Future fermentations will be open, continuous and automatically controlled with fresh water and energy saving. The production strains will be extremophiles engineered by synthetic biology.
Summary
Microbial fermentations produce chemicals, materials, biofuels, foods and medicines for many years. The processes are less competitive compared to chemical industries. To increase its competitiveness, technologies must be developed to address the following issues including fresh water shortage, heavy energy consumption, microbial contaminations, complexity of sterile operations, poor oxygen utilization in the cultures, food‐related ingredients as substrates, low substrate to product conversion efficiency, difficult cells and broth separation, large amount of wastewater, discontinuous processes, heavy labour involvements and expensive bioreactors. Future industrial fermentations should be more effective with the above issues reasonably addressed. Recently, extremophilic bacteria have well addressed the above issues for future fermentation.
Industrial fermentation has been employed to produce chemicals, materials, biofuels, foods and medicines for many years. With the exception of medicines, industrial fermentations are less competitive compared to chemical industries or agricultures. However, due to its safety, green processes and sustainability, industrial fermentation has been rapidly developed to industrial biotechnology for production of more and more products. To increase its competitiveness, technologies must be developed to address the following issues including fresh water shortage, heavy energy consumption, microbial contaminations, complexity of sterile operations, poor oxygen utilization in the cultures, food‐related ingredients as substrates, low substrate to product conversion efficiency, difficult cells and broth separation, large amount of wastewater, discontinuous processes, heavy labour involvements and expensive bioreactors et al. Future industrial fermentations should be more effective with the above issues reasonably addressed (Table 1).
Table 1.
Comparison of current and future fermentation.
| Item | Current fermentation (CF) | Future fermentation (FF) | Note |
|---|---|---|---|
| Microbial chassis: | E. coli, Pseudomonas spp., Bacillus spp., Lactic acid bacteria, Corynebacterium glutamicum, Streptomycetes, Aspergillus, Yeast et al | Extremophilic bacteria, Methylotroph, CO and/or CO2 utilizers, Phototroph, et al | Engineering the chassis cells to grow fast on low‐cost carbon substrates and mineral salts |
| Sterilization | Yes | Not needed | FF reduces energy consumption |
| Contamination | By bacteria, fungi or phages | No | Absence of phage receptor |
| Fresh water | Heavy consumption | Seawater or fresh water recycling | Saving fresh water |
| Growth process | Batch or fed‐batch | Batch or fed‐batch or continuous | Improving process efficiency |
| Substrates (S) | Mostly glucose, fatty acids | Diverse: mixed‐substrates, starch, gases | Reducing substrate cost |
| Product (P) | Usually one product | Intra‐and/or extracellular products | Increase process efficiency |
| S to P ratio | Reasonable | Better | Improve process economy |
| Wastewater | Discharged and treated | Recycled | Reducing wastewater |
| Downstream | Complicated | Convenient via morphology engineering | Reducing downstream cost |
| Aeration | Intensive with high energy demands | Light aeration via haemoglobin expression | FF reduces energy consumption |
| O2 demand | Usually very high | Micro‐ or anaerobic (facultative aerobic) | Increase process efficiency |
| pH for growth | Narrow pH, neutral or weak acidic | Wide pH ranging from 3‐11 | For easy growth control |
| Temperature | 30–40°C | 20–85°C | For easy growth control |
| Osmotic pressure | Low to mild | Low to high | For easy growth control |
| Bioreactor | Made from stainless steel | Plastics, ceramics, cements or stainless steel | FF reduces equipment cost |
| Automation | Half automation | Full automation with AI deep learning | Improving process consistency |
| Aggregation | Usually not | Usually desirable | Easy cells and broth separation |
| Induction | Gene expression induction difficult in late growth | Induction at high cell density possible | Delaying harmful cell processes |
| Cell lysis | Physical process | Inducible cell lysis | Recovery of intracellular P |
| Labour | Well educated and trained | Only simple training required | No‐sterilization and automation |
What microorganisms fit the future industrial fermentation?
Extremophilic or unique substrate selected bacteria are an answer to the above challenges of current industrial fermentations, including acidophilic, alkaliphilc, halophilic, xerophilic, thermophilic, psychrophilic, methylotrophs and gaseous substrates utilizers et al (Chen and Jiang, 2018). These organisms resist growth of others in their culture conditions. Furthermore, the removal of phage receptor genes in the microorganisms is helpful for phage resistance.
Fresh water shortage
The best solution to this issue is the recycle of the fermentation broth, or the use of seawater replacing fresh water (Yue et al., 2014). The fermentation broth containing quorum sensing molecules and macromolecular substances of cell debris such as proteins, polysaccharides, RNA, DNA and lipids is required to conduct an alkali or acidic heat hydrolysis process to be degraded so that these molecules become nutrients for the cell again.
Heavy energy consumption
Aerobic microorganisms require a lot of oxygen to grow or to transform substrates to products, especially in large scale bioreactors in which oxygen is supplied by air compressors. However, oxygen solubility is low in water. Most oxygen escapes to the air passing through the bioreactors. To improve oxygen uptake by the cells, overexpression of bacterial haemoglobin is very helpful (Ouyang et al., 2018). In addition, technologies of turning the above aerobic bacteria into facultative organisms or microaerobic ones are highly welcomed as this saves heavy energy consumption of intensive aerobic processes, especially during the product formation steps. Furthermore, if the production organism is grown in a wide range of temperature, especially from 25 to 50°C, cooling energy can be saved.
Complexity of sterile operations
As the extremophilic chassis bacteria require extreme conditions to grow, they are resistant to microbial contamination especially when the chassis cells can be grown fast under the extreme conditions. No sterile procedure is needed to grow these chassis cells. Therefore, the air filtration system can be removed or simplified to just block the particles in the air to enter the cultures. At the same time, the medium and the fermentation system need not be sterilized during the entire process. No sterilization precaution is required. All of these free the operators from complicated sterilization process and its associated pressure. The non‐sterilization process also allows the fermentative process to turn from batch or fed‐batch ones into continuous ones, permitting continuously feeding the cultures with nutrients and harvesting the products continuously (Tan et al., 2011). It improves the productivity and thus process economy.
Food‐related ingredients as substrates
At the moment, glucose from hydrolyzed starch is commonly used for production of various bioproducts. In the future, waste substrates including molasses, activated sludge, cellulose hydrolyzed sugars or methane, CO + H2, mixed nutrients from kitchen wastes should be used as nutrients for cell growth, avoiding ‘food for chemicals’ issue.
Low substrate to product conversion efficiency
Unlike chemical reactions, substrates are mostly converted to products as designed, cells synthesize our products as a site‐reaction beside the many biochemical pathways serving for cell growth and survival. These biochemical reactions synthesize DNA, RNA, proteins, polysaccharides, lipids and many intermediates with CO2 and H2O generation, leaving only little room for our product synthesis. To increase substrates to product conversion, metabolic engineering approaches must be taken to direct more flux towards our products. In addition, if an aerobic process is turned into anaerobic one, the TCA and electronic transport chain will be turned off, substrates loss to CO2 and H2O production will be reduced, and ratios of substrates to products will be increased.
Difficult cells and broth separation
Smaller cells and heavy fermentation broths are difficult to separate even though disc centrifuges and filtration systems are used. Now synthetic biology approaches allow bacterial cells to change their morphologies from bars or coccus to fibres or large spheres, or particles with large sizes, or even self‐aggregation, all lead to gravity precipitation and thus achieving separation. Future fermentations will depend on controllable morphology changes and self‐aggregation to separate cells and broth via gravity, thus avoiding centrifugations or filtrations (Wang et al., 2019; Ling et al., 2019).
Large amount of wastewater
Fermentation industries generate a lot of wastewater with high COD and BOD containing mostly organic cell debris and inorganic salts. These can be treated to become nutrients for cell growth again. The quorum sensing AHL molecules can be alkali or acidic hydrolysis to become less inhibitory for cell growth. The treated broth can be returned to the bioreactors as nutrients for cell growth, thus avoiding wastewater generation.
Discontinuous processes
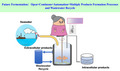
Most of the current fermentation processes are running discontinuously, either in a batch or fed‐batch way, resulting in a low productivity. This is due to the microbial chassis grown under gentle conditions favouring microbial contaminations. The contamination resistant extreme chassis allow continuous growth for a longer period without contamination, which help improve productivity of the processes. For example, Halomonas campaniensis sp. LS21 was reported to grow under non‐sterilization conditions for 65 days in a continuous process for PHB production without contamination (Yue et al., 2014; Fig. 1).
Fig. 1.
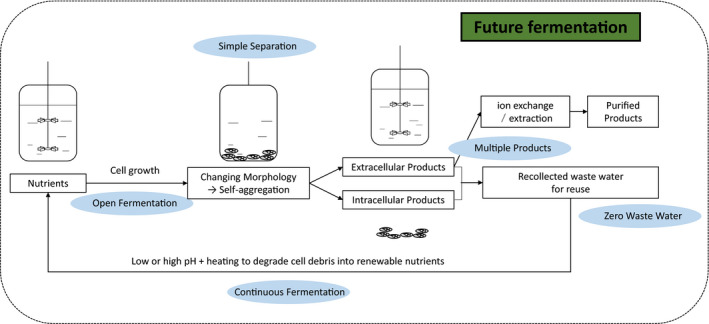
The future fermentation process. Future fermentations will be conducted under non‐sterile and continuous conditions controlled by artificial intelligence with reduced water and energy consumption. Process economy will also be improved by more substrates to product conversions and multiple product generation as well as morphology engineering for easy downstream separations. Investment can also be reduced by using low‐cost materials for bioreactor constructions.
Heavy labour involvements
Most of the current fermentation processes are run batch or fed‐batch way and require some heavy manual manipulations. Since the future fermentation industries can be operated in a continuous way, automation can be installed to reduce human involvement and avoid errors (Chen and Jiang, 2018).
Expensive bioreactors
All of the current bioreactors are built using stainless steel except the light bioreactors that have to use glass or transparent materials. To maintain sterility, all the parts and connections must be well sealed avoiding any pores or cracks that hidden with microbes, this increases the cost of building bioreactors. Future fermentations are not conducted under sterilization conditions, low‐cost materials like plastics, cements and ceramics can be used instead of steel. These will reduce the construction complexity and cost, further lowering the investment. In addition, plastics reduce weights of bioreactors with transparency not possible when using steel. Cements allow construction of very large bioreactors like building skyscrapers.
Intracellular and extracellular products
Except solid state fermentations, all fermentations generate supernatants and solid biomass. Both need be treated before the next step. Future fermentation can design the chassis to produce soluble extracellular small molecular products and insoluble intracellular inclusion body products like bioplastics polyhydroxyalkanoates (PHA), polyphosphates, insoluble proteins, magnetosome, elemental sulfur and glycogen et al (Ma et al., 2020). These allow recovery of at least two products from one process, improving the process economy.
Summary
Future fermentations will be conducted under non‐sterile and continuous conditions controlled by artificial intelligence with reduced water and energy consumption. Process economy will also be improved by more substrates to product conversions and multiple product generation as well as morphology engineering for easy downstream separations. Investment can also be reduced by using low‐cost materials for bioreactor constructions. The costs of producing materials and chemicals will match that generated from the chemical industrial processes. Future fermentation will provide sustainable solution for the chemical industries.
Microbial Biotechnology (2021) 14(1), 18–21
References
- Chen, G.Q. , and Jiang, X.R. (2018) Next generation industry biotechnology based on extremophiles. Curr Opin Biotechnol 50: 94–100. [DOI] [PubMed] [Google Scholar]
- Ling, C. , Qiao, G.Q. , Shuai, B.W. , Song, K.N. , Jiang, X.R. , and Chen, G.Q. (2019) Engineering self‐flocculating Halomonas campaniensis for wastewaterless open and continuous fermentation. Biotechnol Bioeng 116: 805–815. [DOI] [PubMed] [Google Scholar]
- Ma, H. , Zhao, Y.Q. , Huang, W.Z. , Zhang, L.Z. , Wu, F.Q. , Ye, J.W. , and Chen, G.Q. (2020) Rational flux‐tuning Halomonas bluephagenesis for co‐production of PHB and ectoine. Nat Commun 11: 3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang, P.F. , Wang, H. , Hajnal, I. , Wu, Q. , Guo, Y.Y. , and Chen, G.Q. (2018) Increasing oxygen availability for improving PHB production by Halomonas . Metab Eng 45: 20–31. [DOI] [PubMed] [Google Scholar]
- Tan, D. , Xue, Y.S. , Gulsimay, A. , and Chen, G.Q. (2011) Unsterile and continuous production of polyhydroxybutyrate by Halomonas TD01. Biores Technol 102: 8130–8136. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Ling, C. , Chen, Y. , Jiang, X.R. , and Chen, G.Q. (2019) Microbial engineering for easy downstream processing. Biotechnol Adv 37: 107365. [DOI] [PubMed] [Google Scholar]
- Yue, H.T. , Chen, X.B. , Ling, C. , Chen, Y.L. , Deng, H.T. , and Chen, G.Q. (2014) A seawater based open and continuous process for polyhydroxyalkanoates production by Halomonas campaniensis LS21. Biotechnol Biofuels 7: 108. [Google Scholar]


