Abstract
Background:
Nondipping of nocturnal blood pressure (BP) is associated with target organ damage and cardiovascular disease. Obstructive sleep apnea (OSA) is associated with incident nondipping. However, the relationship between disordered breathing during rapid eye movement (REM) sleep and the risk of developing nondipping has not been examined. This study investigates whether OSA during REM sleep is associated with incident nondipping.
Methods:
Our sample included 269 adults enrolled in the Wisconsin Sleep Cohort Study who completed two or more 24-hour ambulatory BP studies over an average of 6.6 years of follow-up. After excluding participants with prevalent nondipping BP or antihypertensive use at baseline, there were 199 and 215 participants available for longitudinal analysis of systolic and diastolic nondipping, respectively. OSA in REM and non-REM sleep were defined by apnea-hypopnea index (AHI) from baseline in-laboratory polysomnograms. Systolic and diastolic nondipping were defined by systolic and diastolic sleep/wake BP ratios > 0.9. Modified Poisson regression models estimated the relative risks for the relationship between REM AHI and incident nondipping, adjusting for non-REM AHI and other covariates.
Results:
There was a dose-response greater risk of developing systolic and diastolic non-dipping BP with greater severity of OSA in REM sleep (p-trend=0.021 for systolic and 0.024 for diastolic nondipping). Relative to those with REM AHI<1 event/hr, those with REM AHI≥15 had higher relative risk of incident systolic non-dipping (2.84, 95% CI 1.10-7.29) and incident diastolic non-dipping (4.27, 95% CI 1.20-15.13).
Conclusions:
Our findings indicate that in a population-based sample, REM OSA is independently associated with incident nondipping of BP.
Keywords: REM sleep apnea, cardiovascular disease, nondipping, hypertension
INTRODUCTION
Blood pressure (BP) follows a diurnal pattern, increasing during the day and dipping during nocturnal sleep. Ambulatory blood pressure monitoring (ABPM) can measure night-day patterns of BP over a 24-hour period and provides more useful prognostic information than clinic measurements of BP.1-3 In healthy individuals BP normally varies during different physiologic states and declines by more than 10-20% at nighttime during sleep compared with daytime waking BP.4 Nondipping is traditionally defined as nocturnal BP decrease less than 10% of daytime BP.2 Nondipping has important clinical implications because it is a marker for future development of hypertension in those who are normotensive and, in hypertensive patients, nocturnal hypertension and nondipping have been associated with worse cardiovascular prognosis and increased target organ damage including left ventricular hypertrophy, myocardial infarction, angina, ischemic stroke and cardiovascular death.5,6
Obstructive sleep apnea (OSA), a highly prevalent chronic condition,7 has been independently associated with cardiovascular morbidity and mortality,8-10 including nondipping nocturnal BP.11,12 Increased sympathetic activity is widely considered to be a major putative mechanism by which OSA increases cardiovascular risk.13,14 It is well established that compared to non-rapid eye movement (NREM) sleep, rapid eye movement (REM) sleep is associated with greater sympathetic activity and cardiovascular instability in healthy humans and in patients with OSA.13,15 Therefore, OSA during REM sleep may have more serious adverse consequences than OSA during NREM sleep. Indeed, obstructive apneas and hypopneas during REM sleep are longer in duration and are associated with significantly greater oxygen desaturation compared to events in NREM sleep.16,17 We recently reported that OSA during REM sleep was cross-sectionally and longitudinally associated with hypertension in the Wisconsin Sleep Cohort.18 The higher relative odds of prevalent and incident hypertension was most evident when the apnea-hypopnea index (AHI) during REM sleep reached 15 events per hour. The longitudinal association between REM-related respiratory events and the risk of developing incident nondipping of nocturnal BP remains largely unexplored. In community-based studies the majority of participants with REM AHI ≥15 would be clinically classified as having no OSA (overall AHI<5) or mild OSA (overall AHI 5 to 14.9).18,19 Although in clinical practice those with mild OSA are deemed at a low risk of future cardiovascular disease, it is important to ascertain whether this low risk also applies to individuals with an overall low AHI but significant OSA during REM sleep (i.e. REM AHI ≥15).
To that end, the objective of this prospective study was to determine the longitudinal association between REM AHI and the risk of developing nondipping nocturnal BP. To address our objective, we performed longitudinal analysis of 24-hour ambulatory BP recordings on a sample of Wisconsin Sleep Cohort Study participants, who were nocturnal BP dippers at baseline, to investigate whether OSA in REM sleep is longitudinally associated with incident nocturnal nondipping over an average 6.6 years of follow-up.
METHODS
Study Sample
The University of Wisconsin Health Sciences Institutional Review Board approved the Wisconsin Sleep Cohort Study (WSCS) protocols and informed consent documents. Details of the WSCS are provided in the online supplement. Since its inception in 1989 the Cohort has provided the opportunity for adding ancillary studies. In this study, a protocol for 24-hour ambulatory BP was added to the protocol in 1991 with sequential enrollment. A total of 817 participants had at least one baseline ambulatory BP study. In our analysis we included subjects who had a minimum of 30 minutes of REM sleep on the baseline polysomnogram to ensure meaningful assessments of OSA during REM sleep and at least two ambulatory BP studies.20 We excluded subjects who used CPAP during the laboratory polysomnogram. We also excluded participants who were on antihypertensive medications or were nondippers at their baseline ABPM study (assessed separately for systolic and diastolic analyses) to create an inception cohort, free of the condition of nocturnal BP nondipping, to follow for the development of new nondipping (Figure 1). Subjects who were hypertensive at baseline but were not on any antihypertensive medications and exhibited a normal dipping pattern at baseline were included in the study.
Figure 1. Flow chart of the study.
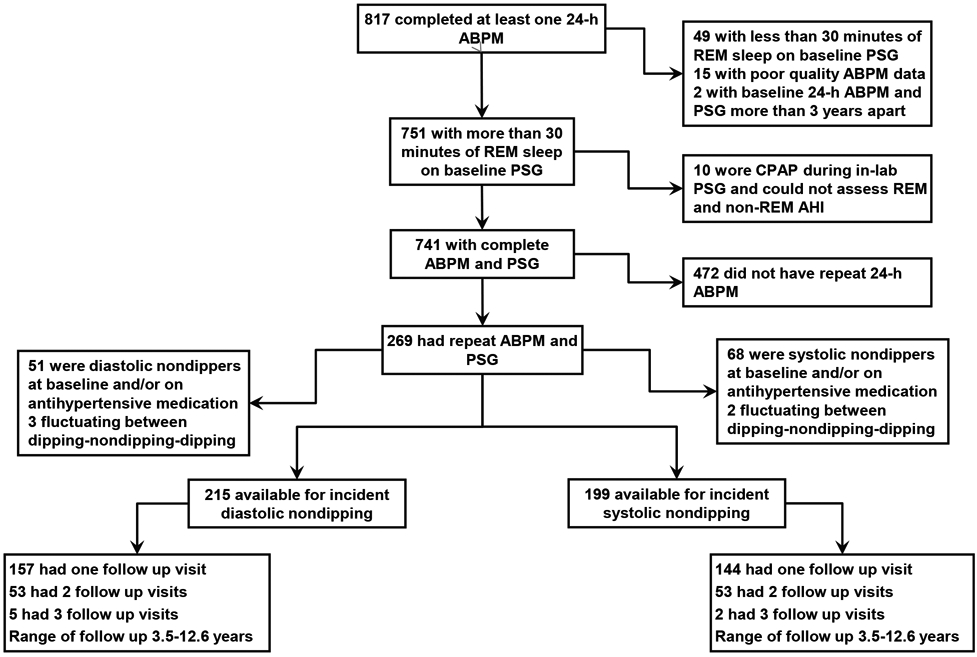
ABPM: Ambulatory blood pressure monitoring, PSG: polysomnogram, REM: rapid eye movement, AHI: apnea-hypopnea index, CPAP: continuous positive airway pressure
Polysomnography
Details of the in-laboratory polysomnography technique are provided in the online supplement.7,21 REM AHI categories were examined as the independent variable: REM AHI<1 (reference category), 1 to 4.9, 5 to 14.9, ≥15.
Ambulatory blood pressure recordings
Typically, the baseline 24-hour ABPM was performed within 6 weeks following the overnight sleep study, with the Accutracker II (SuntechMedical Instruments/Eutectics Electronics, Raleigh, NC), a 24-hour BP monitoring device that uses a modified auscultatory method. ABPM readings were obtained at random intervals averaging every 15 to 20 minutes during wakefulness and every 30 minutes during sleep. Activity, posture, bedtime, and time on awakening from sleep were recorded by participants on diaries. ABPM data were edited using predetermined established criteria.22 Individual mean blood pressures were computed by averaging ABPM measurements during sleep and wake defined by participants’ recorded sleep and wake times, not by preset nighttime and daytime cutoffs.
Systolic BP nocturnal nondipping, assessed from ABPM, was defined as mean systolic sleep BP/mean systolic wake BP ratio>0.9. Diastolic nondipping was defined analogously. An outcome variable was created to represent maintenance of dipping status (no development of nondipping over observed follow-up) or incident nondipping (development of nondipping during observed follow-up).
Covariates
Covariates included in the final models included: age, sex, and race/ethnicity; clinically-measured body mass index (BMI) and waist-to-hip ratio; and questionnaire-assessed smoking status and alcohol intake. For models exploring REM AHI categories, we also adjusted for NREM AHI. For models exploring the association between NREM AHI categories and incident nondipping we also adjusted for REM AHI. Further detail on covariates is provided in the online supplement.
Statistical analysis
All data were analyzed with SAS software, version 9.3 (SAS Institute Inc, Cary, NC) and two-sided p-values of <0.05 were considered to indicate statistical significance. Longitudinal change in nocturnal blood pressure dipping status was modeled using modified Poisson regression to estimate relative risks and 95% confidence intervals (CI) for the relationship between REM AHI severity categories and incident nondipping over an average of 6.6 years follow-up, for systolic nondipping and diastolic nondipping separately.23 We chose to use modified Poisson regression model given that in our cohort the specific time of onset of the binary outcome (i.e. onset of nondipping) was unknown but was known to occur in a broad follow-up interval. Thus we assess risks (probabilities) of outcome, but not rates. The sample for this analysis included those who experienced normal nocturnal dipping (sleep/wake blood pressure <0.9) and were not taking antihypertensive medications at baseline (thus defined, 199 subjects were available to be followed for incident systolic nondipping and 215 for incident diastolic nondipping). These models included REM AHI categories: REM AHI<1 (reference category), 1 to 4.9, 5 to 14.9, ≥15 and were adjusted for log2(NREM AHI+1), age, sex, race/ethnicity, BMI, waist-to-hip ratio, smoking status, and alcohol use based on data from baseline visit. Log2(NREM AHI + 1) allows for the coefficients to be interpreted as the “effect” of a 2-fold increase in NREM AHI (1 was added to NREM AHI in the argument of the logarithm to allow for analysis of zero values). We also constructed models in which individual measures of sleep quality or duration were added to the above-mentioned covariates one at a time to assess the impact on the association between REM AHI categories and incident nondipping. These covariates included self-reported habitual sleep time or several variables from the baseline polysomnogram such as sleep efficiency, wake after sleep onset (WASO) and minutes of slow wave sleep (stage NREM 3). We also explored whether BMI at follow-up, presence of excessive daytime sleepiness, having been started on an antihypertensive medication, or having received treatment for OSA (i.e. CPAP) had any impact on the relationship between REM AHI categories and incident nondipping. Similar analyses were performed using total AHI categories: AHI<1 (reference category), 1 to 4.9, 5 to 14.9, ≥15 and NREM AHI categories: NREM AHI<1 (reference category), 1 to 4.9, 5 to 14.9, ≥15. In the NREM AHI model we also adjusted for Log2(REM AHI+1).
RESULTS
The flow of subjects included in the analytic sample is illustrated in Figure 1. In Table 1 we compare the baseline characteristics of the cohort who had ≥ 30 minutes of REM sleep during the baseline polysomnogram and underwent at least 2 measurements of ABPM (n=269) versus all subjects who had ≥ 30 minutes of REM sleep during the baseline polysomnogram and had at least one measurement of ABPM (n=741). There were no systematic differences between the two groups. The baseline characteristics of the analytic cohort are summarized in Table 2 (systolic nondipping) and Table 3 (diastolic nondipping). In both groups, the mean age was 48 years, mean BMI was 28 kg/m2, 59-60% were men, and 98% were white. The mean scores on the Epworth Sleepiness Scale was 9. The mean total sleep time was 390 minutes and REM sleep represented 19% of total sleep time. As expected, there was an increase in total AHI and NREM AHI across increasing REM AHI severity categories. Participants in the highest REM AHI category had a higher BMI, a higher prevalence of hypertension, and were more likely to be men.
Table 1.
Comparison of baseline characteristics of participants who had repeat 24-h ABPM studies and follow up PSG (n=269) vs. those participants with just one 24-h ABPM study (n=741)
| With follow up ABPM data (n=269) |
Only one ABPM recording (n=741) |
|
|---|---|---|
| Male, % | 62 | 59 |
| Age, years, mean (sd) | 49 (8) | 50 (8) |
| Body mass index, kg/m2, mean (sd) | 29 (5) | 30 (6) |
| Race/Ethnicity, White, % | 97 | 94 |
| Current smoker, % | 17 | 19 |
| Alcohol, number of drinks/wk, (sd) | 4 (5) | 4 (6) |
| Prevalent hypertension, % | 30 | 35 |
| Antihypertensive medication users, % | 13 | 16 |
| Type 2 diabetes, % | 2 | 4 |
| Apnea Hypopnea Index, mean (sd) | 5 (9) | 5(10) |
Table 2.
Sample characteristics by REM AHI severity category for incident systolic nondipping (n=199)
| REM AHI Severity Category | TOTAL | ||||
|---|---|---|---|---|---|
| <1 | 1-4.9 | 5-14.9 | ≥ 15 | ||
| N | 73 | 60 | 38 | 28 | 199 |
| Male, % | 47 | 63 | 66 | 82 | 60 |
| Age, years, mean (sd) | 46 (8) | 49 (7) | 50 (7) | 49 (9) | 48 (8) |
| Body mass index, kg/m2, mean (sd) | 27 (4) | 27 (4) | 30 (4) | 31 (5) | 28 (4) |
| Race/Ethnicity, White, % | 99 | 98 | 95 | 100 | 98 |
| Current smoking, % | 16 | 17 | 18 | 21 | 17 |
| Alcohol, number of drinks/wk, (sd) | 4 (5) | 3 (5) | 5 (6) | 5 (6) | 4 (6) |
| Prevalent hypertension, % | 4 | 23 | 13 | 57 | 19 |
| Type 2 diabetes, % | 0 | 0 | 0 | 0 | 0 |
| Waist-to-hip ratio, mean (sd) | 0.9 (0.1) | 0.9 (0.1) | 0.9 (0.1) | 1.0 (0.1) | 0.9 (0.1) |
| Apnea Hypopnea Index, mean (sd) | 0.6 (1) | 3 (5) | 4 (3) | 18 (19) | 4 (9) |
| NREM Apnea Hypopnea Index, mean (sd) | 0.7 (1) | 3 (6) | 3 (3) | 15 (20) | 4 (9) |
| REM Apnea Hypopnea Index, mean (sd) | 0.2 (0.3) | 3 (1) | 8 (2) | 30 (13) | 7 (11) |
| Minutes total sleep time, mean (sd) | 393 (55) | 393 (54) | 371 (69) | 404 (66) | 391 (60) |
| Minutes REM sleep, mean (sd) | 76 (27) | 77 (28) | 70 (25) | 66 (25) | 74 (27) |
| Percent REM sleep, mean (sd) | 19 (6) | 19 (6) | 19 (5) | 16 (5) | 19 (6) |
| Minutes slow wave sleep, mean (sd) | 43 (29) | 45 (37) | 37 (31) | 36 (35) | 41 (33) |
| Percent slow wave sleep, mean (sd) | 11 (7) | 12 (9) | 9 (7) | 9 (9) | 11 (8) |
| Epworth sleepiness scale*, mean (sd) | 9 (4) | 9 (4) | 9 (3) | 12 (4) | 9 (4) |
| Excessive daytime sleepiness, % | 26 | 12 | 29 | 21 | 22 |
Only available on a subset of studies (n=110)
Table 3.
Sample characteristics by REM AHI severity category for incident diastolic nondipping (n=215)
| REM AHI Severity Category | TOTAL | ||||
|---|---|---|---|---|---|
| <1 | 1-4.9 | 5-14.9 | ≥ 15 | ||
| N | 83 | 61 | 40 | 31 | 215 |
| Male, % | 48 | 61 | 63 | 77 | 59 |
| Age, years, mean (sd) | 46 (8) | 49 (7) | 51 (7) | 49 (9) | 48 (8) |
| Body mass index, kg/m2, mean (sd) | 27 (4) | 28 (4) | 29 (4) | 32 (5) | 28 (5) |
| Race/Ethnicity, White, % | 99 | 98 | 95 | 100 | 98 |
| Current smoking, % | 16 | 16 | 20 | 23 | 18 |
| Alcohol, number of drinks/wk, (sd) | 3 (5) | 3 (4) | 5 (6) | 5 (6) | 4 (5) |
| Prevalent hypertension, % | 7 | 20 | 15 | 52 | 19 |
| Type 2 diabetes, % | 0 | 0 | 0 | 0 | 0 |
| Waist-to-hip ratio, mean (sd) | 0.9 (0.1) | 0.9 (0.1) | 0.9 (0.1) | 0.9 (0.1) | 0.9 (0.1) |
| Apnea Hypopnea Index, mean (sd) | 0.9 (3) | 3 (5) | 4 (3) | 17 (17) | 4 (9) |
| NREM Apnea Hypopnea Index, mean (sd) | 1 (3) | 3 (6) | 3 (3) | 14 (19) | 4 (9) |
| REM Apnea Hypopnea Index, mean (sd) | 0.2 (0.3) | 2 (1) | 8 (3) | 30 (13) | 7 (11) |
| Minutes total sleep time, mean (sd) | 390 (54) | 392 (54) | 373 (66) | 399 (68) | 389 (59) |
| Minutes REM sleep, mean (sd) | 75 (27) | 79 (29) | 70 (26) | 65 (25) | 74 (27) |
| Percent REM sleep, mean (sd) | 19 (6) | 20 (6) | 19 (6) | 16 (5) | 19 (6) |
| Minutes slow wave sleep, mean (sd) | 42 (30) | 45 (36) | 39 (32) | 33 (35) | 41 (33) |
| Percent slow wave sleep, mean (sd) | 11 (8) | 10 (8) | 10 (8) | 8 (9) | 11 (8) |
| Epworth sleepiness scale*, mean (sd) | 9 (4) | 9 (4) | 9 (4) | 11 (5) | 9 (4) |
| Excessive daytime sleepiness, % | 24 | 10 | 33 | 19 | 21 |
Only available on a subset of studies (n=117)
During the follow-up period, 34 subjects (17%) developed systolic nondipping and 20 subjects (9%) developed diastolic nondipping. REM AHI severity at baseline was significantly associated with the onset of nondipping systolic and diastolic BP. Table 4 summarizes the adjusted relative risks for incident systolic and diastolic nondipping based on REM AHI categories. After adjusting for age, sex, race, BMI, waist-to-hip ratio, smoking status, alcohol intake, and NREM AHI, there was a dose-response increased risk of developing systolic and diastolic nondipping with increasing severity of OSA in REM sleep (p-trend 0.021 for systolic and 0.024 for diastolic nondipping) (Figure 2A and 2B). Those with REM AHI ≥ 15 had higher relative risk of incident systolic non-dipping BP (2.84, 95% CI 1.10-7.29) and incident diastolic non-dipping (4.27, 95% CI 1.20-15.13) than subjects with REM AHI < 1. We also assessed the relative risk of incident nondipping by NREM AHI categories and total AHI categories (Figure 2). After adjusting for REM AHI and other covariates, higher NREM AHI categories were not associated with incident systolic or diastolic nondipping (Figure 2C and 2D). In contrast, higher total AHI categories were independently associated with systolic nondipping, but not diastolic nondipping (Figure 2E and 2F).
Table 4.
The association of REM AHI severity category with incident* systolic and diastolic nondipping over an average of 6.6 years of follow up period using modified Poisson regression.
| REM AHI Category |
Total Sample Overall N |
Incident Non-dipping N (%) |
Adjusted Relative Risk (95% Confidence Interval) |
|---|---|---|---|
| Systolic nondipping (n=199) | |||
| <1 | 73 | 8 (11) | 1.0 (reference) |
| 1-4.9 | 60 | 8 (13) | 1.27 (0.52, 3.09) |
| 5-14.9 | 38 | 8 (21) | 2.00 (0.77, 5.19) |
| ≥15 | 28 | 10 (36) | 2.84 (1.10, 7.29) |
| Diastolic nondipping (n=215) | |||
| <1 | 83 | 6 (7) | 1.0 (reference) |
| 1-4.9 | 61 | 2 (3) | 0.48 (0.10, 2.39) |
| 5-14.9 | 40 | 5 (13) | 1.62 (0.46, 5.76) |
| ≥15 | 31 | 7 (23) | 4.27 (1.20, 15.13) |
Among participants with baseline normal blood pressure dipping and no use of antihypertensive medication, followed over an average of 6.6 years. All models adjusted for age, sex, race, body mass index, waist-to-hip ratio, current smoking, alcohol consumption, and NREM AHI. P-trend for incident systolic and diastolic nondipping with increasing REM AHI categories were 0.021 and 0.024, respectively.
Figure 2.
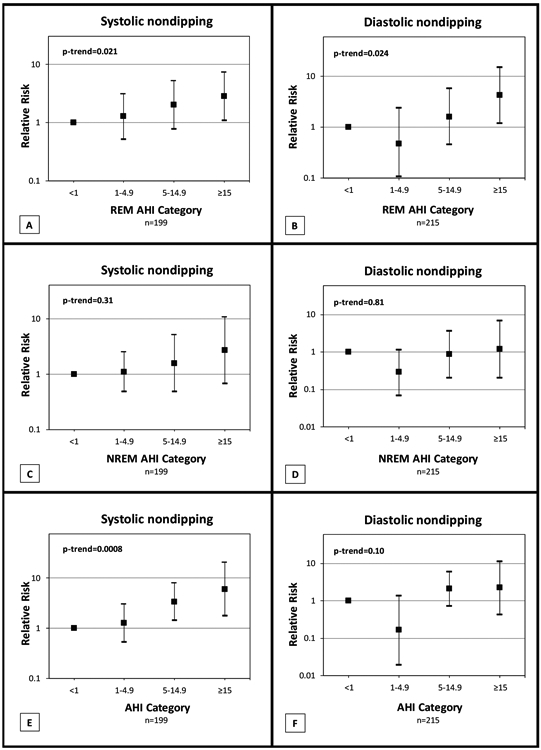
Relative risks and 95% confidence intervals from modified Poisson regression models quantifying the independent association between REM apnea-hypopnea index (AHI) severity categories and incident systolic and diastolic nondipping (panels A and B) over an average follow-up of 6.6 years. Models are adjusted for age, sex, race, body mass index, waist-to-hip ratio, current smoking, alcohol consumption, and Log2(NREM AHI + 1) which allows for the coefficients to be interpreted as the “effect” of a 2-fold increase in NREM AHI (1 was added to NREM AHI in the argument of the logarithm to allow for analysis of zero values). NREM AHI was not significant in these two models. Panels C and D explore the association between NREM AHI severity categories and incident nondipping adjusted for age, sex, race, body mass index, waist-to-hip ratio, current smoking, alcohol consumption, and Log2(REM AHI + 1). NREM AHI categories were not associated with incident systolic or diastolic nondipping. In contrast, doubling of REM AHI had a relative risk of 1.37 (95% CI 1.13-1.65; p=0.0011) for incident systolic nondipping and 1.50 (95% CI 1.17-1.93; p=0.0015) for incident diastolic nondipping. Panels E and F illustrate the association between incident nondipping and total AHI categories adjusted for age, sex, race, body mass index, waist-to-hip ratio, current smoking, and alcohol consumption. Increasing total AHI categories was independently associated with incident systolic nondipping, but not diastolic.
Results were essentially unchanged in models that also adjusted for BMI at follow-up or whether an individual was initiated on antihypertensive medication during the follow-up time period. We also explored whether initiation of home CPAP therapy could alter this relationship. Only 4 subjects in the systolic group and 5 subjects in the diastolic group were initiated on home CPAP therapy during the course of the study. Models that also adjusted for home CPAP therapy were essentially unchanged. Moreover, models that adjusted for various measures of sleep, such as self-reported habitual sleep duration, or several variables from the baseline polysomnogram, including sleep efficiency, wake after sleep onset (WASO) and minutes of slow wave sleep (stage NREM 3), did not substantially change the relationship between and did not change the statistical significance of the AHI categories (REM, NREM and total AHI) and incident nondipping (data not shown). We did not find any significant interaction between REM AHI categories and reported sleepiness (interaction coefficient p-values=0.53 and p=0.75 for systolic and diastolic nondipping outcomes, respectively) or baseline hypertension status (p=0.38 and p=0.65 for systolic and diastolic, respectively).
DISCUSSION
In this longitudinal analysis of a population-based sample, we found that OSA in REM sleep, independent of NREM OSA, is associated with the development of nocturnal systolic and diastolic nondipping of BP over an average follow-up period of 6.6 years among participants who initially were normal nocturnal BP dippers. The association showed a dose response-gradient: individuals with a baseline REM AHI ≥ 15 had an approximately 3 fold greater relative risk of developing systolic nondipping and a 4 fold greater relative risk of developing diastolic nondipping compared to those with REM AHI < 1. To our knowledge, this is the first study to evaluate the association of REM-related OSA and nondipping in a sample selected without regard to the presence of OSA symptoms or other related morbidities. These unique findings suggest a role for OSA in REM sleep in the development of nondipping of nocturnal BP.
The current study demonstrates that REM AHI, but not NREM or total AHI, is significantly associated with nocturnal diastolic BP dipping. Although several cross-sectional studies have reported a higher prevalence of nondipping in moderate to severe OSA,12,24 only one longitudinal population-based study has reported an association between OSA and incident nondipping of systolic BP.11 The direction of causality is further strengthened by studies that have shown CPAP therapy to lower nighttime BP more than daytime BP,13,25-27 particularly in patients with resistant hypertension who are adherent to CPAP therapy.28,29 We previously reported that in the Wisconsin Sleep Cohort, total AHI severity was associated with nocturnal systolic, but not diastolic, dipping; however, that study did not attempt to differentiate between REM and NREM AHI.11 Accordingly, our current findings of a longitudinal association between REM AHI, but not NREM AHI, and diastolic nondipping provides novel insight regarding the potential impact of REM OSA on diastolic BP, an important predictor of cardiovascular risk in middle age.30 This is highly relevant given the mean age of our subjects (i.e., ≤ 50 years).
Findings from the present study as well as prior studies linking OSA to nondipping are clinically relevant because of established associations between nondipping and poor cardiovascular prognosis.5,6 OSA has been independently associated with cardiovascular morbidity and mortality and nondipping may be one of several possible mechanisms by which such outcomes may be initiated or exacerbated in OSA.8-10 The acute and recurrent episodes of complete (apnea) or partial (hypopnea) obstruction of the upper airway in patients with OSA can lead to significant swings in intrathoracic pressure, intermittent hypoxemia and hypercapnia, cortical microarousals, increased oxidative stress, and sleep fragmentation. Moreover, apneas and hypopneas cause temporary and significant elevations of BP (i.e. up to 30-40 mm Hg lasting 20-30 seconds), likely via pathways initiated by sympathetic activation.13 It is possible that these repeated bouts of sympathetic activation prevent complete normalization of BP in the time between apneas and hypopneas, preventing the normal BP dip from occurring.
REM OSA may have a greater impact on sympathetic activation and nondipping of nocturnal BP than NREM OSA. Using beat-to-beat BP measurements and recordings of sympathetic nerve activity via microneurography in patients with OSA, Somers et al found an increase in mean BP from 92±4.5 mm Hg during quiet wakefulness to 116±5 mm Hg in NREM sleep and 127±7 mm Hg during REM sleep. Moreover, sympathetic activity was highest during REM sleep.13 In another study of 16 patients with OSA and untreated hypertension, beat-to-beat BP measured invasively revealed a higher systolic BP in REM sleep compared to NREM sleep (148±29 mm Hg vs. 134±24 mm Hg, respectively).31 The higher BP seen during REM sleep in individuals with OSA may be related to the fact that compared to events in NREM, obstructive apneas and hypopneas during REM sleep last longer and are associated with significantly larger degrees of oxygen desaturation.16,17 Moreover, REM sleep is associated with increased sympathetic activation and reduced vagal tone compared to NREM sleep in normal subjects and even more so in patients with OSA.13,15 Therefore, obstructive respiratory events in REM sleep, a state of sleep that is already under increased sympathetic dominance, could increase the risk for nondipping compared to NREM sleep.
Our findings may have important clinical implications for the duration of CPAP use that is needed to reverse or decrease the risk of nocturnal BP nondipping. In a recent randomized controlled trial of patients with OSA and resistant hypertension, there was a significant positive correlation between hours of CPAP use and the decrease in 24-hour mean and nocturnal blood pressure.32 This positive correlation between hours of CPAP use and the decrease in 24-hour blood pressure was also observed in a meta-analysis.26 Grimaldi et al reported that 3 and 4 hours of CPAP use after lights are turned off would leave the vast majority of obstructive events during REM sleep untreated while 7 hours of CPAP therapy would cover most of REM sleep.33 Indeed, it is plausible that reduced CPAP adherence and the predominantly untreated OSA during REM sleep (which prevails during the latter hours of normal nocturnal sleep) may explain the negative or modest effects of CPAP therapy on BP control in randomized clinical trials. Further research is needed to establish the optimal duration of CPAP use to decrease the risk of developing nondipping and to reverse an already established nondipping pattern in patients with OSA. Moreover, nonintrusive technologies enabling frequent and accurate assessments of vascular function during different stages of sleep can significantly enhance our understanding of the impact of OSA in REM vs. NREM sleep.
Our study has some limitations. First, even though we included NREM AHI as a covariate in our models, there may be residual confounding effects of OSA events not occurring during REM sleep. However, we have previously reported a persistent association between REM AHI and hypertension after excluding subjects with NREM AHI>5.18 Second, in our study hypopneas were classified using ≥4% oxygen desaturation. We do not have data on REM and NREM microarousals for a substantial portion of the cohort. We therefore cannot ascertain whether the observed adverse effect of REM OSA on nondipping would still be detected using hypopnea definitions incorporating ≥3% oxygen desaturation or microarousals. Third, oxygen saturation metrics such as oxygen desaturation index and percent of sleep time below 90% oxygen saturation were not available for the majority of polysomnograms prior to year 2000, when fully digitalized data collection was initiated in the Wisconsin Sleep Cohort Study. Therefore, for the present analyses, we rely only on AHI as the metric of OSA severity. Fourth, our findings may not be generalizable to African Americans or Hispanics since 98% of the subjects included in our cohort were non-Hispanic whites. Lastly, our REM AHI categories were arbitrary and similar to cutoffs used in clinical practice. However, models that used REM AHI categories based on REM AHI quartiles (< 0-0.6, 0.6-2.9, 3.0-12.2, > 12.2) led to very similar results.
Our study has several noteworthy strengths. First, we believe that our conservative inclusion criterion of at least 30 minutes of REM sleep reduces the possibility of exaggerating the effects of REM OSA in individuals with short REM duration. Second, we used actual sleep and wake times recorded by participants and not arbitrary preset times during the 24-hour ABPM. This approach provides additional accuracy in defining sleep and wake BP. Third, ABPM was performed at home and not on the same night of in-laboratory polysomnography thus eliminating the possibility of artificially induced reduction of sleep efficiency and quality related to the discomfort of ABPM measuring BP every 30 minutes. Fourth, our study was performed in a population-based working cohort, enabling us to prospectively determine the association of REM OSA and nondipping in a non-clinical population with occult or undiagnosed OSA. Fifth, with our sample size we were able to account for the confounding effects of several important covariates on the relationship of REM OSA and nondipping. Lastly, our longitudinal analytic approach has the advantage of having the correct temporal sequence and therefore strengthens the evidence that OSA in REM sleep may have a causal role in the development of nocturnal nondipping of BP.
In summary, our findings of a strong longitudinal association of REM OSA with nocturnal nondipping of BP has clinical and public health relevance. Additional studies are needed to establish mechanisms and pathways by which events during REM sleep lead to increased risk of nocturnal nondipping of BP and to explore whether effective treatment that covers most of REM sleep can reduce the risk of nondipping.
Supplementary Material
What is the key question: Does obstructive sleep apnea during rapid eye movement sleep increase the risk of developing nondipping of nocturnal blood pressure?
What is the bottom line: Obstructive sleep apnea in rapid eye movement sleep is longitudinally associated with incident nondipping of systolic and diastolic blood pressure.
Why read on: Since rapid eye movement sleep predominates in the early morning hours before typical awakening, the cardiovascular benefits of CPAP therapy may not be achieved with the typical CPAP use of 3-4 hours per night.
Acknowledgement:
The authors thank Terry Young, PhD, Mari Palta, PhD, Amanda Rasmuson, MS, Kathryn Pluff, Katherine Stanback, Robin Stubbs, Jodi Barnet, MS, Mary Sundstrom, Steven Barczi, MD, and Mihai Teodorescu, MD for their contributions to study implementation and data collection.
Funding support: Supported by National Institutes of Health grants R01HL062252 and 1UL1RR025011. BM is supported by R01HL119161. JRC is supported by R15HL122919.
Footnotes
Competing interests: BM has served as a consultant to Philips/Respironics and has received research support from Philips/Respironics. The other authors do not have any competing interests to report.
REFERENCES
- 1.Pickering TG, White WB, Giles TD, et al. When and how to use self (home) and ambulatory blood pressure monitoring. J Am Soc Hypertens 2010; 4:56–61 [DOI] [PubMed] [Google Scholar]
- 2.Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med 2006; 354:2368–2374 [DOI] [PubMed] [Google Scholar]
- 3.Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension 2005; 46:156–161 [DOI] [PubMed] [Google Scholar]
- 4.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572 [DOI] [PubMed] [Google Scholar]
- 5.Staessen JA, Thijs L, Fagard R, et al. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial Investigators. JAMA 1999; 282:539–546 [DOI] [PubMed] [Google Scholar]
- 6.Boggia J, Li Y, Thijs L, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet 2007; 370:1219–1229 [DOI] [PubMed] [Google Scholar]
- 7.Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342:1378–1384 [DOI] [PubMed] [Google Scholar]
- 9.Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 2012; 307:2169–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, et al. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med 2012; 156:115–122 [DOI] [PubMed] [Google Scholar]
- 11.Hla KM, Young T, Finn L, et al. Longitudinal association of sleep-disordered breathing and nondipping of nocturnal blood pressure in the Wisconsin Sleep Cohort Study. Sleep 2008; 31:795–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seif F, Patel SR, Walia HK, et al. Obstructive sleep apnea and diurnal nondipping hemodynamic indices in patients at increased cardiovascular risk. J Hypertens 2014; 32:267–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Somers VK, Dyken ME, Clary MP, et al. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96:1897–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohler M, Stradling JR. CrossTalk proposal: Most of the cardiovascular consequences of OSA are due to increased sympathetic activity. J Physiol 2012; 590:2813–2815; discussion 2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Somers VK, Dyken ME, Mark AL, et al. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med 1993; 328:303–307 [DOI] [PubMed] [Google Scholar]
- 16.Peppard PE, Ward NR, Morrell MJ. The impact of obesity on oxygen desaturation during sleep-disordered breathing. Am J Respir Crit Care Med 2009; 180:788–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krieger J, Sforza E, Boudewijns A, et al. Respiratory effort during obstructive sleep apnea: role of age and sleep state. Chest 1997; 112:875–884 [DOI] [PubMed] [Google Scholar]
- 18.Mokhlesi B, Finn LA, Hagen EW, et al. Obstructive sleep apnea during REM sleep and hypertension. results of the Wisconsin Sleep Cohort. Am J Respir Crit Care Med 2014; 190:1158–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chami HA, Baldwin CM, Silverman A, et al. Sleepiness, Quality of Life, and Sleep Maintenance in REM versus non-REM Sleep-disordered Breathing. Am J Respir Crit Care Med 2010; 181:997–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mokhlesi B, Punjabi NM. "REM-related" obstructive sleep apnea: an epiphenomenon or a clinically important entity? Sleep 2012; 35:5–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328:1230–1235 [DOI] [PubMed] [Google Scholar]
- 22.Hla KM, Young TB, Bidwell T, et al. Sleep apnea and hypertension. A population-based study. Ann Intern Med 1994; 120:382–388 [DOI] [PubMed] [Google Scholar]
- 23.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159:702–706 [DOI] [PubMed] [Google Scholar]
- 24.Pankow W, Nabe B, Lies A, et al. Influence of sleep apnea on 24-hour blood pressure. Chest 1997; 112:1253–1258 [DOI] [PubMed] [Google Scholar]
- 25.Hla KM, Skatrud JB, Finn L, et al. The effect of correction of sleep-disordered breathing on BP in untreated hypertension. Chest 2002; 122:1125–1132 [DOI] [PubMed] [Google Scholar]
- 26.Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med 2007; 167:757–764 [DOI] [PubMed] [Google Scholar]
- 27.Hu X, Fan J, Chen S, et al. The role of continuous positive airway pressure in blood pressure control for patients with obstructive sleep apnea and hypertension: a meta-analysis of randomized controlled trials. J Clin Hypertens (Greenwich) 2015; 17:215–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbe F, Duran-Cantolla J, Sanchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA 2012; 307:2161–2168 [DOI] [PubMed] [Google Scholar]
- 29.Lloberes P, Sampol G, Espinel E, et al. A randomized controlled study of CPAP effect on plasma aldosterone concentration in patients with resistant hypertension and obstructive sleep apnea. J Hypertens 2014; 32:1650–1657; discussion 1657 [DOI] [PubMed] [Google Scholar]
- 30.Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation 2001; 103:1245–1249 [DOI] [PubMed] [Google Scholar]
- 31.Peter JH, Grote L, Fus E, et al. REM-sleep-hypertension in obstructive sleep apnea. Eur J Med Res 1995; 1:132–136 [PubMed] [Google Scholar]
- 32.Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA 2013; 310:2407–2415 [DOI] [PubMed] [Google Scholar]
- 33.Grimaldi D, Beccuti G, Touma C, et al. Association of obstructive sleep apnea in rapid eye movement sleep with reduced glycemic control in type 2 diabetes: therapeutic implications. Diabetes Care 2014; 37:355–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


