Through laboratory experiments in which treatments were administered singly or in combination to individual insects, we recorded harmful effects of FPF and pathogens on honey bee survival and immune gene expression. Though we found no evidence of synergistic interactions among stressors on either honey bee survival or viral load, the combined treatment SULF and DWV‐B led to a synergistic up‐regulation of dicer‐like gene expression. We conclude that common viral pathogens pose a major threat to honey bees whilst co‐exposure to these novel nACHR insecticides does not significantly exacerbate viral impacts on host survival in the laboratory.
Summary
The decline of insect pollinators threatens global food security. A major potential cause of decline is considered to be the interaction between environmental stressors, particularly between exposure to pesticides and pathogens. To explore pesticide–pathogen interactions in an important pollinator insect, the honey bee, we used two new nicotinic acetylcholine receptor agonist insecticides (nACHRs), flupyradifurone (FPF) and sulfoxaflor (SULF), at sublethal and field‐realistic doses in a fully crossed experimental design with three common viral honey bee pathogens, Black queen cell virus (BQCV) and Deformed wing virus (DWV) genotypes A and B. Through laboratory experiments in which treatments were administered singly or in combination to individual insects, we recorded harmful effects of FPF and pathogens on honey bee survival and immune gene expression. Though we found no evidence of synergistic interactions among stressors on either honey bee survival or viral load, the combined treatment SULF and DWV‐B led to a synergistic upregulation of dicer‐like gene expression. We conclude that common viral pathogens pose a major threat to honey bees, while co‐exposure to these novel nACHR insecticides does not significantly exacerbate viral impacts on host survival in the laboratory.
Introduction
Honey bees (Apis mellifera) play an invaluable role in crop and wild plant pollination (Gallai et al., 2009; Moritz et al., 2010; Al Naggar et al., 2018). Europe and the United States have seen major overwinter losses of honey bee colonies since 2006, with multiple biotic and abiotic factors having been proposed in causing these colony declines (Jacques et al., 2017; Kulhanek et al., 2017; Gray et al., 2019; Neov et al., 2019). Though there is considerable support for the view that viral infection, particularly by Deformed wind virus (DWV) associated with varroa (Varroa destructor) ectoparasitic mite transmission, is a leading cause for elevated overwinter colony mortality (Genersch et al., 2010; Dainat et al., 2012; Francis et al., 2013; McMahon et al., 2016; Natsopoulou et al., 2017), scientific consensus is that exposure to multiple stressors (microbial infections, exposure to pesticides, loss of habitat and improper beekeeping practices) underlies honey bee colony losses (Dechaume Moncharmont et al., 2003; Potts et al., 2010; Gill et al., 2012; Vanbergen et al., 2013; Goulson et al., 2015; Manley et al., 2015; Jacques et al., 2017).
Pesticides are well‐known contributors to declining bee health (Dechaume Moncharmont et al., 2003; Desneux et al., 2007; Gill et al., 2012; Osborne, 2012; Al Naggar et al., 2015; Siviter et al., 2018b; Al Naggar and Baer, 2019). Here, we explore honey bee reactions to two new plant protection products: sulfoxaflor (SULF) and flupyradifurone (FPF), belonging to the chemical groups sulfoximines and butenolides, respectively. These insecticides, which share their mode of action with neonicotinoids as selective agonists of Nicotinic Acetyl Choline Receptors (NAChRs) (Zhu et al., 2011; Sparks et al., 2013), are a more recent entry to the insecticide market. Currently, FPF (Sivanto™ prime) and SULF (Transform®) are, respectively, approved for use in more than 30 and 81 countries worldwide, with effects on insect pollinators potentially comparable to those of neonicotinoid insecticides (Siviter et al., 2018a, 2020b).
Chronic exposure to SULF at field‐realistic concentrations has been shown to reduce egg laying and impair reproductive success in bumble bees (Siviter et al., 2018a, 2020b), though acute exposure to SULF did not affect their learning and memory (Siviter et al., 2019), or the escape response of locusts (Parkinson et al., 2020). Exposure of A. mellifera colonies to SULF in a flight enclosure caused acute toxicity but did not otherwise impact flight activity or long‐term colony development (Cheng et al., 2018). Exposure to high and non‐field‐realistic FPF dosages has been shown to affect the sensory (taste), cognition and motor abilities of honey bees (Hesselbach and Scheiner, 2018, 2019). At field‐realistic, worst‐case FPF doses, a significant reduction in olfactory associative learning performance has also been observed in the closely related bee species Apis cerana (Tan et al., 2017), and more subtle effects of FPF on A. mellifera have also been demonstrated in combination with factors such as bee age, seasonality, nutritional stress and exposure to other chemicals (Tong et al., 2019; Tosi and Nieh, 2019). Hesselbach et al. (2020) recently showed that chronic exposure to FPF can lead to the premature onset of foraging in A. mellifera. Additionally, exposure to field rates of Sivanto™ (FPF) and Transform® (SULF) has also recently been shown to increase oxidative stress and induce apoptosis in A. mellifera (Chakrabarti et al., 2020). Studies have yet to investigate interactions between sublethal doses of these two novel pesticides and viral pathogens that affect honey bee health.
Another important cause of honey bee colony losses is the presence of the ectoparasitic mite V. destructor, which has spread worldwide, with significant impacts on honey bee colony health as a consequence of its transmission of a cocktail of viruses while feeding on honey bee haemolymph and fat bodies (Gisder et al., 2009; Mockel et al., 2011; Martin et al., 2012; Erban et al., 2015; Wilfert et al., 2016; Ramsey et al., 2019). A highly prevalent and relatively virulent virus transmitted by V. destructor and impacting honey bee colony health worldwide is Deformed wing virus (DWV), high titres of which cause developmental deformities and premature ageing in honey bees (Mockel et al., 2011; Natsopoulou et al., 2016; Tehel et al., 2019), and which lead to high overwintering colony losses (Highfield et al., 2009; Genersch et al., 2010; Dainat et al., 2012; Francis et al., 2013; McMahon et al., 2016; Natsopoulou et al., 2017). Recently, a genotypic variant of Deformed wing virus (DWV), genotype B (DWV‐B), has been shown to be more virulent than the original DWV genotype A (DWV‐A) in adult honey bees (McMahon et al., 2016, see also Gisder et al., 2018). In a follow‐up study using the same viral inocula, the same pattern of differential virulence was found between genotypes, but differences in virulence were not statistically significant (Tehel et al., 2020). Another widespread honey bee virus is Black queen cell virus (BQCV), which has been found in collapsing colonies (Mondet et al., 2014) and is potentially very virulent when gaining access to an adult honey bee’s haemocoel (Al Naggar and Paxton, 2020). BQCV also kills developing queen larvae, whose necrotic remains stain their pupal cells black (Spurny et al., 2017).
Scientific attention has been focused on possible interactions between bee pathogens and pesticide exposure that may be synergistic and therefore particularly harmful (James and Xu, 2012; Collison et al., 2016; Sánchez‐Bayo et al., 2016; Al Naggar and Baer, 2019; Feldhaar and Otti, 2020). A synergistic interaction can be defined as occurring when two or more stressors combine to have effect significantly greater than their additive effects. In contrast, an additive (subtractive) effect, or null interaction, is defined when the cumulative effect of two or more factors is not different to the summation (or subtraction) of their separate effects (defined as ‘additive’ and ‘subtractive’, respectively). Lastly, when two or more stressors produce a biological response that is significantly less than their individual effects, their interaction is deemed antagonistic (González‐Varo et al., 2013; Piggott et al., 2015; Maher et al., 2019). Across the few studies that have investigated the effect of exposure to neonicotinoids (imidacloprid, clothianidin and thiacloprid) on viral load, pesticide concentrations of ≥ 1 ppb were shown to result in increased viral titres in honey bee larvae (e.g. Doublet et al., 2015), suggestive of an additive effect. Recently, it has been shown that chronic exposure to FPF affects bees well beyond immediate exposure and is associated with an increased intensity of infection with Nosema ceranae (Al Naggar and Baer, 2019). However, potential interactive effects of the novel nAChRs insecticides FPF and SULF with other pathogens, specifically viruses such as DWV and its genotype variants, on adult bees have not yet been studied.
One means of quantifying the response of a host to a pesticide or a viral challenge is through a change in its gene expression (Aufauvre et al., 2014; Christen et al., 2018). Such studies can also help to highlight the molecular mechanisms by which hosts mount a defence against pathogens and how a pathogen evades those host defence responses (e.g. Galbraith et al., 2015; Doublet et al., 2017). Responses of honey bees to pesticide and viral challenge have been suggested to be underpinned by a common molecular pacemaker (Nazzi et al., 2012; Di Prisco et al., 2013), providing a mechanistic explanation for synergistic pesticide–pathogen interactions in A. mellifera.
Here, using a controlled and fully crossed laboratory experimental design, we tested the effects of chronic exposure to field‐realistic sublethal concentrations of two novel pesticides (FPF or SULF) and three viral pathogens (BQCV, DWV‐A or DWV‐B), individually and in combination, in order to identify their relative impacts as well as potential interactions (i) on the survival of individual honey bee workers, (ii) on pathogen load and (iii) on host expression of key innate immunity and detoxification genes. Our hypothesis was that we would detect a synergistic interaction between pesticide and pathogen, underpinned by a common host gene expression response.
Results
Effects on survival
We exposed adult honey bees in the laboratory to food contaminated with sublethal concentrations of either FPF or SULF for 30 days, well beyond the International Commission for Plant Pollinator Relationships (ICPPR) standard 10‐day test duration (OECD, 2017). Exposure to FPF significantly reduced survival of bees compared to non‐exposed control bees (P < 0.05 after correction for multiple comparisons). There was no significant effect of SULF on the survival of bees at the field‐realistic concentration we provided (P = 0.950) (Fig. 1, Table 1).
Fig. 1.
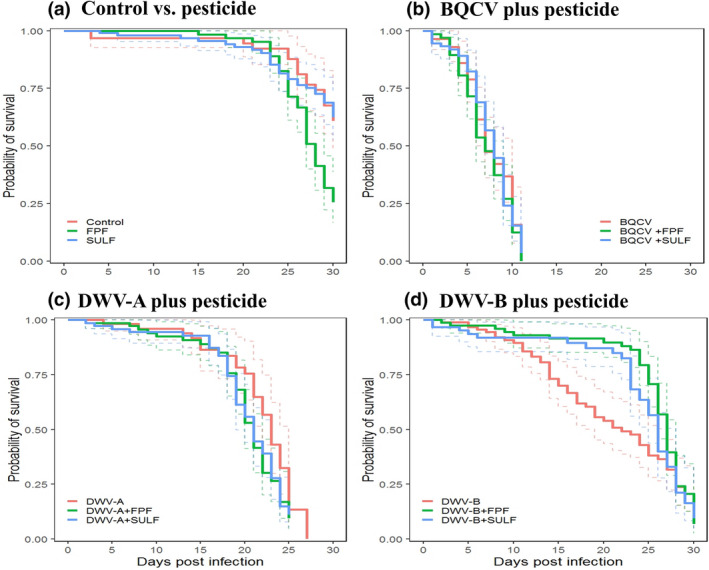
Kaplan–Meier survival curves (solid coloured lines) in days post‐infection and 95% CIs for each fitted curve (dashed coloured lines) of the impact of pesticides (FPF or SULF) and RNA viruses (BQCV, DWV‐A or DWV‐B), alone and in combination, on adult honey bees. Bees (n = 30 bees per cage, n = 3 cages per treatment) were injected with 1 µl of viral inoculum containing 107 BQCV, DWV‐A or DWV‐B and then fed with sublethal concentrations of either FPF (4.300 µg ml−1) or SULF (0.047 µg ml−1) pesticides, or a control solution. Pathogens were inoculated once at day 0 while pesticides were fed ad libitum across the experiment; (A) honey bees treated with either FPF or SULF; (B) honey bees treated with BQCV alone and with either FPF or SULF; (C) honey bees treated with DWV‐A alone and with either FPF or SULF; (D) honey bees treated with DWV‐B alone and with either FPF or SULF. Double treatments (pesticide + virus) in (B), (C) and (D) were all statistically non‐significant compared to virus treatments alone. For statistical details, see Table S1.
Table 1.
Impact of treatments with a single pesticide or a single pathogen on honey bee survival; cage was included as a random variable as it gave a better model fit (lower AIC).
| Treatment | β coefficient* | SE of β coefficient (+/−) | Z | P |
|---|---|---|---|---|
| FPF | 0.954ab | 0.366 | 2.61 | < 0.05 |
| SULF | −0.023a | 0.381 | −0.06 | 0.950 |
| BQCV | 5.074d | 0.472 | 10.76 | < 0.001 |
| DWV‐A | 2.220c | 0.383 | 5.79 | < 0.001 |
| DWV‐B | 1.754bc | 0.349 | 5.02 | < 0.001 |
Model‐averaged β coefficients (standardized effect size of the hazard, where higher β indicates higher risk of death) of the five variables: pesticides (FPF, SULF) and pathogens (BQCV, DWV‐A, DWV‐B) obtained from a Cox proportional hazard model in comparison with control. In bold are treatment effects that were significantly different from control by post hoc Tukey tests (with Bonferroni correction for multiple comparisons).
Different lower case letters following β show significant differences among treatments (P < 0.05, a posteriori Tukey test with Bonferroni correction for multiple comparisons).
As expected, bees also had significantly lower rates of survival when inoculated with honey bee RNA viruses (BQCV: P < 0.001; DWV‐A: P < 0.001; DWV‐B: P < 0.001) compared to non‐infected control‐injected bees (Fig. 1, Table 1). We also found that the survival of bees inoculated with BQCV was significantly lower compared to both DWV‐A‐ and DWV‐B‐infected bees (Table 1, Fig. S1). Whereas all BQCV‐infected bees survived a median of 7 days post‐inoculation (p.i.), DWV‐A‐ or DWV‐B‐infected bees survived a median of 23 and 22 days p.i., respectively, and did not differ in survival (P = 0.654 (before correction for multiple comparisons), Table 1, Fig. S1).
To test for interactions between pesticides and viruses on the survival of bees, we compared the effect of these stressors in combination (‘double treatments’, e.g. ‘BQCV plus FPF’) with the effect of the stressors separately (‘single’ treatments, e.g. ‘BQCV’ or ‘FPF’). All six virus–pesticide double treatments were non‐significant in comparison to virus‐only treatments, indicating a lack of synergistic or antagonistic virus–pesticide interactions in our study; of the six comparisons between survival of virus + pesticide versus virus treatments, three were additive and three were subtractive (Fig. 1, Table S1). For one double treatment, FPF + DWV‐B, the impact of virus on survival seemed lower when bees were also treated with pesticide (Fig. 1), but differences were not significant (Table S1).
From the perspective of pesticide treatments, additionally inoculating with virus increased mortality (Table S1), all virus + pesticide versus pesticide comparisons were positive, five of six significantly so. This result is hardly surprising because pesticide alone had a small (FPF) or zero (SULF) impact on mortality whereas virus had a major impact (Table 1).
Effects on viral load
We found no significant difference in pathogen load (viral genome copy number) in bees when exposed only to virus (BQCV, DWV‐A or DWV‐B) or co‐exposed to virus and either FPF or SULF at 7 and 14 days post‐infection (LM, P > 0.05, Fig. 2, see Table S2 for statistical details). These results indicate that exposure to pesticides had no effect on viral load. Control and pesticide‐only treated bees were devoid of virus at 7 and 14 days p.i..
Fig. 2.
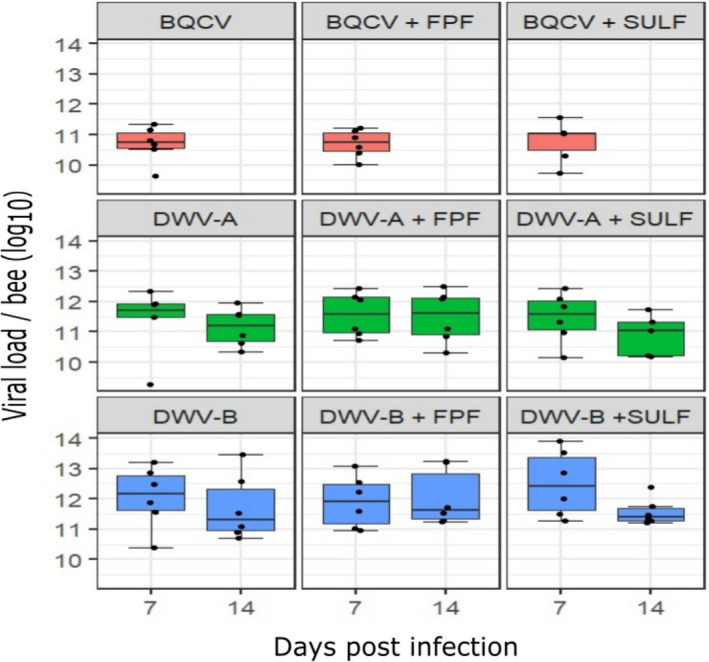
BQCV, DWV‐A and DWV‐B genome equivalents (log10) per adult honey bee (n = 6 bees per treatment and time point) at 7 and 14 days post inoculation (boxplots show median, interquartiles and 95% confidence intervals, with jittered data points). Bees were injected with 107 BQCV, DWV‐A or DWV‐B then treated with sublethal concentrations of either FPF (4.300 µg ml−1) or SULF (0.047 µg ml−1) pesticides or a control solution for 30 days under laboratory conditions. There was no significant difference in viral load between virus‐exposed bees versus those co‐exposed to virus and pesticide at the two time points (LM (ANOVA Type II tests), P > 0.05). Control and pesticide‐only treated bees were devoid of virus at 7 and 14 days post inoculation. For statistical details, see Table S2.
Effects on gene expression
When gene expression levels of bees that were singly exposed to either DWV‐A or DWV‐B were compared to those of control bees, two immune‐related (RNAi pathway) genes: dicer‐like and Argonaute‐2 (AGO2) were significantly upregulated and the two detoxification genes investigated: CYP6AS14 and CYP9Q3 (cytochrome P450 pathway) were significantly downregulated (P < 0.05, Fig. 3; see Tables S3 and S4 for statistical details). In bees that were only exposed to BQCV, we also found significant upregulation of dicer‐like and Argonaute‐2 (AGO2) and significant downregulation for other immune‐ and detoxification‐related genes: tarbp2‐like, CYP6AS14 and CYP9Q3 (P < 0.05, Fig. 3; see Tables S3 and S4 for statistical details).
Fig. 3.
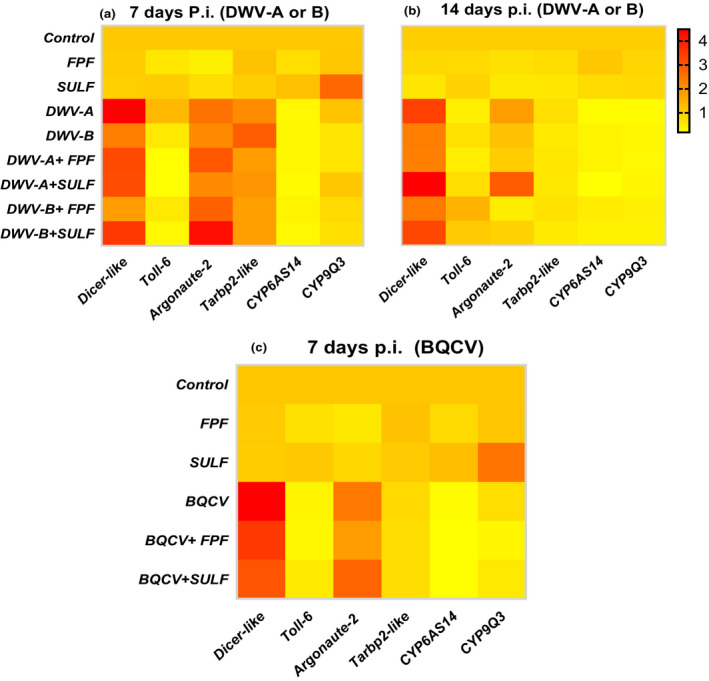
Heat map showing fold‐change in abundance of transcripts of innate immune‐ and detoxification‐related genes in adult honey bees co‐exposed to virus and pesticide at 7 and 14 days post inoculation (p.i.). Bees were inoculated with 107 BQCV, DWV‐A or DWV‐B and then chronically fed with sublethal concentrations of either FPF (4.300 µg ml−1), SULF (0.047 µg ml−1) pesticides or a control solution for 30 days under laboratory conditions; (A) treatments with pesticides and DWV‐A or DWV‐B at day 7 p.i.; (B) treatments with pesticides and DWV‐A or DWV‐B at day 14 p.i.; (C) treatments with pesticides and BQCV at day 7 p.i. Colours indicate the average mRNA titres compared to average mRNA titres in control groups (n = 6 honey bees per treatment and time point): yellow indicates downregulation and red indicates upregulation of transcripts. Each column corresponds to one gene transcript and each row corresponds to the expression profile of a treatment (n = 6 bees per treatment and time point). For statistical details, see Tables S3 and S4.
When we compared the abundance of gene transcripts at 7 and 14 days post exposure across viral treatment groups, a significant interaction term DWV‐B × time was found for two genes: Argonaute‐2 (AGO2) and tarbp2‐like, and a significant interaction term DWV‐A × time for tarbp2‐like (P < 0.05, Fig. 3; see Tables S3 and S4 for statistical details). This indicates that expression changes found in response to DWV‐A or DWV‐B depended on the duration of infection with these viruses.
Gene expression of the four innate immune‐related and the two detoxification‐related genes of bees exposed to either pesticide, virus or both pesticide and virus in combination did not reveal consistent pathogen–pesticide interaction effects. No significant FPF × virus interaction terms were found for any of the virus‐gene interactions investigated (Fig. 3, Table S3). The only significant interaction term we found was for SULF × DWV‐B in dicer‐like gene expression (P < 0.05; see Table S4 for statistical details), suggesting a synergistic (more than additive) interaction between these treatments on dicer‐like gene expression; that is, dicer‐like gene expression was upregulated in response to SULF + DWV‐B compared to either DWV‐B or SULF alone (Fig. S2).
Discussion
Using a controlled experimental design that was fully crossed, we did not find evidence of synergistic interactions between either FPF or SULF insecticides and three common viral pathogens of honey bees (DWV‐A, DWV‐B and BQCV) on honey bee survival and viral load, and only one interactive effect on gene expression.
With regard to individual stressors, we found a significant reduction in survival of bees that were chronically fed on FPF at field‐realistic concentrations. Chronic exposure of adult honey bees to FPF for 30 days, instead of the standard ICPPR 10‐day test (OECD, 2017), revealed a delay in toxicity since most of the toxic effects of FPF on bee survival were observed after 18–20 days. This might reflect an accumulation of pesticide in the insect body. Under natural conditions, honey bees could be exposed to higher concentrations of these pesticides in other bee matrices like pollen pellets, bee bread, wax or royal jelly (Mullin et al., 2010; U.S. EPA, 2014, 2016; Böhme et al., 2018) or/and by dust deposits abraded from treated seeds during sowing (Schnier et al., 2003; Tapparo et al., 2012) and by contaminated water puddles (Samson‐Robert et al., 2014). Further research is therefore required to quantify effects of observed FPF‐induced mortality over longer timespans in the field, not only on individual honey bees but also at the colony level.
Standard methodologies currently require chronic toxicity testing over a 10‐day span (OECD, 2017). This duration seems adequate for testing pesticides with high to moderately acute toxicity for bees. The delay in toxicity of FPF on bee survival, which we observed here, would not however have been detected over a duration of ten days of standard testing. Our findings are also in line with earlier studies that reported a delay in toxicity of some pesticides such as imidacloprid (Dechaume Moncharmont et al., 2003), thiacloprid (Doublet et al., 2015) and boscalid (Simon‐Delso et al., 2018) in bees. A time‐to‐death approach may be more appropriate for determining the risk of chronic exposure to a pesticide, specifically when pesticides are tested at low, sublethal concentrations (Simon‐Delso et al., 2018), as in our experiment. All bees that had been exposed to pathogens, singly or in combination with pesticides, died by day 30, at which point we stopped the experiment. Mortality in control cages by day 30 was only ca. 40% (Fig. 1a); we were not therefore able to perform a robust time‐to‐death analysis, which requires minimally 50% mortality in control treatments (Sánchez‐Bayo, 2009; Sánchez‐Bayo and Tennekes, 2020).
Although we noticed that co‐exposure to either FPF or SULF and DWV‐B increased the survival of bees relative to DWV‐B alone (Fig. 1d), differences were not significant, suggesting a subtractive but not an antagonistic effect of pesticide on viral‐induced mortality. Bees concurrently exposed to a bacterium (Enterococcus faecalis) and the pesticide thiacloprid similarly had significant higher survival rates 11 days post exposure than controls (Dickel et al., 2018). Though Siviter et al. (2020a, 2020b,2020a, 2020b) found an additive impact of SULF exposure and Nosema bombi inoculation on bumble bee larval mortality, they found a subtractive effect of the combined pesticide–pathogen treatment on larval growth. Subtractive or even antagonistic impacts of a combined pesticide–pathogen treatment could be because the detoxification of insecticides can lead to physiological modifications that can counteract the effect of pathogens and parasites on the host (Rivero et al., 2010). This possibility deserves further scrutiny, though clearly it speaks against synergistic pathogen–pesticide interactions.
We found that chronic exposure of bees to either FPF or SULF had no effect on the viral load of both DWV genotypes (A and B) and of BQCV, which might explain why we did not detect a synergistic virus–pesticide effect on bee survival. Exposure to pesticides has sometimes been associated with an increased pathogenic load; a handful of studies have reported high viral titres induced by pesticide exposure in both laboratory tests with individual honey bees (Di Prisco et al., 2013; Doublet et al., 2015) and in field tests with whole colonies (Locke et al., 2012). Other studies have reported no direct effect of pesticides on viral load in either individual‐level or colony‐level tests (Boncristiani et al., 2012; Coulon et al., 2018; Osterman et al., 2019). Indeed, the pesticides fipronil and thiacloprid have been shown to reduce bee pathogen load (Vidau et al., 2011). This lack of consistency in response of bees to combined exposure of pesticide and pathogen suggests the effect of a pesticide on viral load is idiosyncratic and context‐dependent. For example, DWV titres briefly increased immediately after acaricide treatment with tau‐fluvalinate compared to untreated colonies, although this effect was not observed for BQCV and Sacbrood virus (SBV) (Locke et al., 2012). Here, we also found no direct effect of FPF on viral load, although high intensities of infection of another pathogen, Nosema ceranae, have been recorded in bees exposed to FPF at similar concentrations to those we used here (Al Naggar and Baer, 2019). We therefore proposed that interactive effects between pesticides and pathogens on bees could be pathogen‐ or pesticide‐specific and might depend on several factors, such as the dose of either the pesticide or the pathogen, timing of pesticide exposure, age of bees, season, life stage and genetic origin of the bees.
BQCV is currently considered a benign (low virulence) viral pathogen of adult honey bees compared to other viruses such as DWV, possibly because its mode of horizontal transmission is primarily direct, through feeding (Bailey and Woods, 1977; Chen et al., 2006). It has, however, been found at high prevalence and titre in collapsing colonies (Mondet et al., 2014), suggesting that its mode of transmission may not only be direct via ingestion but may also include indirect, vector‐mediate transmission, for example by varroa mites. Recently, we (Al Naggar and Paxton, 2020) investigated the effect of mode of horizontal transmission of BQCV either by feeding (representing direct transmission) or by injection into the haemocoel (analogous to indirect or vector‐mediated transmission) on viral virulence in individual adult honey bees; injecting BQCV directly into haemolymph in the haemocoel resulted in far higher mortality as well as increased viral titre compared to inoculation by feeding (ingestion). Here, we found that BQCV was more virulent than both DWV‐A and DWV‐B when inoculated by injection, regardless of pesticide exposure. This is consistent with our earlier findings (Al Naggar and Paxton, 2020) and suggests that BQCV may pose a future threat to honey bees and apiculture if BQCV transmission becomes primarily vector‐mediated.
Effective immune defence is likely central to honey bee health and colony survival. Individual immunocompetence can be weakened by environmental factors such as pesticides that may render honey bees more vulnerable to parasites and pathogens (Di Prisco et al., 2013; Al Naggar and Baer, 2019). Here, we only observed subtle interactive effects of pesticides and pathogens on the expression of one innate immune gene of honey bees; there was a significant SULF × DWV‐B synergistic interaction (upregulation) on dicer‐like gene expression. These findings are consistent with the lack of consistent interactive effects of pesticides on viral load that we also found. Therefore, our results do not support the hypothesis that additional stressors (e.g. exposure to pesticides) with a potential negative impact on antiviral immunity have the ability to boost unregulated viral replication (cf. Nazzi and Pennacchio, 2018).
Antiviral responses in insects appear to be mediated by two primary pathways, one involving JAK‐STAT (Janus kinase‐signal transducers and activators of transcription) and the other involving the RNA interference (RNAi) pathway, though the latter is better characterized in insects (Merkling and van Rij, 2013). The RNAi pathway functions by cleaving double‐stranded RNA (dsRNA) into small fragments, which are used to target endogenous mRNA transcripts or exogenous virus with the same sequence, and thus prevent translation of mRNA into protein and ultimately decrease the activity of the particular gene, or destroy the virus. Here, we found a pattern of upregulation of RNAi pathway‐related genes: Argonaute‐2 (AGO2) and dicer‐like, that was similar in all virus‐inoculated bees, irrespective of whether or not they were exposed to pesticides. Our findings are consistent with earlier studies which reported significant upregulation for multiple RNAi pathway‐related genes, including Argonaute‐2 (AGO2) and dicer‐like, in honey bees in response to acute infection with Israeli acute paralysis virus (IAPV) (Galbraith et al., 2015). It also supports previous studies, demonstrating that the RNAi pathway plays an important role in mediating antiviral responses in insects, including in honey bees (Maori et al., 2009; Liu et al., 2010; Desai et al., 2012; Flenniken and Andino, 2013).
In BQCV‐inoculated bees, we found significant downregulation of another gene linked to the RNAi pathway: tarbp2‐like, whether or not bees were exposed to pesticide. This interesting result could be one of the reasons why all BQCV‐infected bees in the current study died very quickly, at 11 days p.i. compared to 26 and 30 days for DWV‐A and DWV‐B‐inoculated bees, respectively (Al Naggar and Paxton, 2020).
We also found a significant downregulation of the two cytochrome P450 detoxification genes: CYP6AS14 and CYP9Q3, in virus‐only inoculated bees, regardless of viral target and whether or not bees had been treated with pesticide. That downregulation of these genes did not translate into lowered survival of bees treated with pesticide and virus (compared to virus alone) is surprising. Further research is needed to investigate in greater detail the molecular mechanisms of honey bee detoxification and how gene expression relates to the ability of an individual honey bee to withstand insecticidal challenge.
Conclusions
Here, we investigated for the first time the potential interactive effects of chronic exposure of honey bees to two novel nAChRs insecticides: flupyradifurone and sulfoxaflor, and three common honey bee viral pathogens: BQCV, DWV‐A and DWV‐B. Though FLU and pathogens reduced host survival, co‐exposure to either FPF or SULF insecticides and viral pathogens did not have a synergistic effect on honey bee survival or viral load. The SULF + DWV‐B treatment, though, led to higher dicer‐like gene expression. Importantly, we observed a significant reduction in the survival of bees that were chronically fed with FPF for 30 days. Our data additionally support the role of RNAi pathways in honey bee antiviral responses. Despite the reported harmful effects of sulfoxaflor on bumble bee colony fitness, our data suggest that this insecticide may pose a lesser risk to honey bees at the concentrations and endpoints we assessed.
Experimental procedures
Honey bees
Three colonies of A. mellifera carnica maintained in the General Zoology apiary at Martin Luther University Halle‐Wittenberg, Germany, were used from June to August 2019. They had been treated to control Varroa destructor mites with Bayvarol® strips (Flumethrin, 6.61 g/strip, Bayer Vital GmbH GB, Germany) in November 2018. Prior to any research activities, colonies were inspected visually for V. destructor mites and by quantitative real‐time PCR (qPCR) for seven common viral targets: Acute bee paralysis virus (ABPV), DWV‐A, DWV‐B, BQCV, Chronic bee paralysis virus (CBPV), Sacbrood virus (SBV) and Slow bee paralysis virus (SBPV), using the primers listed in Table S5 and methods described in Tehel et al. (2019). Varroa destructor mites were not seen and viruses were not detected in colonies at a cycle (Ct) of 35, which is a threshold that minimizes the rate of false positives (de Miranda et al., 2013).
Pesticides
We used analytical grade flupyradifurone (Sigma‐Aldrich, catalog# 37050‐100MG, Germany) and Sulfoxaflor (Ms scientific, catalog# 12883‐10MG, Germany). We dissolved FPF or SULF in ddH2O to obtain stock solutions with a concentration of 1μg/μl, which were stored at −20°C to avoid degradation; we did not use acetone as solvent because both compounds are easily soluble in water at the stock concentrations (FPF: 3200 mg l−1 at 20°C; SULF: 809 mg l−1 at 25°C). The feeding solutions were prepared by diluting the stock solution with 50% (w/v) aqueous sugar (sucrose) solution (hereafter: sugar water). Feeding solutions were provided ad libitum to the bees at the beginning of the experiment and renewed each 24 ± 2 h. These dilutions were freshly prepared every 2 days from the stock solutions, were tightly wrapped with aluminium foil to prevent light degradation and stored at 6 ± 2°C to minimize degradation of the active ingredient. When fed to bees, feeding solutions did not show signs of precipitation at any time. Feeding solutions therefore contained either pure sucrose (negative control treatment), flupyradifurone (FPF treatment; 0.0043 μg FPF μl−1) or Sulfoxaflor (SULF treatment; 0.000047 μg SULF μl−1).
Sulfoxaflor (SULF) breaks down quickly in soil but is highly persistent in water, with a half‐life of 37 days to more than a year (U.S. EPA, 2013). It takes about 8–12 days for 90% of the pesticide to dissipate from pollen and nectar (U.S. EPA, 2019). Sivanto (FPF) breaks down slowly and has been found in nectar and honey stored in wax combs for up to five months, and in nectar collected by foragers for more than two weeks (winter oil seed rape fields; U.S. EPA, 2014). Therefore, bees could be exposed to these pesticides for a long time in the field and could also be exposed to higher concentrations in other bee matrices or by dust deposits abraded from treated seeds during sowing and by contaminated water puddles (Krupke et al., 2012; Samson‐Robert et al., 2014; Azpiazu et al., 2019).
To simulate natural exposure to insecticides, we employed a worst‐case exposure scenario for 30 days with sublethal and field relevant concentrations of FPF and SULF to test for cumulative toxicity. Worst‐case field‐realistic concentrations of FPF (4.30 ppm) or SULF (46.97 ppb) found in nectar were given chronically per os to bees via sugar water. We based our dosages on Environmental Protection Agency (EPA) data that reported the residue levels of FPF in nectar and pollen of several plants such as oilseed rape (4.3 ppm in nectar and 21.0 ppm in pollen), apple pollen (39 ppm) and blueberry pollen (68 ppm) (U.S. EPA, 2014). Reported residue levels of SULF ranged between 5.41 and 46.97 ppb in nectar and between 50.12 and 510.95 ppb in pollen of sulfoxaflor‐sprayed cotton across an 11‐day period (U.S. EPA, 2016). Our pesticide exposure levels of FPF or SULF were slightly more conservative (i.e. lower) than many reported field exposures, being an order of magnitude lower than the residues found in pollen and also substantially lower than published LD50 estimates (Glaberman and White, 2014; U.S. EPA, 2016).
Virus inocula and infection
Aliquots of both DWV‐A and DWV‐B were obtained from the propagated inocula of Tehel et al. (2019), which were retrieved from the genotype‐specific inocula of McMahon et al. (2016). The inocula of Tehel et al. (2019) and McMahon et al. (2016) have previously been ultradeep sequenced, revealing one nucleotide difference in the DWV‐A inoculum and three nucleotide differences in the DWV‐B inoculum between the two studies following propagation (details in Tehel et al., 2019), suggesting that inocula remain faithful in genotype and sequence identity during propagation. Aliquots of BQCV inocula were obtained from the propagated inoculum of Doublet et al. (2015). All inocula contained only the viral target and had no detectable contamination with other common honey bee RNA viruses, as determined by reverse transcription quantitative PCR (qPCR) using methods and the primers described above (Table S5).
To inoculate bees, viral inocula were first diluted in 0.5 M of cold potassium phosphate buffer (PPB) (pH 8.0) to final concentrations of 107 genome equivalents per µl, as quantified by qPCR. Then, 1 µl of viral inoculum containing 107 of DWV‐A, DWV‐B or BQCV was injected directly into a bee’s haemolymph between its second and third abdominal tergites using a Hamilton syringe (hypodermic needle outer diameter: 0.235 mm), simulating natural transmission by Varroa mites of DWV‐A, DWV‐B and potentially of BQCV (Chen et al., 2006; Al Naggar and Paxton, 2020).
Exposure to pesticides and honey bee RNA viruses
Combinations of FPF or SULF pesticides and honey bee RNA viruses (DWV‐A, DWV‐B or BQCV) were tested using a fully crossed laboratory experimental design. For each of our three honey bee colonies, we transferred a single frame with sealed worker brood to an incubator kept at 34°C and 80% relative humidity (RH) overnight. The next day we collected 270 newly emerging bees per colony, and then, we randomly assigned them to different treatments. To infect bees, 1 µl of viral inoculum containing 107 of DWV‐A or DWV‐B or BQCV was injected into a bee’s haemocoel. Treatments without viruses were injected with 1 µl of buffer (PPB) devoid of virus. We afterwards kept bees in metal cages (10 × 10 × 6 cm) containing an 8‐cm2 piece of organic beeswax, each with 30 newly emerged worker bees of the same treatment, and provided them with sugar water ad libitum, or sugar water containing FPF (0.0043 µg µl−1) or SULF (0.000047 µg µl−1). Though beeswax may itself contain pesticides (Boi et al., 2016), we used organic beeswax, which helps improve bee survival in cages (Köhler et al., 2013; McQuillan et al., 2014; Doublet et al., 2015; Fleming et al., 2015; Dussaubat et al., 2016; Kairo et al., 2017; Al Naggar and Baer, 2019).
Cages were placed into incubators at 30 ± 1°C and 50% RH. In total, we had 12 treatment groups, with three cages per treatment and 30 bees per cage. Mortality was recorded daily for 30 days. We collected subsamples (3 bees per cage) at 7 and 14 days post‐infection (i.e. 9 bees per treatment) and froze them at −80°C prior to quantifying viral load and gene expression, as described below. We also monitored the consumption of sugar water in each cage for the first 10 days of the experiment, which averaged 24.7 and 25.8 µl per bee per day of FPF and SULF‐laced sugar water for pesticide treatments. Across the 30 days of the experiment, this represents the cumulative consumption of ca. 3.18 µg per bee of FPF and 0.09 µg per bee of SULF, 2.65‐fold and 1.80‐fold greater than the reported LD50 of FPF and SULF, respectively (Glaberman and White, 2014; U.S. EPA, 2016).
RNA extraction and detection of virus
When testing whether our honey bee source colonies were free of RNA virus infection, we collected 10 adult worker honey bees per colony from the brood nest, crushed them in a plastic RNAse‐free mesh bag (BioReba, Reinach, Switzerland) with 5 ml of RLT buffer after snap‐freezing on dry ice, and then recovered 100 µl of homogenate. Viral titres in adult worker bees of the inoculation experiments were determined by crushing whole bees individually in 1 ml of RLT‐buffer (with 1% beta‐mercaptoethanol) using a plastic pestle, of which 100 µl was used for RNA isolation. RNA was extracted from bee homogenates (6 bees per treatment) using an RNeasy mini kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions in a QiaCube robot (Qiagen, Hilden, Germany). cDNA was synthesized from RNA extracts using oligo(dT)18 primers (Thermo Scientific, Schwerte, Germany) and reverse transcriptase (M‐MLV and Revertase, Promega, Mannheim, Germany) following the manufacturer’s instructions. For cDNA synthesis, 800 ng of RNA was used, after which the resultant cDNA was diluted 1:10 prior to use in qPCR.
Real‐time quantitative PCRs (qPCRs) were performed on a Bio‐Rad C1000 thermal cycler using SYBRgreen Sensimix (Bioline, Luckenwalde, Germany) and the primers for DWV‐A, DWV‐B and BQCV listed in Table S5. Amplification steps were as follows: 5 min at 95°C, followed by 40 cycles of 10 s at 95°C and 30 s at 57°C (including a read at each cycle). Following the qPCR, DNA was denatured for 1 min at 95°C then cooled to 55°C in 1 min, and a melting profile was obtained from 55 to 95°C at a 0.5°C increment per second; melt profiles consistently revealed a single product had been amplified and with the expected melt temperature. Absolute quantification of viruses was calculated using standards (ten 10‐fold dilutions of the respective PCR product covering the observed concentrations). To minimize the rate of false positives of viruses, a Ct of 35 cycles was set (de Miranda et al., 2013). Absolute values were log10‐transformed before statistical analysis.
Gene expression
We used qPCR to quantify effects of pesticides and virus exposure on the expression of six key immune and detoxification genes that have previously been used for comparable studies of honey bee response to stressors (Galbraith et al., 2015; Hu et al., 2017). We used four genes with well‐documented involvement in the insect immune response, namely dicer‐like, Argonaute‐2 (AGO2), tarbp2‐like and toll‐6, which are all part of the RNAi or Toll pathways (Brutscher et al., 2015; Galbraith et al., 2015). We selected an additional two genes with well‐documented detoxification activity: CYP6AS14 and CYP9Q3, as representatives of the cytochrome P450 pathway (Hu et al., 2017).
The primers for all genes were taken from the literature (Galbraith et al., 2015; Hu et al., 2017) (Table S6). To quantify gene expression, we used the same synthesized cDNA as for viral quantification mentioned above and qPCRs were performed on the same machine following the same protocols used for viral detection, with slight modification in amplification steps (10 min at 95°C instead of 5 min in viral detection). The qPCR cycle was followed by a dissociation step to validate that only a single product had been amplified in each reaction. For each target gene, the abundance of transcripts was quantified according to the Mean Normalized Expression (MNE) method of Simon (2003), and β‐actin (AMActin) was used as a reference gene. We also determined primer efficiencies using standard curves of serial dilutions of cDNA. We confirmed acceptable reaction conditions for all genes, with efficiencies between 93 and 110% (Table S6).
Statistical analysis
Survival analysis was performed with the R package coxme (Therneau, 2012) in R using mixed‐effects Cox proportional hazard models, with ‘cage’ as a nested random effect; models with ‘cage’ gave a consistently better model fit (lower AIC value) than models without this random effect. Right censored samples (bees removed at days 7 and 14 p.i. for analysis of viral titre and gene expression) were recorded in the dataset and incorporated in the Cox proportional hazard models.
In our first survival model, we tested all single treatments (pesticide or virus) with the control lacking pesticide or virus (with Bonferroni correction for multiple comparisons). To test for differences between single treatments, we performed linear contrasts (Tukey test) of Cox proportional hazard coefficients (hazard ratios) using the R package multcomp (Hothorn et al., 2013), with Bonferroni correction for multiple comparisons.
We subsequently tested for interactions between pesticide and virus in six separate models (one model per pesticide – virus combination) by coding the treatment ‘virus + pesticide’ as an independent, third treatment along with the other two treatments ‘virus’ and ‘pesticide’. Given the known impact of viruses in reducing honey bee survival, we then tested for interactive effects by comparing the treatment ‘virus’ with the treatment ‘virus + pesticide’; a significant linear contrast (Tukey test) was considered to represent a synergistic (significant positive contrast) or antagonistic (significant negative contrast) effect. A non‐significant linear contrast therefore reflected an additive (or subtractive) interaction between virus and pesticide (Piggott et al., 2015). Though use of multiple models may have elevated the Type I statistical error rate, all comparisons of ‘virus’ versus ‘pesticide + virus’ treatments were consistently non‐significant (Table S1), and therefore, adjustment of significance for multiple comparisons would not have altered results.
To test for treatment effects on gene expression, we log10 transformed data to meet the assumptions of normality and used ANOVA (Type II) tests in a linear model (LM). Pesticide exposure, pathogen infection and the day p.i. of bee sampling were used as independent, fixed factors (predictors). To test for significant effects of co‐exposure to pesticides, viruses and the day p.i. of sampling, we inspected the pathogen × pesticide interaction terms by keeping them in all models, independently of whether they were statistically significant or not. Using these linear models, we defined synergistic interactions as those in which the interaction term was significant (and positive) and antagonistic interactions as those in which the interaction term was significant (and negative). A non‐significant interaction term therefore reflected an additive (or subtractive) interaction between two stressors (Piggott et al., 2015).
To compare virus loads between pesticide treatments, we used ANOVA (Type II) tests in a LM, using pesticide exposure and day p.i. of sampling as independent, fixed factors. To test for significant interactive effects of exposure to pesticides and day of sampling p.i., we inspected the pesticide × time interaction terms by keeping them in all models, independently of whether they were statistically significant or not. Synergy and antagonism of interaction terms were again defined as for the analysis of gene expression data.
All statistical analyses and data visualizations were performed using R (R Core Team, 2020).
Conflict of interest
The authors declare no conflict of interest.
Supporting information
Fig. S1. Survival of honey bees inoculated with virus.
Fig. S2. Dicer‐like gene expression in honey bees co‐exposed to virus and pesticide.
Table S1. Statistical contrasts of survival among virus and pesticide treatments.
Table S2. Viral loads of honey bees co‐exposed to virus and pesticide.
Table S3. Effects of flupyradifurone and virus on honey bee gene expression.
Table S4. Effects of sulfoxaflor and virus on honey bee gene expression.
Table S5. PCR primers used for viral quantification.
Table S6. PCR primers used for quantification of honey bee gene expression.
Acknowledgements
This study was financially supported by a postdoctoral fellowship from the Alexander von Humboldt foundation to YA, the German Research Foundation (DFG) grant number Pa632/10‐1 and the European Union H2020 project PoshBee (773921) to RJP. The authors thank Anja Manigk for technical support, Panagiotis Theodorou for statistical advice, Anja Tehel & Tabea Streicher for virus inocula and advice on experimental design, Allyson Ray and Christina Grozinger for gene and primer selection, and the referees for valuable comments that helped improve the manuscript. Open access funding enabled and organized by Projekt DEAL.
Microbial Biotechnology (2021) 14(1), 227–240
Funding information
This study was financially supported by a postdoctoral fellowship from the Alexander von Humboldt foundation to YA, the German Research Foundation (DFG) grant number Pa632/10‐1 and the European Union H2020 project PoshBee (773921) to RJP.
References
- Al Naggar, Y. , and Baer, B. (2019) Consequences of a short time exposure to a sublethal dose of Flupyradifurone (Sivanto) pesticide early in life on survival and immunity in the honeybee (Apis mellifera). Sci Rep 9: 19753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Naggar, Y. , Codling, G. , Giesy, J.P. , and Safer, A. (2018) Beekeeping and the need for pollination from an agricultural perspective in Egypt. Bee World 95: 107–112. [Google Scholar]
- Al Naggar, Y. , and Paxton, R.J. (2020) Mode of transmission determines the virulence of black queen cell virus in adult honey bees, posing a future threat to bees and apiculture. Viruses 12: 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Naggar, Y. , Wiseman, S. , Jianxian, S. , Cutler, G.C. , Aboul‐Soud, M. , Naiem, E. , et al (2015) Effects of environmentally‐relevant mixtures of four common organophosphorus insecticides on the honey bee (Apis mellifera L.). J Insect Physiol 82: 85–91. [DOI] [PubMed] [Google Scholar]
- Aufauvre, J. , Misme‐Aucouturier, B. , Viguès, B. , Texier, C. , Delbac, F. , and Blot, N. (2014) Transcriptome analyses of the honeybee response to Nosema ceranae and insecticides. PLoS One 9: e91686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azpiazu, C. , Bosch, J. , Viñuela, E. , Medrzycki, P. , Teper, D. , and Sgolastra, F. (2019) Chronic oral exposure to field‐realistic pesticide combinations via pollen and nectar: effects on feeding and thermal performance in a solitary bee. Sci Rep 9: 13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, L. , and Woods, R.D. (1977) Two more small RNA viruses from honey bees and further observations on sacbrood and acute bee‐paralysis viruses. J Gen Virol 37: 175–182. [Google Scholar]
- Böhme, F. , Bischoff, G. , Zebitz, C.P.W. , Rosenkranz, P. , and Wallner, K. (2018) From field to food—will pesticide‐contaminated pollen diet lead to a contamination of royal jelly? Apidologie 49: 112–119. [Google Scholar]
- Boi, M. , Serra, G. , Colombo, R. , Lodesani, M. , Massi, S. , and Costa, C. (2016) A 10 year survey of acaricide residues in beeswax analysed in Italy. Pest Manag Sci 72: 1366–1372. [DOI] [PubMed] [Google Scholar]
- Boncristiani, H. , Underwood, R. , Schwarz, R. , Evans, J.D. , Pettis, J. , and VanEngelsdorp, D. (2012) Direct effect of acaricides on pathogen loads and gene expression levels in honey bees Apis mellifera . J Insect Physiol 58: 613–620. [DOI] [PubMed] [Google Scholar]
- Brutscher, L.M. , Daughenbaugh, K.F. , and Flenniken, M.L. (2015) Antiviral defense mechanisms in honey bees. Curr Opin Insect Sci 10: 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti, P. , Carlson, E.A. , Lucas, H.M. , Melathopoulos, A.P. , and Sagili, R.R. (2020) Field rates of SivantoTM (flupyradifurone) and Transform® (sulfoxaflor) increase oxidative stress and induce apoptosis in honey bees (Apis mellifera L.). PLoS One 15: e0233033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Evans, J. , and Feldlaufer, M. (2006) Horizontal and vertical transmission of viruses in the honey bee, Apis mellifera . J Invertebr Pathol 92: 152–159. [DOI] [PubMed] [Google Scholar]
- Cheng, Y. , Bu, Y. , Tan, L. , Wu, W. , Li, J. , Zhou, J. , et al (2018) A semi‐field study to evaluate effects of sulfoxaflor on honey bee (Apis mellifera). Bull Insectology 71: 225–233. [Google Scholar]
- Christen, V. , Schirrmann, M. , Frey, J.E. , and Fent, K. (2018) Global transcriptomic effects of environmentally relevant concentrations of the neonicotinoids clothianidin, imidacloprid, and thiamethoxam in the brain of honey bees (Apis mellifera ). Environ Sci Technol 52: 7534–7544. [DOI] [PubMed] [Google Scholar]
- Collison, E. , Hird, H. , Cresswell, J. , and Tyler, C. (2016) Interactive effects of pesticide exposure and pathogen infection on bee health ‐ a critical analysis. Biol Rev Camb Philos Soc 91: 1006–1019. [DOI] [PubMed] [Google Scholar]
- Coulon, M. , Schurr, F. , Martel, A.‐C. , Cougoule, N. , Bégaud, A. , Mangoni, P. , et al (2018) Metabolisation of thiamethoxam (a neonicotinoid pesticide) and interaction with the Chronic bee paralysis virus in honeybees. Pestic Biochem Physiol 144: 10–18. [DOI] [PubMed] [Google Scholar]
- Dainat, B. , Evans, J.D. , Chen, Y.P. , Gauthier, L. , and Neumann, P. (2012) Dead or alive: deformed wing virus and varroa destructor reduce the life span of winter honeybees. Appl Environ Microbiol 78: 981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechaume Moncharmont, F.‐X. , Decourtye, A. , Hennequet‐Hantier, C. , Pons, O. , and Pham‐Delègue, M.‐H. (2003) Statistical analysis of honeybee survival after chronic exposure to insecticides. Environ Toxicol Chem 22: 3088. [DOI] [PubMed] [Google Scholar]
- Desai, S.D. , Eu, Y.‐J. , Whyard, S. , and Currie, R.W. (2012) Reduction in deformed wing virus infection in larval and adult honey bees (Apis mellifera L.) by double‐stranded RNA ingestion. Insect Mol Biol 21: 446–55. [DOI] [PubMed] [Google Scholar]
- Desneux, N. , Decourtye, A. , and Delpuech, J.‐M. (2007) The Sublethal effects of pesticides on beneficial arthropods. Annu Rev Entomol 52: 81–106. [DOI] [PubMed] [Google Scholar]
- Di Prisco, G. , Cavaliere, V. , Annoscia, D. , Varricchio, P. , Caprio, E. , Nazzi, F. , et al (2013) Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proc Natl Acad Sci USA 110: 18466–18471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickel, F. , Münch, D. , Amdam, G.V. , Mappes, J. , and Freitak, D. (2018) Increased survival of honeybees in the laboratory after simultaneous exposure to low doses of pesticides and bacteria. PLoS One 13: e0191256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doublet, V. , Labarussias, M. , de Miranda, J.R. , Moritz, R.F.A. , and Paxton, R.J. (2015) Bees under stress: sublethal doses of a neonicotinoid pesticide and pathogens interact to elevate honey bee mortality across the life cycle. Environ Microbiol 17: 969–983. [DOI] [PubMed] [Google Scholar]
- Doublet, V. , Poeschl, Y. , Gogol‐Döring, A. , Alaux, C. , Annoscia, D. , Aurori, C. , et al (2017) Unity in defence: honeybee workers exhibit conserved molecular responses to diverse pathogens. BMC Genom 18: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussaubat, C. , Maisonnasse, A. , Crauser, D. , Tchamitchian, S. , Bonnet, M. , Cousin, M. , et al (2016) Combined neonicotinoid pesticide and parasite stress alter honeybee queens’ physiology and survival. Sci Rep 6: 31430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erban, T. , Harant, K. , Hubalek, M. , Vitamvas, P. , Kamler, M. , Poltronieri, P. , et al (2015) In‐depth proteomic analysis of Varroa destructor: Detection of DWV‐complex, ABPV, VdMLV and honeybee proteins in the mite. Sci Rep 5: 13907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldhaar, H. , and Otti, O. (2020) Pollutants and their interaction with diseases of social hymenoptera. Insects 11: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming, J.C. , Schmehl, D.R. , and Ellis, J.D. (2015) Characterizing the impact of commercial pollen substitute diets on the level of Nosema spp. in honey bees (Apis mellifera L.). PLoS One 10: e0132014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flenniken, M.L. , and Andino, R. (2013) Non‐specific dsRNA‐mediated antiviral response in the honey bee. PLoS One 8: e77263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis, R.M. , Nielsen, S.L. , and Kryger, P. (2013) Varroa‐virus interaction in collapsing honey bee colonies. PLoS One 8: e57540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith, D.A. , Yang, X. , Niño, E.L. , Yi, S. , and Grozinger, C. (2015) Parallel epigenomic and transcriptomic responses to viral infection in honey bees (Apis mellifera). PLoS Pathog 11: e1004713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallai, N. , Salles, J.‐M. , Settele, J. , and Vaissière, B.E. (2009) Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol Econ 68: 810–821. [Google Scholar]
- Genersch, E. , von der Ohe, W. , Kaatz, H. , Schroeder, A. , Otten, C. , Büchler, R. , et al (2010) The German bee monitoring project: a long term study to understand periodically high winter losses of honey bee colonies. Apidologie 41: 332–352. [Google Scholar]
- Gill, R.J. , Ramos‐Rodriguez, O. , and Raine, N.E. (2012) Combined pesticide exposure severely affects individual‐ and colony‐level traits in bees. Nature 491: 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisder, S. , Aumeier, P. , and Genersch, E. (2009) Deformed wing virus: replication and viral load in mites (Varroa destructor). J Gen Virol 90: 463–467. [DOI] [PubMed] [Google Scholar]
- Gisder, S. , Möckel, N. , Eisenhardt, D. , and Genersch, E. (2018) In vivo evolution of viral virulence: switching of Deformed wing virus between hosts results in virulence changes and sequence shifts. Environ Microbiol 20: 4612–4628. [DOI] [PubMed] [Google Scholar]
- Glaberman, S. , and White, K. (2014) Environmental fate and ecological risk assessment for foliar, soil drench, and seed treatment uses of the new insecticide flupyradifurone (byi 02960). Environmental Protection Agency Office of Pesticide Programs, Environmental Fate and Effects Division EF. [Google Scholar]
- González‐Varo, J.P. , Biesmeijer, J.C. , Bommarco, R. , Potts, S.G. , Schweiger, O. , Smith, H.G. , et al (2013) Combined effects of global change pressures on animal‐mediated pollination. Trends Ecol Evol 28: 524–530. [DOI] [PubMed] [Google Scholar]
- Goulson, D. , Nicholls, E. , Botias, C. , and Rotheray, E.L. (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347: 1255957. [DOI] [PubMed] [Google Scholar]
- Gray, A. , Brodschneider, R. , Adjlane, N. , Ballis, A. , Brusbardis, V. , Charrière, J.‐D. , et al (2019) Loss rates of honey bee colonies during winter 2017/18 in 36 countries participating in the COLOSS survey, including effects of forage sources. J Apic Res 58: 479–485. [Google Scholar]
- Hesselbach, H. , and Scheiner, R. (2018) Effects of the novel pesticide flupyradifurone (Sivanto) on honeybee taste and cognition. Sci Rep 8: 4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselbach, H. , and Scheiner, R. (2019) The novel pesticide flupyradifurone (Sivanto) affects honeybee motor abilities. Ecotoxicology 28: 354–366. [DOI] [PubMed] [Google Scholar]
- Hesselbach, H. , Seeger, J. , Schilcher, F. , Ankenbrand, M. , and Scheiner, R. (2020) Chronic exposure to the pesticide flupyradifurone can lead to premature onset of foraging in honeybees Apis mellifera. J Appl Ecol 57: 609–618. [Google Scholar]
- Highfield, A.C. , El Nagar, A. , Mackinder, L.C.M. , Noel, L.M.‐L.J. , Hall, M.J. , Martin, S.J. , and Schroeder, D.C. (2009) Deformed wing virus implicated in overwintering honeybee colony losses. Appl Environ Microbiol 75: 7212–7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hothorn, T. , Bretz, F. , Westfal, P. , Heiberger, R.M. , and Schuetzenmeister, A. (2013) multcomp: simultaneous inference in general parametric models. [DOI] [PubMed]
- Hu, Y.‐T. , Wu, T.‐C. , Yang, E.‐C. , Wu, P.‐C. , Lin, P.‐T. , and Wu, Y.‐L. (2017) Regulation of genes related to immune signaling and detoxification in Apis mellifera by an inhibitor of histone deacetylation. Sci Rep 7: 41255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques, A. , Laurent, M. , Ribière‐Chabert, M. , Saussac, M. , Bougeard, S. , Budge, G.E. , et al (2017) A pan‐European epidemiological study reveals honey bee colony survival depends on beekeeper education and disease control. PLoS One 12: e0172591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, R.R. , and Xu, J. (2012) Mechanisms by which pesticides affect insect immunity. J Invertebr Pathol 109: 175–182. [DOI] [PubMed] [Google Scholar]
- Kairo, G. , Poquet, Y. , Haji, H. , Tchamitchian, S. , Cousin, M. , Bonnet, M. , et al (2017) Assessment of the toxic effect of pesticides on honey bee drone fertility using laboratory and semifield approaches: A case study of fipronil. Environ Toxicol Chem 36: 2345–2351. [DOI] [PubMed] [Google Scholar]
- Köhler, A. , Nicolson, S.W. , and Pirk, C.W.W. (2013) A new design for honey bee hoarding cages for laboratory experiments. J Apic Res 52: 12–14. [Google Scholar]
- Krupke, C.H. , Hunt, G.J. , Eitzer, B.D. , Andino, G. , and Given, K. (2012) Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS One 7: e29268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulhanek, K. , Steinhauer, N. , Rennich, K. , Caron, D.M. , Sagili, R.R. , Pettis, J.S. , et al (2017) A national survey of managed honey bee 2015–2016 annual colony losses in the USA. J Apic Res 56: 328–340. [Google Scholar]
- Liu, X. , Zhang, Y. , Yan, X. , and Han, R. (2010) Prevention of Chinese sacbrood virus infection in Apis cerana using RNA interference. Curr Microbiol 61: 422–8. [DOI] [PubMed] [Google Scholar]
- Locke, B. , Forsgren, E. , Fries, I. , and Miranda, J.R. (2012) Acaricide treatment affects viral dynamics in Varroa destructor ‐ infested honey bee colonies via both host physiology and mite control. Appl Environ Microbiol. 78: 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher, R.L. , Rice, M.M. , McMinds, R. , Burkepile, D.E. , and Vega Thurber, R. (2019) Multiple stressors interact primarily through antagonism to drive changes in the coral microbiome. Sci Rep 9: 6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley, R. , Boots, M. , and Wilfert, L. (2015) Emerging viral disease risk to pollinating insects: ecological, evolutionary and anthropogenic factors. J Appl Ecol 52: 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maori, E. , Paldi, N. , Shafir, S. , Kalev, H. , Tsur, E. , Glick, E. , and Sela, I. (2009) IAPV, a bee‐affecting virus associated with colony collapse disorder can be silenced by dsRNA ingestion. Insect Mol Biol 18: 55–60. [DOI] [PubMed] [Google Scholar]
- Martin, S.J. , Highfield, A.C. , Brettell, L. , Villalobos, E.M. , Budge, G.E. , Powell, M. , et al (2012) Global honey bee viral landscape altered by a parasitic mite. Science 336: 1304–1306. [DOI] [PubMed] [Google Scholar]
- McMahon, D.P. , Natsopoulou, M.E. , Doublet, V. , Fürst, M. , Weging, S. , Brown, M.J.F. , et al (2016) Elevated virulence of an emerging viral genotype as a driver of honeybee loss. Proc R Soc B Biol Sci 283: 20160811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillan, H.J. , Nakagawa, S. , and Mercer, A.R. (2014) Juvenile hormone enhances aversive learning performance in 2‐day old worker honey bees while reducing their attraction to queen mandibular pheromone. PLoS One 9: e112740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkling, S.H. , and van Rij, R.P. (2013) Beyond RNAi: antiviral defense strategies in Drosophila and mosquito. J Insect Physiol 59: 159–170. [DOI] [PubMed] [Google Scholar]
- de Miranda, J.R. , Bailey, L. , Ball, B.V. , Blanchard, P. , Budge, G.E. , Chejanovsky, N. , et al (2013) Standard methods for virus research in Apis mellifera . J Apic Res 52: 1–56. [Google Scholar]
- Mockel, N. , Gisder, S. , and Genersch, E. (2011) Horizontal transmission of deformed wing virus: pathological consequences in adult bees (Apis mellifera) depend on the transmission route. J Gen Virol 92: 370–377. [DOI] [PubMed] [Google Scholar]
- Mondet, F. , de Miranda, J.R. , Kretzschmar, A. , Le Conte, Y. , and Mercer, A.R. (2014) On the front line: quantitative virus dynamics in honeybee (Apis mellifera L.) colonies along a new expansion front of the parasite Varroa destructor . PLoS Pathog 10: e1004323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz, R.F.A. , de Miranda, J. , Fries, I. , Le Conte, Y. , Neumann, P. , and Paxton, R.J. (2010) Research strategies to improve honeybee health in Europe. Apidologie 41: 227–242. [Google Scholar]
- Mullin, C.A. , Frazier, M. , Frazier, J.L. , Ashcraft, S. , Simonds, R. , VanEngelsdorp, D. , and Pettis, J.S. (2010) High levels of miticides and agrochemicals in north American apiaries: implications for honey bee health. PLoS One 5: e9754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsopoulou, M.E. , McMahon, D.P. , Doublet, V. , Frey, E. , Rosenkranz, P. , and Paxton, R.J. (2017) The virulent, emerging genotype B of Deformed wing virus is closely linked to overwinter honeybee worker loss. Sci Rep 7: 5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsopoulou, M.E. , McMahon, D.P. , and Paxton, R.J. (2016) Parasites modulate within‐colony activity and accelerate the temporal polyethism schedule of a social insect, the honey bee. Behav Ecol Sociobiol 70: 1019–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazzi, F. , Brown, S.P. , Annoscia, D. , Del Piccolo, F. , Di Prisco, G. , Varricchio, P. , et al (2012) Synergistic parasite‐pathogen interactions mediated by host immunity can drive the collapse of honeybee colonies. PLoS Pathog 8: e1002735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazzi, F. , and Pennacchio, F. (2018) Honey bee antiviral immune barriers as affected by multiple stress factors: a novel paradigm to interpret colony health decline and collapse. Viruses 10: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neov, B. , Georgieva, A. , Shumkova, R. , Radoslavov, G. , and Hristov, P. (2019) Biotic and abiotic factors associated with colonies mortalities of managed honey bee (Apis mellifera). Diversity 11: 237. [Google Scholar]
- OECD (2017) Test No. 245: Honey Bee (Apis Mellifera L.), Chronic Oral Toxicity Test (10‐Day Feeding) OECD Guidelines for the Testing of Chemicals, Section 2. OECD Publishing. [Google Scholar]
- Osborne, J.L. (2012) Bumblebees and pesticides. Nature 491: 43–45. [DOI] [PubMed] [Google Scholar]
- Osterman, J. , Wintermantel, D. , Locke, B. , Jonsson, O. , Semberg, E. , Onorati, P. , et al (2019) Clothianidin seed‐treatment has no detectable negative impact on honeybee colonies and their pathogens. Nat Commun 10: 692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson, R.H. , Zhang, S. , and Gray, J.R. (2020) Neonicotinoid and sulfoximine pesticides differentially impair insect escape behavior and motion detection. Proc Natl Acad Sci USA 117: 5510–5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggott, J.J. , Townsend, C.R. , and Matthaei, C.D. (2015) Reconceptualizing synergism and antagonism among multiple stressors. Ecol Evol 5: 1538–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts, S.G. , Biesmeijer, J.C. , Kremen, C. , Neumann, P. , Schweiger, O. , and Kunin, W.E. (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25: 345–353. [DOI] [PubMed] [Google Scholar]
- R Core Team (2020) R: A Language and Environment for Statistical Computing. [Google Scholar]
- Ramsey, S.D. , Ochoa, R. , Bauchan, G. , Gulbronson, C. , Mowery, J.D. , Cohen, A. , et al (2019) Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc Natl Acad Sci USA 116: 1792–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero, A. , Vézilier, J. , Weill, M. , Read, A.F. , and Gandon, S. (2010) Insecticide control of vector‐borne diseases: when is insecticide resistance a problem? PLoS Pathog 6: e1001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson‐Robert, O. , Labrie, G. , Chagnon, M. , and Fournier, V. (2014) Neonicotinoid‐contaminated puddles of water represent a risk of intoxication for honey bees. PLoS One 9: e108443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez‐Bayo, F. (2009) From simple toxicological models to prediction of toxic effects in time. Ecotoxicology 18: 343–354. [DOI] [PubMed] [Google Scholar]
- Sánchez‐Bayo, F. , Goulson, D. , Pennacchio, F. , Nazzi, F. , Goka, K. , and Desneux, N. (2016) Are bee diseases linked to pesticides? — A brief review. Environ Int 89–90: 7–11. [DOI] [PubMed] [Google Scholar]
- Sánchez‐Bayo, F. , and Tennekes, H.A. (2020) Time‐cumulative toxicity of neonicotinoids: experimental evidence and implications for environmental risk assessments. Int J Environ Res Public Health 17: 1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnier, H.F. , Wenig, G. , Laubert, F. , Simon, V. , and Schmuck, R. (2003) Honey bee safety of imidacloprid corn seed treatment. Bull Insectology 56: 73–75. [Google Scholar]
- Simon, P. (2003) Q‐Gene: processing quantitative real‐time RT‐PCR data. Bioinformatics 19: 1439–1440. [DOI] [PubMed] [Google Scholar]
- Simon‐Delso, N. , San Martin, G. , Bruneau, E. , and Hautier, L. (2018) Time‐to‐death approach to reveal chronic and cumulative toxicity of a fungicide for honeybees not revealed with the standard ten‐day test. Sci Rep 8: 7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siviter, H. , Brown, M.J.F. , and Leadbeater, E. (2018a) Sulfoxaflor exposure reduces bumblebee reproductive success. Nature 561: 109–112. [DOI] [PubMed] [Google Scholar]
- Siviter, H. , Folly, A.J. , Brown, M.J.F. , and Leadbeater, E. (2020a) Individual and combined impacts of sulfoxaflor and Nosema bombi on bumblebee (Bombus terrestris) larval growth. Proc R Soc B Biol Sci 287: 20200935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siviter, H. , Horner, J. , Brown, M.J.F. , and Leadbeater, E. (2020b) Sulfoxaflor exposure reduces egg laying in bumblebees Bombus terrestris . J Appl Ecol 57: 160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siviter, H. , Koricheva, J. , Brown, M.J.F. , and Leadbeater, E. (2018b) Quantifying the impact of pesticides on learning and memory in bees. J Appl Ecol 55: 2812–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siviter, H. , Scott, A. , Pasquier, G. , Pull, C.D. , Brown, M.J.F. , and Leadbeater, E. (2019) No evidence for negative impacts of acute sulfoxaflor exposure on bee olfactory conditioning or working memory. PeerJ 7: e7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks, T.C. , Watson, G.B. , Loso, M.R. , Geng, C. , Babcock, J.M. , and Thomas, J.D. (2013) Sulfoxaflor and the sulfoximine insecticides: Chemistry, mode of action and basis for efficacy on resistant insects. Pestic Biochem Physiol 107: 1–7. [DOI] [PubMed] [Google Scholar]
- Spurny, R. , Přidal, A. , Pálková, L. , Kiem, H.K.T. , de Miranda, J.R. , and Plevka, P. (2017) Virion structure of black queen cell virus, a common honeybee pathogen. J Virol 91: e02100‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, K. , Wang, C. , Dong, S. , Li, X. , and Nieh, J.C. (2017) The pesticide flupyradifurone impairs olfactory learning in Asian honey bees (Apis cerana) exposed as larvae or as adults. Sci Rep 7: 17772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapparo, A. , Marton, D. , Giorio, C. , Zanella, A. , Soldà, L. , Marzaro, M. , et al (2012) Assessment of the environmental exposure of honeybees to particulate matter containing neonicotinoid insecticides coming from corn coated seeds. Environ Sci Technol 46: 2592–2599. [DOI] [PubMed] [Google Scholar]
- Tehel, A. , Streicher, T. , Tragust, S. , and Paxton, R.J. (2020) Experimental infection of bumblebees with honeybee‐associated viruses: no direct fitness costs but potential future threats to novel wild bee hosts. Roy Soc Open Science 7: 200480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehel, A. , Vu, Q. , Bigot, D. , Gogol‐Döring, A. , Koch, P. , Jenkins, C. , et al (2019) The two prevalent genotypes of an emerging infectious disease, deformed wing virus, cause equally low pupal mortality and equally high wing deformities in host honey bees. Viruses 11: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therneau, T. (2012) coxme. R package version 2.2‐5.
- Tong, L. , Nieh, J.C. , and Tosi, S. (2019) Combined nutritional stress and a new systemic pesticide (flupyradifurone, Sivanto®) reduce bee survival, food consumption, flight success, and thermoregulation. Chemosphere 237: 124408. [DOI] [PubMed] [Google Scholar]
- Tosi, S. , and Nieh, J.C. (2019) Lethal and sublethal synergistic effects of a new systemic pesticide, flupyradifurone (Sivanto®), on honeybees. Proc R Soc B Biol Sci 286: 20190433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA (2013) Environmental Fate and Ecological Risk Assessment for Sulfoxaflor Registration.
- U.S. EPA (2014) Environmental fate and ecological risk assessment for foliar, soil drench, and seed treatment uses of the new insecticide flupyradifurone (BYI 02960).
- U.S. EPA (2016) United States Environmental Protection Agency. 2016 Addendum to the Environmental Fate and Ecological Risk Assessment for Sulfoxaflor Registration (2016).
- U.S. EPA (2019) Ecological Risk Assessment for the Registration of Sulfoxaflor.
- Vanbergen, A.J. , and Initiative, the I.P. (2013) Threats to an ecosystem service: pressures on pollinators. Front Ecol Environ 11: 251–259. [Google Scholar]
- Vidau, C. , Diogon, M. , Aufauvre, J. , Fontbonne, R. , Viguès, B. , Brunet, J.‐L. , et al (2011) Exposure to sublethal doses of fipronil and thiacloprid highly increases mortality of honeybees previously infected by Nosema ceranae . PLoS One 6: e21550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfert, L. , Long, G. , Leggett, H.C. , Schmid‐Hempel, P. , Butlin, R. , Martin, S.J.M. , and Boots, M. (2016) Deformed wing virus is a recent global epidemic in honeybees driven by Varroa mites. Science 351: 594–597. [DOI] [PubMed] [Google Scholar]
- Zhu, Y. , Loso, M.R. , Watson, G.B. , Sparks, T.C. , Rogers, R.B. , Huang, J.X. , et al (2011) Discovery and characterization of sulfoxaflor, a novel insecticide targeting sap‐feeding pests. J Agric Food Chem 59: 2950–2957. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Survival of honey bees inoculated with virus.
Fig. S2. Dicer‐like gene expression in honey bees co‐exposed to virus and pesticide.
Table S1. Statistical contrasts of survival among virus and pesticide treatments.
Table S2. Viral loads of honey bees co‐exposed to virus and pesticide.
Table S3. Effects of flupyradifurone and virus on honey bee gene expression.
Table S4. Effects of sulfoxaflor and virus on honey bee gene expression.
Table S5. PCR primers used for viral quantification.
Table S6. PCR primers used for quantification of honey bee gene expression.


