Abstract
Programmed cell death 1 (PD-1)-based immunotherapy has revolutionized the treatment of various cancers. However, only a certain group of patients benefit from PD-1 blockade therapy and many patients succumb to hyperprogressive disease. Although, CD8 T cells and conventional T cells are generally considered to be the primary source of PD-1 in cancer, accumulating evidence suggests that other distinct cell types, including B cells, regulatory T cells, natural killer cells, dendritic cells, tumor-associated macrophages and cancer cells, also express PD-1. Hence, the response of patients with cancer to PD-1 blockade therapy is a cumulative effect of anti-PD-1 antibodies acting on a myriad of cell types. Although, the contribution of CD8 T cells to PD-1 blockade therapy has been well-established, recent studies also suggest the involvement of non-canonical PD-1 signaling in blockade therapy. This review discusses the role of non-canonical PD-1 signaling in distinct cell types and explores how the available knowledge can improve PD-1 blockade immunotherapy, particularly in identifying novel biomarkers and combination treatment strategies.
Keywords: programmed cell death 1 receptor, tumor microenvironment, biomarkers, tumor, immunotherapy
Background
Due to the accumulation of various genetic mutations, cancer cells generally express various neoantigens,1 2 which are released into the tumor microenvironment (TME) following the death of the cancer cells and subsequently initiate the cancer-immunity cycle.3 Ideally, this cycle should result in the generation of abundant tumor-killing lymphocytes, thereby causing the regression of the tumor mass. Unfortunately, the cancer-immunity cycle does not perform optimally in most patients,3 since tumor cells often suppress antitumor immunity by activating a series of negative regulatory pathways.4 This process is known as cancer immunoediting, which includes three phases termed elimination, equilibrium and escape.5 6 Throughout cancer immunoediting, immunosuppressive mechanisms that enable cancer progression are acquired. Among these, programmed cell death 1 (PD-1) signaling is one of the most attractive targets as evidenced by the significant success of PD-1-based immunotherapy in cancer treatment.7 8
PD-1 receptor was first cloned by Ishida et al in 1992.9 It is primarily expressed on T cells on activation. Two tyrosine motifs are present in the cytoplasmic domain of PD-1, including immunoreceptor tyrosine-based switch motif and immunoreceptor tyrosine-based inhibitory motif. Binding of PD-L1 and PD-L2 ligands to PD-1 induces phosphorylation of PD-1 at the tyrosine residues, leading to its interaction with SHP2.10 Historically, it was generally accepted that the recruited SHP2 downregulates T cell receptor (TCR) signaling via the dephosphorylation of downstream signaling regulators, which in turn suppresses the activation, proliferation, cytokine production and survival of T cells.11 However, recent studies suggest PD-1-SHP2 suppresses that T cell function primarily by favoring dephosphorylation of CD28 signaling over dephosphorylation of TCR signaling.12 13 PD-L1 is broadly expressed in somatic cells, while PD-L2 is primarily expressed by antigen-presenting cells (APCs). Driven by hypoxia and inflammatory cytokines, PD-L1 is overexpressed in the TME, along with an elevated expression of PD-1 on tumor-infiltrating lymphocytes, resulting in the disruption of the cancer-immunity cycle.14 Due to the well-established role of PD-1 on tumor-infiltrating cytotoxic T cells and conventional CD4 T cells, we designated this pathway, canonical PD-1 signaling (figure 1). In fact, PD-1 blockade therapy has been developed based on the well-established knowledge regarding canonical PD-1 signaling, and has achieved great success in treating different cancers.15–17
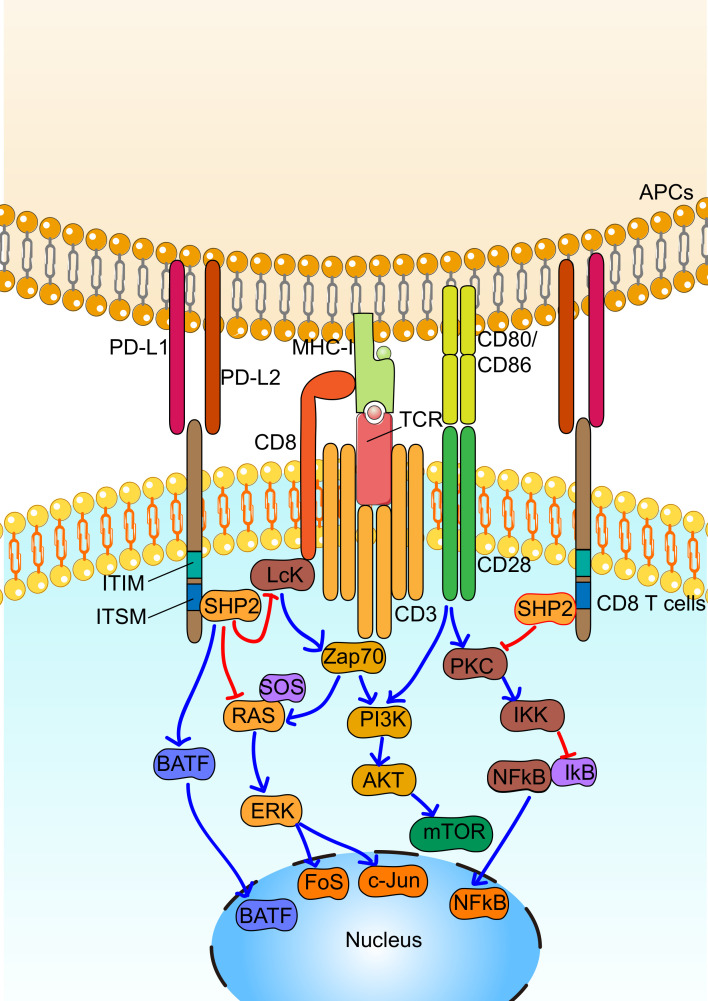
Figure 1.
Canonical programmed cell death 1 (PD-1) signaling in CD8 T cells. By engagement with its ligands, including PD-L1 or PD-L2, PD-1 is phosphorylated at immunoreceptor tyrosine-based switch motif (ITSM) tyrosine residue sites, which leads to the binding of SHP2. Recruited SHP2 directly downregulate T cell receptor (TCR) signaling via dephosphorylation of proximal signaling elements, including PI3K, RAS and PKC, leading to decreased activation, proliferation, cytokine production and survival of CD8+ T cells. In addition, PD-1 signaling increases the expression of basic leucine zipper transcriptional factor ATF-like factor (BATF), which affects differentiation of immune cells. APC, antigen-presenting cell; ITIM, immunoreceptor tyrosine-based inhibitory motif.
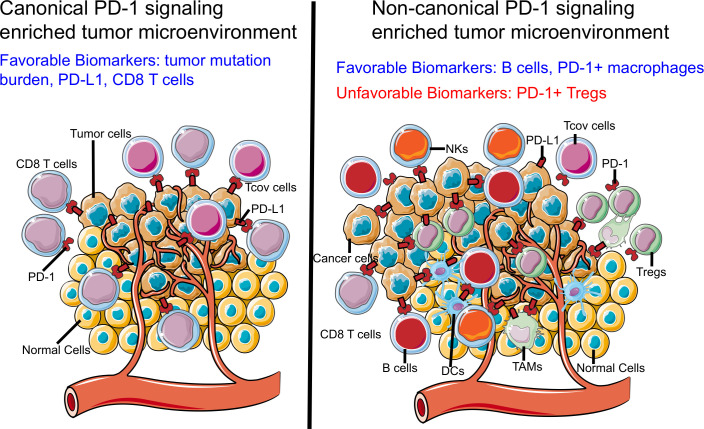
However, canonical PD-1 signaling is not the only type that exists in TME. For instance, in tumors containing tumor-infiltrating lymphocytes expressing heterogenous PD-118 and exhibiting frequent loss of human leukocyte antigen-Ⅰ (HLA-I) expression,19 such as Hodgkin’s lymphoma, PD-1 blockade therapy remains highly responsive.18 Meanwhile, a small fraction of patients with cancer exhibits rapid cancer progression during PD-1 blockade therapy, also known as hyperprogressive disease (HPD).20 Furthermore, increasing evidence indicates that PD-1 is not only expressed by CD8 or CD4 conventional T cells, but also by other cell types, including tumor cells (table 1),21 22 as well as many types of stromal cells (table 2), consisting of regulatory T cells (Tregs),23 24 B cells,25 macrophages,26 natural killer (NK) cells27 and dendritic cells (DCs),28 29 indicating the probable influence of PD-1 blockade therapy on these diverse cell types. Based on the current knowledge, PD-1 signaling in these cell types is distinct from canonical PD-1 signaling, both in terms of function and associated molecular pathways; hence, we termed the PD-1 signaling occurring in these alternate cell types as non-canonical PD-1 signaling. This review focuses on the recent advances on non-canonical PD-1 signaling and aims to broaden the knowledge in the field of oncoimmunology.
Table 1.
Non-canonical programmed cell death 1 (PD-1) signaling in cancer cells
| Cancer type | Biology effect | Potential implication in clinic | Ref |
| Human melanoma (tumor tissue, cell lines), mouse melanoma (cell lines) | Promoting tumorigenesis by activating mTOR signaling | Contribution of melanoma PD-1 to the efficacy of PD-1 blockade therapy | 22 |
| Human hepatoma (tumor tissue, cell lines), mouse hepatoma (cell lines) | Promoting tumorigenesis by activating mTOR signaling | Contribution of hepatoma PD-1 to the efficacy of PD-1 blockade therapy | 131 |
| Human pancreatic cancer (tumor tissue, cell lines) | Promoting proliferation of cancer cells by targeting CYR61/CTGF via hippo pathway | Pancreatic cancer cell-intrinsic PD-1 correlate with poor prognosis | 132 |
| Human NSCLC (tumor tissue, cell lines), mouse NSCLC (cell line, M109) | Inhibiting proliferation of NSCLC cells | Contribution of NSCLC-intrinsic PD-1 signaling to HPD during PD-1 blockade therapy | 133 |
| Human lung cancer (tumor tissue, cell lines), human CRC (cell lines) | Inhibiting proliferation of cancer cells by suppressing AKT and ERK signaling (lung cancer cells and CRC cells) | Contribution of cancer cell-intrinsic PD-1 signaling to HPD during PD-1 blockade therapy | 21 |
CRC, colorectal cancer; HPD, hyperprogressive disease; NSCLC, non-small cell lung cancer.
Table 2.
Non-canonical programmed cell death 1 (PD-1) signaling in stromal cells
| Cell type | Cancer type | Biology effect | Potential implication in clinic | Ref |
| Tregs | Human gastric cancer, mouse implanted tumor model (B16F0) | Promoting Tregs proliferation and immunosuppressive activity | Contribution of PD-1+ Tregs to HPD during PD-1 blockade therapy | 23 |
| Mouse implanted tumor model (B16F10) | PD-1 signaling maintain the expression of FOXP3 through proteolytic pathway | NA | 45 | |
| B cells | Human hepatoma, mouse orthotopic hepatoma (Hepa1-6) | Promoting tumor growth via secretion of IL-10 | Contribution of B cells to efficacy of PD-1 blockade therapy. | 25 |
| NKs | Mouse implanted tumor model (RMA-S, CT26, 4T1) | Suppressing NKs mediated tumor control | Contribution of NK cells to efficacy of PD-1 blockade therapy. | 27 |
| Human head and neck cancer | Inhibiting activation and cytotoxicity of NKs | Contribution of NK cells to efficacy of PD-1 blockade therapy. | 144 | |
| TAMs | Human colorectal cancer and mouse implanted tumor model (CT26) | Inhibiting phagocytic capacity against tumor cells | Contribution of TAMs to efficacy of PD-1 blockade therapy. | 26 |
| Human gastric cancer | Inhibiting phagocytic capacity against tumor cells | PD-1+ TAMs infiltration correlate with unfavorable prognosis in gastric cancer | 118 | |
| Myeloid cells | Mouse implanted tumor model (B16F10, MC38) | Inhibiting differentiation of myeloid cells by restraining cholesterol. | Contribution of myeloid cells to efficacy of PD-1 blockade therapy. | 119 |
| DCs | Human ovarian cancer (tumor tissue and ascites), mouse implanted tumor model (ID8, intraperitoneally) | Inhibiting NF-kB-mediated antigen presentation in a SHP-2-independent manner | Contribution of NKs to efficacy of PD-1 blockade therapy. | 28 |
| Human ovarian cancer | Promoting IL-10 production | Combined PD-1 blockade with IL-10 neutralization shows synergistic effect. | 29 | |
| Mouse implanted tumor model (ID8, intraperitoneally) | Promoting polarization toward an immunosuppressive and immature state by inhibiting NF-kB. | NA | 124 |
DCs, dendritic cells; HPD, hyperprogressive disease; IL, interleukin; NA, not available; NKs, natural killer cells; TAMs, tumor-associated macrophage; Treg, regulatory T cells.
Roles of PD-1 signaling in distinct cell types
Regulatory T cells
FOXP3-expressing Tregs are essential for the maintenance of peripheral tolerance; however, their suppressive effect can help tumor cells evade host immunity.30 31 A growing body of evidence suggests that Tregs are frequently accumulated in various tumor tissues by chemotaxis32 or driver gene alteration.33 34 Their intensity of infiltration is correlated with poor disease prognosis.35 36 Further, the infiltration of Tregs into inflamed tumors along with massive infiltration of CD4 effector T cells and cytotoxic T lymphocytes, has been reported in melanoma.37 38 In such cases, Treg infiltration correlates with a favorable outcome in patients with cancer.39 Furthermore, tumor-infiltrating Tregs are highly heterogeneous in terms of their functional state and stability, which might contribute to their variable correlation with prognosis of patient with cancer.40 41 Although further studies are needed to explore these phenomena, current reports demonstrate that targeting Tregs could be useful for the treatment of cancers.42 However, provided their potent role in the maintenance of peripheral tolerance, it is important to specifically target tumorous Tregs, while leaving the Tregs in other peripheral compartments unaffected, or minimally affected, to minimize the risk of autoimmune disorders.
In addition to conventional CD4 and CD8 T cells, a subset of Tregs also expresses high levels of PD-1.23 43–48 PD-1 on conventional CD4 T cells inhibits TCR signaling, which is essential for the survival and maintenance of the suppressive activity of Tregs,49 indicating that PD-1 expression on Tregs may inhibit their activation and suppressive activity. This hypothesis has been validated in a mouse model of autoimmune pancreatitis, which demonstrated that PD-1-deficient Tregs exhibited enhanced immunosuppressive activity compared with PD-1-sufficient Tregs.50 Furthermore, systemic PD-1 deficiency in mice was shown to cause autoimmunity,11 whereas, conditional PD-1 knockout on Tregs, particularly in the T follicular regulatory subset, resulted in their proliferation and enhanced immunosuppressive activity.51 Moreover, a preclinical study demonstrated that LKB1-deficient Tregs overexpress PD-1, as detected by flow cytometry (antibody clone, J43), while PD-1 blockade promotes the suppressive activity of Tregs, which in turn inhibits T helper 2-mediated immune responses.24 Additionally, a study by Takahiro et al demonstrated that during treatment with anti-PD-1 mAb, 4 of the 36 patients with gastric cancer succumbed to HPD.23 The patients with HPD had a massive infiltration of proliferating activated effector Tregs (eTregs), while patients without HPD had comparatively lower eTreg accumulation. Further, tumorous eTregs exhibited high expression of PD-1, as detected by flow cytometry (antibody clone: MIH4). Using human samples, the authors demonstrated that treatment with anti-PD-1 antibody increased the proliferation and immunosuppressive activity of Tregs in vitro.23 Furthermore, genetic ablation of PD-1 in murine Tregs increases their suppressive activity against antitumor immunity in vivo. Recently, the same group reported that PD-1 blockade reactivates CD28 and TCR signals both in CD8 T cells and Tregs. Intriguingly, they demonstrated that PD-1 expression balance of CD8 T cells and Tregs could predict response to PD-1 blockade therapy.52
The data discussed thus far has suggested that PD-1 suppresses the proliferative and immunosuppressive properties of Tregs. However, opposite effects have also been observed for PD-1 expression on Tregs.43 46 For instance, in the case of tumors and chronic viral infections, PD-1 is essential for maintaining FOXP3 expression on Tregs through a proteolytic pathway.45 As FOXP3 is critical for the maintenance of the suppressive function of Tregs, PD-1 on Tregs is believed to maintain its immunosuppressive functions. Furthermore, using a chronic graft versus-host disease (cGVHD) model, Takeru et al demonstrated that a low dose of interleukin (IL)-2 induces PD-1 expression on Tregs, particularly in the Irving L. Weissman 44+CD62L+ central-memory subset. Moreover, PD-1-deficient Tregs exhibit rapid proliferation following IL-2 administration, however, eventually become proapoptotic.43 In glioblastoma, PD-1high Tregs have been identified as a population of dysfunctional and exhausted Tregs, secreting interferon (IFN)-γ.47 Interestingly, administration of anti-PD-1 antibodies further enhanced the secretion of IFN-γ from Tregs. The contradictory roles of PD-1 on peripheral Tregs indicate that the effects elicited by PD-1 signaling on Tregs are context dependent. For instance, in a cGVHD model, stimulation with IL-2 at a low dose activated PD-1 signaling, while blockade of PD-1 signaling promoted apoptosis of Tregs, suggesting a potential role for PD-1 signaling in promoting, rather than suppressing, the immunosuppressive activity of Tregs in the cGVHD model. Nevertheless, the apoptotic Tregs in tumors exhibit superior immunosuppressive activity,53 indicating that PD-1 signaling blockade might enhance their immunosuppressive activity within the TME. Another possible reason for the differential behavior of Tregs is their heterogeneity. Although, that FOXP3 is highly specific to Tregs,54 studies suggest that FOXP3+CD4+ T cells are functionally and phenotypically heterogeneous, and consist of suppressive and non-suppressive T cells.35 42 55 56 For example, FOXP3+CD4+ T cells in colorectal cancer can be classified into three subsets based on their expression levels of CD45RA and FOXP3: Fraction I (Fr-I, FOXP3lowCD45RA+), Fraction II (Fr-II, FOXP3highCD45RA-), and Fraction III (Fr-III, FOXP3lowCD45RA−). Fr-I cells, representing naïve Tregs, differentiate into highly suppressive and functionally stable effector Tregs or Fr-II cells in response to stimulation by tumor antigen. In contrast, Fr-III cells are not suppressive and secrete inflammatory cytokines.35 Therefore, PD-1 signaling may simultaneously inhibit inflammatory cytokine production by Fr-III cells and impair the immunosuppressive activity and proliferation of Fr-II Tregs. These data highlight a need to accurately explicate the differential roles of PD-1 on various Treg subsets under distinct microenvironments. The clinical benefit of PD-1 blockade therapy needs to be assessed accordingly (figure 2).
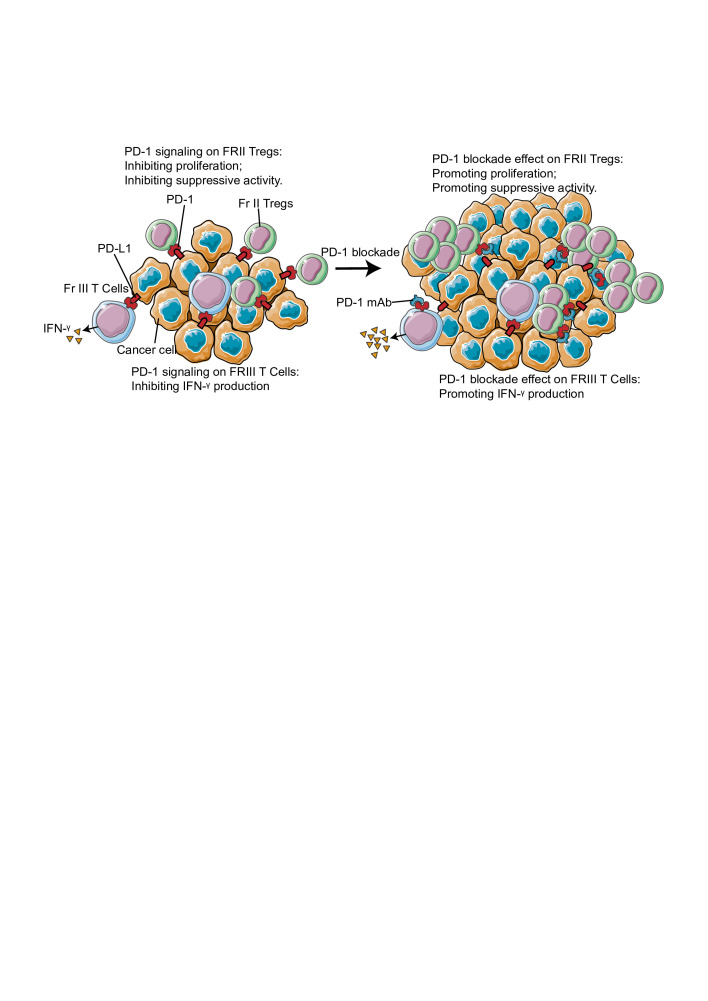
Figure 2.
Role of non-canonical programmed cell death 1 (PD-1) signaling in tumorous Treg. PD-1 signaling inhibit proliferation and suppressive activity of tumorous Fr-II Tregs, while inhibiting IFN-γ production of tumorous Fr-III Tregs. In tumor, the major Treg population is Fr-II Treg. Thus, blockade of PD-1 signaling boost Fr-II Treg by promoting its proliferation and suppressive activity and potentially leads to hyperprogressive disease (HPD). IFN, interferon.
B cells
B cells are abundantly present in the TME.57 58 A recently conducted single-cell RNA-sequencing study on human lung cancer stromal cells demonstrated that B cells were the most enriched cell type in tumor stroma.59 However, the precise role of B cells in tumor immunity is debatable, as paradoxical roles have been reported. On one hand, tumor-infiltrating B cells have been reported to promote tumor growth by producing inhibitory cytokines, including IL-10 and TGF-β60 61 and by interacting with either immune cells via PD-L160 or tumor cells through CD40/CD154 signaling, as shown in hepatocellular carcinoma (HCC).62 On the other hand, tumor-infiltrating B cells reportedly delay tumor growth by secreting antibodies against the tumor,63 producing proinflammatory cytokines, including IL-12,64 antigen presentation,65 or inducible T cell costimulator ligand (ICOSL) / inducible T cell costimulator (ICOS interaction.66 The contrasting roles of tumor-infiltrating B cells may be caused by their high heterogeneity.59 Interestingly, recent studies suggest the involvement of B cells in PD-1 blockade therapy response,67–69 showing clonal expansion, and a unique functional state of B cells as responders to PD-1 blockade therapy.68 Although the underlying mechanism remains largely unknown, these studies suggest a potential role of PD-1 signaling in controlling the functions of tumor-infiltrating B cells.
In addition to T cells, B cells have also been reported to express PD-1.70 71 The role of PD-1 on B cells was first observed in PD-1−/− mice, which exhibited moderate splenomegaly and increased B cell proliferation in response to anti-IgM antibody stimulation.72 Further, resting human B cells express PD-1 at a basal level, while its expression is rapidly induced on stimulation of the toll-like receptor 9 (TLR9) pathway by CpG-B.70 Subsequent PD-1/PD-L1 interaction inhibits the B cell receptor signaling pathway, resulting in the attenuation of cytokine production and proliferation of B cells.71 Recently, a novel tumor-promoting subset of B cells has been identified that expresses high levels of PD-1 in human HCC.25 These PD-1high B cells constitute approximately 10% of the total B cell population in advanced HCC. Unlike conventional regulatory B cells (Bregs), PD-1high B cells exhibit a CD5highCD24−/−CD27high/+CD38dim phenotype. PD-1 expression is highly induced by hyaluronan fragments and TLR agonists, including Pam3CysSK4, lipopolysaccharide (LPS), and oligodeoxynucleotides containing CpG motifs.25 TLR4-induced BCL6 upregulation plays a dominant role in the induction of PD-1 on B cells. The interaction between PD-L1 and PD-1high B cells induces the expression of IL-10, a well-defined immune-suppressive cytokine. These data suggest that the contribution of B cells in PD-1-based immunotherapy should be critically evaluated and PD-1 signaling must be further investigated in different B cell subsets (figure 3). Besides, follicular T-helper cell (Tfh), which exhibit high PD-1 expression, plays a critical role in promoting maturation of B cells.73 Given PD-1 signaling is essential for positioning and function of Tfh, it is reasonable to speculate that PD-1 blockade therapy could affect B cells in an indirect manner.74 In this regard, the role of Tfh on B cells in PD-1 needs blockade therapy which need further study.
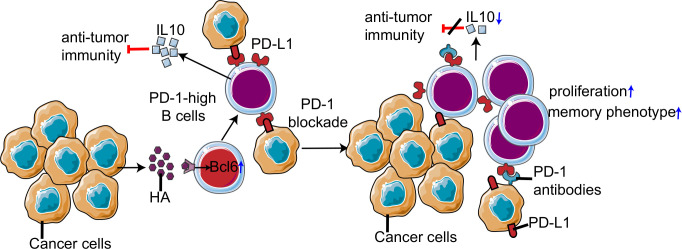
Figure 3.
Non-canonical programmed cell death 1 (PD-1) signaling in tumorous B cells. Hepatocellular carcinoma (HCC) factors induce PD-1high B cells through TLR4-driven Bcl-6 upregulation. By engagement with PD-L1, non-canonical PD-1 signaling in B cells inhibits antitumor immunity by enhancing IL-10 production. PD-1 blockade decreases interleukin (IL)-10 production and promotes proliferation and differentiation into a memory phenotype of B cells, which may influence efficacy of PD-1 blockade therapy.
NK cells
NK cells were first identified in 1975.75 76 Since that time, they have been classified as lymphocytes based on their morphology, phenotype, and origin, and are considered as a part of innate immunity due to the absence of antigen-specific receptors.77 The functional status of NK cells is mediated by the cumulative effect of multiple activating and inhibitory receptors. When activated, NK cells kill virus-infected cells and malignant tumor cells in an major histocompatibility complex (MHC) non-restricted manner.78 NK cells also secrete various inflammatory cytokines with antitumor effects, including IFN-γ and tumor necrosis factor (TNF)-α. Indeed, the presence of NK cells in solid tumors has been described as a good prognostic factor.79–82
Studies have suggested three potential contributions made by PD-1 signaling in NK cells during PD-1 blockade therapy: (1) HLA-I is generally not expressed in human cancer cells, resulting in no interaction between HLA-I and CD8 T cells83 84; however, PD-1-based immunotherapy is effective in these tumors. For instance, 79% (85/108) of the classical Hodgkin’s lymphomas (cHL) exhibit low to no expression of MHC class I molecules.19 Meanwhile, PD-1 blockade therapy is highly effective in the treatment of relapsed or refractory cHL.18 (2) Tumor cells with high tumor mutation burden (TMB) are more likely to be recognized by CD8 T cells, and patients carrying tumors with TMB, such as malignant melanoma85 and non-small cell lung cancer,86 are more likely to benefit from PD-1 blockade therapy.2 However, even in tumors with low TMB, including malignant melanoma and non-small cell lung cancer, a small fraction of patients are responsive to PD-1 blockade therapy.85 86 (3) Human NK cells express PD-1 in various cancers, including Hodgkin’s lymphoma.87–89 Hsu et al27 used different mouse models to investigate PD-1 signaling in NK cells and its role in PD-1-based immunotherapy. Interestingly, PD-1 was found to be expressed by tumor-infiltrating NK cells with an activated but exhausted phenotype. Furthermore, NK cells contributed to the antitumor effect of PD-1-based immunotherapy.27 However, this study had several limitations. For instance, although PD-1 expression on human NK cells has been well characterized, its expression on mouse NK cells remains debatable, as they do not express PD-1 even under robust activation conditions, such as cytomegalovirus infection.90 This study concluded the PD-1 expression on NK cells by flow cytometry, while a recent study suggested that dying immune cells express a nuclear antigen, which cross-reacts with mouse anti-PD-1 monoclonal antibody, leading to a false positive PD-1 staining on NK cells.91 Second, the molecular mechanisms underlying the effect of PD-1-based immunotherapy on NK cells remains unclear. Conversely, a recent study challenged the expression of PD-1 on mouse and human NK cells under diverse conditions.92 Overall, the expression and effect of PD-1 on NK cells remains unclear. However, combination therapies including PD-1 blockade and NK cell activation strategies are currently in clinical trials.93 Further studies are urgently needed to address the expression of PD-1 as well as its precise role in NK cells.
Lastly, considering that NK cells are a small minority of cells in the TME of multiple cancer types, other cell types, such as CD4 T cells94 95 and macrophages96 may function cooperatively with NK cells to exert antitumor effects during PD-1 blockade therapy. In a study of patients with relapsed or refractory cHL, the authors demonstrated that PD-L1 expression and MHC class II positivity on Hodgkin Reed-Sternberg cells are favorable prognostic biomarkers of PD-1 blockade therapy, which potentiate an alternative CD4 T cell-mediated mechanism of response to PD-1 blockade therapy.97 Accordingly, a study suggest that cytotoxic CD4 T cells are essential to the efficacy of PD-1 blockade therapy on MHC class II-expressing tumors.98 Intriguingly, a recent study suggested a tumor-promoting role for PD-L1 reverse signaling in promoting tumor cell growth, proliferation and metabolism in cHL, which may also participate in PD-1 blockade therapy.99
Macrophages
Macrophages that infiltrate solid tumors are referred to as tumor-associated macrophages (TAMs).100 High expression of TAM markers, especially M2 markers, is generally associated with poor prognosis of patients with cancer.100 In most cases, bone marrow monocytes differentiate into TAMs following stimulation by TME factors, including cytokines and hypoxia.101 However, TAMs are also reportedly derived from myeloid progenitors present in the yolk sac.102 103 Additionally, monocyte-derived macrophages can be polarized following stimulation by cytokines and other environmental factors.
Macrophages are categorized as M1 or M2 based on their polarization. M1 refers to a proinflammatory state, which is generally driven by IFN-γ/LPS; while M2 refers to an anti-inflammatory state, generally mediated by IL-4 or IL-13.100 104 However, the M1/M2 model is an oversimplification and cannot precisely describe the polarized state of TAMs.105 In fact, TAMs express both M1 and M2 related markers concurrently.26 106 Therefore, provided the complexity of their origin and polarization state, TAMs exhibit high heterogeneity.107 Generally speaking, TAMs play a dominant role in promoting cancer progression by modulating nearly every aspect of tumor biology, including angiogenesis,108 metastasis,109 proliferation,110 immune suppression,111 112 inflammation113 and stem cell maintenance.114
Macrophages have been recently described as expressing PD-1 under specific conditions, such as tuberculosis,115 sepsis116 and zymosan-induced inflammation.117 However, its expression in macrophages is induced by TLR signaling, whereas in T cells, it is driven primarily by TCR signaling.117 PD-1 signaling in macrophages inhibits M1 polarization in-vitro via attenuation of STAT1/NF-κB phosphorylation.117 Furthermore, PD-1−/− mice are markedly protected from lethal sepsis in vivo; however, the bactericidal effect is reversed, when macrophages are depleted by clodronate liposomes.116 Moreover, PD-1 blockade augments phagocytosis and intracellular killing activity of macrophages against BCG.115 Although these results reveal a potential inhibitory effect for PD-1 signaling on macrophages, the specific role of macrophage-associated PD-1 in tumor immunity remains unclear. In 2017, the research group of Sydney R. Gordon was the first to evaluate the expression and function of PD-1 on TAMs.26 Using flow cytometry and immunofluorescence, they reported that both human and murine TAMs express PD-1. Specifically, PD-1+ TAMs exhibited M2-like phenotype and accumulated in TME over time. Furthermore, in vitro and in vivo studies showed that PD-1+ TAMs had lower phagocytic properties, which were abrogated on PD-L1-knockout in mice. These results suggest that PD-1 is a functionally important M2-like marker. In fact, PD-1+ TAM infiltration correlates with poor prognosis in patients with human gastric cancer.118 Furthermore, PD-1 expression has been reported in murine tumorous myeloid cells using flow cytometry (antibody clone: RMP1-30). Intriguingly, PD-1 deletion in myeloid cells (PD-1f/fLysMcre mice) effectively delays tumor growth, similar to that observed during global deletion of PD-1, whereas deletion of PD-1 in T cells (PD-1f/fCD4cre mice) is less effective.119 These results indicate a crucial role for PD-1 in inhibiting the antitumor immunity of myeloid cells. Similarly, PD-1 expression was also reported in granulocyte/macrophage progenitors (GMPs), which increases during emergency hematopoiesis facilitating differentiation into myeloid-derived suppressor cells (MDSCs). Meanwhile, PD-1 deletion in myeloid cells (PD-1f/fLysMcre mice) inhibits the accumulation of GMPs and MDSCs.119 Further, activation of ERK1/2, as well as the mTOR1 kinase complex by granulocyte colony-stimulating factor in myeloid cells, is inhibited by PD-1 expression. mTOR participates in the regulation of myeloid progenitor cell differentiation, while ERK1/2 controls the differentiation of APCs.119 Taken together, these data indicate that the effect of PD-1 blockade on TAMs should not be neglected, although it remains unclear whether patients with cancer with high PD-1+ TAM infiltration would also benefit from PD-1 blockade therapy.
Dendritic cells
In spite of their paucity, DCs play a central role in the initiation of antigen-specific immunity and tolerance.120 Similar to other tumor-infiltrating stromal cells, DCs exhibit high heterogeneity.120 The functional specificity of DC subpopulations results from the expression of different receptors, including PD-1.28 29 121 122 Furthermore, various inflammatory factors induce PD-1 expression on DCs, which then suppresses innate immunity against bacterial infections by inhibiting IL-12 and TNF-α,122 and promotes apoptosis of activated DCs.123 Since PD-1 expression on DCs is induced by inflammatory factors, it is possible that tumor-infiltrating DCs also express PD-1, driven by chronic inflammation, a hallmark of cancer. By using flow cytometry and immunofluorescence, James et al observed a similar phenomenon of PD-1 expression in tumor-infiltrating DCs using an implanted tumor model of ovarian cancer. In murine species, immature PD-1+ DCs exhibit a classical DC phenotype (CD11c+ CD11b+ CD8-) that is suppressive, and respond weakly to danger signals. PD-1 signaling in mice has also been reported to inhibit NF-κB, a crucial signaling molecule involved in the maturation and activation of DCs.124 Similarly, PD-1+ DCs were also observed in human ovarian cancer, as determined by flow cytometry.28 Similar to its role in murine species, PD-1 expression on human DCs suppresses NF-κB-dependent cytokine release in a SHP-2-dependent manner. Further, PD-1 expression on DCs is induced by IL-10, a well-established suppressive cytokine, while PD-1 blockade leads to increased IL-10 production by DCs. In this regard, combined PD-1 blockade therapy with IL-10 neutralization induced a synergistic antitumor effect.29 These data are in agreement with that of another study, which suggested that PD-1 signaling in monocytes of HIV-infected individuals inhibits IL-10 production.125 Meanwhile, a recent study employed flow cytometry (antibody clone: EH12.2H7) to demonstrate that human tumorous DCs express PD-1. Moreover, PD-1 on DCs neutralizes PD-L1 in cis to inhibit canonical PD-1 signaling in T cells.126 However, these findings were exclusively based on in vitro experiments and, thus, may not reflect actual tumor-infiltrating DCs. Therefore, in vivo studies are warranted to examine the cis effect of PD-1 on DCs to modulate cancer immunity.
Tumor cells
Until 2010, the accepted dogma was that PD-1 is specifically expressed by cells of the hemopoietic lineage. However, by using flow cytometry and immunofluorescence, Tobias et al reported that a subpopulation of melanoma cells express PD-1, and that PD-1+ cancer cells are responsible for tumor initiation.127 In this study, PD-1 was primarily examined as a biomarker, enriched in ABCB5+ malignant melanoma-initiating cells. Meanwhile, the function of PD-1 signaling in melanoma cells remained largely unknown until 2015. Tobias et al further demonstrated that human and murine melanoma cells contain PD-1-expressing subpopulations by using flow cytometry, immunofluorescence, RT-PCR and western blotting.22 In melanoma cells, intrinsic PD-1 signaling plays a key role in tumor initiation in an immunosuppression-independent manner. By interacting with its cognate ligand, melanoma-PD-1 triggers the activation of downstream effectors (eg, ribosomal protein S6) of mTOR signaling, which further accelerate tumor growth. Interestingly, intrinsic PD-1 signaling in melanoma cells activates downstream mTOR signaling in a PI3K/AKT-independent manner, distinct from that observed in canonical PD-1 signaling. In T cells, interaction of PD-1 with PD-L1/PD-L2 inhibits TCR signaling via SHP-2 tyrosine phosphatase.14 SHP-2 expression is reported in a number of cancer cell types, including melanoma,128 breast cancer129 and glioblastoma.130 In cancer cells, SHP-2 may activate mTOR signaling130; hence, the effect of PD-1 signaling on mTOR activation might be tissue specific. Consistent with this, both murine and human HCCs were reported to express PD-1.131 HCC cell-PD-1 promotes phosphorylation of eukaryotic initiation factor 4E (eIF4E) and ribosomal protein S6 (S6), leading to enhanced tumor cell proliferation.131 In addition, intrinsic PD-1 signaling in pancreatic cancer cells promotes tumor proliferation by decreasing the phosphorylation of MOB1, a central component of the Hippo signaling pathway.132 These data demonstrate that tumor cell-intrinsic PD-1 signaling exerts a protumor effect by activating mTOR in melanoma, HCC and pancreatic cancer cells (figure 4).
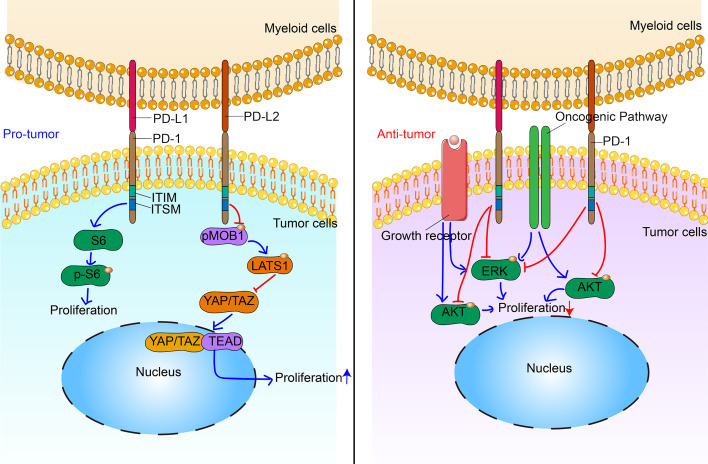
Figure 4.
Multifaceted roles of non-canonical programmed cell death 1 (PD-1) signaling in tumor cells. In malignant melanoma, hepatocellular carcinoma and pancreatic cancer, non-canonical PD-1 signaling promotes proliferation of cancer cell via interacting with mTOR and Hippo signaling. In non-small cell lungcarcinoma (NSCLC), non-canonical PD-1 signaling inhibits proliferation of cancer cell via suppressing AKT and ERK signaling. ITIM, immunoreceptor tyrosine-based inhibitory motif; ITSM, immunoreceptor tyrosine-based switch motif.
On the contrary, tumor cell-intrinsic PD-1 has been reported as a tumor suppressor gene in non-small cell lung carcinoma (NSCLC) and colon cancer.21 133 A previous study reported a 61-year-old woman diagnosed with stage IV NSCLC who was unresponsive to several chemotherapies and experienced rapid disease progression after receiving radiotherapy combined with pembrolizumab.133 PD-1 and PD-L1 expression in the irradiated tumor tissue biopsies obtained prior to pembrolizumab treatment were assessed by immunohistochemistry (IHC). Unexpectedly, the cancer cells displayed diffuse PD-1 staining. Meanwhile, RNA-sequencing data from lung cancer cell lines validated PD-1 expression in only 7 of 236 cell lines considered. Further, treatment with PD-1 antibodies (clone RMP1-14, rat IgG2a) accelerated the growth of M109 murine NSCLC in vitro and in vivo. However, the underlying mechanisms associated with these phenomena remain unclear. In line with this, a recent study shows that tumor cell-intrinsic PD-1 expression suppresses the canonical signaling pathways, including AKT and ERK1/2 in NSCLCs and colon cancer, while mTOR signaling remains unaffected.21 Treatment with either nivolumab (anti-PD-1 antibody) or ateolizumab (anti-PD-L1 antibody) enhances the growth of NCI-H1299 transplanted tumors in NOD-SCID IL-2 receptor gamma null mouse, one of the most immunodeficient mouse strains (figure 4). However, further studies are needed to explore the underlying molecular mechanisms responsible for these contradictory effects mediated by tumor cell-intrinsic PD-1 in melanoma, HCC and NSCLCs.
Furthermore, a recent study reported the potential for obtaining false-positive PD-1 staining in melanoma cells, including B16F1091 due to cross reaction of antibodies with a nuclear antigen. These results raised the question regarding whether epithelial tumor cells truly express PD-1. Although evidence of PD-1 expression in tumor cells has been provided at the mRNA (qRT-PCR and RNA-sequencing), and protein (IHC, immunofluorescence, western blotting, and FACS) levels in previous studies,21 22 127 131 133 we re-evaluated the expression of PDCD1 using the Cancer Cell Line Encyclopedia (Broad, 2019) in lung cancer (NSCLC and SCLC), colorectal, breast, melanoma, kidney, stomach and liver cancer cell lines. Our data suggest that majority of epithelium-derived cancer cells express PDCD1 at extremely low, to undetectable, levels (RPKM <1; an RPKM of 1 is considered as a threshold and is equivalent to one mRNA copy per cell134) (figure 5). The low to undetectable expression of PDCD1 in cancer cell lines may be attributable to its expression only in a subpopulation of cancer cells.21 22 Therefore, single-cell RNA-sequencing of cancer cells might be helpful in validating the expression of PDCD1 in specific cancer cell subpopulations and characterizing the signaling involved in the regulation of PDCD1 in cancer cells.
Figure 5.
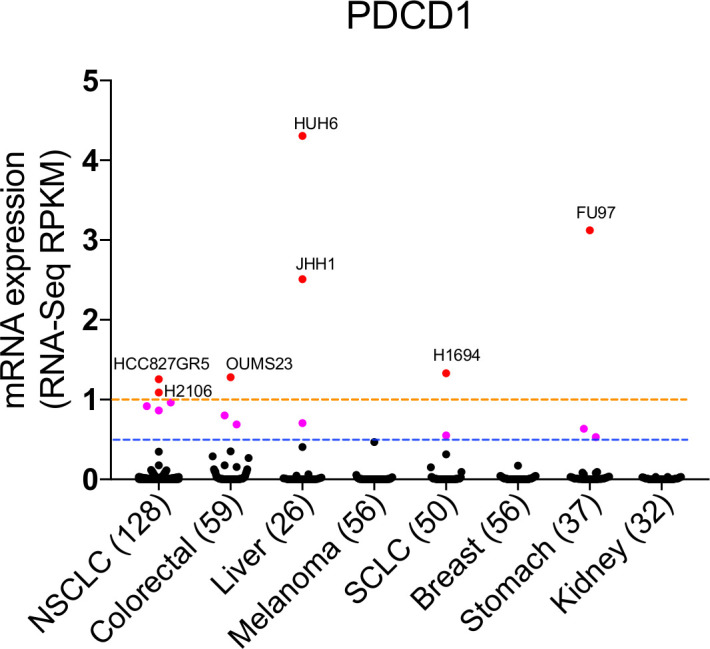
Expression of PDCD1 in cancer cell lines. PDCD1 mRNA expression (RNA-seq, RPKM) data of cancer cell lines (Cancer Cell Line Encyclopedia, Broad, 2019) were downloaded from cBioPortal (https://portals.broadinst-itute.org/ccle/) in 27 March 2020. PDCD1 expression of epithelium-transformed cancer was analyzed. Note: breast cancer cell line (DU4475, RPKM=48.02668) was not included in our panel as it would make this panel less informative.
Implications of non-canonical PD-1 signaling in cancer therapy
PD-1-based immunotherapy has achieved great clinical success and has been approved for the treatment of various cancers.16 17 135 136 However, only a fraction of the patient population benefits from PD-1 blockade therapy.137 Even with the help of validated biomarkers, including PD-L1 and TMB, the response rate remains low (eg, approximately 40% in patients with NSCLC with PD-L1 expression in at least 50% of the cancer cells16). Furthermore, a small fraction of patients with cancer receiving PD-1-based immunotherapy succumbs to HPD.20 Hence, there is a critical need to determine the cause for the beneficial or deleterious effects of PD-1 blockade therapy in patients.
Note, although treatment with anti-PD-1 antibodies affects all PD-1-expressing cells, since conventional CD4 T and CD8 T cells often account for the dominant cells expressing PD-1, effects of PD-1 blockade on other PD-1-expressing cells have been neglected. In fact, the antitumor effect induced by PD-1 blockade therapy is a cumulative effect of its influence on all PD-1-expressing cells. In this regard, cells associated with non-canonical PD-1 signaling should also be evaluated as biomarkers for combinatorial therapeutic strategies.
Potential biomarkers for PD-1 blockade therapy
To date, most of the validated biomarkers related to PD-1 blockade therapy, including PD-L1,138 139 TMB86 136 and CD8 T cell infiltration,140 are based on the mechanisms underlying CD8 T cell-driven antitumor activity. However, even with the help of these biomarkers, it remains difficult to identify responders and non-responders of PD-1 blockade therapy.11 For example, PD-L1 expression does not always correlate with clinical outcomes. Although patients with advanced NSCLC with PD-L1+ tumors (PD-L1 expression on at least 50% of tumor cells) have been reported to respond well to PD-1 blockade therapy, only 44.8% of patients benefit from this therapy.15 Similar results have been reported for TMB as a biomarker for PD-1 blockade therapy, for which a group of patients with low TMB responded well to PD-1 blockade therapy.85 As mentioned earlier, since all PD-1-expressing cells are affected by PD-1 blockade therapy, it is helpful to consider the effect of PD-1 antibodies on these additional PD-1-expressing cells when developing novel biomarkers. For example, Hodgkin’s lymphoma lacks MHC-I expression and has low TMB, making it challenging to generate effective antitumor CD8 T cell responses. Nevertheless, it responds well to PD-1 immunotherapy. These results suggest that other PD-1-expressing cell types, such as B cells and TAMs, may serve as novel biomarkers for PD-1 blockade therapy (figure 6). Interestingly, a few recent studies reveal that B cells are the strongest prognostic factor in patients with melanoma,67 68 sarcoma69 and renal cell carcinoma68 receiving PD-1 blockade therapy, even though the tumors have low level of CD8 T cell infiltration.69 Moreover, patients having tumors with high Treg infiltration should avoid anti-PD-1 antibody monotherapy, as it may lead to HPD due to increased expansion and immunosuppressive activity of Tregs.23 Further, the effect of PD-1 blockade therapy in such patients can be determined using patient derived xenograft models. Lastly, given a recent study demonstrated that PD-1 expression balance of T cells could predict efficacy of PD-1 blockade therapy,52 it would be valuable to determine the association between PD-1 expression balance of all PD-1-expressing cell subsets and clinical response of patients receiving PD-1 blockade therapy.
Figure 6.
Tumor microenvironment (TME) characterized by canonical or non-canonical programmed cell death 1 (PD-1) signaling. Based on the different status of PD-1 signaling, we classified TME into two types: canonical PD-1 signaling-enriched TME (left) and non-canonical PD-1 signaling-enriched TME (right). Canonical PD-1 signaling-enriched TME is characterized by high infiltration of CD8 T cells and conventional CD4 T cells. Biomarkers for PD-1 blockade therapy including PD-L1 and TMB may work optimally in this type TME. However, biomarkers for non-canonical PD-1 signaling-enriched TME remains largely unknown. DC, dendritic cell; NK, natural killer; TAM, tumor-associated macrophages.
Combination therapy
As the response rate to PD-1 blockade monotherapy remains low, combination therapy has emerged as a recent trend in cancer treatment.141 In this context, targeting non-canonical PD-1 signaling may be considered as a novel strategy. For example, expansion of PD-1-expressing Tregs during PD-1 blockade therapy serves as a potential cause of HPD. Hence, targeting Tregs could be an important strategy for the prevention of HPD, while enhancing the efficacy of PD-1 blockade therapy. In fact, combination of nivolumab (anti-PD-1 antibody) and ipilimumab (anti-CTLA4 antibody) has resulted in higher response rates and longer progression-free survival in malignant melanoma patients than nivolumab or ipilimumab alone.135 Further, the HPD rate was also reduced in the combination group,135 which was likely due to the tumorous Treg-depleting effect of ipilimumab.142 In addition, a previous study reported that CCR4 is expressed specifically on the surface of tumorous effector Tregs, and an anti-CCR4 mAb (mogamulizumab) effectively depletes tumorous Tregs.42 Further, a recent phase I clinical trial reported an acceptable safety profile for mogamulizumab and nivolumab combination therapy.143
Conclusions
Due to the well-established role of canonical PD-1 signaling in T cells, PD-1-based immunotherapy has achieved great clinical success. However, the response rate remains low, while a small group of patients succumb to HPD during PD-1 blockade therapy. Hence, canonical PD-1 signaling does not provide a complete explanation for the effect. Multiple studies have reported the expression and function of PD-1 on B cells, TAMs, DCs, NKs, Tregs and cancer cells, although the role of PD-1 in these cells remains unclear. Furthermore, it has been shown that non-canonical PD-1 signaling plays an important role in PD-1 blockade therapy response and HPD. Thus, a detailed understanding of non-canonical PD-1 signaling may provide novel biomarkers for identifying responders and non-responders to PD-1 blockade therapy. Additionally, it may inform the directed exploration of strategies for combinational therapy to markedly enhance the efficacy of PD-1-based immunotherapy.
Acknowledgments
Special thanks to Wang Chunjuan for her critical reading of the manuscript and kindly help during the COVID-19 pandemic.
Footnotes
HZ and YJ contributed equally.
Contributors: BZ, HC and ZL provided direction and guidance throughout the preparation of this manuscript. HZ and YJ collected and interpreted studies and was a major contributor to the writing and editing of the manuscript. XW, NW, JS, WZ, LY and ZL collected and interpreted studies. JA and XZ reviewed and made significant revisions in language to the manuscript. All authors read and approved the final manuscript.
Funding: This work was supported by the National Natural Science Foundation of China (No. 81472648, No. 81620108023 to Zhu Bo, and No. 31900627 to Zha Haoran).
Competing interests: No, there are no competing interests.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480:480–9. 10.1038/nature10673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015;348:69–74. 10.1126/science.aaa4971 [DOI] [PubMed] [Google Scholar]
- 3.Chen DS, Mellman I. Oncology meets immunology: the Cancer-Immunity cycle. Immunity 2013;39:1–10. 10.1016/j.immuni.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 4.Chen DS, Mellman I. Elements of cancer immunity and the cancer–immune set point. Nature 2017;541:321–30. 10.1038/nature21349 [DOI] [PubMed] [Google Scholar]
- 5.Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002;3:991–8. 10.1038/ni1102-991 [DOI] [PubMed] [Google Scholar]
- 6.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol 2004;22:329–60. 10.1146/annurev.immunol.22.012703.104803 [DOI] [PubMed] [Google Scholar]
- 7.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359:1350–5. 10.1126/science.aar4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren D, Hua Y, Yu B, et al. Predictive biomarkers and mechanisms underlying resistance to PD1/PD-L1 blockade cancer immunotherapy. Mol Cancer 2020;19:19 10.1186/s12943-020-1144-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishida Y, Agata Y, Shibahara K, et al. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. Embo J 1992;11:3887–95. 10.1002/j.1460-2075.1992.tb05481.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, et al. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. Journal of Experimental Medicine 2012;209:1201–17. 10.1084/jem.20112741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol 2018;18:153–67. 10.1038/nri.2017.108 [DOI] [PubMed] [Google Scholar]
- 12.Hui E, Cheung J, Zhu J, et al. T cell costimulatory receptor CD28 is a primary target for PD-1–mediated inhibition. Science 2017;355:1428–33. 10.1126/science.aaf1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamphorst AO, Wieland A, Nasti T, et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science 2017;355:1423–7. 10.1126/science.aaf0683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 2018;8:1069–86. 10.1158/2159-8290.CD-18-0367 [DOI] [PubMed] [Google Scholar]
- 15.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med Overseas Ed 2013;369:122–33. 10.1056/NEJMoa1302369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–Positive Non–Small-Cell lung cancer. New England Journal of Medicine 2016;375:1823–33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 17.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. New England Journal of Medicine 2015;373:1803–13. 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin’s Lymphoma. New England Journal of Medicine 2015;372:311–9. 10.1056/NEJMoa1411087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roemer MGM, Advani RH, Redd RA, et al. Classical Hodgkin Lymphoma with Reduced β 2 M/MHC Class I Expression Is Associated with Inferior Outcome Independent of 9p24.1 Status. Cancer Immunol Res 2016;4:910–6. 10.1158/2326-6066.CIR-16-0201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Champiat S, Ferrara R, Massard C, et al. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nat Rev Clin Oncol 2018;15:748–62. 10.1038/s41571-018-0111-2 [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Yang X, Zhang C. Tumor cell-intrinsic PD-1 receptor is a tumor suppressor and mediates resistance to PD-1 blockade therapy. Proceedings of the National Academy of Sciences of the United States of America, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleffel S, Posch C, Barthel SR, et al. Melanoma cell-intrinsic PD-1 receptor functions promote tumor growth. Cell 2015;162:1242–56 https://pubpeer.com/publications/EF751634AC1B70FBAAC2A4649D8EC4?utm_source=Firefox&utm_medium=BrowserExtension&utm_campaign=Firefox 10.1016/j.cell.2015.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamada T, Togashi Y, Tay C, et al. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci U S A 2019;116:9999–10008. 10.1073/pnas.1822001116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang K, Blanco DB, Neale G, et al. Homeostatic control of metabolic and functional fitness of Treg cells by LKB1 signalling. Nature 2017;548:602–6. 10.1038/nature23665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao X, Lao X-M, Chen M-M, et al. PD-1hi identifies a novel regulatory B-cell population in human hepatoma that promotes disease progression. Cancer Discov 2016;6:546–59. 10.1158/2159-8290.CD-15-1408 [DOI] [PubMed] [Google Scholar]
- 26.Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017;545:495–9. 10.1038/nature22396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu J, Hodgins JJ, Marathe M, et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J Clin Invest 2018;128:4654–68. 10.1172/JCI99317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karyampudi L, Lamichhane P, Krempski J, et al. PD-1 blunts the function of ovarian tumor-infiltrating dendritic cells by inactivating NF-κB. Cancer Res 2016;76:239-50. 10.1158/0008-5472.CAN-15-0748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamichhane P, Karyampudi L, Shreeder B, et al. IL10 release upon PD-1 blockade sustains immunosuppression in ovarian cancer. Cancer Res 2017;77:6667–78. 10.1158/0008-5472.CAN-17-0740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu C, Workman CJ, Vignali DAA. Targeting regulatory T cells in tumors. Febs J 2016;283:2731–48. 10.1111/febs.13656 [DOI] [PubMed] [Google Scholar]
- 31.Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression - implications for anticancer therapy. Nat Rev Clin Oncol 2019;16:356–71. 10.1038/s41571-019-0175-7 [DOI] [PubMed] [Google Scholar]
- 32.Facciabene A, Peng X, Hagemann IS, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells. Nature 2011;475:226–30. 10.1038/nature10169 [DOI] [PubMed] [Google Scholar]
- 33.Sugiyama E, Togashi Y, Takeuchi Y, et al. Blockade of EGFR improves responsiveness to PD-1 blockade in EGFR -mutated non–small cell lung cancer. Sci Immunol 2020;5:eaav3937 10.1126/sciimmunol.aav3937 [DOI] [PubMed] [Google Scholar]
- 34.Kumagai S, Togashi Y, Sakai C, et al. An oncogenic alteration creates a microenvironment that promotes tumor progression by conferring a metabolic advantage to regulatory T cells. Immunity 2020;53:187–203. 10.1016/j.immuni.2020.06.016 [DOI] [PubMed] [Google Scholar]
- 35.Saito T, Nishikawa H, Wada H, et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med 2016;22:679–84. 10.1038/nm.4086 [DOI] [PubMed] [Google Scholar]
- 36.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 2005;102:18538–43. 10.1073/pnas.0509182102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spranger S, Spaapen RM, Zha Y, et al. Up-Regulation of PD-L1, IDO, and Tregs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci Transl Med 2013;5:200ra116 10.1126/scitranslmed.3006504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams JB, Horton BL, Zheng Y, et al. The EGR2 targets LAG-3 and 4-1BB describe and regulate dysfunctional antigen-specific CD8+ T cells in the tumor microenvironment. Journal of Experimental Medicine 2017;214:381–400. 10.1084/jem.20160485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salama P, Phillips M, Grieu F, et al. Tumor-Infiltrating FOXP3 + T Regulatory Cells Show Strong Prognostic Significance in Colorectal Cancer. Journal of Clinical Oncology 2009;27:186–92. 10.1200/JCO.2008.18.7229 [DOI] [PubMed] [Google Scholar]
- 40.Delgoffe GM, Woo S-R, Turnis ME, et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature 2013;501:252–6. 10.1038/nature12428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wing JB, Tanaka A, Sakaguchi S. Human FOXP3(+ regulatory T cell heterogeneity and function in autoimmunity and cancer. Immunity 2019;50:302–16. 10.1016/j.immuni.2019.01.020 [DOI] [PubMed] [Google Scholar]
- 42.Sugiyama D, Nishikawa H, Maeda Y, et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci U S A 2013;110:17945–50. 10.1073/pnas.1316796110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asano T, Meguri Y, Yoshioka T, et al. PD-1 modulates regulatory T-cell homeostasis during low-dose interleukin-2 therapy. Blood 2017;129:2186–97. 10.1182/blood-2016-09-741629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montler R, Bell RB, Thalhofer C, et al. OX40, PD-1 and CTLA-4 are selectively expressed on tumor-infiltrating T cells in head and neck cancer. Clin Trans Immunol 2016;5:e70 10.1038/cti.2016.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stathopoulou C, Gangaplara A, Mallett G, et al. PD-1 inhibitory receptor downregulates asparaginyl endopeptidase and maintains FOXP3 transcription factor stability in induced regulatory T cells. Immunity 2018;49:247–63. 10.1016/j.immuni.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park HJ, Park JS, Jeong YH, et al. PD-1 upregulated on regulatory T cells during chronic virus infection enhances the suppression of CD8+ T cell immune response via the interaction with PD-L1 expressed on CD8+ T cells. J Immunol 2015;194:5801–11. 10.4049/jimmunol.1401936 [DOI] [PubMed] [Google Scholar]
- 47.Lowther DE, Goods BA, Lucca LE, et al. PD-1 marks dysfunctional regulatory T cells in malignant gliomas. JCI Insight 2016;1 10.1172/jci.insight.85935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta S, Thornley TB, Gao W, et al. Allograft rejection is restrained by short-lived TIM-3+PD-1+Foxp3+ Tregs. J Clin Invest 2012;122:2395–404. 10.1172/JCI45138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levine AG, Arvey A, Jin W, et al. Continuous requirement for the TCR in regulatory T cell function. Nat Immunol 2014;15:1070–8. 10.1038/ni.3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang B, Chikuma S, Hori S, et al. Nonoverlapping roles of PD-1 and FoxP3 in maintaining immune tolerance in a novel autoimmune pancreatitis mouse model. Proc Natl Acad Sci U S A 2016;113:8490–5. 10.1073/pnas.1608873113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sage PT, Francisco LM, Carman CV, et al. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol 2013;14:152–61. 10.1038/ni.2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumagai S, Togashi Y, Kamada T, et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol 2020;21:1346–58. 10.1038/s41590-020-0769-3 [DOI] [PubMed] [Google Scholar]
- 53.Maj T, Wang W, Crespo J, et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol 2017;18:1332–41. 10.1038/ni.3868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sakaguchi S Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol 2005;6:345–52. 10.1038/ni1178 [DOI] [PubMed] [Google Scholar]
- 55.Plitas G, Konopacki C, Wu K, et al. Regulatory T cells exhibit distinct features in human breast cancer. Immunity 2016;45:1122–34. 10.1016/j.immuni.2016.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 2009;30:899–911. 10.1016/j.immuni.2009.03.019 [DOI] [PubMed] [Google Scholar]
- 57.Nelson BH CD20 + B Cells: The Other Tumor-Infiltrating Lymphocytes. J.i. 2010;185:4977–82. 10.4049/jimmunol.1001323 [DOI] [PubMed] [Google Scholar]
- 58.Liu R-X, Wei Y, Zeng Q-H, et al. Chemokine (C-X-C motif) receptor 3-positive B cells link interleukin-17 inflammation to protumorigenic macrophage polarization in human hepatocellular carcinoma. Hepatology 2015;62:1779–90. 10.1002/hep.28020 [DOI] [PubMed] [Google Scholar]
- 59.Lambrechts D, Wauters E, Boeckx B, et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat Med 2018;24:1277–89. 10.1038/s41591-018-0096-5 [DOI] [PubMed] [Google Scholar]
- 60.Shalapour S, Font-Burgada J, Di Caro G, et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature 2015;521:94–8. 10.1038/nature14395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yuen GJ, Demissie E, Pillai S. B lymphocytes and cancer: a Love–Hate relationship. Trends in Cancer 2016;2:747–57. 10.1016/j.trecan.2016.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shao Y, Lo CM, Ling CC, et al. Regulatory B cells accelerate hepatocellular carcinoma progression via CD40/CD154 signaling pathway. Cancer Lett 2014;355:264–72. 10.1016/j.canlet.2014.09.026 [DOI] [PubMed] [Google Scholar]
- 63.Guillem EB, Sampsel JW. Antitumor-associated antigens IgGs: dual positive and negative potential effects for cancer therapy. Adv Exp Med Biol 2006;587:341–74. 10.1007/978-1-4020-5133-3_26 [DOI] [PubMed] [Google Scholar]
- 64.Pham-Nguyen KB, Yang W, Saxena R, et al. Role of NK and T cells in IL-12-induced anti-tumor response against hepatic colon carcinoma. International Journal of Cancer 1999;81:813–9. [DOI] [PubMed] [Google Scholar]
- 65.Shen SN, Xu Z, Qian XP, et al. RNA-electroporated CD40-activated B cells induce functional T-cell responses against HepG2 cells. Eur J Cancer Care 2008;17:404–11. 10.1111/j.1365-2354.2007.00841.x [DOI] [PubMed] [Google Scholar]
- 66.Lu Y, Zhao Q, Liao J-Y, et al. Complement signals determine opposite effects of B cells in chemotherapy-induced immunity. Cell 2020;180:1081–97 https://pubpeer.com/publications/05F89A4B34B41917D0F43813CBE86A?utm_source=Firefox&utm_medium=BrowserExtension&utm_campaign=Firefox 10.1016/j.cell.2020.02.015 [DOI] [PubMed] [Google Scholar]
- 67.Cabrita R, Lauss M, Sanna A, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 2020;577:561–5. 10.1038/s41586-019-1914-8 [DOI] [PubMed] [Google Scholar]
- 68.Helmink BA, Reddy SM, Gao J, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 2020;577:549–55. 10.1038/s41586-019-1922-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Petitprez F, de Reyniès A, Keung EZ, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 2020;577:556–60. 10.1038/s41586-019-1906-8 [DOI] [PubMed] [Google Scholar]
- 70.Thibult M-L, Mamessier E, Gertner-Dardenne J, et al. Pd-1 is a novel regulator of human B-cell activation. Int Immunol 2013;25:129–37. 10.1093/intimm/dxs098 [DOI] [PubMed] [Google Scholar]
- 71.Okazaki T, Maeda A, Nishimura H, et al. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting Src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A 2001;98:13866–71. 10.1073/pnas.231486598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nishimura H, Minato N, Nakano T. Immunological studies on PD-1 deficient mice: implication of PD-1 as a negative regulator for B cell responses. Int Immunol 1998;10:1563–72. 10.1093/intimm/10.10.1563 [DOI] [PubMed] [Google Scholar]
- 73.Wan Z, Lin Y, Zhao Y, et al. TFH cells in bystander and cognate interactions with B cells. Immunol Rev 2019;288:28–36. 10.1111/imr.12747 [DOI] [PubMed] [Google Scholar]
- 74.Shi J, Hou S, Fang Q, et al. PD-1 controls follicular T helper cell positioning and function. Immunity 2018;49:264–74. 10.1016/j.immuni.2018.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. distribution of reactivity and specificity. Int J Cancer 1975;16:216–29. 10.1002/ijc.2910160204 [DOI] [PubMed] [Google Scholar]
- 76.Kiessling R, Klein E, Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol 1975;5:112–7. 10.1002/eji.1830050208 [DOI] [PubMed] [Google Scholar]
- 77.Vivier E, Raulet DH, Moretta A, et al. Innate or adaptive immunity? The example of natural killer cells. Science 2011;331:44–9. 10.1126/science.1198687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Knorr DA, Bachanova V, Verneris MR, et al. Clinical utility of natural killer cells in cancer therapy and transplantation. Semin Immunol 2014;26:161–72. 10.1016/j.smim.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ghazarian AA, Kelly SP, Altekruse SF, et al. Future of testicular germ cell tumor incidence in the United States: forecast through 2026. Cancer 2017;123:2320–8. 10.1002/cncr.30597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ishigami S, Natsugoe S, Tokuda K, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer 2000;88:577–83. [DOI] [PubMed] [Google Scholar]
- 81.Schleypen JS, Baur N, Kammerer R, et al. Cytotoxic markers and frequency predict functional capacity of natural killer cells infiltrating renal cell carcinoma. Clinical Cancer Research 2006;12:718–25. 10.1158/1078-0432.CCR-05-0857 [DOI] [PubMed] [Google Scholar]
- 82.McKay K, Moore PC, Smoller BR, et al. Association between natural killer cells and regression in melanocytic lesions. Hum Pathol 2011;42:1960–4. 10.1016/j.humpath.2011.02.019 [DOI] [PubMed] [Google Scholar]
- 83.Garrido F, Algarra I. Mhc antigens and tumor escape from immune surveillance. Adv Cancer Res 2001;83:117–58. 10.1016/s0065-230x(01)83005-0 [DOI] [PubMed] [Google Scholar]
- 84.Garrido F, Cabrera T, Lopez-Nevot MA, et al. HLA class I antigens in human tumors. Adv Cancer Res 1995;67:155–95. 10.1016/s0065-230x(08)60713-7 [DOI] [PubMed] [Google Scholar]
- 85.Hugo W, Zaretsky JM, Sun L, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell 2016;165:35–44 https://pubpeer.com/publications/588F97B02637A1737313639C635EE5?utm_source=Firefox&utm_medium=BrowserExtension&utm_campaign=Firefox 10.1016/j.cell.2016.02.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science 2015;348:124–8. 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu Y, Cheng Y, Xu Y, et al. Increased expression of programmed cell death protein 1 on NK cells inhibits NK-cell-mediated anti-tumor function and indicates poor prognosis in digestive cancers. Oncogene 2017;36:6143–53. 10.1038/onc.2017.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pesce S, Greppi M, Tabellini G, et al. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: A phenotypic and functional characterization. J Allergy Clin Immunol 2017;139:335–46. 10.1016/j.jaci.2016.04.025 [DOI] [PubMed] [Google Scholar]
- 89.Vari F, Arpon D, Keane C, et al. Immune evasion via PD-1/PD-L1 on NK cells and monocyte/macrophages is more prominent in Hodgkin lymphoma than DLBCL. Blood 2018;131:1809–19. 10.1182/blood-2017-07-796342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dunai C, Murphy WJ. Nk cells for PD-1/PD-L1 blockade immunotherapy: pinning down the NK cell. J Clin Invest 2018;128:4251–3. 10.1172/JCI123121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Metzger P, Kirchleitner SV, Koenig LM, et al. Dying cells expose a nuclear antigen cross-reacting with anti-PD-1 monoclonal antibodies. Sci Rep 2018;8:8810 10.1038/s41598-018-27125-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Judge SJ, Dunai C, Aguilar EG, et al. Minimal PD-1 expression in mouse and human NK cells under diverse conditions. J Clin Invest 2020;130:3051–68. 10.1172/JCI133353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lin M, Luo H, Liang S, et al. Pembrolizumab plus allogeneic NK cells in advanced non-small cell lung cancer patients. J Clin Invest 2020;130:2560–9 https://pubpeer.com/publications/3D91A0371CE3C38AB24707757D896D?utm_source=Firefox&utm_medium=BrowserExtension&utm_campaign=Firefox 10.1172/JCI132712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cader FZ, Schackmann RCJ, Hu X, et al. Mass cytometry of Hodgkin lymphoma reveals a CD4+ regulatory T-cell-rich and exhausted T-effector microenvironment. Blood 2018;132:825–36. 10.1182/blood-2018-04-843714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang Z-Z, Kim HJ, Villasboas JC, et al. Mass Cytometry Analysis Reveals that Specific Intratumoral CD4+ T Cell Subsets Correlate with Patient Survival in Follicular Lymphoma. Cell Rep 2019;26:2178–93. 10.1016/j.celrep.2019.01.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carey CD, Gusenleitner D, Lipschitz M, et al. Topological analysis reveals a PD-L1-associated microenvironmental niche for Reed-Sternberg cells in Hodgkin lymphoma. Blood 2017;130:2420–30. 10.1182/blood-2017-03-770719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roemer MGM, Redd RA, Cader FZ, et al. Major histocompatibility complex class II and programmed death ligand 1 expression predict outcome after programmed death 1 blockade in classic Hodgkin lymphoma. J Clin Oncol 2018;36:942–50. 10.1200/JCO.2017.77.3994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nagasaki J, Togashi Y, Sugawara T, et al. The critical role of CD4+ T cells in PD-1 blockade against MHC-II-expressing tumors such as classic Hodgkin lymphoma. Blood Adv 2020;4:4069–82. 10.1182/bloodadvances.2020002098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jalali S, Price-Troska T, Bothun C, et al. Reverse signaling via PD-L1 supports malignant cell growth and survival in classical Hodgkin lymphoma. Blood Cancer J 2019;9:22 10.1038/s41408-019-0185-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell 2015;27:462–72. 10.1016/j.ccell.2015.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Franklin RA, Liao W, Sarkar A, et al. The cellular and molecular origin of tumor-associated macrophages. Science 2014;344:921–5. 10.1126/science.1252510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhu Y, Herndon JM, Sojka DK, et al. Tissue-Resident macrophages in pancreatic ductal adenocarcinoma originate from embryonic hematopoiesis and promote tumor progression. Immunity 2017;47:e326:323–38. 10.1016/j.immuni.2017.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bowman RL, Klemm F, Akkari L, et al. Macrophage ontogeny underlies differences in tumor-specific education in brain malignancies. Cell Rep 2016;17:2445–59. 10.1016/j.celrep.2016.10.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mantovani A, Marchesi F, Malesci A, et al. Tumour-Associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 2017;14:399–416. 10.1038/nrclinonc.2016.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res 2006;66:605–12. 10.1158/0008-5472.CAN-05-4005 [DOI] [PubMed] [Google Scholar]
- 106.Kaneda MM, Messer KS, Ralainirina N, et al. Pi3Kγ is a molecular switch that controls immune suppression. Nature 2016;539:437–42. 10.1038/nature19834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Qian B-Z, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010;141:39–51. 10.1016/j.cell.2010.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang Q, Li Y, Miao C, et al. Anti-Angiogenesis effect of neferine via regulating autophagy and polarization of tumor-associated macrophages in high-grade serous ovarian carcinoma. Cancer Lett 2018;432:144–55. 10.1016/j.canlet.2018.05.049 [DOI] [PubMed] [Google Scholar]
- 109.Zhang Y-L, Li Q, Yang X-M, et al. SPON2 promotes M1-like macrophage recruitment and inhibits hepatocellular carcinoma metastasis by distinct Integrin-Rho GTPase-Hippo pathways. Cancer Res 2018;78:2305–17. 10.1158/0008-5472.CAN-17-2867 [DOI] [PubMed] [Google Scholar]
- 110.Schmall A, Al-Tamari HM, Herold S, et al. Macrophage and cancer cell cross-talk via CCR2 and CX3CR1 is a fundamental mechanism driving lung cancer. Am J Respir Crit Care Med 2015;191:437–47. 10.1164/rccm.201406-1137OC [DOI] [PubMed] [Google Scholar]
- 111.Ruffell B, Chang-Strachan D, Chan V, et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell 2014;26:623–37. 10.1016/j.ccell.2014.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zha H, Wang X, Zhu Y, et al. Intracellular activation of complement C3 leads to PD-L1 antibody treatment resistance by modulating tumor-associated macrophages. Cancer Immunol Res 2019;7:193–207. 10.1158/2326-6066.CIR-18-0272 [DOI] [PubMed] [Google Scholar]
- 113.Li X, Liu R, Su X, et al. Harnessing tumor-associated macrophages as AIDS for cancer immunotherapy. Mol Cancer 2019;18:177. 10.1186/s12943-019-1102-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Müller L, Tunger A, Plesca I, et al. Bidirectional crosstalk between cancer stem cells and immune cell subsets. Front Immunol 2020;11:140. 10.3389/fimmu.2020.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shen L, Gao Y, Liu Y, et al. PD-1/PD-L pathway inhibits M.tb-specific CD4+ T-cell functions and phagocytosis of macrophages in active tuberculosis. Sci Rep 2016;6:38362. 10.1038/srep38362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huang X, Venet F, Wang YL, et al. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A 2009;106:6303–8. 10.1073/pnas.0809422106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen W, Wang J, Jia L, et al. Attenuation of the programmed cell death-1 pathway increases the M1 polarization of macrophages induced by zymosan. Cell Death Dis 2016;7:e2115. 10.1038/cddis.2016.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kono Y, Saito H, Miyauchi W, et al. Increased PD-1-positive macrophages in the tissue of gastric cancer are closely associated with poor prognosis in gastric cancer patients. BMC Cancer 2020;20:175. 10.1186/s12885-020-6629-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Strauss L, Mahmoud MAA, Weaver JD, et al. Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci Immunol 2020;5. 10.1126/sciimmunol.aay1863. [Epub ahead of print: 03 01 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Broz ML, Binnewies M, Boldajipour B, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 2014;26:638–52. 10.1016/j.ccell.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wculek SK, Cueto FJ, Mujal AM, et al. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol 2020;20:7–24. 10.1038/s41577-019-0210-z [DOI] [PubMed] [Google Scholar]
- 122.Yao S, Wang S, Zhu Y, et al. PD-1 on dendritic cells impedes innate immunity against bacterial infection. Blood 2009;113:5811–8. 10.1182/blood-2009-02-203141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Park SJ, Namkoong H, Doh J, et al. Negative role of inducible PD-1 on survival of activated dendritic cells. J Leukoc Biol 2014;95:621–9. 10.1189/jlb.0813443 [DOI] [PubMed] [Google Scholar]
- 124.Krempski J, Karyampudi L, Behrens MD, et al. Tumor-Infiltrating programmed death receptor-1+ dendritic cells mediate immune suppression in ovarian cancer. J Immunol 2011;186:6905–13. 10.4049/jimmunol.1100274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Said EA, Dupuy FP, Trautmann L, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med 2010;16:452–9. 10.1038/nm.2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhao Y, Harrison DL, Song Y, et al. Antigen-Presenting cell-intrinsic PD-1 neutralizes PD-L1 in cis to attenuate PD-1 signaling in T cells. Cell Rep 2018;24:e376:379–90. 10.1016/j.celrep.2018.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schatton T, Schütte U, Frank NY, et al. Modulation of T-cell activation by malignant melanoma initiating cells. Cancer Res 2010;70:697–708. 10.1158/0008-5472.CAN-09-1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ostman A, Hellberg C, Böhmer FD. Protein-Tyrosine phosphatases and cancer. Nat Rev Cancer 2006;6:307–20. 10.1038/nrc1837 [DOI] [PubMed] [Google Scholar]
- 129.Aceto N, Sausgruber N, Brinkhaus H, et al. Tyrosine phosphatase SHP2 promotes breast cancer progression and maintains tumor-initiating cells via activation of key transcription factors and a positive feedback signaling loop. Nat Med 2012;18:529–37. 10.1038/nm.2645 [DOI] [PubMed] [Google Scholar]
- 130.Liu K-W, Feng H, Bachoo R, et al. SHP-2/PTPN11 mediates gliomagenesis driven by PDGFRA and INK4A/ARF aberrations in mice and humans. J Clin Invest 2011;121:905–17. 10.1172/JCI43690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li H, Li X, Liu S, et al. Programmed cell death-1 (PD-1) checkpoint blockade in combination with a mammalian target of rapamycin inhibitor restrains hepatocellular carcinoma growth induced by hepatoma cell-intrinsic PD-1. Hepatology 2017;66:1920–33. 10.1002/hep.29360 [DOI] [PubMed] [Google Scholar]
- 132.Pu N, Gao S, Yin H, et al. Cell-Intrinsic PD-1 promotes proliferation in pancreatic cancer by targeting CYR61/CTGF via the Hippo pathway. Cancer Lett 2019;460:42–53. 10.1016/j.canlet.2019.06.013 [DOI] [PubMed] [Google Scholar]
- 133.Du S, McCall N, Park K, et al. Blockade of Tumor-Expressed PD-1 promotes lung cancer growth. Oncoimmunology 2018;7:e1408747. 10.1080/2162402X.2017.1408747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mortazavi A, Williams BA, McCue K, et al. Mapping and quantifying mammalian transcriptomes by RNA-seq. Nat Methods 2008;5:621–8. 10.1038/nmeth.1226 [DOI] [PubMed] [Google Scholar]
- 135.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–20. 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pitt JM, Vétizou M, Daillère R, et al. Resistance mechanisms to Immune-Checkpoint blockade in cancer: tumor-intrinsic and -Extrinsic factors. Immunity 2016;44:1255–69. 10.1016/j.immuni.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 138.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018–28. 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 139.Herbst RS, Soria J-C, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563–7. 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568–71. 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chowdhury PS, Chamoto K, Honjo T. Combination therapy strategies for improving PD-1 blockade efficacy: a new era in cancer immunotherapy. J Intern Med 2018;283:110–20. 10.1111/joim.12708 [DOI] [PubMed] [Google Scholar]
- 142.Arce Vargas F, Furness AJS, Litchfield K, et al. Fc effector function contributes to the activity of human anti-CTLA-4 antibodies. Cancer Cell 2018;33:649–63. 10.1016/j.ccell.2018.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Doi T, Muro K, Ishii H, et al. A phase I study of the Anti-CC chemokine receptor 4 antibody, Mogamulizumab, in combination with nivolumab in patients with advanced or metastatic solid tumors. Clin Cancer Res 2019;25:6614–22. 10.1158/1078-0432.CCR-19-1090 [DOI] [PubMed] [Google Scholar]
- 144.Concha-Benavente F, Kansy B, Moskovitz J, et al. PD-L1 Mediates Dysfunction in Activated PD-1+ NK Cells in Head and Neck Cancer Patients. Cancer Immunol Res 2018;6:1548–60. 10.1158/2326-6066.CIR-18-0062 [DOI] [PMC free article] [PubMed] [Google Scholar]