Abstract
Aim:
Chemotherapy-induced peripheral neuropathy (CIPN) is a long-term neurological health issue in patients diagnosed with multiple myeloma (MM). The aim of this study was to assess CIPN symptoms and health-related quality of life (HRQOL) among MM patients.
Methods:
A cross-sectional survey was conducted among patients diagnosed with MM in a tertiary care hospital using a self-reported Arabic questionnaire, European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire for CIPN scale (QLQ-CIPN20). The HRQOL was assessed using EORTC multiple myeloma module (QLQ-MY20). Categorical variables were reported in frequency tables and percentages. Age and duration of MM diagnosis were reported as mean and standard deviation. Survey responses were presented using descriptive statistics.
Results:
In total, 62 patients had participated. Males were 60%. The average age was 58.74 ± 11.49 years. On sensory scale, 20% reported “quite a bit”/”very much” tingling in fingers/hands, 23% in toes/feet, 39% numbness in fingers/hands, 37% in toes/feet, and 43% reported trouble standing or walking. On motor scale, 40% reported trouble walking and 60% had difficulty in climbing stairs/standing up from chair. On autonomic scale, 27% reported orthostatic hypotension and only 13/37 (46%) males reported erectile dysfunction. For HRQOL, 50% reported bone aches/pain, 42% reported back pain, 57% reported feeling ill, 33% reported lost hair, 35% had been thinking about their illness, whereas 28% were worried about future health and 22% had reported being worried about dying.
Conclusion:
MM patients encounter CIPN symptoms with impaired HRQOL. Capturing CIPN as a patient-reported outcome needs to be considered in routine clinical practice.
Keywords: Chemotherapy, European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire for Chemotherapy-Induced Peripheral Neuropathy 20-item scale, EORTC multiple myeloma module, health-related quality of life, multiple myeloma, peripheral neuropathy
INTRODUCTION
Advances in cancer therapy have led to a gradual shift in long-term treatment effects, patient-reported outcomes, and patient experience.[1] Chemotherapy-induced peripheral neuropathy (CIPN) is a common long-term neurologic side effect after chemotherapy,[2] with debilitating symptoms that negatively impact on a patient's quality of life (QoL).[3] The incidence of CIPN ranges between 30% and 70%,[4,5] with the wide variation likely due to the use of different methods to assess CIPN assessment. CIPN incidence rate depends on the type and dose of the chemotherapy,[6,7] and the prevalence decreases with time since starting chemotherapy: 68% of patients experience it in the 1st month, 60% at 3 months, and 30% at 6 months.[8]
Multiple myeloma (MM) is cancer of clonal plasma cells, and it makes up 10% of all hematological malignancies.[9,10] Usual therapeutic management includes immunomodulatory drugs (thalidomide) and proteasome inhibitor (bortezomib).[10] CIPN more commonly occurs in patients receiving platinum-derived compounds (cisplatin, carboplatin, and oxaliplatin), taxanes (paclitaxel and docetaxel), vinca alkaloids (vincristine and vinblastine), and proteasome inhibitors (bortezomib).[11,12,13,14] The first-line treatment for patients diagnosed with MM, bortezomib, causes CIPN in 75% of patients.[12,15]
The development of CIPN symptoms depends on the used chemotherapy. The symptoms affect upper and lower limbs, in a “stocking-and-glove” pattern, and the orofacial region.[6,16,17,18] Onset of symptoms is gradual, starting in the lower limbs and migrating to the upper limbs.[2] Sensory symptoms such as pain, dysesthesia, numbness, and tingling in the hands and feet are more prevalent and predominant than autonomic symptoms (constipation, urinary retention, and erectile dysfunction).[6,13,16,19] Clinical examination reveals impairment of perception of touch vibration and proprioception.[1] The symptoms can be debilitating and impair QoL.[2,20] Several tools have been reported in literature to assess CIPN.[7,13,21,22,23,24,25] The European Organization for Research and Treatment of Cancer (EORTC) QoL Questionnaire for CIPN 20-item scale (QLQ-CIPN20) is reported to have good reliability and internal validity (Cronbach's α: 0.88).[24,26,27]
CIPN needs to be recognized as a serious health issue among patients diagnosed with MM. The study aims to assess self-reported CIPN symptoms and the effect of these on health-related QoL (HRQOL) in patients diagnosed with MM.
METHODS
A cross-sectional survey to assess CIPN and HRQOL in patients diagnosed with MM was conducted in August 2016 in a tertiary care hospital. Ethical approval was obtained from the Institutional Review Board (IRB) with the approval number (RSS16/005).
Study participants
A convenient sample of patients aged 18 years and above, male/female, diagnosed with MM since 2010, started on chemotherapeutic agents and attending the hematology clinics/ward at a tertiary care hospital were invited to participate in the study. Patients were enrolled after signing written informed consent.
Instruments
CIPN was assessed using the Arabic version of self-reported tool, EORTC QLQ-CIPN20, composed of 20 items: three subscales examining sensory (nine items), motor (eight items), and autonomic symptoms (three items). Items were rated on a Likert scale: 1, not at all; 2, a little; 3, quite a bit; and 4, very much. Final scores were linearly transformed from 0 to 100, with a higher score denoting increased symptom burden.[24] HRQOL was assessed using the Arabic version of EORTC Multiple Myeloma Module, QLQ-MY20. It consists of two subscales: a functional domain (4 items) and a symptoms domain (16 items). Scores were linearly transformed to a 0–100 scale, with a higher score denoting a better QoL.
The Arabic validated versions of EORTC QLQ-CIPN20 and EORTC multiple myeloma module (QLQ-MY20) were distributed to the participants during their follow-up visit in the hematology clinics or ward. The participants could read and write Arabic. On an average, participants took 5 min to complete the questionnaire. The self-completed questionnaire was collected in an envelope to maintain the confidentiality of the responses. Participants' demographic data and clinical information were collected from electronic health records.
Statistical analysis
Categorical variables, i.e., gender, comorbidities, MM type, disease stage, chemotherapy received, history of chemotherapy/radiotherapy, family history of MM, and treatment outcomes, were reported in frequency tables and percentages. Age and duration of MM diagnosis were reported as mean and standard deviation. Results from the QLQ-CIPN20 and QLQ-MY20 were presented using descriptive statistics. The QLQ-CIPN20 results were compared across subscales for diabetic and nondiabetic patients using the Wilcoxon rank-sum test. Significance was taken as α < 0.05. Analyses were carried out using SAS version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Patients characteristics
Sixty-two patients filled in the questionnaire, 37 men (60%) and 25 women (40%), with a mean age of 58.74 ± 11.49 years [Table 1]. The mean duration of MM was 3.08 ± 2.68 years. Almost two-thirds of patients (49/62, 79%) received active chemotherapy, with bortezomib-cyclophosphamide -dexamethasone including bortezomib as the most common regimen 31 (63%) (n = 49). In total, 12 (25%) patients received thalidomide. Some patients have received more than one neurotoxic agent 6 (12%). Twelve (22%) patients had received radiotherapy. Half of the patients received autologous stem cell transplantation (31 patients). In terms of comorbidities, 18 (34%) were known to have diabetes mellitus, 18 (34%) were known to have hypertension, and 3 (6%) had ischemic heart disease. Overall, 5 (9.0%) patients had prior malignancy and 5 (10%) had myelodysplastic syndrome. Information on response to treatment data was available for only 23 patients: 9 (39%) patients had relapse, 8 (35%) had partial response, and 6 (26%) had complete response [Table 1].
Table 1.
Demographics and baseline clinical characteristics of the study cohort
| Variables (n=62) | n (%)* |
|---|---|
| Age (mean±SD) (n=58) | 58.74±11.49 |
| Sex (n=62) | |
| Males | 37 (59.67) |
| Females | 25 (40.32) |
| MM diagnosis duration years (mean±SD) (n=50) | 3.08±2.68 |
| Comorbidities | |
| Diabetes mellitus (n=52) | 18 (34.62) |
| Hypertension (n=52) | 18 (34.62) |
| Ischemic heart disease (n=51) | 3 (5.88) |
| Autologous stem cell transplant (n=39) | 31 (79.49) |
| Prior malignancy (n=53) | 5 (9.43) |
| History of chemotherapy (n=53) | 19 (35.85) |
| History of radiotherapy (n=53) | 12 (22.64) |
| Family history of multiple myeloma (n=53) | 8 (15.09) |
| Myelodysplastic syndrome (n=51) | 5 (9.8) |
| Treatment outcome (n=23) | |
| Relapse | 9 (39.13) |
| Partial response | 8 (34.78) |
| Complete response | 6 (26.09) |
| Receiving active chemotherapy (n=49) | 49 (79.03) |
| Chemotherapy type (n=49)** | |
| VCD | 31 (63.27) |
| VTD | 11 (22.45) |
| VD | 5 (10.2) |
| LEN-DEX | 4 (8.16) |
| RVD | 2 (4.08) |
| Bortezomib | 2 (4.08) |
| Thalidomide | 1 (2.04) |
*Data are shown for n=62, Unless otherwise specified, **The percentage is exceeding 100 since multiple treatments have been received. MM: Multiple myeloma, SD: Standard deviation, VCD: Bortezomib-cyclophosphamide-dexamethasone, VTD: Bortezomib-thalidomide-dexamethasone, VD: Bortezomib-dexamethasone, LEN-DEX: Lenalidomide-dexamethasone, RVD: Lenalidomide-bortezomib-dexamethasone
Chemotherapy-induced peripheral neuropathy symptoms among multiple myeloma patients
The CIPN symptoms are summarized below for participants who had reported (“quite a bit”/”very much”).
On the sensory scale, of the 62 participants, 20% had reported tingling in fingers/hands and 23% in toes/feet. Numbness was reported by 39% in fingers/hands and 37% in toes/feet. Shooting or burning pain was reported by 14% in fingers/hands and 26% in toes/feet [Figure 1]. Trouble standing or walking was reported by 43%. Hearing difficulties were reported by 17%, whereas only 3% had reported difficulty distinguishing between hot and cold water.
Figure 1.
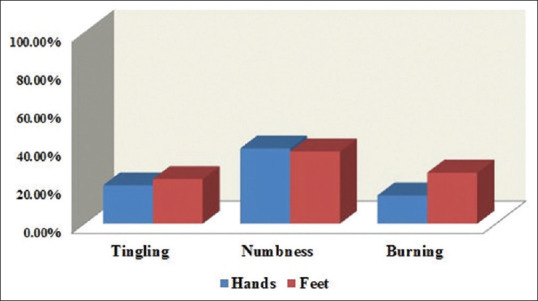
Patients reporting “quite a bit” or “very much” for numbness, tingling, and shooting/burning pain on the European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire for Chemotherapy-Induced Peripheral Neuropathy 20-item sensory scale
On the motor scale, the most commonly reported symptoms were trouble walking 40% and difficulty in climbing stairs and standing up from a chair 60%. The driving question was analyzed for the 25 participants who drove: 44% reported “quite a bit” or “very much” for the question about trouble using pedals when driving.
On the autonomic scale, participants who reported “quite a bit” or “very much” were summarized as follows: 27% reported symptoms of orthostatic hypotension, 31% had blurred vision, and 46% reported erectile dysfunction [Table 2].
Table 2.
Participants’ responses to the European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire for Chemotherapy-Induced Peripheral Neuropathy 20-item scale (QLQ-CIPN20)
| CIPN subscale | Total number responding, n (%) | Response to question, n (%) | |||
|---|---|---|---|---|---|
| Not at all | A little bit | Quite a bit | Very much | ||
| Sensory scale | |||||
| Did you have tingling fingers or hands? | 60 (96.77) | 31 (51.67) | 17 (28.33) | 8 (13.33) | 4 (6.67) |
| Did you have tingling toes or feet? | 60 (96.77) | 28 (46.67) | 18 (30) | 10 (16.67) | 4 (6.67) |
| Did you have numbness in your fingers or hands? | 61 (98.39) | 12 (19.67) | 25 (40.98) | 19 (31.15) | 5 (8.2) |
| Did you have numbness in your toes or feet? | 61 (98.39) | 14 (22.95) | 24 (39.34) | 16 (26.23) | 7 (11.48) |
| Did you have shooting or burning pain in your fingers or hands? | 61 (98.39) | 40 (65.57) | 12 (19.67) | 5 (8.2) | 4 (6.56) |
| Did you have shooting or burning pain in your toes or feet? | 60 (96.77) | 35 (58.33) | 9 (15) | 9 (15) | 7 (11.67) |
| Did you have problems standing or walking because of difficulty feeling the ground under your feet? | 60 (96.77) | 27 (45) | 7 (11.67) | 18 (30) | 8 (13.33) |
| Did you have difficulty distinguishing between hot and cold water? | 61 (98.39) | 57 (93.44) | 2 (3.28) | 1 (1.64) | 1 (1.64) |
| Did you have difficulty hearing? | 58 (93.55) | 37 (63.79) | 11 (18.97) | 7 (12.07) | 3 (5.17) |
| Motor scale | |||||
| Did you have cramps in your hands? | 60 (96.77) | 42 (70) | 10 (16.67) | 6 (10) | 2 (3.33) |
| Did you have cramps in your feet? | 61 (98.39) | 40 (65.57) | 11 (18.03) | 8 (13.11) | 2 (3.28) |
| Did you have a problem holding a pen? Which made writing difficult? | 55 (88.71) | 42 (76.36) | 7 (12.73) | 4 (7.27) | 2 (3.64) |
| Did you have a difficulty manipulating small objects with your fingers (e.g., fastening small buttons)? | 60 (96.77) | 43 (71.67) | 5 (8.33) | 3 (5) | 9 (15) |
| Did you have difficulty opening a jar or bottle because of weakness in your hands? | 60 (96.77) | 38 (63.33) | 8 (13.33) | 4 (6.67) | 10 (16.67) |
| Did you have difficulty walking because your feet dropped downwards? | 60 (96.77) | 26 (43.33) | 10 (16.67) | 13 (21.67) | 11 (18.33) |
| Did you have difficulty climbing stairs or getting up out of chair because of weakness in your legs? | 60 (96.77) | 14 (23.33) | 10 (16.67) | 13 (21.67) | 23 (38.33) |
| Did you have using the pedals?a | 25 (40.32) | 12 (48) | 2 (8) | 5 (20) | 6 (24) |
| Autonomic scale | |||||
| Were you dizzy when standing up from a sitting or lying position? | 61 (98.39) | 27 (44.26) | 17 (27.87) | 12 (19.67) | 5 (8.2) |
| Did you have blurred vision? | 60 (96.77) | 29 (48.33) | 12 (20) | 16 (26.67) | 3 (5) |
| Did you have difficulty getting or maintaining an erection?b | 13 (20.97) | 5 (38.46) | 2 (15.38) | 3 (23.08) | 3 (23.08) |
Data are shown for n=62 unless otherwise specified. aOnly for those driving cars, bOnly for males. CIPN: Chemotherapy-induced peripheral neuropathy
Comparing mean scores on the CIPN subscales between patients with/without diabetes did not show statistically significant differences for the sensory (P = 0.162), motor (P = 0.866), or autonomic (P = 0.474) scales [Figure 2].
Figure 2.
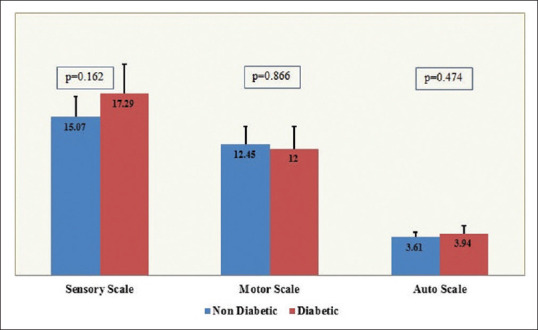
Comparison of chemotherapy-induced peripheral neuropathy subscale scores between diabetics and nondiabetics
Quality of life among multiple myeloma patients
The proportions of the 62 participants who reported “quite a bit” or “very much” on QoL scales are as follows.
Half of the participants reported aches and pain. Back pain was reported by 42%, with an increase in pain on activity reported by 49%. More than half (57%) reported that they had felt ill, 43% reported having a dry mouth, and 33% reported losing hair. Half of the respondents reported having tingling hands or feet. Just over one-third (35%) reported that they had been thinking about their illness, and 28% were worried about their health in the future [Table 3].
Table 3.
Participants’ responses to the European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire for (QLQ-MY20)
| QoL question | Total number responding, n (%) | Response to question, n (%) | |||
|---|---|---|---|---|---|
| Not at all | A little bit | Quite a bit | Very much | ||
| Have you had bone aches or pain | 62 (100) | 22 (35.48) | 9 (14.52) | 20 (32.26) | 11 (17.74) |
| Have you had pain in your back | 61 (98.39) | 20 (32.79) | 15 (24.59) | 15 (24.59) | 11 (18.03) |
| Have you had pain in your hip | 61 (98.39) | 24 (39.34) | 14 (22.95) | 15 (24.59) | 8 (13.11) |
| Have you had pain in your arm or shoulder | 61 (98.39) | 26 (42.62) | 15 (24.59) | 7 (11.48) | 13 (21.31) |
| Have you had pain in your chest | 61 (98.39) | 38 (62.3) | 14 (22.95) | 4 (6.56) | 5 (8.2) |
| If you had pain did it increase with activity | 59 (95.16) | 16 (27.12) | 14 (23.73) | 16 (27.12) | 13 (22.03) |
| Did you feel drowsy | 61 (98.39) | 26 (42.62) | 17 (27.87) | 11 (18.03) | 7 (11.48) |
| Did you feel thirsty | 61 (98.39) | 33 (54.1) | 11 (18.03) | 11 (18.03) | 6 (9.84) |
| Have you felt ill | 61 (98.39) | 10 (16.39) | 16 (26.23) | 26 (42.62) | 9 (14.75) |
| Have you had a dry mouth | 60 (96.77) | 22 (36.67) | 12 (20) | 15 (25) | 11 (18.33) |
| Have you lost any hair | 60 (96.77) | 20 (33.33) | 20 (33.33) | 14 (23.33) | 6 (10) |
| Were you upset by the loss of your hair | 56 (90.32) | 30 (53.57) | 15 (26.79) | 7 (12.5) | 4 (7.14) |
| Did you have tingling hands or feet | 61 (98.39) | 13 (21.31) | 17 (27.87) | 23 (37.7) | 8 (13.11) |
| Did you feel restless or agitated | 60 (96.77) | 19 (31.67) | 18 (30) | 14 (23.33) | 9 (15) |
| Have you had acid indigestion or heartburn | 59 (95.16) | 21 (35.59) | 14 (23.73) | 16 (27.12) | 8 (13.56) |
| Have you had burning or sore eyes | 59 (95.16) | 24 (40.68) | 18 (30.51) | 14 (23.73) | 3 (5.08) |
| Have you felt physically less attractive as a result of your disease or treatment | 58 (93.55) | 20 (34.48) | 22 (37.93) | 14 (24.14) | 2 (3.45) |
| Have you been thinking about your illness | 57 (91.94) | 20 (35.09) | 17 (29.82) | 18 (31.58) | 2 (3.51) |
| Have you been worried about dying | 55 (88.71) | 34 (61.82) | 10 (18.18) | 6 (10.91) | 5 (9.09) |
| Have you worried about your health in the future | 53 (85.48) | 28 (52.83) | 10 (18.87) | 7 (13.21) | 8 (15.09) |
Data are shown for n=62, unless otherwise specified. QoL: Quality of life
Chemotherapy-induced peripheral neuropathy between chemotherapy with and without bortezomib
The CIPN symptoms were compared across patients who had received chemotherapy with and without bortezomib. The symptoms are not statistically different between two groups, except blurry vision which is significant (P = 0.03) [Supplemental Table 1].
Supplemental Table 1.
Response to the European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire for Chemotherapy-Induced Peripheral Neuropathy 20-item scale comparison across different chemotherapy groups (QLQ-CIPN20)
| CIPN questions, n (%) | Chemo without Bortezomib (n=7), n (%) | Chemo with Bortezomib (n=49), n (%) | P* |
|---|---|---|---|
| Sensory scale | |||
| Tingling fingers or hands? 47 (95.92) | 3 (25) | 6 (17.14) | 0.67 |
| Tingling toes or feet? 48 (97.96) | 3 (25) | 7 (19.44) | 0.69 |
| Numbness in fingers or hands? 48 (97.96) | 4 (33.33) | 15 (41.67) | 0.73 |
| Numbness in toes or feet? 48 (97.96) | 4 (33.33) | 15 (41.67) | 0.73 |
| Aching or burning pain in fingers or hands? 48 (97.96) | 2 (16.67) | 4 (11.11) | 0.63 |
| Aching or burning pain in toes or feet? 47 (95.92) | 4 (33.33) | 7 (20) | 0.43 |
| Trouble standing or walking? 47 (95.92) | 5 (41.67) | 13 (37.14) | 1 |
| Trouble distinguishing temperature of hot and cold water? 48 (97.96) | 0 (0) | 1 (2.78) | 1 |
| Trouble hearing? 46 (93.88) | 2 (16.67) | 5 (14.71) | 1 |
| Motor Scale | |||
| Cramps in hands? 47 (95.92) | 3 (25) | 4 (11.43) | 0.34 |
| Cramps in feet? 48 (97.96) | 3 (25) | 6 (16.67) | 0.67 |
| Trouble holding a pen which made writing difficult? 45 (91.84) | 1 (8.33) | 4 (12.12) | 1 |
| Trouble handling small objects (e.g., buttoning a blouse)? 48 (97.96) | 1 (8.33) | 7 (19.44) | 0.66 |
| Trouble opening jar/bottle due to loss of strength in hands? 48 (97.96) | 1 (8.33) | 9 (25) | 0.41 |
| Trouble walking because your feet come down to hard? 48 (97.96) | 4 (33.33) | 16 (44.44) | 0.74 |
| Trouble walking stairs or standing up from a chair due to weakness in legs? 48 (97.96) | 9 (75) | 18 (50) | 0.18 |
| Only for those driving cars: Trouble driving due to use of pedals? 22 (44.9) | 4 (80) | 6 (35.29) | 0.14 |
| Autonomic scale | |||
| Dizziness after standing up? 48 (97.96) | 4 (33.33) | 9 (25) | 0.71 |
| Blurry vision? 48 (97.96) | 7 (58.33) | 8 (22.22) | 0.03 |
| Only for males: Trouble getting or maintaining an erection? 12 (24.49) | 1 (50) | 5 (50) | 1 |
Only for patients who had answered “quite a bit” or “very much” for above answers. *P is based on Fisher’s exact test. CIPN: Chemotherapy-induced peripheral neuropathy
DISCUSSION
Peripheral neuropathy is the most common side effect of chemotherapy among MM patients. However, the neuropathy symptoms are not necessarily related to chemotherapy, and it can be age related and with other comorbid conditions such as diabetes. This study shows that EORTC QLQ-CIPN20 is a valid instrument in identifying CIPN in MM patients. Participants took, on average, 5 min to complete the Arabic questionnaire, making the use of the tool feasible in the routine care of MM patients.
CIPN associated with the use of bortezomib and thalidomide is reversible.[28,29] When bortezomib is discontinued, 80% of patients become asymptomatic.[28] However, symptoms can continue after discontinuation of chemotherapy,[1] and cure is not guaranteed once chemotherapy stops.[2] Capturing CIPN symptoms and change overtime is crucial for chemotherapy dose monitoring.
A comparison of these results with those of other studies is challenging. The QLQ-CIPN20 is not commonly used for myeloma patients, except few studies.[27,30] The sensory symptoms reported by participants in this study occur less frequently than in the study reported by Wolf et al.:[27] tingling in fingers/hands (20% vs. 63%), numbness in fingers/hands (39% vs. 57%), and shooting/burning pain in fingers/hands (14% vs. 18%).[27] The symptom frequencies are also lower than those reported by Beijers et al.:[30] tingling (37%), shooting/burning pain (37%), and numbness (27%) in upper and/or lower extremities. However, erectile dysfunction was reported more commonly in the current study participants (46% vs. 28%).[30]
CIPN can be difficult to differentiate from other forms of peripheral neuropathies, for example, diabetic and paraneoplastic neuropathies.[1,31] In the current study sample, 86.9% of participants reported at least one symptom that bothered them “quite a bit” or “very much” and impacted their daily life activity, which is higher than that reported in an earlier study (53%).[30]
Patients with MM experience low HRQOL. Several tools have been reported to capture HRQOL among MM patients; however, the EORTC QLQ-MY20 is a disease-specific instrument.[32] Floortje et al. reported worse QoL scores among MM patients compared with healthy individuals using the EORTC QLQ-C30; the scores in the MM respondents deteriorated over time. The symptoms reported in this patient group were less frequent than in the current study sample for nearly all measures: tingling hands/feet (32% vs. 50%), back pain (28% vs. 42%), bone aches/pain (26% vs. 50%), worried about future health (34% vs. 28%), and worried about dying (21% vs. 20%).[33] However, the duration of MM diagnosis was 10 years compared with the 5-year duration in the current study participants.[33]
The hearing difficulty based on EORTC QLQ-CIPN20 was high 17% in our study compared to earlier studies (5%) with different cancer diagnosis[34] and 11% among colorectal cancer patients.[33] A case report had reported bilateral hearing loss with four doses of bortezomib among a MM patient.[35] Whether the hearing difficulty is due to bortezomib, age related or due to complication of myeloma itself is beyond the scope of this study.
Limitations
The baseline disease status of participants could not be captured; therefore, an overlap with preexisting peripheral neuropathy symptoms due to diabetes could not be excluded. As there was no control group, it would be difficult to assess whether the observed frequencies differ from those in the wider population. Although we have captured all the patients diagnosed with MM over the 5 years, the sample size is not reasonable enough to draw the conclusion that peripheral neuropathy is caused by a specific chemotherapy.
CONCLUSION
The self-reported EORTC QLQ-CIPN20 is a reasonable tool to capture CIPN among MM patients. CIPN is associated with impaired HRQOL. Chemotherapy dose modification or discontinuation according to symptom severity is needed. Capturing CIPN as a patient-reported outcome needs to be considered in routine clinical practice.
Ethical approval
This is an observational study. The study was approved by King Abdullah International Medical Research Center, Institutional Review Board (IRB), with approval number (RSS16/005).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors thank all the participants who had participated in the study and the nurses at the Department of Hematology for their support.
REFERENCES
- 1.Park SB, Goldstein D, Krishnan AV, Lin CS, Friedlander ML, Cassidy J, et al. Chemotherapy-induced peripheral neurotoxicity: A critical analysis. CA Cancer J Clin. 2013;63:419–37. doi: 10.3322/caac.21204. [DOI] [PubMed] [Google Scholar]
- 2.Iżycki D, Niezgoda AA, Kaźmierczak M, Piorunek T, Iżycka N, Karaszewska B, et al. Chemotherapy-induced peripheral neuropathy – Diagnosis, evolution and treatment. Ginekol Pol. 2016;87:516–21. doi: 10.5603/GP.2016.0036. [DOI] [PubMed] [Google Scholar]
- 3.Cavaletti G, Argyriou AA, Frigeni B, Lanzani F, Piatti M, Rota S, et al. The Total Neuropathy Score as an assessment tool for grading the course of chemotherapy-induced peripheral neurotoxicity: Comparison with the National Cancer Institute-Common Toxicity Scale. J Peripher Nerv Syst. 2007;12:210–5. doi: 10.1111/j.1529-8027.2007.00141.x. [DOI] [PubMed] [Google Scholar]
- 4.Cavaletti, Guido, Jann S, Pace A, Plasmati R, Siciliano G, et al. Multi-center assessment of the Total Neuropathy Score for chemotherapy-induced peripheral neurotoxicity. J Peripher Nerv Syst. 2006;11:135–41. doi: 10.1111/j.1085-9489.2006.00078.x. [DOI] [PubMed] [Google Scholar]
- 5.Mantyh PW. Cancer pain and its impact on diagnosis, survival and quality of life.Nature Reviews. Neuroscience. 2006;7:797–809. doi: 10.1038/nrn1914. [DOI] [PubMed] [Google Scholar]
- 6.Argyriou, Andreas A, Bruna J, Marmiroli P, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): An update. Crit Rev Oncol Hematol. 2012;82:51–77. doi: 10.1016/j.critrevonc.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Chen WW, Huang WJ. Chemotherapy-induced peripheral neuropathy. Biomed Rep. 2017;6:267–71. doi: 10.3892/br.2017.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain. 2014;155:2461–70. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860–73. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- 10.Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111:2962–72. doi: 10.1182/blood-2007-10-078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhagra A, Rao RD. Chemotherapy-induced neuropathy. Curr Oncol Rep. 2007;9:290–9. doi: 10.1007/s11912-007-0036-x. [DOI] [PubMed] [Google Scholar]
- 12.Cavaletti, Guido, Nobile-Orazio E. Bortezomib-induced peripheral neurotoxicity: Still far from a painless gain. Haematologica. 2007;92:1308–10. doi: 10.3324/haematol.11752. [DOI] [PubMed] [Google Scholar]
- 13.Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol. 2006;33:15–49. doi: 10.1053/j.seminoncol.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Velasco R, Bruna J. Chemotherapy-induced peripheral neuropathy: An unresolved issue. Neurologia. 2010;25:116–31. [PubMed] [Google Scholar]
- 15.Argyriou, Andreas A, Iconomou G, Kalofonos HP. Bortezomib-induced peripheral neuropathy in multiple myeloma: A comprehensive review of the literature. Blood. 2008;112:1593–9. doi: 10.1182/blood-2008-04-149385. [DOI] [PubMed] [Google Scholar]
- 16.Argyriou AA, Zolota V, Kyriakopoulou O, Kalofonos HP. Toxic peripheral neuropathy associated with commonly used chemotherapeutic agents. J BUON. 2010;15:435–46. [PubMed] [Google Scholar]
- 17.LoMonaco M, Milone M, Batocchi AP, Padua L, Restuccia D, Tonali P. Cisplatin neuropathy: Clinical course and neurophysiological findings. J Neurol. 1992;239:199–204. doi: 10.1007/BF00839140. [DOI] [PubMed] [Google Scholar]
- 18.Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13:27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- 19.Visovsky C, Daly BJ. Clinical evaluation and patterns of chemotherapy-induced peripheral neuropathy. J Am Acad Nurse Pract. 2004;16:353–9. doi: 10.1111/j.1745-7599.2004.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 20.Wampler M, Miaskowski C, Byl N, Rugo H, Topp K. The modified total neuropathy score: A clinically feasible and valid measure of taxane-induced peripheral neuropathy in women with breast cancer. J Support Oncol. 2006;4:W9–16. [Google Scholar]
- 21.da Silva Simao DA, Teixeira AL, Souza RS, de Paula Lima ED. Evaluation of the Semmes-Weinstein filaments and a questionnaire to assess chemotherapy-induced peripheral neuropathy. Support Care Cancer. 2014;22:2767–73. doi: 10.1007/s00520-014-2275-3. [DOI] [PubMed] [Google Scholar]
- 22.Hershman DL, Weimer LH, Wang A, Kranwinkel G, Brafman L, Fuentes D, et al. Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res Treat. 2011;125:767–74. doi: 10.1007/s10549-010-1278-0. [DOI] [PubMed] [Google Scholar]
- 23.Hershman DL, Unger JM, Crew KD, Minasian LM, Awad D, Moinpour CM, et al. Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for the prevention of taxane-induced neuropathy in women undergoing adjuvant breast cancer therapy. J Clin Oncol. 2013;31:2627–33. doi: 10.1200/JCO.2012.44.8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Postma TJ, Aaronson NK, Heimans JJ, Muller MJ, Hildebrand JG, Delattre JY, et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: The QLQ-CIPN20. Eur J Cancer. 2005;41:1135–9. doi: 10.1016/j.ejca.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Tofthagen C, McAllister RD, McMillan SC. Peripheral neuropathy in patients with colorectal cancer receiving oxaliplatin. Clin J Oncol Nurs. 2011;15:182–8. doi: 10.1188/11.CJON.182-188. [DOI] [PubMed] [Google Scholar]
- 26.Cavaletti G, Cornblath DR, Merkies IS, Postma TJ, Rossi E, Frigeni B, et al. The chemotherapy-induced peripheral neuropathy outcome measures standardization study: From consensus to the first validity and reliability findings. Ann Oncol. 2013;24:454–62. doi: 10.1093/annonc/mds329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolf SL, Barton DL, Qin R, Wos EJ, Sloan JA, Liu H, et al. The relationship between numbness, tingling, and shooting/burning pain in patients with chemotherapy-induced peripheral neuropathy (CIPN) as measured by the EORTC QLQ-CIPN20 instrument, N06CA. Support Care Cancer. 2012;20:625–32. doi: 10.1007/s00520-011-1141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaudhry V, Cornblath DR, Polydefkis M, Ferguson A, Borrello I. Characteristics of bortezomib- and thalidomide-induced peripheral neuropathy. J Peripher Nerv Syst. 2008;13:275–82. doi: 10.1111/j.1529-8027.2008.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dimopoulos MA, Mateos MV, Richardson PG, Schlag R, Khuageva NK, Shpilberg O, et al. Risk factors for, and reversibility of, peripheral neuropathy associated with bortezomib-melphalan-prednisone in newly diagnosed patients with multiple myeloma: Subanalysis of the phase 3 VISTA study. Eur J Haematol. 2011;86:23–31. doi: 10.1111/j.1600-0609.2010.01533.x. [DOI] [PubMed] [Google Scholar]
- 30.Beijers AJ, Vreugdenhil G, Oerlemans S, Eurelings M, Minnema MC, Eeltink CM, et al. Chemotherapy-induced neuropathy in multiple myeloma: Influence on quality of life and development of a questionnaire to compose common toxicity criteria grading for use in daily clinical practice. Support Care Cancer. 2016;24:2411–20. doi: 10.1007/s00520-015-3032-y. [DOI] [PubMed] [Google Scholar]
- 31.Titulaer MJ, Soffietti R, Dalmau J, Gilhus NE, Giometto B, Graus F, et al. Screening for tumours in paraneoplastic syndromes: Report of an EFNS task force. Eur J Neurol. 2011;18:19–e3. doi: 10.1111/j.1468-1331.2010.03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gadoó K, Domjaán G. Quality of Life Issues of Patients with Multiple Myeloma. Ch 14. London, UK: IntechOpen; 2013. [Google Scholar]
- 33.Mols F, Oerlemans S, Vos AH, Koster A, Verelst S, Sonneveld P, et al. Health-related quality of life and disease-specific complaints among multiple myeloma patients up to 10 yr after diagnosis: Results from a population-based study using the PROFILES registry. Eur J Haematol. 2012;89:311–9. doi: 10.1111/j.1600-0609.2012.01831.x. [DOI] [PubMed] [Google Scholar]
- 34.Kwak MK, Kim EJ, Lee ER, Kwon IG, Hwang MS. Characteristics and quality of life in patients with chemotherapy-induced peripheral neuropathy. J Korean Oncol Nurs. 2010;10:231–9. [Google Scholar]
- 35.Engelhardt M, Müller AM, Maier W, Wäsch R. Severe irreversible bilateral hearing loss after bortezomib (VELCADE®) therapy in a multiple myeloma (MM) patient. Leukemia. 2005;19:869–70. doi: 10.1038/sj.leu.2403723. [DOI] [PubMed] [Google Scholar]


