Three seminal papers published in JEM between 1995 and 2000 laid the grounds for the Nobel prize–winning discovery of immune checkpoint inhibitors for the treatment of cancer.
Abstract
Three papers by James Allison and Tasuku Honjo published in JEM between 1995 and 2000 crystallized seminal insights into the role of CTLA-4 and PD-1 in immunosuppression (Krummel and Allison. 1995. J. Exp. Med. https://doi.org/10.1084/jem.182.2.459; van Elsas et al. 1999. J. Exp. Med. https://doi.org/10.1084/jem.190.3.355; Freeman et al. 2000. J. Exp. Med. https://doi.org/10.1084/jem.192.7.1027). These papers laid the basis for modern cancer immunotherapy and led to a shared 2018 Nobel Prize.
Two decades ago, JEM published three landmark papers that paved the way to modern cancer immunotherapy by immune checkpoint inhibitors (ICIs): two papers by James Allison’s team (Krummel and Allison, 1995; van Elsas et al., 1999) and another by an American–Japanese network coordinated by Tasuku Honjo (Freeman et al., 2000). These articles dealt with three different molecules that would become the preferential targets of ICIs until the present day: cytotoxic T lymphocyte–associated protein 4 (CTLA-4; Krummel and Allison, 1995; van Elsas et al., 1999), programmed death 1 (PD-1), and programmed death ligand 1 (PD-L1; Freeman et al., 2000). Each of these molecules is targeted by several clinically approved antibodies, which are currently used for the treatment of >15 cancer types.

Insights from Guido Kroemer and Laurence Zitvogel.
In 1995, Krummel and Allison reported that a monoclonal antibody raised against mouse CTLA-4 blocked the inhibitory interaction of CTLA-4 receptors with small amounts of B7.2 ligands expressed on T lymphocytes to stimulate proliferation and IL-2 secretion in response to stimulation of T cell receptors and CD28-mediated costimulation (Krummel and Allison, 1995). This firmly established that CD28 and CTLA-4 deliver opposing signals that are integrated by T cells to dictate the magnitude of their response.
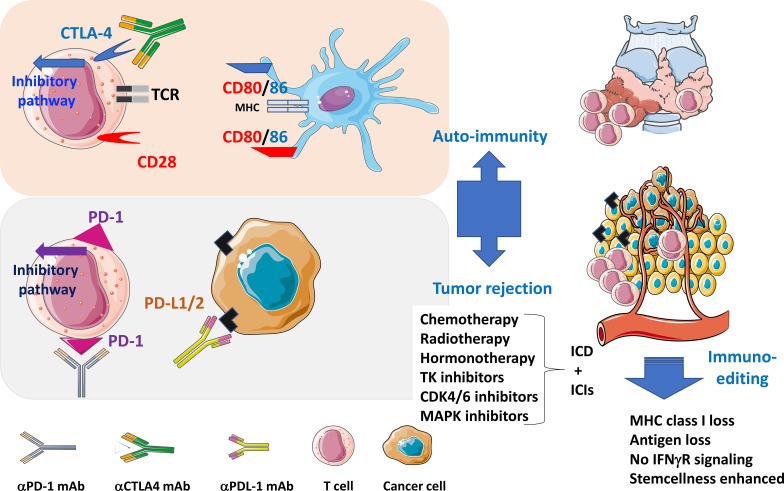
Immune checkpoints in tumor immunology. During the cognate interaction between MHC class I and II complexes (on dendritic cells or tumor antigen–presenting cells) with T cell receptors in the context of a costimulatory signal through CD80/CD86 and CD28 (elicited when effective anticancer therapies inducing an ICD are administered), CTLA-4 and PD-1 are up-regulated and will bind CD86 (and CD80) and PD-L1 (expressed on dendritic, myeloid, and neoplastic cells), respectively, to block the activation signaling cascade in T lymphocytes. Therapeutic monoclonal antibodies blocking PD-1, CTLA-4, or PD-L1 (ICIs) interfere with this natural coinhibitory pathways to reinstate T cell proliferation, and cytokine secretion, culminating in improved tumor immunosurveillance and autoimmune side effects. The selective pressure of CD8+ T lymphocytes on tumor cells leads to “immunoediting,” i.e., the selection of cancer cells lacking tumor antigens, MHC class I expression, or IFNγ responses.
Later, James Allison’s group reported in 1999 (van Elsas et al., 1999) the possibility to treat mice bearing established B16-BL6 melanomas by a combination of CTLA-4 blockade and immunization with irradiated tumor cells engineered to express GM-CSF. Although this type of combination therapy has not been Food and Drug Administration approved, each of the elements of this strategy has separately led to three medical applications: (1) CTLA-4 blockade as the first example of clinically approved ICI for the treatment of stage III and IV melanoma (Hodi et al., 2010); (2) the a posteriori realization that radiotherapy (and thereafter other cytotoxic compounds) can induce immunogenic cell death (ICD) in malignant cells, thus causing anticancer immune responses that explain its long-term benefits and synergy with ICI (Apetoh et al., 2007); and (3) the use of a GM-CSF–producing oncolytic virus (talimogene laherparepvec, best known under the acronym TVEC) that has been clinically approved for the treatment of inoperable melanoma (Pol et al., 2016). Moreover, clinical studies suggest that the combination of CTLA-4 blockade and radiotherapy is particularly efficient in inducing abscopal effects (Postow et al., 2012). Signals of superior clinical activity of the combination of CTLA-4 blockade and GM-CSF have also been detected by meta-analysis.
The paper by Tasuku Honjo published in 2000 (Freeman et al., 2000) deals with the discovery of PD-L1 as the ligand of PD-1, which is expressed by T lymphocytes. The authors demonstrate that PD-L1 is present on the surface of activated dendritic cells and macrophages, as well as on epithelia (keratinocytes), and cells from parenchyma (heart and lung). By interacting with PD-1, PD-L1 avoids activating the costimulatory effects mediated by CD80 and CD86 (which both act on CD28), hence controlling T lymphocyte responses. In this way, PD-L1 joined a club of immunologically relevant B7 family members that include the costimulatory molecules CD80 (which interacts with CD28, CTLA-4, and PD-L1), CD86 (which interacts with CD28 and CTLA-4), ICOSL (a ligand of ICOS), but also coinhibitory molecules such as PD-L1, PD-L2 (both ligands of PD-1), and others. As a consequence of these findings, multiple PD-L1 antibodies have been developed and introduced into the oncological armamentarium with a success that is only comparable to those of PD-1–specific antibodies (Hellmann et al., 2019). Antibodies targeting the PD-1/PD-L1 interaction have been advantageously combined with anti–CTLA-4 antibodies, for instance, for the treatment of melanoma (Wolchok et al., 2013) and nonsmall cell lung cancer (Hellmann et al., 2019). Moreover, combination therapies designed to inhibit PD-1 or PD-L1 together with other ICIs (such as LAG3, TIGIT, TIM3, B7H3, CD39, CD73, and adenosine A2A receptor), disrupting negative regulation between neoplastic, myeloid, and T cells, and immunostimulatory agents (such as agonistic ICOS-specific antibodies) are in clinical and preclinical development. There are currently >3,000 active clinical trials evaluating T cells modulators, representing the majority of all oncology trials.
In retrospect, the pioneering work by Allison and Honjo was groundbreaking. Two decades ago, cancer was considered a merely cell-autonomous genetic and epigenetic disease that had to be tackled by cancer cell–specific antibiotics including general cytotoxicants and so-called targeted therapies. Immunologists could conceive the importance of cancer immunosurveillance, but drug developers and clinical oncologists did not care about anticancer immunity. It took more than a decade until the widespread recognition that cancer cells can only strive and form diagnosable tumors if they fail to be eliminated by the immune system, meaning that the etiology of malignant disease involves a stepwise process: first, the malignant transformation of (epi)genetically unstable cells and, second, the subversion, or evasion from, immunosurveillance.
The preclinical work done by James Allison was largely ignored by the oncological research community, in line with the widespread neglect of the discoveries that conventional antineoplastic therapies including chemotherapy and radiotherapy are only efficient if they are able to induce ICD and hence succeed in eliciting an anticancer immune response (Apetoh et al., 2007; Casares et al., 2005), that cancers developing in immunodeficient mice would not form tumors if transplanted into histocompatible, immunocompetent hosts (Koebel et al., 2007) and that signs of anticancer immune responses detectable by immunohistochemistry predicted a favorable prognosis in human colorectal cancer (Galon et al., 2006) and many other cancer types. Thus, in spite of overwhelming evidence pleading in favor of the practical and clinical implications of the cancer-immune dialog, basic cancer researchers, clinical oncologists, and drug developers preferred to apply Occam’s razor to avoid including immune parameters in the etiology of cancer. Occam’s razor, also called lex parsimoniae (the law of briefness), constitutes a principle from philosophy according to which explanations of natural phenomena should be as simple as possible, hence requiring the smallest number of assumptions.
The immune control of cancer was only acknowledged when the first phase III clinical trial demonstrating anti-melanoma effects of anti–CTLA-4 blockade was reported (Hodi et al., 2010). The development of this first-in-class drug required implementing new primary endpoints, such as overall survival (rather than objective response rates), and enforcing recognition of immune-related response criteria to best evaluate the retarded effects of immunotherapy (Hoos et al., 2010). However, immuno-oncology became a truly fashionable subject one step later, when the first clinical trials started to reveal the vast anticancer activity of PD-1 blockade (Hellmann et al., 2019). One of the most impressive successes of ICIs has been long-term remission in spite of treatment discontinuation, raising hope for cure for some patients, specifically in melanoma. Beyond metastatic stages, ICIs now show efficacy for the adjuvant (preoperative) treatment of melanoma, decreasing the risk of recurrence (Eggermont et al., 2018).
At the beginning of the third decade of the 21st century, the basic concept of immuno-oncology has been assimilated by a vast majority of the specialized community. Most of the investments effectuated by the pharmaceutical and biotechnology industry now focus on the development of immunotherapies, alone or in combination with chemotherapies and targeted therapies. Unfortunately, lex parsimoniae is still applied. Immunologists interested in immunopathology know that the prime purpose of the immune system is not to cause autoimmune or allergic diseases. Rather, the immune system has been built during evolution as a defense system against pathogenic invaders. Immuno-oncologists, however, were incredulous when they learned that anticancer immunotherapies become inefficient in patients treated with broad-spectrum antibiotics that grossly perturb the intestinal microbiota (Zitvogel et al., 2018). Indeed, an ever-expanding body of evidence indicates that intestinal dysbiosis negatively affects the capacity of immunotherapies to restore an efficient state of antineoplastic immunosurveillance.
The work performed by James Allison and Tasuku Honjo illustrates how the addition of knowledge to an established field, creating a sort of interdisciplinarity, in this case between oncology and immunology, can have far-reaching implications for biomedicine. Although ICIs have revolutionized the clinical management of cancer patients, complete and definitive cure of advanced neoplasia is still a rarity, perhaps because cure requires autoimmune reactions that are only affordable when they affect superfluous cell types. Thus, one of the three historical papers that we comment on in this forum (van Elsas et al., 1999) demonstrates that the cure of established melanoma is tied to vitiligo, i.e., the autoimmune destruction of normal melanocytes. The question arises whether an arsenal of conventional anticancer therapies combined with multiple and novel ICIs, perhaps together with interventions on the microbiota, will be able to increase the fraction of oncological patients that experience complete and durable remissions at the cost of manageable side effects. In any case, lex parsimoniae should be abandoned in favor of innovate approaches that transcend the current limits of knowledge.
Acknowledgments
L. Zitvogel and G. Kroemer were supported by the Ligue contre le Cancer (équipe labelisée); Agence Nationale de la Recherche Projets blancs; Agence Nationale de la Recherche under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer; Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix); Fondation pour la Recherche Médicale; a donation by Elior; the European Commission (Horizon 2020: Oncobiome); the European Research Council; Fondation Carrefour; High-end Foreign Expert Program in China (GDW20171100085 and GDW20181100051); Institut National du Cancer; Institut National de la Santé et de la Recherche Médicale (HTE); Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology; the RHU Torino Lumière; the Seerave Foundation; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); ONCOBIOME H2020 network; the SIRIC Cancer Research and Personalized Medicine (CARPEM); and RHU Torino Lumière (ANR-16-RHUS-0008).
L. Zitvogel and G. Kroemer are the scientific co-founders of everImmune. G. Kroemer is a scientific co-founder of Samsara Therapeutics and Therafast Bio.
References
- Apetoh, L., et al. 2007. Nat. Med. 10.1038/nm1622 [DOI] [Google Scholar]
- Casares, N., et al. 2005. J. Exp. Med. 10.1084/jem.20050915 [DOI] [Google Scholar]
- Eggermont, A.M.M., et al. 2018. N. Engl. J. Med. 10.1056/NEJMoa1802357 [DOI] [Google Scholar]
- Freeman, G.J., et al. 2000. J. Exp. Med. 10.1084/jem.192.7.1027 [DOI] [Google Scholar]
- Galon, J., et al. 2006. Science. 10.1126/science.1129139 [DOI] [Google Scholar]
- Hellmann, M.D., et al. 2019. N. Engl. J. Med. 10.1056/NEJMoa1910231 [DOI] [Google Scholar]
- Hodi, F.S., et al. 2010. N. Engl. J. Med. 10.1056/NEJMoa1003466 [DOI] [Google Scholar]
- Hoos, A., et al. 2010. J. Natl. Cancer Inst. 10.1093/jnci/djq310 [DOI] [Google Scholar]
- Koebel, C.M., et al. 2007. Nature. 10.1038/nature06309 [DOI] [Google Scholar]
- Krummel, M.F., and Allison J.P.. 1995. J. Exp. Med. 10.1084/jem.182.2.459 [DOI] [Google Scholar]
- Pol, J., Kroemer G., and Galluzzi L.. 2016. OncoImmunology. 10.1080/2162402X.2015.1115641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow, M.A., et al. 2012. N. Engl. J. Med. 10.1056/NEJMoa1112824 [DOI] [Google Scholar]
- van Elsas, A., et al. 1999. J. Exp. Med. 10.1084/jem.190.3.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolchok, J.D., et al. 2013. N. Engl. J. Med. 10.1056/NEJMoa1302369 [DOI] [Google Scholar]
- Zitvogel, L., et al. 2018. Science. 10.1126/science.aar6918 [DOI] [Google Scholar]