Mass‐production of clean (i.e. in vitro produced), safe and robust inoculum of arbuscular mycorrhizal fungi (AMF) at affordable costs remains a critical challenge. Engineering plants for enhanced delivery of lipids to AMF could represent an innovative avenue to produce a novel generation of high‐quality and cost‐effective bio‐fortified AMF inoculants for application in agro‐ecosystems.
Summary
Arbuscular mycorrhizal fungi (AMF) are among the most ancient, widespread and functionally important symbioses on Earth that help feed the world. Yet, mass‐production of clean (i.e. in vitro produced), safe and robust inoculum at affordable costs remains a critical challenge. Very recently, Luginbuehl et al. (2017) found that plants supply lipids to the symbiotic partner, thus ‘providing the AMF with a robust source of carbon for their metabolic needs’. Hence, engineering plants for enhanced delivery of lipids to AMF could represent an innovative avenue to produce a novel generation of high‐quality and cost‐effective bio‐fortified AMF inoculants for application in agro‐ecosystems.
Introduction
Arbuscular mycorrhizal fungi (AMF) are ubiquitous soil microorganisms forming a mutualistic symbiosis with an estimate of 72% of terrestrial plant taxa. They are key players in agro‐ecosystems, improving plant nutrition (Smith and Read, 2008) and increasing their resistance/tolerance to biotic and abiotic stresses (Pozo et al., 2013; Plouznikoff et al., 2016). Unfortunately, overuse of chemical fertilizers and pesticides and intensive agricultural practices have recklessly decreased mycorrhizal diversity in modern agricultural systems (Sosa‐Hernández et al., 2019), a situation which is exacerbated under climate changes believed to impart dramatic effects on plant–soil–AMF interactions by altering the community structure, abundance, composition and functional activity of plant‐associated microbial taxa (Sergaki et al., 2018). Because of that, applying agricultural practices favouring/restoring AMF diversity or introducing selected mass‐produced AMF inoculants into soils have become key challenges of the next big revolution in the development of sustainable agricultural systems.
Towards engineered in vitro AMF production systems: a novel generation of AMF mass‐produced inoculants
The large‐scale production of AMF is not possible in the absence of a suitable host and most production systems are conducted on substrate‐based (e.g. in greenhouse) or substrate‐free (e.g. in hydroponics, aeroponics) conditions which often require large surfaces and cannot guarantee the absence of unwanted microbes. Promising alternatives are the production of AMF on transformed root organ cultures (ROC) which correspond to a successful established Petri plate culture of AMF with Ri T‐DNA transformed root organs on a gelled medium. Different sub‐production systems have been derived from this basic ROC system for mass‐production, by cultivation of root organs and AMF in small containers (Declerck et al., 2005), in airlift bioreactors (Jolicoeur et al., 1999) or in bioreactors containing solid support elements [US Pat. No. 5554530 (1996)]. In parallel, in vitro culture systems based on autotrophic plants have been developed with shoots developing outside the Petri plate while roots and AMF were associated inside the Petri plate on gelled medium (Voets et al., 2005). In vitro cultivation on root organs or whole plants are the only systems that could guarantee the propagation of contaminant‐free material, making them prior candidates for large‐scale production of high‐quality controlled inoculum. However, these systems remain technologically costly, are limited to a restricted number of AMF species and production remains low as compared to in vivo systems. There is thus a need for the development of innovative technologies allowing the production of high density, safe and robust spore inoculum with reduced costs.
Several attempts have been conducted to tackle this challenge with the AMF Rhizophagus irregularis by using dual‐compartment culture systems (Rosikiewicz et al., 2017) or by the addition of stimulating chemical molecules to compensate for lack of microbial associates and trigger gene activation for secondary metabolite production. The addition of fatty acids (myristate) in the growth medium was recently shown to induce hyphal branching, colonization ability and sporulation in the asymbiotic stage of R. irregularis (Kameoka et al., 2019). The addition of chemical molecules is, however, very costly, but suggests that engineering fatty acid metabolism in host plants may be an innovative approach for a novel generation of in vitro AMF produced spores.
The prospective to engineer plants–microorganisms interaction has been conducted successfully in different studies but these synthetic biology approaches have been focused only on filamentous fungi such as Aspergillus niger and Penicillium chrysogenum (Guzmán‐Chávez et al., 2018). Currently, there is no research on the use of AMF colonized plants in synthetic biology to improve the symbiotic establishment and large‐scale spore production. Moreover, AMF are multinucleated organisms not amenable for engineering (Dubey et al., 2019). Technological advances need to focus on synthetic biology with AMF colonized plants. Lipid engineering in plants is expected to fuel the AMF with high value carbon sources, leading to a novel generation of high quality and quantity in vitro produced AMF inoculants.
Combinatorial metabolic engineering strategy
Transcriptomic analysis showed that the development of arbuscules is accompanied by the activation of an AMF‐specific operational unit of lipid biosynthesis and transport of fatty acids (FA) from plant cells to the FA auxotroph AMF (Fig. 1), governed by transcription factors and genes found to be absent from genomes of non‐mycotrophic plants (Luginbuehl et al., 2017). Such information is presumed to be essential to the development of a novel generation of mass‐produced AMF inoculants, giving rise to consistently increasing TAG content of spores (spore bio‐fortification) that would enhance spore production as well as spore fitness to increase overall colonization efficiency. Further analyses need to be undertaken with engineered plants to characterize the key AMF‐specific genes that promote TAG accumulation in fortified spores and/or increase their number. As known, gene modification analyses demonstrated that single‐gene manipulations typically only have marginal effects on TAG content in vegetative tissues (Vanhercke et al., 2019). However, recent combinatorial metabolic engineering strategy focused on the simultaneous optimization of oil synthesis, packaging and degradation pathways have a great power with engineered TAG levels in plants. This strategy has been coined in plants as ‘Push, Pull, Package and Protect’ (PPPP strategy) (Vanhercke et al., 2019). The ultimate aim of the use of PPPP strategy is to mimic the lipid metabolic fluxes occurring in oilseeds plants into non‐seed plant tissues, where carbon is efficiently shunted into TAG for longer term storage in photosynthetic sources tissues. By analogy, the PPPP strategy could be applied in the symbiotic interface to generate mycorrhized transgenic lines with boosting de novo FA biosynthesis in plant root cells, shunt more FA pool from root cells to arbuscule cells, increase transport of neutral lipids to hypha structure, and storage of neutral lipids in AMF spore (Fig. 1). Such approach could open the way to develop an engineered host plant for the growth benefits of the non‐engineered AMF symbiont, thereby effectively changing host plant into a ‘factory’ delivering essential energy dense lipids for the production of bio‐fortified AMF cells. The proposed engineering approach should, when coupled with a series of conventional enzymatic assays and continuous pulse and pulse‐chase metabolic isotopic labelling (Allen et al., 2015), provide a holistic understanding of the redistribution of acyl groups between various pathways of membrane lipids and TAG production (Zhou et al., 2020) in the symbiotic interface. Furthermore, functional oriented approaches of the bio‐fortified AMF spores could be applied to characterize their role in plant growth, development, physiology and stress tolerance under non‐optimal field growing conditions and environmental changes. In the long term, such information could become essential to the development of the next generation of bio‐fertilizer inoculants, giving rise to consistently functioning microbial communities that restore soil health and enhance agriculture sustainability and crop productivity.
Fig. 1.
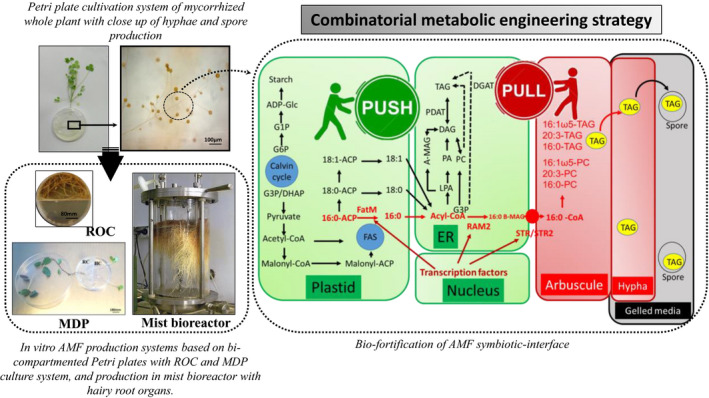
Combinatorial lipid engineering strategy of mycorrhized host plants for mass‐production of bio‐fortified AMF in Petri plates or bioreactors using ‘Push, Pull, Package and Protect’ strategy (Vanhercke, et al., 2019). AMF‐specific operational unit of lipid biosynthesis and transport pathways are represented by red arrows and lipid biosynthesis pathways in root cell are represented by black arrows. Model of the proposed route for biosynthesis of fatty acyl groups stored in fungal triacylglycerols (TAG) was adapted from Luginbuehl et al. (2017). ROC correspond to Root Organ Culture (Ijdo, et al., 2011). MDP correspond to Mycorrhizal Donor Plant in vitro culture system with (RC) root compartment and (HC) hyphal compartment (Lalaymia and Declerck, 2020). The mist bioreactor photograph was extracted from Urbańska et al. (2014).
Domestication of AMF ‘bio‐fortifed’ spores
Until today, the in vitro cultivation of AMF is mostly restricted to species in the genus Rhizophagus (formerly Glomus for most of them) and to a few individuals belonging to the Gigasporaceae. Moreover, with the exception of R. irregularis and R. Intraradices and a few others species, biomass production remains low and for some species can even await several months to produce only a limited number of spores (e.g. the Gigasporaceae). The reason for the failure to grow a broad set of AMF remains enigmatic, probably partly related to their life history strategy, r‐strategists being presumably more prone for in vitro cultivation than K strategists. Similarly, the reasons for their relative limited biomass production are manifold and may be related to the limited volume of Petri plates, the short period of growth of root organs and to the limited capacity of the host to sustain high levels of spore production. Modulating growth media and volume have been proposed for mass‐production and achieved for a very limited number of species in bioreactors. Similarly, moving from ROC to photosynthetically active plants have been suggested to increase the number of species grown in vitro but should now been demonstrated. Metabolic engineering for lipid production and delivery in whole plants or hairy roots lines combined with bioreactor technologies could represent an innovative approach either to access AMF species that until now have evaded in vitro cultivation (e.g. Fulleniformis mosseae, etc.) or for mass‐production. Several studies have reported the culture of hairy roots in various bioreactor configurations for large‐scale cultivation of AMF. Hairy roots have been grown in solid support elements (Fortin et al., 1996), mist‐ and airlift (Jolicoeur et al., 1999) bioreactors, which were evolved to ensure cultivation in a low‐stress environment, homogenous growth distribution as well as adequate oxygen and nutrient supply. However, none of them involved the production of AMF using engineered plant host for enhancing a low‐cost mass production of AMF. Hence, the development of a bioreactor could be a step forward for the optimization process of bio‐fortified spores production by high lipid producing and delivery plants under strict in vitro cultivation conditions. This approach is likely to reduce the cost per item and labour time.
Concluding remarks
We envision that future efforts to extend the number of AMF species grown in vitro and to achieve mass‐production of these organisms could focus on lipid engineering of the symbiotic interface. Success in this attempt will inevitably necessitate intense methodological research including combinatorial lipid metabolic engineering, selection of mycorrhized TAG‐accumulating host, lipid flux analysis and spores domestication. This new concept will offer versatile and multi‐beneficial options: (i) increase TAG‐based carbon sources in the AMF, with vesicles, intra‐ and extra‐radical spores accumulating more lipids for a higher germination and root‐colonization potential (bio‐fortification = best quality), ii) stimulate the asexual reproduction machinery to produce more spores in Petri plates and bioreactors (biomass production = high quantity), decreasing costs of in vitro spore production systems (cost‐efficiency = industry profitable). Ultimately, it is expected that research will transform this concept into a novel generation of high‐quality and cost‐effective bio‐inoculant for large‐scale application in agro‐ecosystems.
Microbial Biotechnology (2021) 14(1), 31–34
References
- Allen, D.K. , Bates, P.D. , and Tjellström, H. (2015) Tracking the metabolic pulse of plant lipid production with isotopic labeling and flux analyses: past, present and future. Prog Lipid Res 58: 97–120. [DOI] [PubMed] [Google Scholar]
- Declerck, S. , Strullu, D.‐G. , and Fortin, A. (2005) In Vitro Culture of Mycorrhizas. Berlin Heidelberg: Springer‐Verlag, XXIV, p. 392. [Google Scholar]
- Dubey, A. , Malla, M.A. , Khan, F. , Chowdhary, K. , Yadav, S. , Kumar, A. , et al (2019) Soil microbiome: a key player for conservation of soil health under changing climate. Biodivers Conserv 28: 2405–2429. [Google Scholar]
- Fortin, J. , St‐Arnaud, M. , Hamel, C. , Chaverie, C. , and Jolicoeur, M. (1996) Aseptic in vitro endomycorrhizal spore mass production [US Pat. No. 5554530].
- Guzmán‐Chávez, F. , Zwahlen, R.D. , Bovenberg, R.A.L. , and Driessen, A.J.M. (2018) Engineering of the filamentous fungus penicillium chrysogenum as cell factory for natural products. Front Microbiol 9: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijdo, M. , Cranenbrouck, S. , and Declerck, S. (2011) Methods for large‐scale production of AM fungi: past, present, and future. Mycorrhiza 21: 1–16. [DOI] [PubMed] [Google Scholar]
- Jolicoeur, M. , Williams, R.D. , Chavarie, C. , Fortin, J.A. , and Archambault, J. (1999) Production of Glomus intraradices propagules, an arbuscular mycorrhizal fungus, in an airlift bioreactor. Biotechnol Bioeng 63: 224–232. [DOI] [PubMed] [Google Scholar]
- Kameoka, H. , Tsutsui, I. , Saito, K. , Kikuchi, Y. , Handa, Y. , Ezawa, T. , et al (2019) Stimulation of asymbiotic sporulation in arbuscular mycorrhizal fungi by fatty acids. Nat Microbiol 4: 1654–1660. [DOI] [PubMed] [Google Scholar]
- Lalaymia, I. , and Declerck, S. (2020) The Mycorrhizal Donor Plant (MDP) in vitro culture system for the efficient colonization of whole plants In Arbuscular Mycorrhizal Fungi: Methods and Protocols. Ferrol, N. , and Lanfranco, L. (eds). New York, NY: Springer US, pp. 19–31. [DOI] [PubMed] [Google Scholar]
- Luginbuehl, L.H. , Menard, G.N. , Kurup, S. , Van Erp, H. , Radhakrishnan, G.V. , Breakspear, A. , et al (2017) Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science 356: 1175–1178. [DOI] [PubMed] [Google Scholar]
- Plouznikoff, K. , Declerck, S. , and Calonne‐Salmon, M. (2016) Mitigating abiotic stresses in crop plants by arbuscular mycorrhizal fungi In Belowground Defence Strategies in Plants. Vos, C.M.F. , and Kazan, K. (eds). Cham: Springer International Publishing, pp. 341–400. [Google Scholar]
- Pozo, M.J. , Jung, S.C. , Martínez‐Medina, A. , López‐Ráez, J.A. , Azcón‐Aguilar, C. , and Barea, J.‐M. (2013) Root allies: arbuscular mycorrhizal fungi help plants to cope with biotic stresses In Symbiotic Endophytes. Aroca, R. (ed). Berlin, Heidelberg: Springer, pp. 289–307. [Google Scholar]
- Rosikiewicz, P. , Bonvin, J. , and Sanders, I.R. (2017) Cost‐efficient production of in vitro Rhizophagus irregularis. Mycorrhiza 27: 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergaki, C. , Lagunas, B. , Lidbury, I. , Gifford, M.L. , and Schäfer, P. (2018) Challenges and approaches in microbiome research: from fundamental to applied. Front Plant Sci 9: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S.E. , and Read, D. (2008) In Mycorrhizal Symbiosis (3rd ed.). Smith, S.E. , and Read, D. (eds). London: Academic Press, pp. 1–800. [Google Scholar]
- Sosa‐Hernández, M.A. , Leifheit, E.F. , Ingraffia, R. , and Rillig, M.C. (2019) Subsoil arbuscular mycorrhizal fungi for sustainability and climate‐smart agriculture: a solution right under our feet? Front Microbiol 10: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbańska, N. , Giebułtowicz, J. , Olszowska, O. , and Szypuła, J. (2014) The growth and saponin production of Platycodon grandiflorum (Jacq.) A. DC. (Chinese bellflower) hairy roots cultures maintained in shake flasks and mist bioreactor. Acta Soc Bot Pol 83: 229–237. [Google Scholar]
- Vanhercke, T. , Dyer, J.M. , Mullen, R.T. , Kilaru, A. , Rahman, M.M. , Petrie, J.R. , et al (2019) Metabolic engineering for enhanced oil in biomass. Prog Lipid Res 74: 103–129. [DOI] [PubMed] [Google Scholar]
- Voets, L. , Dupré de Boulois, H. , Renard, L. , and Declerck, S. (2005) Development of an autotrophic culture system for the in vitro mycorrhization of potato plantlets. FEMS Microbiol Lett 248: 111–118. [DOI] [PubMed] [Google Scholar]
- Zhou, X.‐R. , Bhandari, S. , Johnson, B.S. , Kotapati, H.K. , Allen, D.K. , Vanhercke, T. , and Bates, P.D. (2020) Reorganization of acyl flux through the lipid metabolic network in oil‐accumulating tobacco leaves. Plant Physiol 182: 739–755. [DOI] [PMC free article] [PubMed] [Google Scholar]


