Introduction
Incidence, Prevalence and Temporal Trends
Pregnancy and puerperium confer an increased risk for ischemic as well as hemorrhagic stroke, with incidence rates being three-fold higher as compared to non-pregnant women. A meta-analysis of the epidemiological characteristics and risk factors for stroke in pregnancy found that the mean age ranged from 22 to 33 years, and the crude incidence rate was 30/100,000 (95% CI, 18.8–49.4/100,000), which was nearly three times that of non-pregnant women of childbearing age suffering a stroke.1 The rate of ischemic and hemorrhagic stroke was 12.2/100,000 pregnancies, while for cerebral venous sinus thrombosis (CVST) the rate was 9.1/100,000 pregnancies. In this meta-analysis the rates of pregnancy-associated stroke remained unchanged from 1990 to 2017.1 Subsequently, using hospital discharge data from the US Nationwide Inpatient Sample, Kuklina and colleagues reported that the rate of stroke from 1994–1995 to 2006–2007 increased by 47% for antenatal stroke, and by 83% for post-natal strokes. Changes in the prevalence of hypertensive disorders of pregnancy (HDP) and heart disease from 1994–1995 to 2006–2007 explained almost all the increase in postpartum hospitalizations.2 Such changes in the incidence of stroke were not replicated in other studies;2 Furthermore, a more recent study of data from the US National Inpatient Sample reported a decrease in the incidence of stroke during the delivery period from 10/100000 to 6/100000 deliveries from 2004 to 2014, despite an increase in the prevalence of HDP from 8.4% to 10.9% during that timeframe.3
Timing
The rate of pre-partum/peripartum stroke is 18.3/100,000 pregnancies, whereas for post-partum stroke the rate is 14.7/100,000. Given the short duration of the post-partum period, the daily rate of stroke is higher in the postpartum as compared to pre- or peri-partum periods.1 Concordantly, a recent study has shown that the majority of readmissions for postpartum stroke occur in the first 10 days after hospital discharge.4 Different rates have been reported in England: 10.7/100,000 person-years, antepartum, but 9-fold higher peripartum (161.1/100,000 person-years) and 3-fold higher early postpartum (47.1/100,000 person-years).5 It is postulated that these differences could be due to variances in access to obstetrical care between the United States and England. The post-partum period with highest risk for thrombotic events is the first 6 weeks. While the period of 7–12 weeks post-partum also has an increased, albeit lower, risk of thrombotic events compared to weeks 0–6, there is no significant increase in stroke risk.6
Hemorrhage rates in pregnancy are also significantly higher during the third trimester (2.9/100,000 pregnancies) and in the first 12 weeks post-partum (4.4/100,000).7
Risk Factors
Patient Characteristics
A study of the Nationwide Inpatient Sample showed that the absolute risk of stroke increased with age: compared to patients < 20 years, those age 35–39 years had an odds ratio (OR) for stroke of 2.0 (95% confidence interval (CI) 1.4–2.7, P <0.01), and those ≥ 40 years had OR of 3.1 (95% CI 2–4.6).8 This was confirmed in a later study of the Nationwide Inpatient Sample, which showed a substantially higher risk of acute stroke during pregnancy/puerperium for women ≥ 45 years.9 Interestingly, one study found that younger women, but not older women, had an increased stroke risk during pregnancy and the post-partum state.10 Pregnancy at older age may, however, have negative implications for cerebrovascular health later in life. The risk of stroke in relation to age of prior pregnancy/delivery was analyzed in women aged 59–70 years from the observational cohort of the Women’s Health Study. Older age at delivery (age ≥ 40 years) was associated with a small but significant increased risk of hemorrhagic stroke in multivariate analyses (OR 1.5, 95% CI 1.0–2.1) compared to women < 40 years at delivery. Compared to younger women at the time of delivery, women ≥ 40 years at delivery had higher mean systolic blood pressure and higher rates of diabetes mellitus, heart failure, atrial fibrillation and any alcohol use later in life.11
Data from the Nationwide Inpatient Sample also showed that race/ethnicity influences the incidence of pregnancy-associated stroke: African-American women have higher risk of stroke than White women (OR 1.5, 95% CI 1.2–1.9) and Hispanic women.8 Additionally, minority women (black, Hispanic, Asian/Pacific Islander) with chronic hypertension and pregnancy-induced hypertension are at substantially higher risk of stroke during delivery admissions compared to white women with the same conditions.12
Medical Risk Factors
The presence of vascular risk factors at the time of pregnancy contribute to the risk of stroke. Medical conditions that are strongly associated with stroke include: migraine, odds ratio (OR) 16.9 (95% CI 9.7–29.5); thrombophilia, OR 16.0 (95% CI 9.4–27.2); systemic lupus erythematosus, OR 15.2 (95% CI 7.4–31.2); heart disease, OR 13.2 (95% CI 10.2–17.0); sickle cell disease, OR 9.1 (95% CI 3.7–22.2); hypertension, OR 6.1 (95% CI 4.5–8.1); thrombocytopenia, OR 6.0 (95% CI 1.5–24.1); and diabetes, OR 2.5 (95% CI 1.3–4.6). Migraine-associated stroke in pregnancy may be mediated by HDP.13 Additionally, pregnancy-associated complications such as transfusion, postpartum infections, and any type of infection at the time of delivery admission, especially genitourinary infections and sepsis, are strong predictors of pregnancy-associated stroke.8,14 Infection during delivery admission was shown to be a risk factor for stroke readmission at a median of 6.7 days. This relationship was significant for readmissions due to post-partum ischemic stroke, but not for hemorrhagic stroke.15 Longer length of stay during delivery hospitalization, a potential marker of increased morbidity, is an independent risk factor for maternal stroke.4 Furthermore, smoking is highly prevalent in women who suffer strokes during pregnancy and is also an independent risk factor for maternal stroke.16,4 Other pregnancy-specific causes, such as peripartum cardiomyopathy, choriocarcinoma, and embolization of amniotic fluid or air, are rare. It is postulated that ischemic strokes secondary to trans cardiac embolism through a patent foramen ovale may occur during pregnancy because of the higher rates pregnancy-associated venous thrombosis. However, a review article showed only a small number of such cases, most of which occurred in the first and second trimesters of pregnancy.17
For hemorrhagic stroke during pregnancy/puerperium, aneurysms, arteriovenous malformations, HDP, advanced maternal age, black race, coagulopathy and smoking are the most important risk factors.1,7 Furthermore, chronic hypertension or pregnancy-induced hypertension strongly modify the effect of race and ethnicity on the risk of hemorrhagic stroke: minority women (black, Hispanic, Asian/Pacific Islander) with these conditions are at substantially higher risk of hemorrhagic stroke compared to white women.12
Assisted Reproductive Therapies
Conflicting data have been reported regarding the risk of cardiovascular disease and stroke after hormonally-assisted reproductive therapies. A meta-analysis involving 30,477 women who received fertility therapy and 1,296,734 women who did not, who were respectively followed for a median of 9.7 and 8.6 years, showed a trend towards an increased risk of stroke or transient ischemic attack (TIA) in women after ovulation induction compared to women who did not receive fertility therapy (hazard ratio (HR), 1.25, 95% CI 0.96 to 1.63; I20%). It is noteworthy that the patients included in the studies were relatively young, with mean age of 28.5–34 years at the time of delivery, which could have biased the results toward a more favorable outcome.18
A prospective cohort study analyzed the risk of cardiovascular disease after failed assisted reproductive therapy (defined as women who did not give birth one year after their last cycle of assisted reproductive therapy) in 28442 women who received fertility therapy, after a median of 8.4 years of follow-up. A total of 67% failed reproductive therapy and had a 21% increased annual risk of cardiovascular disease (95% CI 13–30%), an increased risk of ischemic stroke (adjusted relative rate ratio (ARR) 1.33, 95% CI 1.22–1.46) but not hemorrhagic stroke (ARR 0.88, 95% CI 0.80–0.96).19
The mechanisms by which assisted reproduction increases the risk of cardiovascular disease are not fully understood. It is postulated that ovarian hyperstimulation activates the renin-angiotensin system, thus modifying sodium balance, blood pressure regulation, and promoting fluid shifts with intravascular volume depletion, leading to hypercoagulability.
Cesarean Delivery
In a study of data from the Healthcare Cost and Utilization Project from 1993 and 1994, risk factors for peripartum stroke and venous sinus thrombosis were assessed among 1,408,015 sampled deliveries. In that study, the risk of peripartum stroke was 34.3/100,000 births for women who had a cesarean birth, compared with risk of 7.1/100,000 births for vaginal delivery (p<0.001). The risk of intracranial venous thrombosis was also increased for cesarean delivery: 26.6/100,000 births for cesarean birth, versus 7.4/100,000 births for vaginal delivery (p <0.001).20 Similar findings were observed in a population-based study from Taiwan, in which the increased risk of postpartum stroke in women undergoing cesarean delivery compared to vaginal delivery persisted up to 12 months post-partum.21 The possibility of cesarean section being a consequence of pregnancies complicated by HDP, for which the risk of stroke is higher due to hypertension, or due to the actual occurrence of a cerebrovascular event is a potential confounder. However, surgery itself promotes increased risk of clotting through various mechanisms, which may account for the increased stroke risk.20
Pathophysiology of Pregnancy-Associated Stroke
Hemodynamic Changes
During pregnancy, there is high metabolic demand. To account for this, cardiovascular changes occur to allow the maternal circulation to meet new physiological requirements. One of the initial changes is an increase in the plasmatic volume beginning early in the first trimester, secondary to an increase in renin activity as stimulated by estrogen and other circulating hormones. There is also development of mild hemodilutional anemia and substantial increases in heart rate and cardiac output, with elevation up to 45% above the non-pregnancy state by 24 weeks, and even further during labor and birth.22,23 Systemic vascular resistance drops in early gestation. With this, blood pressure (BP) drops as well, with nadir around 20–32 weeks. This promotes relative venous stasis, heightened by vena cava compression in the supine position by the progressively growing uterus, and reduced physical activity during late pregnancy and puerperium. Therefore, the combination of hypervolemia, increased circulatory demand, decreased BP and increased venous stasis predispose women to circulatory complications. These changes persist until 6–12 weeks post-partum.
Vascular and Connective Tissue Changes
Pregnancy causes remodeling of the heart and blood vessels, with increased vascular distensibility in the first trimester. In late pregnancy, however, there is loss of distensibility due to a reduction in collagen and elastin content in the systemic arterial walls, which persists for months after delivery.24 It is not clear how these vascular changes promote the development of stroke. However, it is postulated that these somewhat vulnerable vessel walls could be subject to greater hemodynamic stress in the setting of increased blood volume and cardiac output during pregnancy and delivery, and hence susceptible to rupture and resultant hemorrhagic stroke.16,23 Recent studies indicate that the reversible cerebral vasoconstriction syndrome is the most frequent cause of pregnancy associated stroke.
Changes in the Coagulation System
Gestation is a hypercoagulable state, with a 4 to 10-fold increased risk of thrombosis during pregnancy and puerperium. The increased hypercoagulability is in part due to elevations of procoagulant factors VII, IX, X, XII, XIII, fibrinogen and von Willebrand factor. Further, physiologic anticoagulation is partly impaired by reductions in protein S activity, reductions of antithrombin III levels (nadir in the third trimester) and development of acquired activated protein C resistance in the second and third trimesters in 1/3 of pregnant women. Fibrinolysis is also reduced because of increases in serum plasminogen activator inhibitor (PAI) type 1 and placental derived PAI type 2. Iron deficiency also contributes to a procoagulant state. All the above, combined with venous stasis, congestion, and compression of the inferior vena cava and aorta by the pregnant uterus promote an enhanced hypercoagulable state predominantly in the third trimester and puerperium. Further contributing to the risk of thrombosis in the puerperium is the acute phase response to surgical trauma and hemorrhage of delivery.25,23,16
Pathological Conditions, Condition-Specific Management and Outcomes
Hypertensive Disorders of Pregnancy
HDP are a group of conditions occurring in pregnancy and puerperium with a common background of hypertension, defined as BP ≥ 140/90 mmHg. Included in this group are gestational hypertension, preeclampsia, severe preeclampsia (eclampsia and hemolysis elevated liver enzymes and low platelets [HELLP] syndrome), and chronic hypertension with superimposed preeclampsia. HDP are of clinical relevance given their prevalence and strong risk of cardiovascular disease and stroke during pregnancy and the puerperium and later in life, with associated increased risk of cardiovascular mortality.26,27,28,29,30 Preeclampsia is associated with a 2 to 4-fold increase in future incident heart failure and a 2-fold increased risk in coronary heart disease, stroke, and cardiovascular death.28,29 HDP lead to a loss of women’s pre-menopausal cardiovascular advantage, and cardiovascular disease may occur as early as the first year after a first pre-eclamptic pregnancy, and up to a decade earlier than expected for HDP. Due to these important findings, HDP should be considered as a risk factor for future cardiovascular disease and should prompt earlier screening for such conditions.29
The prevalence of HDP, gestational hypertension and preeclampsia is 5.2–8.2%, 1.8–4.4% and 0.2–9.2%, respectively. HDP is more common in developing countries, although its prevalence has increased substantially in the United States in the past 20 years.31,32 HDP is also more common in African-Americans.31
Modifiable risk factors for HDP include increased body mass index, anemia, and lower education. Non-modifiable risk factors for HDP include older and younger age (age > 35 years and < 20 years, respectively); primiparity and multiparity; previous HDP; gestational diabetes mellitus; preexisting hypertension; preexisting type 2 diabetes mellitus; preexisting urinary tract infection; and a family history of hypertension, type 2 diabetes mellitus and preeclampsia.31 Additionally, coagulopathies and underlying prothrombotic conditions also increase the risk of pregnancy-associated stroke in women with preeclampsia.33
HDP are associated with 26% of maternal deaths in Latin America/Caribbean and 16% of maternal deaths in developing countries. HDP also account for 10% of preterm births, and 1/50 stillbirths.34
HDP are associated with a 1.7–5.2-fold increase in the risk of stroke. Furthermore, preeclampsia occurs in 21–47% of pregnancy-associated strokes.8,35 Importantly, the rate of stroke associated with HDP increased 103% in the United States between 1994–1995 and 2010–2011.30
Hypertension in pregnancy may predate the gestation or may be diagnosed de novo during pregnancy.
De Novo Hypertension36
Gestational hypertension: diagnosed when hypertension develops in a woman at ≥ 20 weeks gestation, in the absence of proteinuria or other metabolic abnormalities. It may be mild, when BP ranges 140–149/90–99 mmHg; moderate when BP is 150–159/100–109 mmHg; and severe when BP ≥ 160/110 mmHg. Gestational hypertension is usually a relatively benign condition. However, 25% of women with gestational hypertension may develop pre-eclampsia, and those that have gestational hypertension before 34 weeks’ gestation are at highest risk.
Preeclampsia: a multisystem disorder of mid- to late pregnancy diagnosed by the presence of de novo hypertension after 20 weeks gestation accompanied by proteinuria (≥ 300 mg protein/24 h urine) or evidence of maternal acute kidney injury, liver dysfunction, neurological changes (encephalopathy, seizures), hemolysis or thrombocytopenia, or fetal growth restriction. Preeclampsia may occur intrapartum and less frequently early postpartum, usually within 48 hours. Eclampsia, one of the most dreaded consequences of preeclampsia, is the development of seizures in a patient with preeclampsia. This occurs in about 0.1% of all pregnancies, and is 10 to 30 time more common in developing countries.37,31
The pathophysiology of preeclampsia is not fully understood. There is evidence that immune maladaptation is central to its cause. Abnormal placentation, leading to restricted placental blood flow, may be the key factor promoting a cascade of events culminating with preeclampsia. Restricted placental blood flow leads to development of a necrotizing spiral artery arteriopathy, coined acute atherosis, which resembles the early stages of systemic atherosclerosis. The resultant placental oxidative and endoplasmic reticulum stress leads to the release of components from the intervillous space into the maternal circulation, promoting maternal intravascular inflammatory response (increase in TNF alpha, IL-6, ICAM-1, CRP, IL-2), generalized endothelial dysfunction, immune and clotting activation, decreased intravascular volume and increased vascular reactivity. Microthrombi may be seen in multiple organs.34,38 The endothelial dysfunction may lead to disruption of the blood-brain barrier, leading to cerebral edema and hemorrhage, as well as possible disruption of vascular cerebral autoregulation.39
The importance of diagnosis and early management of preeclampsia cannot be overstated. The maternal mortality rate in preeclampsia-eclampsia is 6.4/10,000 cases at delivery, with nearly 20-fold increased risk of maternal mortality when preeclampsia develops before 32 weeks gestation.40 A recent study has shown that mortality attributed to stroke in preeclampsia occurs in approximately 60% of cases. Furthermore, the ability to modify a patient’s outcome by more rapidly attending to clinical warning signs and symptoms of stroke occurred in 91%, and opportunities for improved medical management occurred in 76%.41 The most common type of stroke occurring after preeclampsia is hemorrhagic stroke, usually present in the post-partum period.35 Additionally, there is a clear overlap syndrome of postpartum angiopathy or the reversible cerebral vasoconstriction syndrome (RCVS), posterior reversible encephalopathy syndrome (PRES), and preeclampsia/eclampsia.37 The relative risk of stroke occurring later in life after preeclampsia is 1.76 (95% CI, 1.40–2.22) for non-fatal stroke, and RR=2.98 (95% CI, 1.11–7.96) for fatal stroke.42 For women with preeclampsia, those who have severe preeclampsia or eclampsia, hypercoagulable states, chronic hypertension, peripartum infections, and coagulopathies are at highest risk for stroke.33
Management (Table 1)
Table 1.
Key treatment points for stroke in pregnancy and puerperium.
| Pregnancy-Associated Disease | T reatment |
|---|---|
| Gestational Hypertension | Blood pressure control to <140/85 mmHg, preferably with oral or intravenous labetalol, hydralazine, and nicardipine. |
| Preeclampsia/Eclampsia | Prevention of pre-term preeclampsia: aspirin 150 mg daily from weeks 11–14 to week 36 of gestation. |
| Definitive treatment: delivery of the fetus and diseased placenta. | |
| Arterial Ischemic Stroke | Intravenous tissue plasminogen activator should be considered within 4.5 hours after symptom onset. |
| Mechanical thrombectomy should be offered to patients with large-vessel occlusion strokes. | |
| Hemorrhagic Stroke | Treatment involves blood pressure control. Treatment of the underlying vascular lesion as appropriate. |
| Cerebral Venous Sinus Thrombosis | Treatment: unfractionated or low molecular weight heparin. |
Delivery: The definitive treatment for preeclampsia is delivery of the baby and of the diseased placenta. For women with onset of preeclampsia ≥ 37 weeks, delivery should be performed. For women with preeclampsia that begins < 37 weeks of gestation, they should be managed with an expectant approach (preferably with maternal fetal medicine involvement for gestational age < 34 weeks), unless features of severe disease develop.36
-
Aspirin: Aspirin was first shown to reduce the risk of preeclampsia in women in 1979.43 Thereafter, it has been studied in multiple clinical trials. A recent study showed that for patients at high risk of developing preeclampsia, aspirin 150 mg daily, from weeks 11–14 to week 36 of gestation, was superior to placebo for prevention of pre-term preeclampsia (1.6% vs. 4.3%, respectively, OR 0.38, 95% CI 0.20 – 0.74), without increasing the risk of poor neonatal outcomes.44 This therapy has been recommended in the recently published consensus statement on hypertensive disorders of pregnancy from the International Society for the Study of Hypertension in Pregnancy (ISSHP).36
Recent data from a prospective cohort study showed that for women < 60 years old with a prior history of HDP, those taking aspirin had a lower risk of stroke compared to nonusers of aspirin. These thought-provoking findings prompt consideration of clinical trials of aspirin use for stroke prevention in women with prior HDP.45
Calcium: High calcium intake during pregnancy has been shown to be associated with a reduced incidence of pre-eclampsia. The mechanism whereby calcium might mediate this effect is through reduction of parathyroid hormone release and reduction of intracellular calcium, leading to decreased smooth muscle contractility. Additionally, calcium supplementation could also reduce uterine smooth muscle contractility and prevent preterm labor and delivery.46,47,48 Calcium supplementation, 1.2–2.5 g/day is also recommended, in addition to aspirin, for women at high risk of preeclampsia who have low daily calcium intake, or in whom the daily intake of calcium cannot be estimated.36
Hypertension management: For patients with severe hypertension (BP ≥ 160/110) during pregnancy/puerperium, BP should be controlled in a monitored setting, with agents including intravenous labetalol or hydralazine, or oral nifedipine. Management recommendations of moderate hypertension vary by medical society. For example, the American College of Obstetricians and Gynecologists does not recommend treatment of mild or moderate hypertension.49 Conversely, a 2014 statement from the American Heart Association (AHA) suggests consideration of treatment of moderate hypertension in pregnancy.50 The divergent opinions stem from concerns regarding a) fetal outcomes, because aggressive BP control may lead to low birth weight due to relative placental insufficiency; and b) maternal outcomes, given increased maternal stroke risk and risk of development of severe hypertension during gestation. A 2015 randomized controlled clinical trial addressed this question. In that study, 987 pregnant women at 14–33 weeks gestation, with non-proteinuric hypertension or gestational hypertension, were randomized to BP control with target diastolic BP 100 mmHg (control group) or target diastolic BP 85 mmHg. The primary outcome (pregnancy loss or high-level neonatal care > 48 hours in the first 28 postnatal days) occurred in 31.4% of controls and in 30.7% receiving aggressive BP control (adjusted OR 1.02, 95% CI 0.77 to 1.35). There was also no significant difference in the percentage of infants with low birth weight between the two groups. Serious maternal complications did not differ between the study arms.51 Since the reporting of this study’s results, the ISSHP has recommended treatment of any type of maternal hypertension, aiming for target diastolic BP of 85 mmHg.36
Magnesium sulfate: this is recommended for women with preeclampsia who have proteinuria and severe hypertension, or hypertension with neurological signs or symptoms.36 The use of magnesium sulfate in preeclamptic women with severe features has been shown to reduce the rate of progression to eclampsia by 58%, as compared to placebo.52 Magnesium sulfate is also superior to diazepam and phenytoin for prevention of recurrent seizures in eclampsia.53,54
Regular exercise during pregnancy to maintain appropriate body weight is recommended, as that will reduce the likelihood of hypertension.36
Posterior Reversible Encephalopathy
PRES refers to a syndrome of hypertensive encephalopathy characterized by reversible brain edema. This usually occurs in the setting of elevated BP or relative hypertension (compared to a patient’s baseline BP). Patients typically presents with headache, visual symptoms referable to the occipital lobes, and seizures. The disease may be complicated by cerebral hemorrhages and ischemic strokes. There is an overlap between PRES and severe preeclampsia/eclampsia.55 A small single center retrospective cohort study showed radiological features suggestive of PRES in 98% of women with eclampsia.56 Similarly, another small retrospective cohort study reported radiological features suggestive of PRES in 92% of women with eclampsia and in 19% of women with preeclampsia.57
The typical radiographic appearance of PRES is that of subcortical white matter and cortical edema in the bilateral parietal and occipital lobes. In less typical PRES, there can be extension to the frontal lobes, inferior temporal lobes, cerebellar hemispheres, brainstem and deep white matter.58 MRI diffusion-weighted imaging can also show restricted diffusion and intracranial hemorrhages. Atypical radiological features and intracranial hemorrhages are less commonly seen in obstetrical PRES.59
The goals of therapy are to normalize systemic BP, control seizures, and minimize vasospasm and risk of secondary infarct and hemorrhage.
Outcomes in non-obstetrical PRES may be more severe than that of patients with obstetrical PRES, which may be driven by the severity of the underlying comorbidities in that population.60
Reversible Cerebral Vasoconstriction Syndrome (RCVS)
RCVS denotes a group of diseases characterized by segmental narrowing and dilatation of multiple intracranial arteries of days to weeks duration.61–64 RCVS is relatively rare, with a female to male ratio of 2:10 to 10:1, and mean age of 42–44 years.63,65,66 RCVS may be the most frequent cause of pregnancy-associated stroke. The most common presenting sign is recurrent thunderclap headache over days to weeks, sometimes progressing to persistent moderate headache. Focal neurological deficits, such as hemiparesis, aphasia, and altered mental status, occur in 8–43%.63,65,67 Visual changes are common, including blurriness, cortical visual loss, and Balint syndrome.65 Generalized tonic-clonic seizures occur in 1–17% at disease onset.
The pathophysiology is uncertain. RCVS may occur due to spontaneous or provoked failed regulation of cerebral vascular tone, likely related to serotoninergic pathways and sympathetic overactivity.68,69 RCVS has been associated with exposure to vasoactive drugs (antidepressants, nasal decongestants, triptans, cannabis, nicotine patches, cocaine, methamphetamine, amphetamine, lysergic acid), catecholamine secreting tumors, immunosupressants, blood products, uncontrolled hypertension, head trauma, and sexual activity. This condition has been seen in the postpartum state, with or without preeclampsia/eclampsia.63,65,68–77 There may be overlapping mechanisms between RCVS and posterior reversible leukoencephalopathy syndrome (PRES) given the presence of reversible cerebral edema in 8–38% of RCVS cases, angiographic changes in patients with PRES, and similar clinical presentation between the two conditions.63,65,70,78,79
Brain parenchymal imaging is often normal on admission, but changes may be seen over time in 81%, including ischemic strokes, intraparenchymal hemorrhages (42%), and convexal non-aneurysmal subarachnoid hemorrhages.63,66,65,78,80 Vasogenic edema suggestive of PRES is seen in 28%. Vascular imaging (computer tomographic angiography, magnetic resonance angiography, or conventional transfemoral angiography) demonstrates multifocal areas of smooth arterial tapering and alternating dilatation. Initial angiographic studies may be normal in RCVS. Angiographic findings typically resolve within 90 days.66 RCVS can be difficult to distinguish from primary angiitis of the central nervous system (PACNS). The presence of recurrent thunderclap headaches; or the presence of a single thunderclap headache combined with normal neuroimaging, borderzone ischemic strokes or vasogenic edema suggestive of PRES, are highly specific for a diagnosis of RCVS.66 The recent RCVS2 score also has high accuracy for prediction of RCVS. In this score, the variables that predict RCVS are: recurrent or single thunderclap headache, presence of a trigger for vasoconstriction (drugs, postpartum state, orgasm), female sex, and subarachnoid hemorrhage. Involvement of the intracranial internal carotid artery is a negative predictor for RCVS.81
RCVS and PRES may co-occur: radiological feature of PRES has been reported in 9% of patients with RCVS.82 Additionally, cases have been reported of women with clinico-radiological features of RCVS overlapping with PRES. In such cases, imaging shows posterior white and often gray matter change consistent with vasogenic cerebral edema, or segmental narrowing and dilation of large and medium-sized cerebral arteries, the findings typical of RCVS.70
RCVS is best managed conservatively, with discontinuation of the offending agent; symptomatic treatment for headache and agitation; bed rest and stool softeners to avoid the Valsalva maneuver; and avoidance of physical exertion. Antiplatelets are not recommended. BP should be allowed to autorregulate, and BP drops avoided. Calcium channel blockers can be used for headache relief although they are often administered to treat the ‘vasospasm’. Logically, patients should avoid exposure to precipitants of the disease as far as possible however there is no evidence that re-exposure precipitates RCVS.62,69 There is no role for the use of glucocorticoids in RCVS, which can lead to worse clinical outcomes.83
The outcome in RCVS is favorable, with > 90% of patients having no disability. However, less than 5% have an aggressive course, with progression of vasospasm, severe neurological disability or death. This might be more common in post-partum RCVS.75,84,85 Recurrent RCVS (thunderclap headache, but without any focal deficits) has been reported in up to 5.8% of patients; post-partum RVCS was reported to occur after a subsequent delivery in 9% of patients.86
Cerebral Venous Sinus Thrombosis
CVST is an uncommon cerebrovascular disease characterized by thrombosis of the dural sinuses and cerebral veins, with resultant venous congestion, focal cerebral edema, and ultimately hemorrhagic venous infarctions. CVST accounts for 0.5–1% of all strokes.87 CVST is most commonly seen in patients below the age of 50, and 75% are women.88 The most common risk factor is oral contraceptive use (54%). Genetic and acquired thrombophilias are seen in 34%. Other conditions include malignancy, hematologic disorders, pregnancy in 6.3%, puerperium in 13.8%, and infections. 44% of patients may have a combination of risk factors.88 Obstetrical CVST is most commonly associated with hypertension, cesarean delivery and infection.20 A recent case-control study showed that the risk of gestational CVST is mostly attributable to the puerperium, with an aOR for CVST of 18.7 (95% CI, 8/3–41.9) during the first 6 weeks post-delivery, as opposed to aOR of 1.2 (95% CI, 0.6–2.3) during pregnancy.89
The most common presenting symptoms include headache (88%),90–92 seizures (40%), papilledema (30%), visual loss, diplopia, fluctuating motor or sensory deficits, stupor and coma.88 The disease affects most commonly the superior sagittal sinus (62%), the transverse sinus (41–45%), straight sinus (18%), cortical veins (17%), and deep venous system (11%).88
The diagnosis of CVST requires a high degree of clinical suspicion. Cerebral venous imaging is required for diagnosis, preferably performed with CT venography and MR venography. MRI susceptibility-weighted imaging is useful for the diagnosis of CVST, especially for cortical vein thrombosis.93 Indirect parenchymal signs may be seen in CVST, which are most conspicuous on brain MRI. These vary from normal appearing parenchyma to focal edema, cerebral hemorrhages, and increased or decreased water diffusivity.94 (Figure 1)
Figure 1.
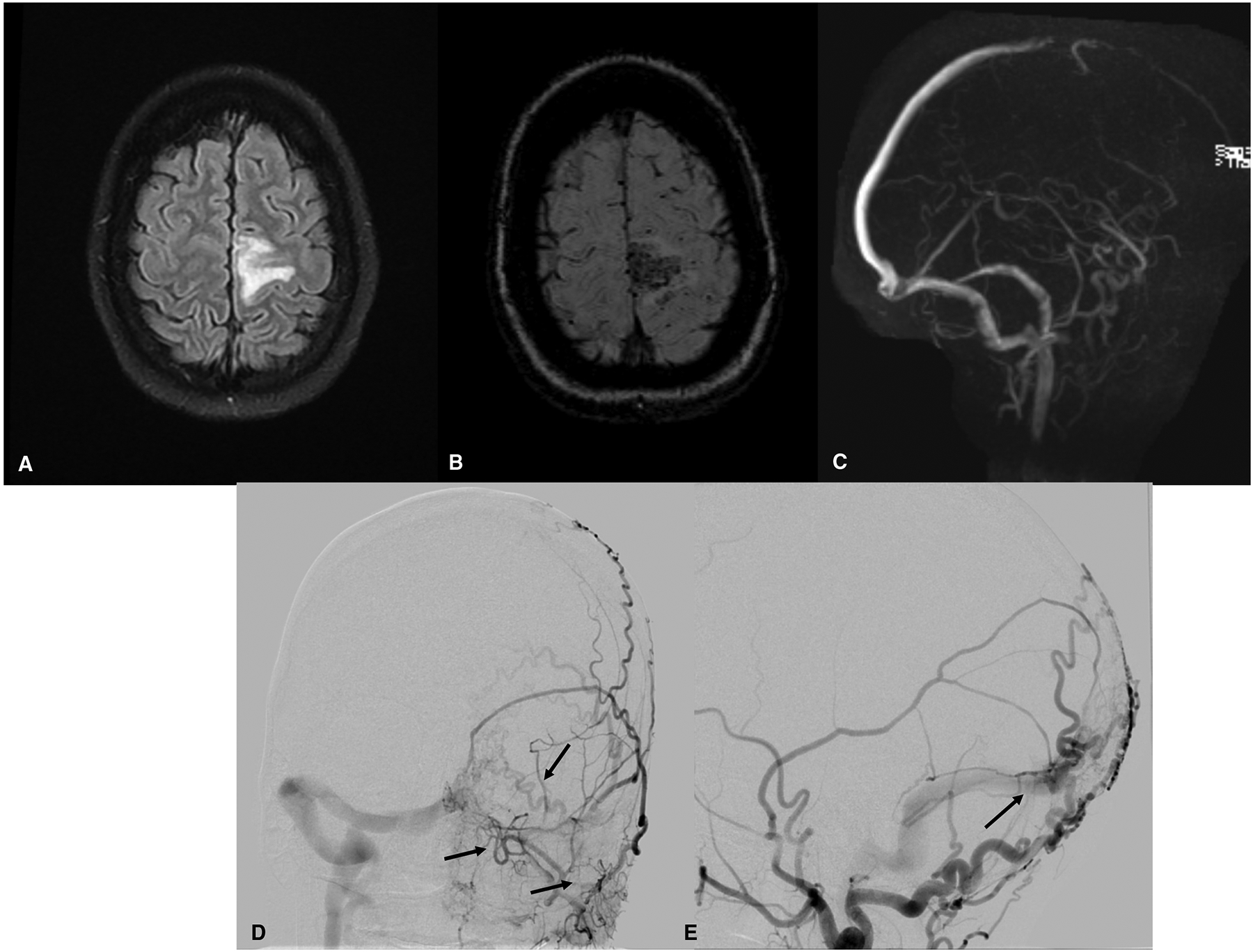
Brain and vascular imaging findings in a 34-year primigravida at 14 weeks gestation, presenting with right leg weakness for hours, followed by a focal motor seizure of the right leg and arm that progressed to a generalized tonic-clonic seizure. (A) Brain magnetic resonance imaging (MRI, fluid-attenuated inversion recovery (FLAIR) sequence) shows cerebral edema in the left frontal lobe. (B) Susceptibility-weighted MRI shows hemorrhage in the same region. (C) Magnetic resonance venography shows thrombo-occlusion in the anterior portion of the superior sagittal sinus and decreased flow-related enhancement of cortical veins overlying the left hemisphere. The patient was diagnosed with cerebral venous sinus and cortical vein thrombosis. She received therapeutic anticoagulation with low-molecular-weight heparin. A subsequent digital subtraction angiogram of the brain revealed a dural arterial-venous fistula of the brain, with extensive arterial supply from the bilateral occipital arteries and middle meningeal arteries (D, E). Because the patient was clinically stable, treatment of the vascular malformation was postponed until after delivery.
The mainstay of treatment of CVST is clot dissolution with anticoagulation. In the acute phase, unfractionated heparin or low-molecular-weight heparin are recommended, followed by oral anticoagulation with warfarin or dabigatran for at least 3–6 months.95 The duration of anticoagulation depends on the underlying condition. Anticoagulation is considered safe in the presence of intracranial hemorrhage. However, in the peri-partum period this may be complicated by risk of bleeding post-delivery or cesarean surgery. Because of its safety profile, warfarin and direct acting oral anticoagulants should be avoided during pregnancy. After delivery women may be transitioned from low molecular weight heparin to warfarin, even if nursing. Direct acting oral anticoagulants are not considered safe to use while breastfeeding.96 Thrombectomy is not routinely performed in CVST and has not shown to improve clinical outcomes when compared to medical management.97 (Table 1)
CVST is usually a benign condition, whereby 87% of patients will have none to mild deficits. Death occurs in 8.3%.88,98 Recurrent CVST is uncommon (2.2%), and recurrent venous thromboembolic disease in recurrent pregnancies is infrequent.99 Seizures can occur in 10.6% of patients. Any venous thromboembolic event can occur in 5.8% of patients, especially in the first year after CVST. Severe headaches can unfortunately persist after CVST in 14% of cases.88
Hemorrhagic Stroke (Table 1)
Pregnancy increases the risk for hemorrhagic much more than for ischemic stroke, with relative risk 2.5 during pregnancy and 28.5 post-partum.100 Hemorrhagic stroke is an important cause of maternal and fetal pregnancy-related mortality.7 The major established causes of pregnancy-related cerebral hemorrhage are preeclampsia-eclampsia, followed by arteriovenous malformations and aneurysms. The risk of aneurysmal rupture increases with gestational age, peaking at 30–34 weeks. A recent study showed an increase in the incidence of spontaneous subarachnoid hemorrhages during pregnancy in the US from 2002 to 2014, with the greatest increase affecting African-American pregnant women.101 Cerebral aneurysmal rupture during pregnancy is associated with high maternal (35%) and fetal (17%) mortality.102 If a ruptured aneurysm is left unsecured surgically, there is a very high rate of recurrent hemorrhage and maternal and fetal mortality, 63% and 27%, respectively. These mortalities were lowered to 11% and 5%, respectively, by early surgery.102 Thus, if aneurysmal subarachnoid hemorrhage occurs during pregnancy, then surgical control of the aneurysm should be performed immediately during pregnancy, when possible.103 If urgent obstetric issues prevent immediate neurosurgery, then urgent cesarean delivery is recommended, followed by surgical control of the cerebral aneurysm. There is increased risk of aneurysmal rupture near term. Therefore, it is recommended that unruptured aneurysms at significant risk of rupture be secured before pregnancy, when possible.
Hemorrhage is the most common presenting manifestation of arterio-venous malformations (AVM), followed by seizures or focal neurologic deficits. Whether AVMs bleed more during pregnancy has been debated. A study from 1990 showed an annual rate of hemorrhage of 3.5% in women with AVM and no prior hemorrhage, and 5.8% in those with prior hemorrhage, with no increase secondary to pregnancy.104 However, when considering the daily risk of rupture, there was a several-fold increase in risk on the day of delivery.105,106 More recent studies have shown an increase in the rate of intracranial hemorrhage in women during pregnancy and puerperium. A retrospective Chinese study of 264 women with AVM showed an annualized rate of AVM rupture and hemorrhage of 5.59% in pregnant women as compared to 2.52% in non-pregnant women (p=0.002).107 Similar results were seen in an American retrospective cohort of 270 women, showing an annual hemorrhage rate of 5.7% in pregnant women versus 1.3% in nonpregnant women (P<0.001).108 Further, AVM bleeding during pregnancy is associated with a higher rebleeding rate than in non-pregnant women (26% vs 6%, respectively).
Regarding management of AVMs in pregnancy, based on the above considerations, expert recommendation are that 1) if a woman with known AVM anticipates pregnancy, the AVM should be treated before pregnancy, 2) if an AVM is discovered during pregnancy and has not bled during the pregnancy, then conservative observation is usually recommended with plans to proceed to definitive treatment after delivery, 3) if an AVM bleeds during pregnancy, then consideration should be given to treatment during the pregnancy, taking into account the grade of the lesion and the expected timing of benefit in lowering risk (immediate for low grade lesions amenable to complete surgical excision or embolization) but delayed by 1–3 years for higher grade lesions requiring radiosurgery and combination therapies.109 Cesarean delivery is usually favored over vaginal delivery due to higher rates of hemorrhage on the day of delivery.
Blood pressure control is key to the acute management of patients with hemorrhagic stroke, irrespective of its etiology. This allows for reduction of hematoma growth. However, this must be balanced against the competing concern for severe hypotension, which can promote placental hypoperfusion.110
Cervical Artery Dissection (CAD)
It is not completely understood if gestation and delivery can be complicated by cervical artery dissection, especially for women undergoing vaginal delivery, and if CAD can lead to pregnancy-associated stroke. To address this question, a recent case control study showed that pregnancy may be accompanied by a higher risk of CAD (incidence RR 2.2, 95% CI 1.3–3.5). This risk was particularly high during the post-partum period, on average 21 days after delivery, but not during the pre-partum period. Of the patients developing CAD during pregnancy, 45% had HDP.111
Management of Stroke in Pregnancy
Acute Ischemic Stroke Management (Table 1)
For patients presenting with acute focal neurological deficits concerning for cerebrovascular disease, neuroimaging should be obtained expeditiously. Non-contrast head computerized tomography (CT), the initial imaging modality of choice, can be performed with abdominal shielding to protect the developing fetus. Non-contrast brain magnetic resonance imaging (MRI) is also considered safe during pregnancy. For vascular imaging, CT angiogram of the head and neck and or/CT venography can be safely performed with iodinated contrast, which does not cross the placenta. Head and neck MRA can be performed with time-of-flight techniques, which also do not require contrast. However, gadolinium-based MR contrast should not be administered to pregnant patients because it may cross the placenta and deposit in fetal tissues.112
For non-pregnant patients with acute ischemic stroke, early thrombolytic therapy and endovascular clot retrieval are the recommended hyperacute therapies to improve long term clinical outcomes. However, these therapies have not been studied in randomized trials involving pregnant women. Further, this therapy is often withheld in many women given concerns for life-threatening maternal and placental hemorrhages, including risk of fetal demise. The most widespread used thrombolytic, tissue plasminogen activator, does not cross the placenta and therefore, does not cause direct fetal harm. There have been numerous case reports and small case series of use of thrombolytic therapy for arterial ischemic stroke and other systemic indications, without clear evidence of harm or benefit.113 A recent study sought to address this question. Using data from the AHA Get with the Guidelines Registry, the authors compared 338 pregnant or post-partum women to 24303 women, all acute ischemic stroke sufferers who received reperfusion therapy (intravenous thrombolytic or catheter-based thrombolysis/clot retrieval). Pregnant women were less likely to receive intravenous thrombolysis than the non-pregnant women, but the overall rates of reperfusion therapy were similar between the two groups. Furthermore, patients in both groups had similar rates of discharge to home. There was a non-significant trend of increased rates of symptomatic intracranial hemorrhage in pregnant women who received intravenous thrombolysis as compared to non-pregnant women. Fetal outcomes were not reported in this study. However, this study did show that it may be reasonable to offer reperfusion to pregnant/post-partum women who suffered an ischemic stroke. Intra-arterial therapy is an especially interesting approach to acute stroke management in this population.114 A subsequent smaller study reported on 34 patients from the literature who received thrombolytic therapy for acute stroke: most of the cases reported favorable maternal and fetal outcomes.115
A multi-center case series of seven patients with acute ischemic stroke during pregnancy who received acute endovascular therapy showed promising results, with successful revascularization in all cases, no symptomatic hemorrhages, favorable NIHSS scores at discharge, and good outcomes at 3 months.116
Delivery
The timing of delivery for women who have suffered a stroke during pregnancy is determined by the severity of the mother’s medical condition and fetal stability. For women who suffer a stroke at < 24 weeks’ gestation, decisions regarding continuing pregnancy versus therapeutic termination rely on the clinical state of the patient and the need for use of thrombolysis, which can increase the risk of fetal loss. For women who suffer a stroke at 24–32 weeks’ gestation, antenatal glucocorticoids may be given to accelerate fetal lung maturation. If mother and fetus are clinically stable, then pregnancy may be continued, aiming for controlled induction at 34–39 weeks gestation. Cesarean section should be avoided if possible.113,117 In regards to antithrombotic management, heparinoids should be discontinued 24 hours before induction and labor, and may be resumed 24 hours after delivery.118
Secondary Stroke Prevention of Ischemic Stroke in Pregnancy/Puerperium
The choice of antithrombotic will be determined by the stroke mechanism and patient preferences. Aspirin is by the far the most widely used antithrombotic for secondary stroke prevention. It is deemed safe for use in the second and third trimesters for fetus and mother. In the first trimester, however, aspirin use has been reported to have an association with increased incidence of fetal gastroschisis and other fetal malformations.96 Other studies and metanalyses of aspirin use for prevention of preeclampsia even before 11 weeks do not support the same findings. A large meta-analysis of studies of antiplatelets use in women at high risk for preeclampsia showed safety of antiplatelets without adverse fetal outcomes, increase in fetal systemic or intracranial hemorrhages, adverse effects on fetal weight, and with more favorable maternal outcomes.119 The safety of other antiplatelets during pregnancy has not been established in clinical trials.
Regarding anticoagulants, warfarin is contraindicated in pregnancy due to its teratogenic effects and risk for fetal hemorrhages. It should be avoided in early pregnancy, and if at all also throughout the entire pregnancy because it may also cause central nervous system abnormalities in the 2nd and 3rd trimesters. Preference should be given to heparins. Unfractionated heparin (UFH) and low molecular heparins (LMWH) do not cross the placenta, although UFH can increase the risk of maternal thrombocytopenia and osteoporosis. The novel oral anticoagulants should not be used during pregnancy given their uncertain safety profile. Post-delivery, nursing mothers may continue to take low dose aspirin, warfarin, UFH and LMWH. However, novel oral anticoagulants are not recommended.120,96
Statins are often used for secondary prevention of ischemic stroke. There is currently insufficient data to assess the safety of statins during pregnancy.121 Furthermore, statins are classified as category X by the US Food and Drug Administration. Therefore, they should be avoided during gestation.
Importance of Multi-Disciplinary Care
A multidisciplinary collaboration in the care of the pregnant patient with stroke is key to success. In the emergency setting, healthcare providers should be well versed in the diagnosis and management of headaches associated with cerebrovascular diseases in pregnancy. In the emergency department, systems of care should be in place and readily activated in the case of an acute stroke syndrome. For success of such treatments, teams should include emergency medicine practitioners, neurologists, neuroradiologists, neuro-interventional radiologists and anesthesiologists, among others. The guidance of high-risk obstetricians is important for management decisions regarding thrombolysis and thrombectomy for acute ischemic stroke. After a stroke, patients benefit from care in highly specialized stroke units, which have been shown to improve outcomes when compared to standard inpatient care. In such units, stroke-specific care is offered by stroke neurologists and nurses, and patients receive specialized therapy for post-stroke recovery from physical and occupational therapist, and speech and language pathologists.
Hematologists may be involved when coagulation dyscrasias are identified. Interventional cardiologists may be consulted when cardiac procedures are required to reduce the risk of subsequent stroke. After a stroke, collaborations with social workers are important to facilitate transitions into the community. In certain cases, vocational counselors may help guide professional transitions into the community.
Stroke Outcomes
Morbidity and Mortality
Stroke morbidity is determined by the type of stroke, its severity, and therapies received for early management and secondary prevention. Mortality rates for stroke in pregnancy are reported at 2.7 to 20.4%.1,9 Mortality related to acute stroke during pregnancy was almost 385 times higher than that of pregnant women without acute stroke in one study.9 While previous studies had not shown a significant decrease in stroke-related mortality, a recent study of the National Inpatient Sample showed that the rates of in-hospital mortality for acute stroke during pregnancy and puerperium declined from 5.5% in 2007 to 2.7% in 2015, despite unchanged rates of acute stroke during that period. Independent predictors of in-house stroke-related mortality included age ≥ 40 years, black or Asian race, hemorrhagic stroke, anemia, heart failure, preeclampsia/eclampsia, gestational diabetes, and cesarean delivery.9
Risk of Recurrent Stroke
For women of child-bearing age who have a stroke or VST, the risk of a recurrent stroke during pregnancy is not substantially high. A study of 441 women with a first ever stroke followed for 5 years showed that only 13 strokes occurred in that time; only two strokes occurred during pregnancy/puerperium. Puerperium was the period of highest risk for an obstetrical ischemic stroke. Therefore, having had a previous ischemic stroke should not preclude women of child-bearing age to seek pregnancy.122 A subsequent study of 252 women with pregnancy-associated stroke confirmed that the risk of recurrent stroke or venous thrombosis during a subsequent pregnancy is low (2%).123 However, women with prior stroke who become pregnant appear to have a significantly higher risk of miscarriages, fetal loss, and pregnancy complications than pregnant women without prior stroke. These studies did not determine the cause of this association, although it is postulated that the underlying etiology of the index stroke could impact fertility, such as in patients with antiphospholipid syndrome or other hypercoagulable states.124
Synopsis.
Pregnancy confers a substantially increased risk of stroke, especially during the 3rd trimester and until 6 weeks post-partum. Hypertensive disorders of pregnancy and gestational hypercoagulability are important contributors to obstetrical stroke. Pre-eclampsia and eclampsia confer risk for future cardiovascular disease. Hemorrhagic stroke is the most common type of obstetrical stroke. Ischemic stroke can result from cardiomyopathy, paradoxical embolism, posterior reversible encephalopathy, reversible cerebral vasoconstriction syndrome, and dissections. Cerebral venous sinus thrombosis is a frequent complication of pregnancy.
Key Points.
Pregnancy and puerperium confer a substantially increased risk of ischemic and hemorrhagic stroke, with rates increasing approximately 60% from 2003–2004 to 2015–2016. The period of highest risk is from the 3rd trimester until 6 weeks post-partum, coinciding with the highest risk for hypertensive disorders of pregnancy and the peak risk of gestational hypercoagulability.
Hemorrhagic stroke is the most common type of obstetrical stroke, most commonly associated with hypertensive disorders of pregnancy. Additionally, increased risk of rupture of arteriovenous malformations and cerebral aneurysms during pregnancy contribute to hemorrhagic stroke.
Hypertensive disorders of pregnancy are among the most important contributors to obstetrical stroke and may predispose women to premature cardiovascular disease later in life. Common risk factors for pregnancy associated stroke include older age, African American race, congenital and acquired heart disease, pre-eclampsia/eclampsia, infection, diabetes, and c-section.
Conditions associated with ischemic stroke in pregnancy include peripartum cardiomyopathy, posterior reversible encephalopathy, reversible cerebral vasoconstriction syndrome, cerebral venous sinus thrombosis, paradoxical cardioembolism across a patent foramen ovale, and cervical arterial dissection. Cerebral venous sinus thrombosis is a common complication.
Acute ischemic stroke therapies such as intravenous thrombolysis with tissue plasminogen activator or endovascular clot retrieval appear safe during pregnancy and should not be withheld.
Disclosures
Dr. Singhal received research grant support from NIH-NINDS; consulting fees from Omniox, Deck Therapeutics, and Medicolegal firms; authorship royalty payments from UptoDate and Medlink, Inc; and his wife is an employee and owns stock in Biogen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Erica C. Camargo, Department of Neurology, Massachusetts General Hospital, Instructor in Neurology, Harvard Medical School, Boston, Massachusetts.
Aneesh B. Singhal, Department of Neurology, Director, Comprehensive Stroke Center, Massachusetts General Hospital, Associate Professor of Neurology, Harvard Medical School, Boston, Massachusetts.
References
- 1.Swartz RH, Cayley ML, Foley N, et al. The incidence of pregnancy-related stroke: A systematic review and meta-analysis. Int J Stroke. 2017;12(7):687–697. [DOI] [PubMed] [Google Scholar]
- 2.Kuklina EV, Tong X, Bansil P, George MG, Callaghan WM. Trends in pregnancy hospitalizations that included a stroke in the United States from 1994 to 2007: reasons for concern? Stroke. 2011;42(9):2564–2570. [DOI] [PubMed] [Google Scholar]
- 3.Wu P, Jordan KP, Chew-Graham CA, et al. Temporal Trends in Pregnancy-Associated Stroke and Its Outcomes Among Women With Hypertensive Disorders of Pregnancy. J Am Heart Assoc. 2020;9(15):e016182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Too G, Wen T, Boehme AK, et al. Timing and Risk Factors of Postpartum Stroke. Obstet Gynecol. 2018;131(1):70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ban L, Sprigg N, Abdul Sultan A, et al. Incidence of First Stroke in Pregnant and Nonpregnant Women of Childbearing Age: A Population-Based Cohort Study From England. J Am Heart Assoc. 2017;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamel H, Navi BB, Sriram N, Hovsepian DA, Devereux RB, Elkind MS. Risk of a thrombotic event after the 6-week postpartum period. N Engl J Med. 2014;370(14):1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meeks JR, Bambhroliya AB, Alex KM, et al. Association of Primary Intracerebral Hemorrhage With Pregnancy and the Postpartum Period. JAMA Netw Open. 2020;3(4):e202769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.James AH, Bushnell CD, Jamison MG, Myers ER. Incidence and risk factors for stroke in pregnancy and the puerperium. Obstet Gynecol. 2005;106(3):509–516. [DOI] [PubMed] [Google Scholar]
- 9.Elgendy IY, Gad MM, Mahmoud AN, Keeley EC, Pepine CJ. Acute Stroke During Pregnancy and Puerperium. J Am Coll Cardiol. 2020;75(2):180–190. [DOI] [PubMed] [Google Scholar]
- 10.Miller EC, Gatollari HJ, Too G, et al. Risk of Pregnancy-Associated Stroke Across Age Groups in New York State. JAMA Neurol. 2016;73(12):1461–1467. [DOI] [PubMed] [Google Scholar]
- 11.Qureshi AI, Saeed O, Malik AA, Suri MF. Pregnancy in advanced age and the risk of stroke in postmenopausal women: analysis of Women’s Health Initiative Study. Am J Obstet Gynecol. 2017;216(4):409 e401–409 e406. [DOI] [PubMed] [Google Scholar]
- 12.Miller EC, Zambrano Espinoza MD, Huang Y, et al. Maternal Race/Ethnicity, Hypertension, and Risk for Stroke During Delivery Admission. J Am Heart Assoc. 2020;9(3):e014775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bandoli G, Baer RJ, Gano D, Pawlowski LJ, Chambers C. Migraines During Pregnancy and the Risk of Maternal Stroke. JAMA Neurol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller EC, Gallo M, Kulick ER, Friedman AM, Elkind MSV, Boehme AK. Infections and Risk of Peripartum Stroke During Delivery Admissions. Stroke. 2018;49(5):1129–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller EC, Wen T, Elkind MSV, Friedman AM, Boehme AK. Infection During Delivery Hospitalization and Risk of Readmission for Postpartum Stroke. Stroke. 2019;50(10):2685–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanders BD, Davis MG, Holley SL, Phillippi JC. Pregnancy-Associated Stroke. J Midwifery Womens Health. 2018;63(1):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L, Deng W, Palacios I, et al. Patent foramen ovale (PFO), stroke and pregnancy. J Investig Med. 2016;64(5):992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dayan N, Filion KB, Okano M, et al. Cardiovascular Risk Following Fertility Therapy: Systematic Review and Meta-Analysis. J Am Coll Cardiol. 2017;70(10):1203–1213. [DOI] [PubMed] [Google Scholar]
- 19.Udell JA, Lu H, Redelmeier DA. Failure of fertility therapy and subsequent adverse cardiovascular events. CMAJ. 2017;189(10):E391–E397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanska DJ, Kryscio RJ. Risk factors for peripartum and postpartum stroke and intracranial venous thrombosis. Stroke. 2000;31(6):1274–1282. [DOI] [PubMed] [Google Scholar]
- 21.Lin SY, Hu CJ, Lin HC. Increased risk of stroke in patients who undergo cesarean section delivery: a nationwide population-based study. Am J Obstet Gynecol. 2008;198(4):391 e391–397. [DOI] [PubMed] [Google Scholar]
- 22.Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation. 2014;130(12):1003–1008. [DOI] [PubMed] [Google Scholar]
- 23.Feske SK, Singhal AB. Cerebrovascular disorders complicating pregnancy. Continuum (Minneap Minn). 2014;20(1 Neurology of Pregnancy):80–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poppas A, Shroff SG, Korcarz CE, et al. Serial assessment of the cardiovascular system in normal pregnancy. Role of arterial compliance and pulsatile arterial load. Circulation. 1997;95(10):2407–2415. [DOI] [PubMed] [Google Scholar]
- 25.Brenner B Haemostatic changes in pregnancy. Thromb Res. 2004;114(5–6):409–414. [DOI] [PubMed] [Google Scholar]
- 26.Coutinho T, Lamai O, Nerenberg K. Hypertensive Disorders of Pregnancy and Cardiovascular Diseases: Current Knowledge and Future Directions. Curr Treat Options Cardiovasc Med. 2018;20(7):56. [DOI] [PubMed] [Google Scholar]
- 27.Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet. 2005;366(9499):1797–1803. [DOI] [PubMed] [Google Scholar]
- 28.Wu P, Haththotuwa R, Kwok CS, et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ Cardiovasc Qual Outcomes. 2017;10(2). [DOI] [PubMed] [Google Scholar]
- 29.Leon LJ, McCarthy FP, Direk K, et al. Preeclampsia and Cardiovascular Disease in a Large UK Pregnancy Cohort of Linked Electronic Health Records: A CALIBER Study. Circulation. 2019;140(13):1050–1060. [DOI] [PubMed] [Google Scholar]
- 30.Leffert LR, Clancy CR, Bateman BT, Bryant AS, Kuklina EV. Hypertensive disorders and pregnancy-related stroke: frequency, trends, risk factors, and outcomes. Obstet Gynecol. 2015;125(1):124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Umesawa M, Kobashi G. Epidemiology of hypertensive disorders in pregnancy: prevalence, risk factors, predictors and prognosis. Hypertens Res. 2017;40(3):213–220. [DOI] [PubMed] [Google Scholar]
- 32.Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113(6):1299–1306. [DOI] [PubMed] [Google Scholar]
- 33.Miller EC, Gatollari HJ, Too G, et al. Risk Factors for Pregnancy-Associated Stroke in Women With Preeclampsia. Stroke. 2017;48(7):1752–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376(9741):631–644. [DOI] [PubMed] [Google Scholar]
- 35.Bateman BT, Schumacher HC, Bushnell CD, et al. Intracerebral hemorrhage in pregnancy: frequency, risk factors, and outcome. Neurology. 2006;67(3):424–429. [DOI] [PubMed] [Google Scholar]
- 36.Brown MA, Magee LA, Kenny LC, et al. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension. 2018;72(1):24–43. [DOI] [PubMed] [Google Scholar]
- 37.Bushnell C, Chireau M. Preeclampsia and Stroke: Risks during and after Pregnancy. Stroke Res Treat. 2011;2011:858134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodie VA, Freeman DJ, Sattar N, Greer IA. Pre-eclampsia and cardiovascular disease: metabolic syndrome of pregnancy? Atherosclerosis. 2004;175(2):189–202. [DOI] [PubMed] [Google Scholar]
- 39.Hammer ES, Cipolla MJ. Cerebrovascular Dysfunction in Preeclamptic Pregnancies. Curr Hypertens Rep. 2015;17(8):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet Gynecol. 2001;97(4):533–538. [DOI] [PubMed] [Google Scholar]
- 41.Judy AE, McCain CL, Lawton ES, Morton CH, Main EK, Druzin ML. Systolic Hypertension, Preeclampsia-Related Mortality, and Stroke in California. Obstet Gynecol. 2019;133(6):1151–1159. [DOI] [PubMed] [Google Scholar]
- 42.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335(7627):974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crandon AJ, Isherwood DM. Effect of aspirin on incidence of pre-eclampsia. Lancet. 1979;1(8130):1356. [DOI] [PubMed] [Google Scholar]
- 44.Rolnik DL, Wright D, Poon LC, et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med. 2017;377(7):613–622. [DOI] [PubMed] [Google Scholar]
- 45.Miller EC, Boehme AK, Chung NT, et al. Aspirin reduces long-term stroke risk in women with prior hypertensive disorders of pregnancy. Neurology. 2019;92(4):e305–e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hofmeyr GJ, Lawrie TA, Atallah AN, Duley L, Torloni MR. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst Rev. 2014(6):CD001059. [DOI] [PubMed] [Google Scholar]
- 47.Bassaw B, Roopnarinesingh S, Roopnarinesingh A, Homer H. Prevention of hypertensive disorders of pregnancy. J Obstet Gynaecol. 1998;18(2):123–126. [DOI] [PubMed] [Google Scholar]
- 48.Villar J, Repke JT. Calcium supplementation during pregnancy may reduce preterm delivery in high-risk populations. Am J Obstet Gynecol. 1990;163(4 Pt 1):1124–1131. [DOI] [PubMed] [Google Scholar]
- 49.American College of O, Gynecologists, Task Force on Hypertension in P. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131. [DOI] [PubMed] [Google Scholar]
- 50.Bushnell C, McCullough LD, Awad IA, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(5):1545–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magee LA, von Dadelszen P, Rey E, et al. Less-tight versus tight control of hypertension in pregnancy. N Engl J Med. 2015;372(5):407–417. [DOI] [PubMed] [Google Scholar]
- 52.Altman D, Carroli G, Duley L, et al. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359(9321):1877–1890. [DOI] [PubMed] [Google Scholar]
- 53.Which anticonvulsant for women with eclampsia? Evidence from the Collaborative Eclampsia Trial. Lancet. 1995;345(8963):1455–1463. [PubMed] [Google Scholar]
- 54.Lucas MJ, Leveno KJ, Cunningham FG. A comparison of magnesium sulfate with phenytoin for the prevention of eclampsia. N Engl J Med. 1995;333(4):201–205. [DOI] [PubMed] [Google Scholar]
- 55.McDermott M, Miller EC, Rundek T, Hurn PD, Bushnell CD. Preeclampsia: Association With Posterior Reversible Encephalopathy Syndrome and Stroke. Stroke. 2018;49(3):524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brewer J, Owens MY, Wallace K, et al. Posterior reversible encephalopathy syndrome in 46 of 47 patients with eclampsia. Am J Obstet Gynecol. 2013;208(6):468 e461–466. [DOI] [PubMed] [Google Scholar]
- 57.Mayama M, Uno K, Tano S, et al. Incidence of posterior reversible encephalopathy syndrome in eclamptic and patients with preeclampsia with neurologic symptoms. Am J Obstet Gynecol. 2016;215(2):239 e231–235. [DOI] [PubMed] [Google Scholar]
- 58.Bartynski WS, Boardman JF. Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2007;28(7):1320–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liman TG, Bohner G, Heuschmann PU, Scheel M, Endres M, Siebert E. Clinical and radiological differences in posterior reversible encephalopathy syndrome between patients with preeclampsia-eclampsia and other predisposing diseases. Eur J Neurol. 2012;19(7):935–943. [DOI] [PubMed] [Google Scholar]
- 60.Postma IR, Slager S, Kremer HP, de Groot JC, Zeeman GG. Long-term consequences of the posterior reversible encephalopathy syndrome in eclampsia and preeclampsia: a review of the obstetric and nonobstetric literature. Obstet Gynecol Surv. 2014;69(5):287–300. [DOI] [PubMed] [Google Scholar]
- 61.Call GK, Fleming MC, Sealfon S, Levine H, Kistler JP, Fisher CM. Reversible cerebral segmental vasoconstriction. Stroke. 1988;19(9):1159–1170. [DOI] [PubMed] [Google Scholar]
- 62.Calabrese LH, Dodick DW, Schwedtt T, Singhal AB. Reversible Cerebral Vasoconstriction Syndromes. Annals Int Med. 2007;in press. [DOI] [PubMed] [Google Scholar]
- 63.Ducros A, Boukobza M, Porcher R, Sarov M, Valade D, Bousser MG. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain. 2007;130(Pt 12):3091–3101. [DOI] [PubMed] [Google Scholar]
- 64.Singhal AB. Cerebral vasoconstriction syndromes. Top Stroke Rehabil. 2004;11(2):1–6. [DOI] [PubMed] [Google Scholar]
- 65.Singhal AB, Hajj-Ali RA, Topcuoglu MA, et al. Reversible cerebral vasoconstriction syndromes: analysis of 139 cases. Arch Neurol. 2011;68(8):1005–1012. [DOI] [PubMed] [Google Scholar]
- 66.Singhal AB, Topcuoglu MA, Fok JW, et al. RCVS and PACNS: Clinical, imaging, and angiographic comparison. Ann Neurol. 2016. [DOI] [PubMed] [Google Scholar]
- 67.Chen SP, Fuh JL, Lirng JF, Chang FC, Wang SJ. Recurrent primary thunderclap headache and benign CNS angiopathy: spectra of the same disorder? Neurology. 2006;67(12):2164–2169. [DOI] [PubMed] [Google Scholar]
- 68.Calabrese LH, Dodick DW, Schwedt TJ, Singhal AB. Narrative review: reversible cerebral vasoconstriction syndromes. Ann Intern Med. 2007;146(1):34–44. [DOI] [PubMed] [Google Scholar]
- 69.Ducros A Reversible cerebral vasoconstriction syndrome. Lancet Neurol. 2012;11(10):906–917. [DOI] [PubMed] [Google Scholar]
- 70.Singhal AB. Postpartum angiopathy with reversible posterior leukoencephalopathy. Arch Neurol. 2004;61(3):411–416. [DOI] [PubMed] [Google Scholar]
- 71.Singhal AB, Caviness VS, Begleiter AF, Mark EJ, Rordorf G, Koroshetz WJ. Cerebral vasoconstriction and stroke after use of serotonergic drugs. Neurology. 2002;58(1):130–133. [DOI] [PubMed] [Google Scholar]
- 72.Nighoghossian N, Derex L, Trouillas P. Multiple intracerebral hemorrhages and vasospasm following antimigrainous drug abuse. Headache. 1998;38(6):478–480. [DOI] [PubMed] [Google Scholar]
- 73.Razavi M, Bendixen B, Maley JE, et al. CNS pseudovasculitis in a patient with pheochromocytoma. Neurology. 1999;52(5):1088–1090. [DOI] [PubMed] [Google Scholar]
- 74.Verillaud B, Ducros A, Massiou H, Huy PT, Bousser MG, Herman P. Reversible cerebral vasoconstriction syndrome in two patients with a carotid glomus tumour. Cephalalgia. 2010;30(10):1271–1275. [DOI] [PubMed] [Google Scholar]
- 75.Fugate JE, Wijdicks EF, Parisi JE, et al. Fulminant postpartum cerebral vasoconstriction syndrome. Arch Neurol. 2012;69(1):111–117. [DOI] [PubMed] [Google Scholar]
- 76.Lee JH, Martin NA, Alsina G, et al. Hemodynamically significant cerebral vasospasm and outcome after head injury: a prospective study. J Neurosurg. 1997;87(2):221–233. [DOI] [PubMed] [Google Scholar]
- 77.Wolff V, Lauer V, Rouyer O, et al. Cannabis use, ischemic stroke, and multifocal intracranial vasoconstriction: a prospective study in 48 consecutive young patients. Stroke. 2011;42(6):1778–1780. [DOI] [PubMed] [Google Scholar]
- 78.Ducros A, Fiedler U, Porcher R, Boukobza M, Stapf C, Bousser MG. Hemorrhagic manifestations of reversible cerebral vasoconstriction syndrome: frequency, features, and risk factors. Stroke. 2010;41(11):2505–2511. [DOI] [PubMed] [Google Scholar]
- 79.Dodick DW. Reversible segmental cerebral vasoconstriction (Call-Fleming syndrome): the role of calcium antagonists. Cephalalgia. 2003;23(3):163–165. [DOI] [PubMed] [Google Scholar]
- 80.Kumar S, Goddeau RP Jr., Selim MH, et al. Atraumatic convexal subarachnoid hemorrhage: clinical presentation, imaging patterns, and etiologies. Neurology. 2010;74(11):893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rocha EA, Topcuoglu MA, Silva GS, Singhal AB. RCVS2 score and diagnostic approach for reversible cerebral vasoconstriction syndrome. Neurology. 2019;92(7):e639–e647. [DOI] [PubMed] [Google Scholar]
- 82.Chen SP, Fuh JL, Wang SJ, et al. Magnetic resonance angiography in reversible cerebral vasoconstriction syndromes. Ann Neurol. 2010;67(5):648–656. [DOI] [PubMed] [Google Scholar]
- 83.Singhal AB, Topcuoglu MA. Glucocorticoid-associated worsening in reversible cerebral vasoconstriction syndrome. Neurology. 2017;88(3):228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hajj-Ali RA, Furlan A, Abou-Chebel A, Calabrese LH. Benign angiopathy of the central nervous system: cohort of 16 patients with clinical course and long-term followup. Arthritis Rheum. 2002;47(6):662–669. [DOI] [PubMed] [Google Scholar]
- 85.Singhal AB, Kimberly WT, Schaefer PW, Hedley-Whyte ET. Case records of the Massachusetts General Hospital. Case 8–2009. A 36-year-old woman with headache, hypertension, and seizure 2 weeks post partum. N Engl J Med. 2009;360(11):1126–1137. [DOI] [PubMed] [Google Scholar]
- 86.Boitet R, de Gaalon S, Duflos C, et al. Long-Term Outcomes After Reversible Cerebral Vasoconstriction Syndrome. Stroke. 2020;51(2):670–673. [DOI] [PubMed] [Google Scholar]
- 87.Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007;6(2):162–170. [DOI] [PubMed] [Google Scholar]
- 88.Ferro JM, Canhao P, Stam J, Bousser MG, Barinagarrementeria F. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35(3):664–670. [DOI] [PubMed] [Google Scholar]
- 89.Silvis SM, Lindgren E, Hiltunen S, et al. Postpartum Period Is a Risk Factor for Cerebral Venous Thrombosis. Stroke. 2019;50(2):501–503. [DOI] [PubMed] [Google Scholar]
- 90.Biousse V, Ameri A, Bousser MG. Isolated intracranial hypertension as the only sign of cerebral venous thrombosis. Neurology. 1999;53(7):1537–1542. [DOI] [PubMed] [Google Scholar]
- 91.Ferro JM, Correia M, Pontes C, Baptista MV, Pita F. Cerebral vein and dural sinus thrombosis in Portugal: 1980–1998. Cerebrovasc Dis. 2001;11(3):177–182. [DOI] [PubMed] [Google Scholar]
- 92.Cumurciuc R, Crassard I, Sarov M, Valade D, Bousser MG. Headache as the only neurological sign of cerebral venous thrombosis: a series of 17 cases. J Neurol Neurosurg Psychiatry. 2005;76(8):1084–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Idbaih A, Boukobza M, Crassard I, Porcher R, Bousser MG, Chabriat H. MRI of clot in cerebral venous thrombosis: high diagnostic value of susceptibility-weighted images. Stroke. 2006;37(4):991–995. [DOI] [PubMed] [Google Scholar]
- 94.Yuh WT, Simonson TM, Wang AM, et al. Venous sinus occlusive disease: MR findings. AJNR Am J Neuroradiol. 1994;15(2):309–316. [PMC free article] [PubMed] [Google Scholar]
- 95.Ferro JM, Coutinho JM, Dentali F, et al. Safety and Efficacy of Dabigatran Etexilate vs Dose-Adjusted Warfarin in Patients With Cerebral Venous Thrombosis: A Randomized Clinical Trial. JAMA Neurol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Caso V, Falorni A, Bushnell CD, et al. Pregnancy, Hormonal Treatments for Infertility, Contraception, and Menopause in Women After Ischemic Stroke: A Consensus Document. Stroke. 2017;48(2):501–506. [DOI] [PubMed] [Google Scholar]
- 97.Coutinho JM, Zuurbier SM, Bousser MG, et al. Effect of Endovascular Treatment With Medical Management vs Standard Care on Severe Cerebral Venous Thrombosis: The TO-ACT Randomized Clinical Trial. JAMA Neurol. 2020;77(8):966–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Canhao P, Ferro JM, Lindgren AG, Bousser MG, Stam J, Barinagarrementeria F. Causes and predictors of death in cerebral venous thrombosis. Stroke. 2005;36(8):1720–1725. [DOI] [PubMed] [Google Scholar]
- 99.Aguiar de Sousa D, Canhao P, Crassard I, et al. Safety of Pregnancy After Cerebral Venous Thrombosis: Results of the ISCVT (International Study on Cerebral Vein and Dural Sinus Thrombosis)-2 PREGNANCY Study. Stroke. 2017;48(11):3130–3133. [DOI] [PubMed] [Google Scholar]
- 100.Kittner SJ, Stern BJ, Feeser BR, et al. Pregnancy and the risk of stroke. New England Journal of Medicine. 1996;335:768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Limaye K, Patel A, Dave M, et al. Secular Increases in Spontaneous Subarachnoid Hemorrhage during Pregnancy: A Nationwide Sample Analysis. J Stroke Cerebrovasc Dis. 2019;28(4):1141–1148. [DOI] [PubMed] [Google Scholar]
- 102.Dias MS, Sekhar LN. Intracranial hemorrhage from aneurysms and arteriovenous malformations during pregnancy and the puerperium. Neurosurgery. 1990;27:855–865. [DOI] [PubMed] [Google Scholar]
- 103.Meyers PM, Halbach VV, Malek AM, et al. Endovascular treatment of cerebral artery aneurysms during pregnancy: report of three cases. AJNR American Journal of Neuroradiology. 2000;21:1306–1311. [PMC free article] [PubMed] [Google Scholar]
- 104.Horton JC, Chambers WA, Lyons SL, et al. Pregnancy and the risk of hemorrhage from cerebral arteriovenous malformations. Neurosurgery. 1990;27:867–872. [DOI] [PubMed] [Google Scholar]
- 105.Parkinson D, Bachers G. Arteriovenous malformations: summary of 100 consecutive supratentorial cases. Journal of Neurosurgery. 1980;53:285–299. [DOI] [PubMed] [Google Scholar]
- 106.Weir B, MR L. Management of intracranial aneurysms and arteriovenous malformations during pregnancy In: Wilkins RH, Rengachary SS, eds. Neurosurgery. New York: McGraw-Hill; 1996:2421–2427. [Google Scholar]
- 107.Zhu D, Zhao P, Lv N, et al. Rupture Risk of Cerebral Arteriovenous Malformations During Pregnancy and Puerperium: A Single-Center Experience and Pooled Data Analysis. World Neurosurg. 2018;111:e308–e315. [DOI] [PubMed] [Google Scholar]
- 108.Porras JL, Yang W, Philadelphia E, et al. Hemorrhage Risk of Brain Arteriovenous Malformations During Pregnancy and Puerperium in a North American Cohort. Stroke. 2017;48(6):1507–1513. [DOI] [PubMed] [Google Scholar]
- 109.Ogilvy CS, Stieg PE, Awad I, et al. Recommendations for the management of intracranial arteriovenous malformations: a statement for healthcare professionals for a special writing group of the Stroke Council, American Stroke Association. Stroke. 2001;32:1458–1471. [DOI] [PubMed] [Google Scholar]
- 110.Toossi S, Moheet AM. Intracerebral Hemorrhage in Women: A Review with Special Attention to Pregnancy and the Post-Partum Period. Neurocrit Care. 2019;31(2):390–398. [DOI] [PubMed] [Google Scholar]
- 111.Salehi Omran S, Parikh NS, Poisson S, et al. Association between Pregnancy and Cervical Artery Dissection. Ann Neurol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chansakul T, Young GS. Neuroimaging in Pregnant Women. Semin Neurol. 2017;37(6):712–723. [DOI] [PubMed] [Google Scholar]
- 113.Shainker SA, Edlow JA, O’Brien K. Cerebrovascular emergencies in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2015;29(5):721–731. [DOI] [PubMed] [Google Scholar]
- 114.Leffert LR, Clancy CR, Bateman BT, et al. Treatment patterns and short-term outcomes in ischemic stroke in pregnancy or postpartum period. Am J Obstet Gynecol. 2016;214(6):723 e721–723 e711. [DOI] [PubMed] [Google Scholar]
- 115.Ryman KM, Pace WD, Smith S, Fontaine GV. Alteplase Therapy for Acute Ischemic Stroke in Pregnancy: Two Case Reports and a Systematic Review of the Literature. Pharmacotherapy. 2019;39(7):767–774. [DOI] [PubMed] [Google Scholar]
- 116.Limaye K, Van de Walle Jones A, Shaban A, et al. Endovascular management of acute large vessel occlusion stroke in pregnancy is safe and feasible. J Neurointerv Surg. 2020;12(6):552–556. [DOI] [PubMed] [Google Scholar]
- 117.Lee M-JH,S. Cerebrovascular disorders complicating pregnancy. In: Biller JL, C.J., ed. UpToDate. Waltham, MA: UpToDate 2018. [Google Scholar]
- 118.Barghouthi T, Bushnell C. Prevention and Management of Stroke in Obstetrics and Gynecology. Clin Obstet Gynecol. 2018;61(2):235–242. [DOI] [PubMed] [Google Scholar]
- 119.Duley L, Henderson-Smart D, Knight M, King J. Antiplatelet drugs for prevention of pre-eclampsia and its consequences: systematic review. BMJ. 2001;322(7282):329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e691S–e736S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Karalis DG, Hill AN, Clifton S, Wild RA. The risks of statin use in pregnancy: A systematic review. J Clin Lipidol. 2016;10(5):1081–1090. [DOI] [PubMed] [Google Scholar]
- 122.Lamy C, Hamon JB, Coste J, Mas JL. Ischemic stroke in young women: risk of recurrence during subsequent pregnancies. French Study Group on Stroke in Pregnancy. Neurology. 2000;55(2):269–274. [DOI] [PubMed] [Google Scholar]
- 123.Karjalainen L, Tikkanen M, Rantanen K, Laivuori H, Gissler M, Ijas P. Pregnancy-associated stroke -a systematic review of subsequent pregnancies and maternal health. BMC Pregnancy Childbirth. 2019;19(1):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.van Alebeek ME, de Vrijer M, Arntz RM, et al. Increased Risk of Pregnancy Complications After Stroke: The FUTURE Study (Follow-Up of Transient Ischemic Attack and Stroke Patients and Unelucidated Risk Factor Evaluation). Stroke. 2018;49(4):877–883. [DOI] [PubMed] [Google Scholar]


