concentrations of oxytocin (which, compared with basal concentrations, are typically four orders of magnitude higher in intravenous administration studies) 114 may lead to competitive inhibition, altering the binding of vasopressin to its specific receptors. Oxytocin may also bind and activate vasopressin receptors given their similar molecular structures 62, 71, 77. Vasopressin has also been shown to increase insulin and glucagon production in β‐cells 77; therefore, more studies regarding the cross‐reactivity of vasopressin receptors, particularly at supraphysiological concentrations of oxytocin, will be needed to enhance our current understanding of oxytocin in glucose homeostasis.
Oxytocin and insulin sensitivity
Insulin sensitivity, at least as far as glucose metabolism is concerned, is typically defined as the ability of insulin to stimulate glucose uptake in peripheral tissues (predominantly muscle) and inhibit glucose production (predominantly liver). Lowering of insulin sensitivity (i.e. insulin resistance) is an important pathophysiological mechanism in the development of obesity‐related diabetes. To date, the effect of pharmacological doses of oxytocin on peripheral insulin sensitivity is still largely unknown. A small improvement in insulin sensitivity assessed by oral minimal modelling was reported in normal‐weight men administered acute intranasal oxytocin (24 IU) 85. However, the relative contribution of glucose uptake and glucose production or glucose effectiveness (i.e. the ability of glucose itself to promote its own uptake) to the improved overall glucose tolerance is unclear as tracer studies were not conducted. In an earlier euglycaemic glucose clamp study, no effect on peripheral insulin sensitivity was reported in men receiving continuous oxytocin infusion (0.2 U min−1) 76. The question of whether oxytocin can be effectively evaluated in studies of insulin sensitivity has to be carefully considered, as oxytocin appears to elicit physiological responses parallel to those of insulin and glucagon 62, 64, 71, 76.
Oxytocin system in weight loss and exercise
Oxytocin administration has been shown in a number of murine studies to induce significant weight loss and attenuate weight gain in both high fat diet‐induced and genetic models of obesity 52, 80, 82. Specifically, oxytocin administration was linked to a significant decrease in fat mass and a concomitant increase in lean body mass independent of changes in food intake 52, 82. However, the effects of oxytocin on weight loss in humans are still unclear. In a pilot study by Zhang et al. 86, four times daily intranasal oxytocin (24 IU) in nine Asian men and women with obesity (BMI ≥28 kg m−2) for 8 weeks led to a mean BMI reduction of 3.2 ± 1.9 kg m−2. The magnitude of weight loss was also greater in subjects with higher degrees of obesity. Oxytocin treatment also led to decreases in waist and hip circumferences. However, it is not known whether the observed effects of oxytocin were associated with reductions of food intake, changes in physical activity or increases in metabolic rate. Further study limitations include small sample size (9 treated and 11 placebo) and poor matching of baseline characteristics. At the time of this review, a few ongoing studies on the effects of longer term administration of oxytocin in adults with obesity have been recorded on http://clinicaltrials.gov (http://clinicaltrials.gov Identifiers: NCT03043053/NCT03119610). It is anticipated that more data on its effects on calorie intake, resting energy expenditure and body composition will soon be available.
Little is still known about the oxytocin system in exercise; most studies in the literature to date were found to have involved trained subjects. Plasma oxytocin concentrations were shown to be heightened following ~60 min of treadmill running at 80% of maximal oxygen uptake (VO2max), albeit only in three of the five trained males tested 122. In contrast, plasma oxytocin concentrations did not change in response to 20–25 min of high‐intensity exercise in trained male cyclists exercising to exhaustion 123, or in healthy females during 20 min of graded exercise up to 90% of VO2max 124. To add, test plasma values for oxytocin were significantly lower in the experienced cyclists compared with sedentary controls, although it was noted that plasma oxytocin concentrations measured in the cyclists and controls were much lower than reference oxytocin values obtained from measurements using RIA with extraction (typically 1–5 pg mL−1) 123. Nocturnal plasma oxytocin concentration was also lower in amenorrheic female athletes compared with non‐athletes; significant positive correlations between plasma oxytocin concentrations and resting energy expenditure, body fat and hormones regulating energy expenditure and satiety (irisin and PYY) were also found in female athletes 125, 126. In a recent study of untrained, nondiabetic women with obesity, 10 weeks of low frequency, low‐intensity treadmill sessions did not have any effect on fasting plasma oxytocin concentrations 127. Collectively, it would suggest that plasma oxytocin is unaffected by exercise, although further studies will be needed to establish the relationship between chronic exercise and plasma oxytocin.
In pharmacological trials, 24 IU of intranasal oxytocin attenuated the rise in cortisol levels in response to 1 h of vigorous (70% of maximum heart rate) treadmill running 128. However, this effect was not observed with a higher dose (48 IU). In a separate study, adrenocorticotropic hormone (ACTH) and cortisol responses to graded exercise testing were also significantly reduced by intravenous administration of oxytocin (2 IU bolus with 32 mIU min−1 infusion) in healthy men 129. Evidence suggests that oxytocin reduces ACTH and cortisol production by competing with vasopressin for V1b receptors in the anterior pituitary – at equimolar concentrations, the stimulation of ACTH production by oxytocin was shown to be reduced in comparison with stimulation with vasopressin 130. Although oxytocin has been also shown to up‐regulate ACTH production via binding with OTRs, oxytocin alone exhibits lower activity compared with co‐stimulation with vasopressin, further supporting that oxytocin blunts the ACTH response in the anterior pituitary 130. In addition, human studies involving simultaneous administration of oxytocin with vasopressin showed a reduction in ACTH and cortisol response compared with vasopressin and saline 131. Also, naloxone (a drug that antagonises opioid effects by competing for opiate receptor sites in the central nervous system) inhibited the effects of oxytocin on the stress hormone response in the aforementioned exercise testing study, suggesting that oxytocin mediates its effects through the release of endogenous opioids. In support of this hypothesis, endogenous plasma oxytocin concentrations were highly correlated with plasma levels of β‐endorphin 127. Of interest, orexin‐A, which is positively associated with the amount of spontaneous physical activity 132, also showed remarkable positive correlation with plasma oxytocin 127, 132.
Conclusion
Animal studies far outnumber the amount of human evidence on the physiological effects of oxytocin; therefore, it is important to note that major differences exist between the human and animal oxytocin systems. To name a few, parasympathetic innervation of human islets differs from that of murine islets and do not conform to existing models of islet autonomic control 133. Distribution of the OTRs in the murine and human brain was also shown to differ 9, and significant diurnal variation in cerebrospinal fluid oxytocin concentrations has been observed in humans and rats 134. In addition, murine OTR has two consensus sequences for N‐linked glycosylation, while human OTR has three potential glycosylation sites, although the significance of these glycosylation sites has not yet been elucidated (site‐directed mutagenesis studies indicated that elimination of these sites had no influence on ligand binding and selectivity). Therefore, the translation of present findings from animal models to the human system can be difficult; accordingly, the design and ethical consideration of human studies should accommodate for unexpected outcomes. From our review of the existing literature, there also exists a stark knowledge gap on the sexual dimorphic effects of pharmacological oxytocin in glucose and lipid homeostasis. This is of particular interest as differences in the effects of oxytocin have been observed in men and women; e.g. endogenous opioids modulate the release of oxytocin during insulin‐induced hypoglycaemia in male subjects, but this was not the case in females 135. OTR affinity also appears to be modulated by oestradiol and progesterone 9. It has been demonstrated that intranasal oxytocin differentially activates brain networks involved in social and emotional information processing in women when compared with men, suggesting important sexual differences in the central effects of oxytocin 136. Moreover, sexual dimorphism has also long been observed in the pathophysiology of diabetes and obesity 137. More studies are therefore needed to shed light on the oxytocin system to appreciate its effects on cardiometabolic risk in the wider community.
We noted that the majority of interventional studies assessed outcomes from immediately after to 60 min after administration of oxytocin 11, 13, 14, 85, 120. As significant increases of oxytocin concentration in the cerebrospinal fluid were previously detected only after 60–75 min after administration of intranasal oxytocin in two studies, one involving humans and another in rhesus macaques 115, 138, future studies that measure outcomes beyond this brief period may more concretely establish the relationship between increases in central or peripheral oxytocin levels and measured outcomes. It is clear however that the effects of oxytocin may manifest even before significant increases in its concentration can be detected centrally or in the periphery.
There also appears to be a large discordance in the reporting of physiological plasma oxytocin concentrations, with measured concentrations ranging from ~0.1 to ~1,000 pg mL−1 in normal subjects, with no clear relationship to gender, BMI or metabolic health status 13, 100, 102, 123, 127, 138. The use of plasma samples with or without extraction, co‐detection of other molecules with OT‐like immunoreactivity 101 and heterophilic interference 139 have been suggested as possible reasons for the apparent inconsistencies in data. There appears to be a need to standardise and validate methods for measuring oxytocin in human samples, and highly sensitive liquid chromatography–mass spectrometry protocols developed recently offer promise as a robust and precise candidate for assaying oxytocin in human biological fluids 140.
These limitations notwithstanding, the oxytocin system has emerged as an attractive pharmacotherapy target for treating metabolic dysfunction related to the development of obesity and diabetes (Fig. 1). In addition, oxytocin administered intranasally has so far demonstrated significant central effects accompanied with an excellent safety profile with minimal side effects and adverse reactions 141. It should be mentioned that rapid, high‐dose bolus injections of oxytocin has been previously linked to electrocardiogram changes and subjective signs suggestive for myocardial ischaemia 142, 143. The possibility of hyponatraemia should also be considered in human studies, particularly with continuous oxytocin infusion studies. Although oxytocin could potentially improve tissue glucose and lipid handling, we have yet to garner an adequate understanding of its distinct effects on glucose and lipid homeostasis in the system as a whole, and evaluation of its safety during chronic use needs to be assessed. Furthermore, the pharmacodynamics of oxytocin appear to be altered significantly with supraphysiological doses, and its structural similarity and interdependent relationship with vasopressin further add to the complexity of its biological action. Therefore, it is proposed that a systems approach may be key to understanding oxytocin physiology and developing oxytocin pharmacotherapy regimens in obesity and diabetes management.
Figure 1.
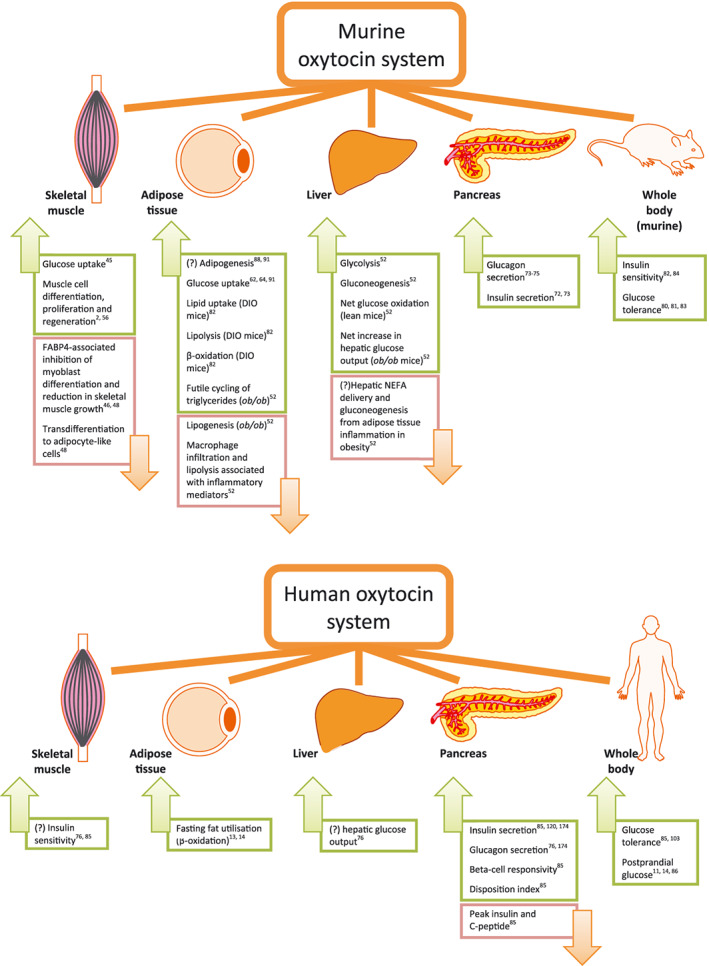
Identified tissue targets in the murine and human oxytocin system for treatment of metabolic dysfunction associated with the development of obesity and diabetes. Abbreviations: DIO, diet‐induced obesity; FABP4, fatty acid binding protein 4; NEFA, non‐esterified fatty acids; ob/ob, obese leptin‐deficient mouse model. [Colour figure can be viewed at wileyonlinelibrary.com]
Conflict of interest statement
The authors have no conflicts of interest relevant to the content of this article.
Author contributions
All authors were involved in manuscript writing and review. All authors approved the version submitted for publication.
Acknowledgement
This review was supported by award BMSI/16‐07803C‐R20H from the Singapore Institute for Clinical Sciences (SICS), Agency for Science, Technology and Research (A*STAR), Singapore.
Ding, C. , Leow, M. K.‐S. , and Magkos, F. (2019) Oxytocin in metabolic homeostasis: implications for obesity and diabetes management. Obesity Reviews, 20: 22–40. 10.1111/obr.12757.
References
- 1. Dale HH. On some physiological actions of ergot. J Physiol. 1906; 34: 163–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu Y, Conboy I. Unexpected evolutionarily conserved rapid effects of viral infection on oxytocin receptor and TGF‐beta/pSmad3. Skelet Muscle. 2017; 7: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kamm O, Aldrich TB, Grote IW, Rowe LW, Bugbee EP. The active principles of the posterior lobe of the pituitary gland.1i. The demonstration of the presence of two active principles. Ii. The separation of the two principles and their concentration in the form of potent solid preparations. J Am Chem Soc. 1928; 50: 573–601. [Google Scholar]
- 4. Pinkerton JHM. Advances in Oxytocin Research. Pergamon Press: Oxford, 1965. [Google Scholar]
- 5. Burt RL, Leake NH, Dannenburg WN. Effect of synthetic oxytocin on plasma nonesterified fatty acids, triglycerides, and blood glucose. Obstet Gynecol. 1963; 21: 708–712. [PubMed] [Google Scholar]
- 6. Fairweather DV. Changes in serum non‐esterified fatty acid levels in spontaneous and in oxytocin induced labour. J Obstet Gynaecol Br Commonw. 1965; 72: 408–415. [DOI] [PubMed] [Google Scholar]
- 7. Kashyap ML, Sivasamboo R, Sothy SP, Cheah JS, Gartside PS. Carbohydrate and lipid metabolism during human labor: free fatty acids, glucose, insulin, and lactic acid metabolism during normal and oxytocin‐induced labor for postmaturity. Metabolism. 1976; 25: 865–875. [DOI] [PubMed] [Google Scholar]
- 8. Mirsky IA. Metabolic effects of the neurohypophyseal hormones and related polypeptides In: Berde B (ed.). Neurohypophysial Hormones and Similar Polypeptides. Springer Berlin Heidelberg: Berlin, Heidelberg, 1968, pp. 613–624. [Google Scholar]
- 9. Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001; 81: 629–683. [DOI] [PubMed] [Google Scholar]
- 10. WHO Global Survey on Maternal and Perinatal Health . Induction of Labour Data. World health Organization: Geneva, 2010. Available at: http://www.who.int/reproductivehealth/topics/maternal_perinatal/globalsurvey/en/. [Google Scholar]
- 11. Ott V, Finlayson G, Lehnert H et al Oxytocin reduces reward‐driven food intake in humans. Diabetes. 2013; 62: 3418–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arletti R, Benelli A, Bertolini A. Influence of oxytocin on feeding behavior in the rat. Peptides. 1989; 10: 89–93. [DOI] [PubMed] [Google Scholar]
- 13. Lawson EA, Marengi DA, DeSanti RL, Holmes TM, Schoenfeld DA, Tolley CJ. Oxytocin reduces caloric intake in men. Obesity. 2015; 23: 950–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thienel M, Fritsche A, Heinrichs M et al Oxytocin's inhibitory effect on food intake is stronger in obese than normal‐weight men. Int J Obes. 2016; 40: 1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blevins JE, Ho JM. Role of oxytocin signaling in the regulation of body weight. Rev Endocr Metab Disord. 2013; 14: 311–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Olszewski PK, Klockars A, Levine AS. Oxytocin: a conditional anorexigen whose effects on appetite depend on the physiological, behavioural and social contexts. J Neuroendocrinol 2016; 28. [DOI] [PubMed] [Google Scholar]
- 17. Leng G, Sabatier N. Oxytocin – the sweet hormone? Trends Endocrinol Metab. 2017; 28: 365–376. [DOI] [PubMed] [Google Scholar]
- 18. Chaves VE, Tilelli CQ, Brito NA, Brito MN. Role of oxytocin in energy metabolism. Peptides. 2013; 45: 9–14. [DOI] [PubMed] [Google Scholar]
- 19. Lawson EA. The effects of oxytocin on eating behaviour and metabolism in humans. Nat Rev Endocrinol. 2017; 13: 700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barberis C, Mouillac B, Durroux T. Structural bases of vasopressin/oxytocin receptor function. J Endocrinol. 1998; 156: 223–229. [DOI] [PubMed] [Google Scholar]
- 21. Burbach JP, Luckman SM, Murphy D, Gainer H. Gene regulation in the magnocellular hypothalamo‐neurohypophysial system. Physiol Rev. 2001; 81: 1197–1267. [DOI] [PubMed] [Google Scholar]
- 22. Fabian M, Forsling ML, Jones JJ, Pryor JS. The clearance and antidiuretic potency of neurohypophysial hormones in man, and their plasma binding and stability. J Physiol. 1969; 204: 653–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chapter VH. Five – human lactation and breast feeding In: Larson BL (ed.). The Mammary Gland/Human Lactation/Milk Synthesis. Academic Press, 1978, pp. 181–280. [Google Scholar]
- 24. Rydæn G. Cystine aminopeptidase and oxytocinase activity in pregnancy: a comparative study in human and rat tissues. Acta Obstet Gynecol Scand. 1966; 45: 3–105. [PubMed] [Google Scholar]
- 25. Laustsen PG, Rasmussen TE, Petersen K et al The complete amino acid sequence of human placental oxytocinase. Biochim Biophys Acta Gene Struct Expr. 1997; 1352: 1–7. [DOI] [PubMed] [Google Scholar]
- 26. Matsumoto H, Nagasaka T, Hattori A et al Expression of placental leucine aminopeptidase/oxytocinase in neuronal cells and its action on neuronal peptides. Eur J Biochem. 2001; 268: 3259–3266. [DOI] [PubMed] [Google Scholar]
- 27. Rogi T, Tsujimoto M, Nakazato H, Mizutani S, Tomoda Y. Human placental leucine aminopeptidase/oxytocinase. A new member of type II membrane‐spanning zinc metallopeptidase family. J Biol Chem. 1996; 271: 56–61. [DOI] [PubMed] [Google Scholar]
- 28. Kimura T, Tanizawa O, Mori K, Brownstein MJ, Okayama H. Structure and expression of a human oxytocin receptor. Nature. 1992; 356: 526. [DOI] [PubMed] [Google Scholar]
- 29. Kimura T, Makino Y, Bathgate R et al The role of N‐terminal glycosylation in the human oxytocin receptor. Mol Hum Reprod. 1997; 3: 957–963. [DOI] [PubMed] [Google Scholar]
- 30. Busnelli M, Sauliere A, Manning M, Bouvier M, Gales C, Chini B. Functional selective oxytocin‐derived agonists discriminate between individual G protein family subtypes. J Biol Chem. 2012; 287: 3617–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morrison JJ, Dearn SR, Smith SK, Ahmed A. Activation of protein kinase C is required for oxytocin‐induced contractility in human pregnant myometrium. Hum Reprod. 1996; 11: 2285–2290. [DOI] [PubMed] [Google Scholar]
- 32. Kim SH, MacIntyre DA, Firmino Da Silva M et al Oxytocin activates NF‐κB‐mediated inflammatory pathways in human gestational tissues. Mol Cell Endocrinol. 2015; 403: 64–77. [DOI] [PubMed] [Google Scholar]
- 33. Gimpl G, Postina R, Fahrenholz F, Reinheimer T. Binding domains of the oxytocin receptor for the selective oxytocin receptor antagonist barusiban in comparison to the agonists oxytocin and carbetocin. Eur J Pharmacol. 2005; 510: 9–16. [DOI] [PubMed] [Google Scholar]
- 34. Elands J, Barberis C, Jard S et al 125I‐labelled d (CH2)5[Tyr (Me)2,Thr4,Tyr‐NH29]OVT: a selective oxytocin receptor ligand. Eur J Pharmacol. 1988; 147: 197–207. [DOI] [PubMed] [Google Scholar]
- 35. Albizu L, Balestre MN, Breton C et al Probing the existence of G protein‐coupled receptor dimers by positive and negative ligand‐dependent cooperative binding. Mol Pharmacol. 2006; 70: 1783–1791. [DOI] [PubMed] [Google Scholar]
- 36. Cottet M, Albizu L, Perkovska S et al Past, present and future of vasopressin and oxytocin receptor oligomers, prototypical GPCR models to study dimerization processes. Curr Opin Pharmacol. 2010; 10: 59–66. [DOI] [PubMed] [Google Scholar]
- 37. Busnelli M, Kleinau G, Muttenthaler M et al Design and characterization of superpotent bivalent ligands targeting oxytocin receptor dimers via a channel‐like structure. J Med Chem. 2016; 59: 7152–7166. [DOI] [PubMed] [Google Scholar]
- 38. Amrani Y, Syed F, Huang C et al Expression and activation of the oxytocin receptor in airway smooth muscle cells: regulation by TNFα and IL‐13. Respir Res. 2010; 11: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Richter ON, Kubler K, Schmolling J et al Oxytocin receptor gene expression of estrogen‐stimulated human myometrium in extracorporeally perfused non‐pregnant uteri. Mol Hum Reprod. 2004; 10: 339–346. [DOI] [PubMed] [Google Scholar]
- 40. Grazzini E, Guillon G, Mouillac B, Zingg HH. Inhibition of oxytocin receptor function by direct binding of progesterone. Nature. 1998; 392: 509. [DOI] [PubMed] [Google Scholar]
- 41. Schmid B, Wong S, Mitchell BF. Transcriptional regulation of oxytocin receptor by interleukin‐1β and interleukin‐6. Endocrinology. 2001; 142: 1380–1385. [DOI] [PubMed] [Google Scholar]
- 42. Maggi M, Peri A, Baldi E et al Interferon‐alpha downregulates expression of the oxytocin receptor in cultured human myometrial cells. Am J Physiol. 1996; 271: E840–E846. [DOI] [PubMed] [Google Scholar]
- 43. Jeng YJ, Soloff SL, Anderson GD, Soloff MS. Regulation of oxytocin receptor expression in cultured human myometrial cells by fetal bovine serum and lysophospholipids. Endocrinology. 2003; 144: 61–68. [DOI] [PubMed] [Google Scholar]
- 44. Jarvis AA, Cain C, Dennis EA. Purification and characterization of a lysophospholipase from human amnionic membranes. J Biol Chem. 1984; 259: 15188–15195. [PubMed] [Google Scholar]
- 45. Lee ES, Uhm K‐O, Lee YM, Kwon J, Park S‐H, Soo KH. Oxytocin stimulates glucose uptake in skeletal muscle cells through the calcium–CaMKK–AMPK pathway. Regul Pept. 2008; 151: 71–74. [DOI] [PubMed] [Google Scholar]
- 46. Berio E, Divari S, Starvaggi Cucuzza L, Biolatti B, Cannizzo FT. 17beta‐estradiol upregulates oxytocin and the oxytocin receptor in C2C12 myotubes. PeerJ. 2017; 5: e3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang L, Zhao Y, Ning Y, Wang H, Zan L. Ectopical expression of FABP4 gene can induce bovine muscle‐derived stem cells adipogenesis. Biochem Biophys Res Commun. 2017; 482: 352–358. [DOI] [PubMed] [Google Scholar]
- 48. Ryan KJP, Daniel ZCTR, Craggs LJL, Parr T, Brameld JM. Dose‐dependent effects of vitamin D on transdifferentiation of skeletal muscle cells to adipose cells. J Endocrinol. 2013; 217: 45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gagliardi AD, Kuo EY, Raulic S, Wagner GF, DiMattia GE. Human stanniocalcin‐2 exhibits potent growth‐suppressive properties in transgenic mice independently of growth hormone and IGFs. Am J Physiol Endocrinol Metab. 2005; 288: E92–E105. [DOI] [PubMed] [Google Scholar]
- 50. Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008; 9: 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest. 2016; 126: 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Altirriba J, Poher A‐L, Caillon A et al Divergent effects of oxytocin treatment of obese diabetic mice on adiposity and diabetes. Endocrinology. 2014; 155: 4189–4201. [DOI] [PubMed] [Google Scholar]
- 53. Shi H, Halvorsen Y‐D, Ellis PN, Wilkison WO, Zemel MB. Role of intracellular calcium in human adipocyte differentiation. Physiol Genomics. 2000; 3: 75–82. [DOI] [PubMed] [Google Scholar]
- 54. Jager ND, Hudson NJ, Reverter A et al Chronic exposure to anabolic steroids induces the muscle expression of oxytocin and a more than fiftyfold increase in circulating oxytocin in cattle. Physiol Genomics. 2011; 43: 467–478. [DOI] [PubMed] [Google Scholar]
- 55. Kongsuwan K, Knox MR, Allingham PG, Pearson R, Dalrymple BP. The effect of combination treatment with trenbolone acetate and estradiol‐17β on skeletal muscle expression and plasma concentrations of oxytocin in sheep. Domest Anim Endocrinol. 2012; 43: 67–73. [DOI] [PubMed] [Google Scholar]
- 56. Elabd C, Cousin W, Upadhyayula P et al Oxytocin is an age‐specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat Commun. 2014; 5: 4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Breton C, Haenggeli C, Barberis C et al Presence of functional oxytocin receptors in cultured human myoblasts. J Clin Endocrinol Metab. 2002; 87: 1415–1418. [DOI] [PubMed] [Google Scholar]
- 58. Nervi C, Benedetti L, Minasi A, Molinaro M, Adamo S. Arginine‐vasopressin induces differentiation of skeletal myogenic cells and up‐regulation of myogenin and Myf‐5. Cell Growth Differ. 1995; 6: 81–89. [PubMed] [Google Scholar]
- 59. Tsuda T, Ueno Y, Yoshikawa T, Kojo H, Osawa T. Microarray profiling of gene expression in human adipocytes in response to anthocyanins. Biochem Pharmacol. 2006; 71: 1184–1197. [DOI] [PubMed] [Google Scholar]
- 60. Gajdosechova L, Krskova K, Segarra AB et al Hypooxytocinaemia in obese Zucker rats relates to oxytocin degradation in liver and adipose tissue. J Endocrinol. 2014; 220: 333–343. [DOI] [PubMed] [Google Scholar]
- 61. Schwartz Y, Goodman HM, Yamaguchi H. Refractoriness to growth hormone is associated with increased intracellular calcium in rat adipocytes. Proc Natl Acad Sci U S A. 1991; 88: 6790–6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Egan JJ, Saltis J, Wek SA, Simpson IA, Londos C. Insulin, oxytocin, and vasopressin stimulate protein kinase C activity in adipocyte plasma membranes. Proc Natl Acad Sci U S A. 1990; 87: 1052–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mühlbacher C, Karnieli E, Schaff P et al Phorbol esters imitate in rat fat‐cells the full effect of insulin on glucose‐carrier translocation, but not on 3‐O‐methylglucose‐transport activity. Biochem J. 1988; 249: 865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mukherjee SP, Mukherjee C. Stimulation of pyruvate dehydrogenase activity in adipocytes by oxytocin: evidence for mediation of the insulin‐like effect by endogenous hydrogen peroxide independent of glucose transport. Arch Biochem Biophys. 1982; 214: 211–222. [DOI] [PubMed] [Google Scholar]
- 65. Cortright RN, Azevedo JL Jr, Zhou Q et al Protein kinase C modulates insulin action in human skeletal muscle. Am J Physiol Endocrinol Metab. 2000; 278: E553–E562. [DOI] [PubMed] [Google Scholar]
- 66. Überall F, Hellbert K, Kampfer S et al Evidence that atypical protein kinase C‐λ and atypical protein kinase C‐ζ participate in Ras‐mediated reorganization of the F‐actin cytoskeleton. J Cell Biol. 1999; 144: 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bandyopadhyay G, Sajan MP, Kanoh Y et al PKC‐zeta mediates insulin effects on glucose transport in cultured preadipocyte‐derived human adipocytes. J Clin Endocrinol Metab. 2002; 87: 716–723. [DOI] [PubMed] [Google Scholar]
- 68. Szeto A, Nation DA, Mendez AJ et al Oxytocin attenuates NADPH‐dependent superoxide activity and IL‐6 secretion in macrophages and vascular cells. Am J Physiol Endocrinol Metab. 2008; 295: E1495–E1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Roberts ZS, Wolden‐Hanson T, Matsen ME et al Chronic hindbrain administration of oxytocin is sufficient to elicit weight loss in diet‐induced obese rats. Am J Physiol Regul Integr Comp Physiol. 2017; 313: R357–R371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wu L, Meng J, Shen Q et al Caffeine inhibits hypothalamic A1R to excite oxytocin neuron and ameliorate dietary obesity in mice. Nat Commun. 2017; 8: 15904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Arino J, Bosch F, Gomez‐Foix AM, Guinovart JJ. Oxytocin inactivates and phosphorylates rat hepatocyte glycogen synthase. Biochem J. 1989; 261: 827–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gao ZY, Drews G, Henquin JC. Mechanisms of the stimulation of insulin release by oxytocin in normal mouse islets. Biochem J. 1991; 276: 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Björkstrand E, Eriksson M, Uvnäs‐Moberg K. Evidence of a peripheral and a central effect of oxytocin on pancreatic hormone release in rats. Neuroendocrinology. 1996; 63: 377–383. [DOI] [PubMed] [Google Scholar]
- 74. Dunning BE, Moltz JH, Fawcett CP. Modulation of insulin and glucagon secretion from the perfused rat pancreas by the neurohypophysial hormones and by desamino‐D‐arginine vasopressin (DDAVP). Peptides. 1984; 5: 871–875. [DOI] [PubMed] [Google Scholar]
- 75. Widmaier EP, Shah PR, Lee G. Interactions between oxytocin, glucagon and glucose in normal and streptozotocin‐induced diabetic rats. Regul Pept. 1991; 34: 235–249. [DOI] [PubMed] [Google Scholar]
- 76. Paolisso G, Pizza G, De Riu S et al Effects of oxytocin upon the endocrine pancreas secretion and glucose turnover in normal man. Eur J Endocrinol. 1990; 123: 504–510. [DOI] [PubMed] [Google Scholar]
- 77. Lee B, Yang C, Chen TH. al‐Azawi N, Hsu WH. Effect of AVP and oxytocin on insulin release: involvement of V1b receptors. Am J Physiol. 1995; 269: E1095–E1100. [DOI] [PubMed] [Google Scholar]
- 78. Camerino C. Low sympathetic tone and obese phenotype in oxytocin‐deficient mice. Obesity. 2009; 17: 980–984. [DOI] [PubMed] [Google Scholar]
- 79. Watanabe S, Wei F‐Y, Matsunaga T, Matsunaga N, Kaitsuka T, Tomizawa K. Oxytocin protects against stress‐induced cell death in murine pancreatic β‐cells. Sci Rep. 2016; 6: 25185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Maejima Y, Iwasaki Y, Yamahara Y, Kodaira M, Sedbazar U, Yada T. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging (Albany NY). 2011; 3: 1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhang G, Cai D. Circadian intervention of obesity development via resting‐stage feeding manipulation or oxytocin treatment. Am J Physiol Endocrinol Metab. 2011; 301: E1004–E1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Deblon N, Veyrat‐Durebex C, Bourgoin L et al Mechanisms of the anti‐obesity effects of oxytocin in diet‐induced obese rats. PLoS One. 2011; 6: e25565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mohan S, Khan D, Moffett RC, Irwin N, Flatt PR. Oxytocin is present in islets and plays a role in beta‐cell function and survival. Peptides. 2018; 100: 260–268. [DOI] [PubMed] [Google Scholar]
- 84. Plante E, Menaouar A, Danalache BA et al Oxytocin treatment prevents the cardiomyopathy observed in obese diabetic male db/db mice. Endocrinology. 2015; 156: 1416–1428. [DOI] [PubMed] [Google Scholar]
- 85. Klement J, Ott V, Rapp K et al Oxytocin improves beta‐cell responsivity and glucose tolerance in healthy men. Diabetes 2016. [DOI] [PubMed] [Google Scholar]
- 86. Zhang H, Wu C, Chen Q et al Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PLoS One. 2013; 8: e61477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yi KJ, So KH, Hata Y et al The regulation of oxytocin receptor gene expression during adipogenesis. J Neuroendocrinol. 2015; 27: 335–342. [DOI] [PubMed] [Google Scholar]
- 88. Elabd C, Basillais A, Beaupied H et al Oxytocin controls differentiation of human mesenchymal stem cells and reverses osteoporosis. Stem Cells. 2008; 26: 2399–2407. [DOI] [PubMed] [Google Scholar]
- 89. Liu G‐S, Chan EC, Higuchi M, Dusting GJ, Jiang F. Redox mechanisms in regulation of adipocyte differentiation: beyond a general stress response. Cells. 2012; 1: 976–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Drehmer DL, de Aguiar AM, Brandt AP et al Metabolic switches during the first steps of adipogenic stem cells differentiation. Stem Cell Res. 2016; 17: 413–421. [DOI] [PubMed] [Google Scholar]
- 91. Eckertova M, Ondrejcakova M, Krskova K, Zorad S, Jezova D. Subchronic treatment of rats with oxytocin results in improved adipocyte differentiation and increased gene expression of factors involved in adipogenesis. Br J Pharmacol. 2011; 162: 452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Draznin B, Sussman KE, Eckel RH, Kao M, Yost T, Sherman NA. Possible role of cytosolic free calcium concentrations in mediating insulin resistance of obesity and hyperinsulinemia. J Clin Invest. 1988; 82: 1848–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rodbell M. Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem. 1964; 239: 375–380. [PubMed] [Google Scholar]
- 94. Nye C, Kim J, Kalhan SC, Hanson RW. Reassessing triglyceride synthesis in adipose tissue. Trends Endocrinol Metab. 2008; 19: 356–361. [DOI] [PubMed] [Google Scholar]
- 95. Maejima Y, Aoyama M, Sakamoto K et al Impact of sex, fat distribution and initial body weight on oxytocin's body weight regulation. Sci Rep. 2017; 7: 8599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhang G, Bai H, Zhang H et al Neuropeptide exocytosis involving synaptotagmin‐4 and oxytocin in hypothalamic programming of body weight and energy balance. Neuron. 2011; 69: 523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Stock S, Granstrom L, Backman L, Matthiesen AS, Uvnas‐Moberg K. Elevated plasma levels of oxytocin in obese subjects before and after gastric banding. Int J Obes. 1989; 13: 213–222. [PubMed] [Google Scholar]
- 98. Schorr M, Marengi DA, Pulumo RL et al Oxytocin and its relationship to body composition, bone mineral density, and hip geometry across the weight spectrum. J Clin Endocrinol Metab. 2017; 102: 2814–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yuan G, Qian W, Pan R et al Reduced circulating oxytocin and high‐molecular‐weight adiponectin are risk factors for metabolic syndrome. Endocr J. 2016; 63: 655–662. [DOI] [PubMed] [Google Scholar]
- 100. Qian W, Zhu T, Tang B et al Decreased circulating levels of oxytocin in obesity and newly diagnosed type 2 diabetic patients. J Clin Endocrinol Metab. 2014; 99: 4683–4689. [DOI] [PubMed] [Google Scholar]
- 101. McCullough ME, Churchland PS, Mendez AJ. Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neurosci Biobehav Rev. 2013; 37: 1485–1492. [DOI] [PubMed] [Google Scholar]
- 102. Al‐Rawashdeh A, Kasabri V, Bulatova N et al The correlation between plasma levels of oxytocin and betatrophin in non‐diabetic and diabetic metabolic syndrome patients: a cross sectional study from Jordan. Diabetes Metab Syndr. 2017; 11: 59–67. [DOI] [PubMed] [Google Scholar]
- 103. Eisenberg Y, Dugas LR, Akbar A, Reddivari B, Layden BT, Barengolts E. Oxytocin is lower in African American men with diabetes and associates with psycho‐social and metabolic health factors. PLoS One. 2018; 13: e0190301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Amico JA, Ulbrecht JS, Robinson AG. Clearance studies of oxytocin in humans using radioimmunoassay measurements of the hormone in plasma and urine. J Clin Endocrinol Metab. 1987; 64: 340–345. [DOI] [PubMed] [Google Scholar]
- 105. Ruth F, Ilanit G, Orna ZS. Maternal and paternal plasma, salivary, and urinary oxytocin and parent–infant synchrony: considering stress and affiliation components of human bonding. Developmental Science. 2011; 14: 752–761. [DOI] [PubMed] [Google Scholar]
- 106. Szulc P, Amri EZ, Varennes A et al High serum oxytocin is associated with metabolic syndrome in older men – the MINOS study. Diabetes Res Clin Pract. 2016; 122: 17–27. [DOI] [PubMed] [Google Scholar]
- 107. Binay Ç, Paketçi C, Güzel S, Samancı N. Serum irisin and oxytocin levels as predictors of metabolic parameters in obese children. J Clin Res Pediatr Endocrinol. 2017; 9: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Chiodera P, Volpi R, Capretti L et al Oxytocin response to challenging stimuli in elderly men. Regul Pept. 1994; 51: 169–176. [DOI] [PubMed] [Google Scholar]
- 109. Ishunina TA, Swaab DF. Vasopressin and oxytocin neurons of the human supraoptic and paraventricular nucleus: size changes in relation to age and sex. J Clin Endocrinol Metab. 1999; 84: 4637–4644. [DOI] [PubMed] [Google Scholar]
- 110. Arsenijevic Y, Dreifuss JJ, Vallet P, Marguerat A, Tribollet E. Reduced binding of oxytocin in the rat brain during aging. Brain Res. 1995; 698: 275–279. [DOI] [PubMed] [Google Scholar]
- 111. Keck ME, Hatzinger M, Wotjak CT, Landgraf R, Holsboer F, Neumann ID. Ageing alters intrahypothalamic release patterns of vasopressin and oxytocin in rats. Eur J Neurosci. 2000; 12: 1487–1494. [DOI] [PubMed] [Google Scholar]
- 112. Yu BL, Zhao SP, Hu JR. Cholesterol imbalance in adipocytes: a possible mechanism of adipocytes dysfunction in obesity. Obes Rev. 2010; 11: 560–567. [DOI] [PubMed] [Google Scholar]
- 113. Borg J, Simrén M, Ohlsson B. Oxytocin reduces satiety scores without affecting the volume of nutrient intake or gastric emptying rate in healthy subjects. Neurogastroenterol Motil. 2011; 23: 56–e5. [DOI] [PubMed] [Google Scholar]
- 114. Leng G, Ludwig M. Intranasal oxytocin: myths and delusions. Biol Psychiatry. 2016; 79: 243–250. [DOI] [PubMed] [Google Scholar]
- 115. Lee MR, Scheidweiler KB, Diao XX et al Oxytocin by intranasal and intravenous routes reaches the cerebrospinal fluid in rhesus macaques: determination using a novel oxytocin assay. Mol Psychiatry. 2017; 23: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sabatier N, Caquineau C, Dayanithi G et al Alpha‐melanocyte‐stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. J Neurosci. 2003; 23: 10351–10358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Dallman MF, Pecoraro N, Akana SF et al Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci U S A. 2003; 100: 11696–11701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Plessow F, Marengi DA, Perry SK et al Effects of intranasal oxytocin on the blood oxygenation level‐dependent signal in food motivation and cognitive control pathways in overweight and obese men. Neuropsychopharmacology. 2018; 43: 638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. van der Klaauw AA, Ziauddeen H, Keogh JM et al Oxytocin administration suppresses hypothalamic activation in response to visual food cues. Sci Rep. 2017; 7: 4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Chiodera P, Coiro V, Camellini L et al Effect of pharmacological doses of oxytocin on insulin response to glucose in normal man. Horm Res Paediatr. 1984; 20: 150–154. [DOI] [PubMed] [Google Scholar]
- 121. Akerlund M, Bossmar T, Brouard R et al Receptor binding of oxytocin and vasopressin antagonists and inhibitory effects on isolated myometrium from preterm and term pregnant women. Br J Obstet Gynaecol. 1999; 106: 1047–1053. [DOI] [PubMed] [Google Scholar]
- 122. Landgraf R, Hacker R, Buhl H. Plasma vasopressin and oxytocin in response to exercise and during a day‐night cycle in man. Endokrinologie. 1982; 79: 281–291. [PubMed] [Google Scholar]
- 123. Chicharro JL, Hoyos J, Bandrés F, Gómez Gallego F, Pérez M, Lucía A. Plasma oxytocin during intense exercise in professional cyclists. Horm Res Paediatr. 2001; 55: 155–159. [DOI] [PubMed] [Google Scholar]
- 124. Altemus M, Roca C, Galliven E, Romanos C, Deuster P. Increased vasopressin and adrenocorticotropin responses to stress in the midluteal phase of the menstrual cycle. J Clin Endocrinol Metab. 2001; 86: 2525–2530. [DOI] [PubMed] [Google Scholar]
- 125. Lawson EA, Ackerman KE, Slattery M, Marengi DA, Clarke H, Misra M. Oxytocin secretion is related to measures of energy homeostasis in young amenorrheic athletes. J Clin Endocrinol Metab. 2014; 99: E881–E885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Lawson EA, Ackerman KE, Estella NM et al Nocturnal oxytocin secretion is lower in amenorrheic athletes than nonathletes and associated with bone microarchitecture and finite element analysis parameters. Eur J Endocrinol. 2013; 168: 457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Godwin EM, Uglialoro AD, Ali A, Yearwood L, Banerji MA, Kral JG. Cardio‐ and neurometabolic effects of lower‐body pressure supported exercise in obese non‐diabetic women: resetting autonomic imbalance? bioRxiv 2017.
- 128. Cardoso C, Ellenbogen MA, Orlando MA, Bacon SL, Joober R. Intranasal oxytocin attenuates the cortisol response to physical stress: a dose–response study. Psychoneuroendocrinology. 2013; 38: 399–407. [DOI] [PubMed] [Google Scholar]
- 129. Coiro V, Passeri M, Davoli C et al Oxytocin reduces exercise‐induced ACTH and cortisol rise in man. Acta Endocrinol (Copenh). 1988; 119: 405–412. [DOI] [PubMed] [Google Scholar]
- 130. Nakamura K, Fujiwara Y, Mizutani R et al Effects of vasopressin V1b receptor deficiency on adrenocorticotropin release from anterior pituitary cells in response to oxytocin stimulation. Endocrinology. 2008; 149: 4883–4891. [DOI] [PubMed] [Google Scholar]
- 131. Legros JJ, Chiodera P, Demey‐Ponsart E. Inhibitory influence of exogenous oxytocin on adrenocorticotropin secretion in normal human subjects. J Clin Endocrinol Metab. 1982; 55: 1035–1039. [DOI] [PubMed] [Google Scholar]
- 132. Kotz CM, Perez‐Leighton CE, Teske JA, Billington CJ. Spontaneous physical activity defends against obesity. Curr Obes Rep. 2017; 6: 362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Rodriguez‐Diaz R, Abdulreda Midhat H, Formoso Alexander L et al Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab. 2011; 14: 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Amico JA, Tenicela R, Johnston J, Robinson AG. A time‐dependent peak of oxytocin exists in cerebrospinal fluid but not in plasma of humans. J Clin Endocrinol Metab. 1983; 57: 947–951. [DOI] [PubMed] [Google Scholar]
- 135. Johnson MR, Bower M, Seckl JR, Lightman SL. Neurohypophyseal secretion to insulin‐induced hypoglycemia and its regulation by endogenous opioids in women. ACTA ENDOCRINOL BUCH. 1990; 122: 467–471. [DOI] [PubMed] [Google Scholar]
- 136. Domes G, Lischke A, Berger C et al Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010; 35: 83–93. [DOI] [PubMed] [Google Scholar]
- 137. Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol. 2015; 402: 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Striepens N, Kendrick KM, Hanking V et al Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci Rep. 2013; 3: 3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Leng G, Sabatier N. Measuring oxytocin and vasopressin: bioassays, immunoassays and random numbers. J Neuroendocrinol. 2016; 28 10.1111/jne.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Brandtzaeg OK, Johnsen E, Roberg‐Larsen H et al Proteomics tools reveal startlingly high amounts of oxytocin in plasma and serum. Sci Rep. 2016; 6: 31693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. MacDonald E, Dadds MR, Brennan JL, Williams K, Levy F, Cauchi AJ. A review of safety, side‐effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology. 2011; 36: 1114–1126. [DOI] [PubMed] [Google Scholar]
- 142. Jonsson M, Hanson U, Lidell C, Norden‐Lindeberg S. ST depression at caesarean section and the relation to oxytocin dose. A randomised controlled trial. BJOG. 2010; 117: 76–83. [DOI] [PubMed] [Google Scholar]
- 143. Svanström MC, Biber B, Hanes M, Johansson G, Näslund U, Bålfors EM. Signs of myocardial ischaemia after injection of oxytocin: a randomized double‐blind comparison of oxytocin and methylergometrine during Caesarean section. Br J Anaesth. 2008; 100: 683–689. [DOI] [PubMed] [Google Scholar]
- 144. Chibbar R, Miller FD, Mitchell BF. Synthesis of oxytocin in amnion, chorion, and decidua may influence the timing of human parturition. J Clin Invest. 1993; 91: 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Blanks AM, Vatish M, Allen MJ et al Paracrine oxytocin and estradiol demonstrate a spatial increase in human intrauterine tissues with labor. J Clin Endocrinol Metab. 2003; 88: 3392–3400. [DOI] [PubMed] [Google Scholar]
- 146. Mechsner S, Bartley J, Loddenkemper C, Salomon DS, Starzinski‐Powitz A, Ebert AD. Oxytocin receptor expression in smooth muscle cells of peritoneal endometriotic lesions and ovarian endometriotic cysts. Fertil Steril. 2005; 83(Suppl 1): 1220–1231. [DOI] [PubMed] [Google Scholar]
- 147. Filippi S, Vannelli GB, Granchi S et al Identification, localization and functional activity of oxytocin receptors in epididymis. Mol Cell Endocrinol. 2002; 193: 89–100. [DOI] [PubMed] [Google Scholar]
- 148. Kinsey CG, Bussolati G, Bosco M et al Constitutive and ligand‐induced nuclear localization of oxytocin receptor. J Cell Mol Med. 2007; 11: 96–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Amico JA, Rauk PN, H‐m C. Estradiol and progesterone regulate oxytocin receptor binding and expression in human breast cancer cell lines. Endocrine. 2002; 18: 79–84. [DOI] [PubMed] [Google Scholar]
- 150. Kimura T, Ito Y, Einspanier A et al Expression and immunolocalization of the oxytocin receptor in human lactating and non‐lactating mammary glands. Hum Reprod. 1998; 13: 2645–2653. [DOI] [PubMed] [Google Scholar]
- 151. Kimura T, Takemura M, Nomura S et al Expression of oxytocin receptor in human pregnant myometrium. Endocrinology. 1996; 137: 780–785. [DOI] [PubMed] [Google Scholar]
- 152. Szukiewicz D, Bilska A, Mittal TK et al Myometrial contractility influences oxytocin receptor (OXTR) expression in term trophoblast cells obtained from the maternal surface of the human placenta. BMC Pregnancy Childbirth. 2015; 15: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Fuchs AR, Behrens O, Helmer H et al Oxytocin and vasopressin binding sites in human and bovine ovaries. Am J Obstet Gynecol. 1990; 163: 1961–1967. [DOI] [PubMed] [Google Scholar]
- 154. Ivell R, Furuya K, Brackmann B, Dawood Y, Khan‐Dawood F. Expression of the oxytocin and vasopressin genes in human and baboon gonadal tissues. Endocrinology. 1990; 127: 2990–2996. [DOI] [PubMed] [Google Scholar]
- 155. Maas S, Jarry H, Teichmann A, Rath W, Kuhn W, Wuttke W. Paracrine actions of oxytocin, prostaglandin F2 alpha, and estradiol within the human corpus luteum. J Clin Endocrinol Metab. 1992; 74: 306–312. [DOI] [PubMed] [Google Scholar]
- 156. Wathes DC, Swann RW, Pickering BT, Porter DG, Hull MG, Drife JO. Neurohypophysial hormones in the human ovary. Lancet. 1982; 2: 410–412. [DOI] [PubMed] [Google Scholar]
- 157. Kim S‐C, Lee J‐E, Kang SS, Yang H‐S, Kim SS, An B‐S. The regulation of oxytocin and oxytocin receptor in human placenta according to gestational age. J Mol Endocrinol. 2017; 59: 235–243. [DOI] [PubMed] [Google Scholar]
- 158. Whittington K, Assinder S, Gould M, Nicholson H. Oxytocin, oxytocin‐associated neurophysin and the oxytocin receptor in the human prostate. Cell Tissue Res. 2004; 318: 375–382. [DOI] [PubMed] [Google Scholar]
- 159. Nicholson HD, Swann RW, Burford GD, Wathes DC, Porter DG, Pickering BT. Identification of oxytocin and vasopressin in the testis and in adrenal tissue. Regul Pept. 1984; 8: 141–146. [DOI] [PubMed] [Google Scholar]
- 160. Frayne J, Nicholson HD. Localization of oxytocin receptors in the human and macaque monkey male reproductive tracts: evidence for a physiological role of oxytocin in the male. Mol Hum Reprod. 1998; 4: 527–532. [DOI] [PubMed] [Google Scholar]
- 161. Quintana DS, Rokicki J, van der Meer D, Alnaes D, Kaufmann T, Palomera AC , et al Oxytocin gene networks in the human brain: a gene expression and large‐scale fMRI meta‐analysis study. bioRxiv 2017.
- 162. Monstein HJ, Grahn N, Truedsson M, Ohlsson B. Oxytocin and oxytocin‐receptor mRNA expression in the human gastrointestinal tract: a polymerase chain reaction study. Regul Pept. 2004; 119: 39–44. [DOI] [PubMed] [Google Scholar]
- 163. Deing V, Roggenkamp D, Kühnl J et al Oxytocin modulates proliferation and stress responses of human skin cells: implications for atopic dermatitis. Exp Dermatol. 2013; 22: 399–405. [DOI] [PubMed] [Google Scholar]
- 164. Geenen V, Legros J, Franchimont P, Baudrihaye M, Defresne M, Boniver J. The neuroendocrine thymus: coexistence of oxytocin and neurophysin in the human thymus. Sci. 1986; 232: 508–511. [DOI] [PubMed] [Google Scholar]
- 165. Geenen V, Legros J‐J, Franchimont P et al The thymus as a neuroendocrine organ. Synthesis of vasopressin and oxytocin in human thymic epithelium. Ann N Y Acad Sci. 1987; 496: 56–66. [DOI] [PubMed] [Google Scholar]
- 166. Thibonnier M, Conarty DM, Preston JA, Plesnicher CL, Dweik RA, Erzurum SC. Human vascular endothelial cells express oxytocin receptors*. Endocrinology. 1999; 140: 1301–1309. [DOI] [PubMed] [Google Scholar]
- 167. Yamaguchi Y, Yamada K, Suzuki T et al Induction of uPA release in human peripheral blood lymphocytes by [deamino‐Cysl,D‐Arg8]‐vasopressin (dDAVP). Am J Physiol Endocrinol Metab. 2004; 287: E970–E976. [DOI] [PubMed] [Google Scholar]
- 168. Krause S, Boeck C, Gumpp AM et al Child maltreatment is associated with a reduction of the oxytocin receptor in peripheral blood mononuclear cells. Front Psychol. 2018; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Copland JA, Ives KL, Simmons DJ, Soloff MS. Functional oxytocin receptors discovered in human osteoblasts. Endocrinology. 1999; 140: 4371–4374. [DOI] [PubMed] [Google Scholar]
- 170. Colaianni G, Di Benedetto A, Zhu L‐L et al Regulated production of the pituitary hormone oxytocin from murine and human osteoblasts. Biochem Biophys Res Commun. 2011; 411: 512–515. [DOI] [PubMed] [Google Scholar]
- 171. Colucci S, Colaianni G, Mori G, Grano M, Zallone A. Human osteoclasts express oxytocin receptor. Biochem Biophys Res Commun. 2002; 297: 442–445. [DOI] [PubMed] [Google Scholar]
- 172. Copland JA, Jeng Y‐J, Strakova Z, Ives KL, Hellmich MR, Soloff MS. Demonstration of functional oxytocin receptors in human breast Hs578T cells and their up‐regulation through a protein kinase C‐dependent pathway*. Endocrinology. 1999; 140: 2258–2267. [DOI] [PubMed] [Google Scholar]
- 173. Copland JA, Zlatnik MG, Ives KL, Soloff MS. Oxytocin receptor regulation and action in a human granulosa‐lutein cell line. Biol Reprod. 2002; 66: 1230–1236. [DOI] [PubMed] [Google Scholar]


