Abstract
Lepidium meyenii (maca), a plant indigenous to the Peruvian Andes, recently has been utilized globally for claimed health or recreational benefits. The search for natural products that inhibit soluble epoxide hydrolase (sEH), with therapeutically relevant potencies and concentrations, led to the present study on bioactive amide secondary metabolites found in L. meyenii, the macamides. Based on known and suspected macamides, 19 possible macamides were synthesized and characterized. The majority of these amides displayed excellent inhibitory potency (IC50 ≈ 20–300 nM) towards the recombinant mouse, rat and human sEH. Quantitative analysis of commercial maca products revealed that certain products contain known macamides (1–5, 8–12) at therapeutically relevant total concentrations (≥ 3.29 mg/g of root), while the inhibitory potency of L. meyenii extracts directly correlates with the sum of concentration/IC50 ratios of macamides present. Considering both its in vitro efficacy and high abundance in commercial products, N-benzyl-linoleamide (4) was identified as a particularly relevant macamide that can be utilized for in vivo studies. Following oral administration in the rat, compound 4 not only displayed acceptable pharmacokinetic characteristics, but it effectively reduced lipopolysaccharide-induced inflammatory pain. Inhibition of sEH by macamides provides a plausible biological mechanism of action to account for several beneficial effects previously observed with L. meyenii treatments.
Graphical Abstract
Epoxy fatty acids (EpFAs), derived from cytochrome P450 oxidation of polyunsaturated fatty acids, are lipid mediators with primarily anti-inflammatory, analgesic, antihypertensive and antiapoptotic activities.1–4 However, they are rapidly degraded to less bioactive dihydroxy fatty acids by soluble epoxide hydrolase (sEH). sEH inhibitors (sEHI) stabilize endogenous levels of EpFAs, enhancing their bioavailability and biological functions. Hence, inhibition of sEH is a novel and powerful therapeutic approach that could be utilized to tackle a number of unmet clinical needs. Thousands of highly potent urea, amide and carbamate-based sEHI have been synthesized and characterized. Despite this, only a handful have entered clinical trials and, currently, no drug on the market is used intentionally as an sEH inhibitor. One huge barrier is the extremely costly and time-consuming approval process for synthetic drugs due to very stringent regulatory requirements.
Alternatively, sEHI from natural sources, such as botanicals, can act as possible nutraceuticals and, through dietary supplementation, might provide a significantly faster and inexpensive means to treat patients. This could be particularly important in developing countries. Previously, a number of chemicals from a variety of natural sources have been identified as sEHI,5–12 including urea and amide-based inhibitors from plants in the order Brassicales.13,14 However, a large number of these compounds have either a low potency or their concentrations in the plant are insufficient for clinical application.
Lepidium meyenii Walp.
(L. meyenii), commonly known as maca or “Peruvian ginseng”, belongs to the family Brassicaceae and is indigenous to the high altitude of central Peruvian Andes. Its root is a native food crop and there is rising global interest in L. meyenii products as herbal remedies.15–17 Characterization of L. meyenii has revealed several secondary metabolites including glucosinolates, flavonolignans, and macamides.18,19 Macamides are N-benzylamides of long-chain fatty acids (LCFAs) and are the key bioactive components of L. meyenii. They have demonstrated therapeutic potential against neurological disorders, including antidepressant effects20 and protective action in models of Parkinson’s disease.21,22 Improvements in neural cell viability are attributed to the prevention of mitochondrial membrane depolarization and reduced induction of reactive oxygen species (ROS). Attenuation of oxidative stress has also been shown to mediate non-neurological effects including protection against erythrocyte hemolysis,23 antifatigue effects in muscles,24 and decreased lipid peroxidation in a diabetic rat model.25 Moreover, combining a Chinese clive seed extract with a maca extract significantly synergized beneficial effects on male sexual health.26
Macamides have been characterized as inhibitors of fatty acid amide hydrolase (FAAH),27,28 an enzyme that degrades neuroprotective endocannabinoids such as anandamide.29 Hence, FAAH inhibition has been proposed as a mechanism of action for macamides in the central nervous system. However, the potency is poor (IC50 >10 μM), insufficient for the degree of FAAH inhibition typically required to exert biological effects such as analgesia.30 Thus, FAAH inhibition is unlikely to fully account for the neuroprotective activity of macamides. sEH inhibition stabilizes the mitochondrial dysfunction-ROS-Endoplasmic Reticulum stress axis and, consequently, underlies the amelioration of several pathologies.3 Therefore, potentially it provides an alternative mode of action that captures macamide bioactivity. sEHI have also been shown to dramatically synergize with and expand the biological activity of FAAH inhibitors.31
Considering their structural similarities to previously identified amide pharmacophore containing sEHI,32–34 19 possible macamides were synthesized, characterized, and tested as novel inhibitors of sEH in vitro. Additionally, macamide content in a number of commercial maca products was quantified to investigate compound distribution and abundance among products. Finally, the most pertinent macamides were studied in vivo to assess pharmacokinetic parameters and analgesic effects in an inflammatory pain model.
RESULTS AND DISCUSSION
Macamide Design and Synthesis.
A proposed route of biosynthesis of macamides involves LCFAs and benzylamine derivatives as substrates, which accumulate during traditional post-harvest treatments as catabolic processes accelerate.35–38 LCFAs are released from membrane lipids while glucosinolates are broken down to benzyl isothiocyanates, converted to benzylamines and enzymatically linked to the fatty acids, forming macamides (Figure 1). Thus, the macamides designed include a benzylamine moiety, which was either unsubstituted or possessed methoxy groups at meta/para positions, similar to certain parent glucosinolates. They also contain a fatty acid portion and a range of common saturated, mono- or polyunsaturated LCFAs were selected to account for effects of varying tail lengths and unsaturation sites.
Figure 1.
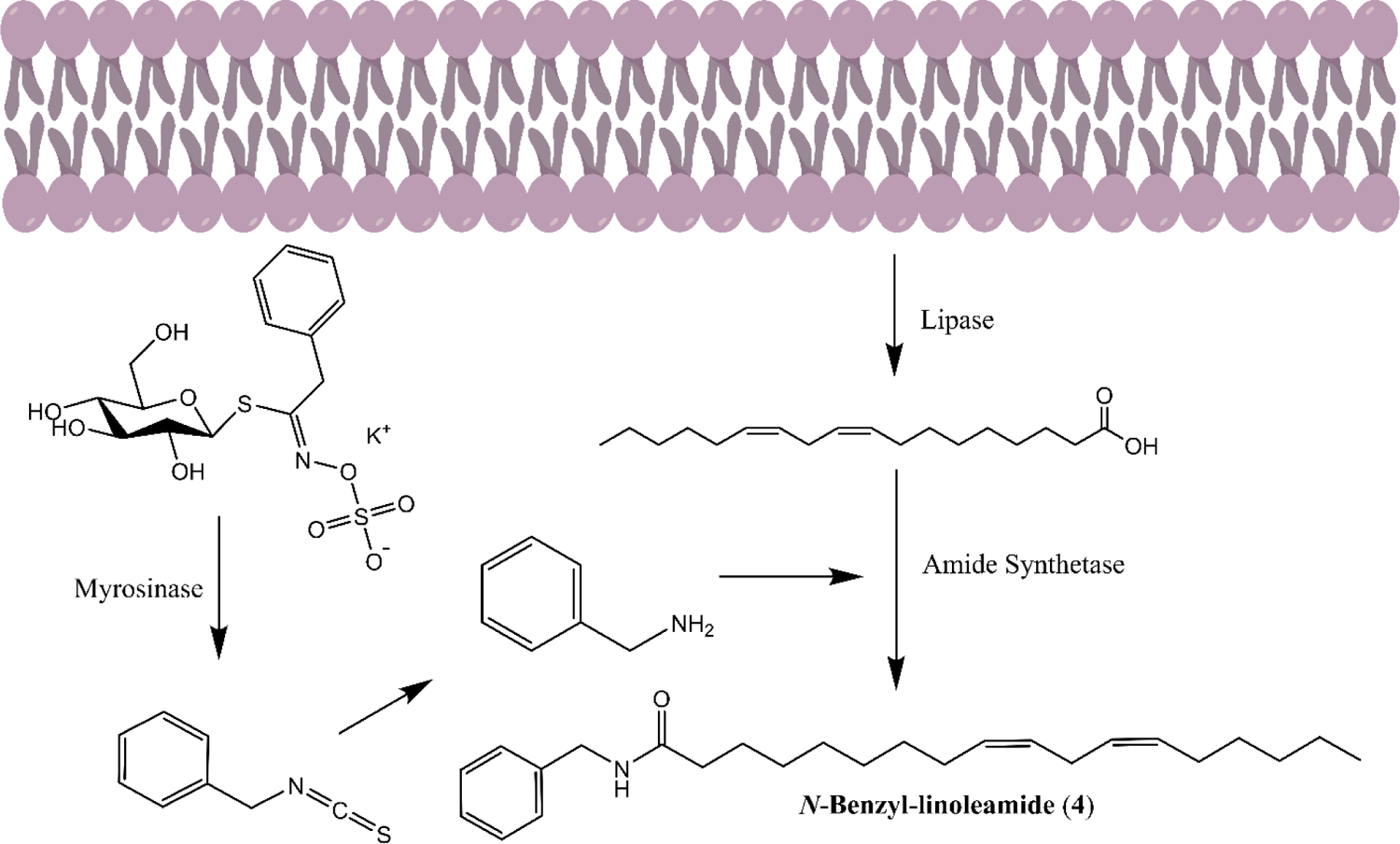
Schematic of hypothesized route for biosynthesis of macamides.35–38 Shown with the representative N-Benzylamide of linoleic acid (4).
Accordingly, the central synthetic method employed was amide synthesis (Scheme 1). In general, the appropriate LCFA was activated with 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide, and a catalytic amount of 4-dimethylaminopyridine, under dry and inert conditions. This was followed by addition of benzylamine to generate compounds 2–7, 3-methoxybenzylamine to generate compounds 8–14, 3,4-dimethoxybenzylamine to generate compounds 15–17, and phenylethylamine to generate compounds 18 and 19.
Scheme 1.
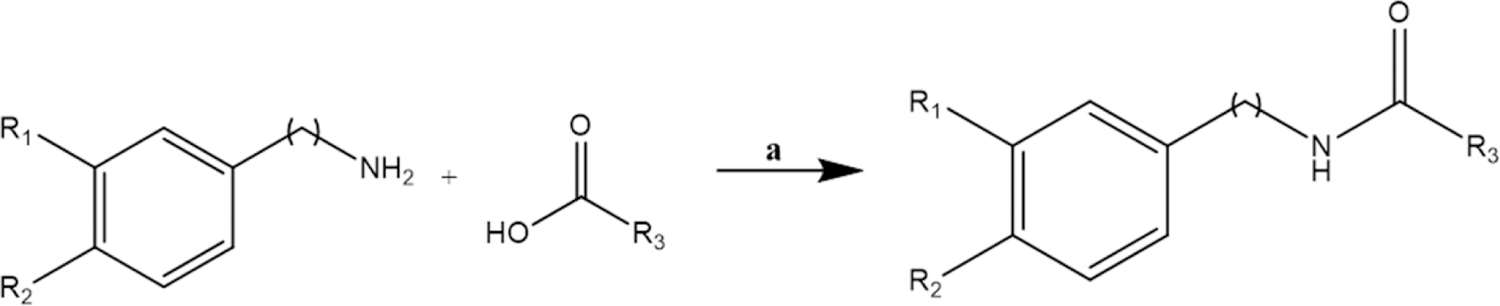
General Synthesis of Compounds 2–19. a: EDC, DMAP (cat.), CH2Cl2
Inhibition of Recombinant Human, Rat, and Mouse sEH.
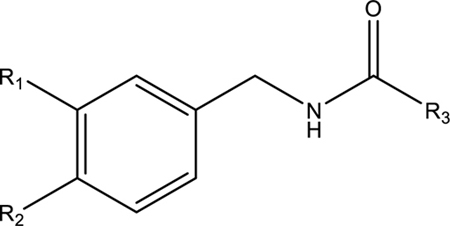
Inhibitory potency of the synthesized amides was tested against recombinant human, rat, and mouse sEH (Table 1). Compounds with polyunsaturated fatty acid tails were more potent than the corresponding benzylamide analogue with saturated tails (4–7 vs. 1–2 and 11–14 vs. 8–9). The difference was especially prominent relative to the saturated 18-carbon chains (2 and 9), which were between two and three orders of magnitude less potent and were the weakest of the characterized inhibitors. The addition of a single double bond at the C-9-C-10 position (3 and 10) increased potency by almost two orders of magnitude. These observations suggest olefinic bonds are important for the inhibitory potential of macamides with longer fatty acid tails. However, within inhibitor groups with two or more double bonds in their tails (4–7 and 11–14), there was little discernible difference in potencies. The addition of a meta-methoxy to the phenyl ring (8–14) generally decreased IC50 values compared to unsubstituted analogs (1–7). This indicated that methoxy groups at meta positions can enhance sEH inhibition, as has been demonstrated with benzyl-containing urea-based natural sEH inhibitors.14 A second, para-methoxy (15–17), however, did not improve inhibitory potency. In fact, it significantly reduced potency of compounds with a saturated 16-carbon tail (15 vs. 1 and 8) and also lessened the efficacy of compounds with unsaturated tails (16 and 17 vs. 4 and 5, and 11 and 12). Insertion of a methylene between the phenyl ring and amide function (18 and 19) increased inhibitory potency towards the human enzyme by almost one order of magnitude, compared to analogues 4–5. The influence might be due to reduced steric hindrance in the active site.
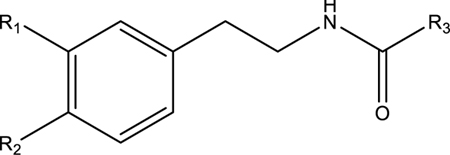
Table 1.
Effects of Phenyl Substituents and Fatty Acid Chains on the sEH Inhibitory Potency of 19 Macamide-like Compounds
 |
||||||||
|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | Natural Product (+/−) | Human sEH IC50 (nM) |
Rat sEH IC50 (nM) |
Mouse sEH IC50 (nM) |
Human FAAH IC50a (nM) | |
| 1 | H | H | + | 524 | 370 | 422 | >100,000 | |
| 2 | H | H | + | >100,000 | 35,800 | 59,600 | >100,000 | |
| 3 | H | H | + | 893 | 198 | 265 | 16,700 | |
| 4 | H | H | + | 155 | 41 | 44 | 10,800 | |
| 5 | H | H | + | 134 | 23 | 28 | 41,800 | |
| 6 | H | H | − | 123 | 40 | 50 | N/A | |
| 7 | H | H | − | 111 | 29 | 43 | N/A | |
| 8 | OCH3 | H | + | 235 | 113 | 159 | N/A | |
| 9 | OCH3 | H | + | 16,077 | 28,676 | 89,850 | N/A | |
| 10 | OCH3 | H | + | 241 | 84 | 126 | 11,000 | |
| 11 | OCH3 | H | + | 92 | 43 | 38 | 10,300 | |
| 12 | OCH3 | H | + | 76 | 9.3 | 27 | 13,700 | |
| 13 | OCH3 | H | − | 55 | 22 | 73 | N/A | |
| 14 | OCH3 | H | − | 63 | 18.6 | 49 | N/A | |
| 15 | OCH3 | OCH3 | + | 7,059 | 15,910 | >100,000 | N/A | |
| 16 | OCH3 | OCH3 | − | 104 | 111 | 148 | N/A | |
| 17 | OCH3 | OCH3 | − | 84 | 90 | 122 | N/A | |
 |
||||||||
| 18 | H | H | − | 28 | 33 | 91 | N/A | |
| 19 | H | H | − | 15 | 15 | 39 | N/A | |
Literature values.27
Macamide Levels in L. meyenii Products and Inhibitory Potency of Extracts.
Thirteen commercially available L. meyenii root products were extracted and analyzed to quantify the amount of macamides present. Product types and sources are described in the Supporting Information (Table S1). Total macamide concentration estimates were based only on compounds with synthetic standards. Levels of macamides present varied significantly (> two orders of magnitude) from product to product (Table 2), with an average of around 1.24 mg/g of root. The key determinant for differences is likely variability in post-harvest treatment factors such as drying temperature, storage time and air exposure, since these conditions dictate substrate release and subsequent biosynthesis efficiency.35–38 Certain extracts (e.g., E2 and E9) contained concentrations (≥ 3.29 mg/g of root) that were nearly two orders of magnitude greater than those of urea sEHI (< 0.058 mg/g of root) found in plants such as Pentadiplandra brazzeana Baill.13 This suggests that, under the right post-harvest conditions, dried L. meyenii root extracts might have sufficiently high macamide concentrations to be of nutraceutical value as a source of naturally derived sEHI. To support this claim, the inhibitory potency of the L. meyenii extracts was measured for the human and mouse sEH (Table 2). Biological activity of extracts also varied dramatically, but the IC50 values obtained inversely correlated with the amount of macamides in the extracts (Spearman’s rank correlation coefficient ρhuman sEH = −0.928 and ρmouse sEH = −0.956), suggesting that the sEH inhibition was mostly due to the presence of macamides in the extracts.
Table 2:
Macamide Content and Inhibitory Potency of Thirteen L. meyenii Product Extracts
| L. meyenii Extracts | Total Macamide Concentrations (μg/g of root) | Human sEH IC50 (ng/mL) |
Mouse sEH IC50 (ng/mL) |
|---|---|---|---|
| E1 | 22.4 | 11,700 | 12,600 |
| E2 | 3,602 | 329 | 138 |
| E3 | 1,804 | 377 | 169 |
| E4 | 1,780 | 450 | 139 |
| E5 | 210 | 6,380 | 6,530 |
| E6 | 217 | 4,550 | 3,400 |
| E7 | 66.5 | 5,085 | 6,290 |
| E8 | 2,480 | 422 | 207 |
| E9 | 3,290 | 287 | 157 |
| E10 | 301 | 2,620 | 1,701 |
| E11 | 1,490 | 980 | 456 |
| E12 | 870 | 1,606 | 869 |
| E13 | 16.0 | 4,380 | > 14,000 |
Ten out of the 19 synthesized compounds were detected in all extracts, and these are known natural products,26,27 specifically N-benzylamides of palmitic acid (1), stearic acid (2), oleic acid (3), linoleic acid (4) and α-linolenic acid (5) and their 3-methoxy substituted analogues (8–12). Compound 15, the 3,4-dimethoxy substituted analogue of N-benzylpalmitamide (1) has also been detected previously in L. meyenii.25 However, it was below the limit of quantitation (Table S2, Supporting Information) in the analyzed samples, possibly due to the difference in origin of maca. Amides with fatty acid tails containing 20 carbons, i.e. arachidonic acid (6, 13) and eicosapentaenoic acid (7, 14), were below the limit of quantitation (Table S2, Supporting Information), likely due to the relatively low concentrations of 20- and 22-carbon fatty acids in plant membrane lipids.39,40 In the most abundant extract (E2), levels of compound 4 were the highest, with concentrations nearly three and four times greater than those of the next two most abundant macamides, 1 and 5, respectively (Table 3). In order to gauge inhibitory contributions of individual macamides within the extract, the ratio of concentrations to the corresponding IC50 values was determined (Table 3), to obtain a metric that accounted for both abundance in L. meyenii and potency towards sEH. The ratio was easily the greatest for macamide 4, suggesting it is likely the most biologically relevant macamide found in L. meyenii roots to date. Macamide 5 was the next most relevant product, while the potential activity of the 3-methoxy substituted analogues of 4 and 5 (i.e. 11 and 12) as well as macamide 1 was also noteworthy. Higher abundance would largely account for the contribution of 1 while, conversely, the excellent potency of 11 and 12 (Table 1) would compensate for their relatively lower levels in extracts.
Table 3.
Concentrations and Abundance/IC50 Ratios of Individual Macamides in Extract 2
| Compound | Concentration (μg/g of Root) | Abundance/hsEH IC50 (μg/nM) | Abundance/msEH IC50 (μg/nM) |
|---|---|---|---|
| 1 | 680 | 1.30 | 1.61 |
| 2 | 269 | <0.003 | 0.005 |
| 3 | 275 | 0.308 | 1.04 |
| 4 | 1,640 | 10.6 | 37.3 |
| 5 | 464 | 3.46 | 16.6 |
| 8 | 39.6 | 0.168 | 0.249 |
| 9 | 24.0 | 0.001 | <0.001 |
| 10 | 21.6 | 0.090 | 0.171 |
| 11 | 146 | 1.59 | 3.84 |
| 12 | 42.8 | 0.563 | 1.58 |
Furthermore, the potency of extracts E1–13 directly correlated with the sum (Table S3, Supporting Information) of detected macamide abundance/IC50 ratios (Spearman’s rank correlation coefficient ρhuman sEH = 0.929 and ρmouse sEH = 0.887), signifying that the inhibitory potency towards sEH is likely a function of the concentrations and bioactivity of individual macamides present in the L. meyenii product.
Pharmacokinetics.
Based on a combination of strong inhibitory potency and abundance in L. meyenii samples (Table 3), compounds 4 and 5 were selected for in vivo studies. To assess oral bioavailability of 4 and 5, a pharmacokinetic study in rats was conducted to study their fate following oral administration of 100 mg/kg of each compound. A 96-h time-course of plasma concentrations of 4 and 5 was generated to assess key pharmacokinetic characteristics and is displayed in the Supporting Information (Figure S1).
Pharmacokinetic parameters (Table 4) indicated that the concentration of compound 4 peaked in the blood within 3 h and was around one order of magnitude greater than its rat sEH IC50 (Table 1). However, the pharmacokinetic profile of compound 5 was poorer than that of 4. It took double the time to reach its maximum blood concentration, which was nearly one order of magnitude smaller, relative to 4. The total body exposure to the macamides (i.e., AUC) was also approximately five times lower for 5, compared to 4. It is possible the additional olefin bond in 5 increases susceptibility to secondary metabolism, autoxidation, and allylic and bisallylic hydroxylation,41,42 decreasing in vivo stability.
Table 4.
Pharmacokinetic Parameters for a 100 mg/kg Oral Dose of Compounds 4 and 5a
| Compound | Cmax (nM) | Tmax (hours) | AUC (nM × hours) | t1/2 (hours) |
|---|---|---|---|---|
| 4 | 519 ± 149 | 3 | 3690 ± 664 | 9 |
| 5 | 54 ± 11 | 6 | 723 ± 230 | 14.6 |
Results are the mean ± SEM (n=4/group).
Analgesia in Inflammatory Pain Model.
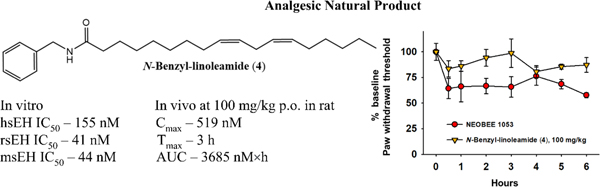
Compound 4 demonstrated antinociceptive effects in a lipopolysaccharide (LPS)-induced inflammatory pain model in rat. Baseline paw withdrawal thresholds were scored before administration of compounds and normalized to 100%. LPS results in lower than baseline scores, indicating a painful state. Oral administration of 4 (100 mg/kg) significantly increased paw withdrawal thresholds (interpreted as pain relief), relative to the vehicle control (Figure 2). Therapeutic effects persisted over a 6-h time-course, similar to activity of classic, synthetic sEHI against LPS induced allodynia.43 Effects of 5 were not significant (Figure S2, Supporting Information), probably due to its poor bioavailability.
Figure 2.
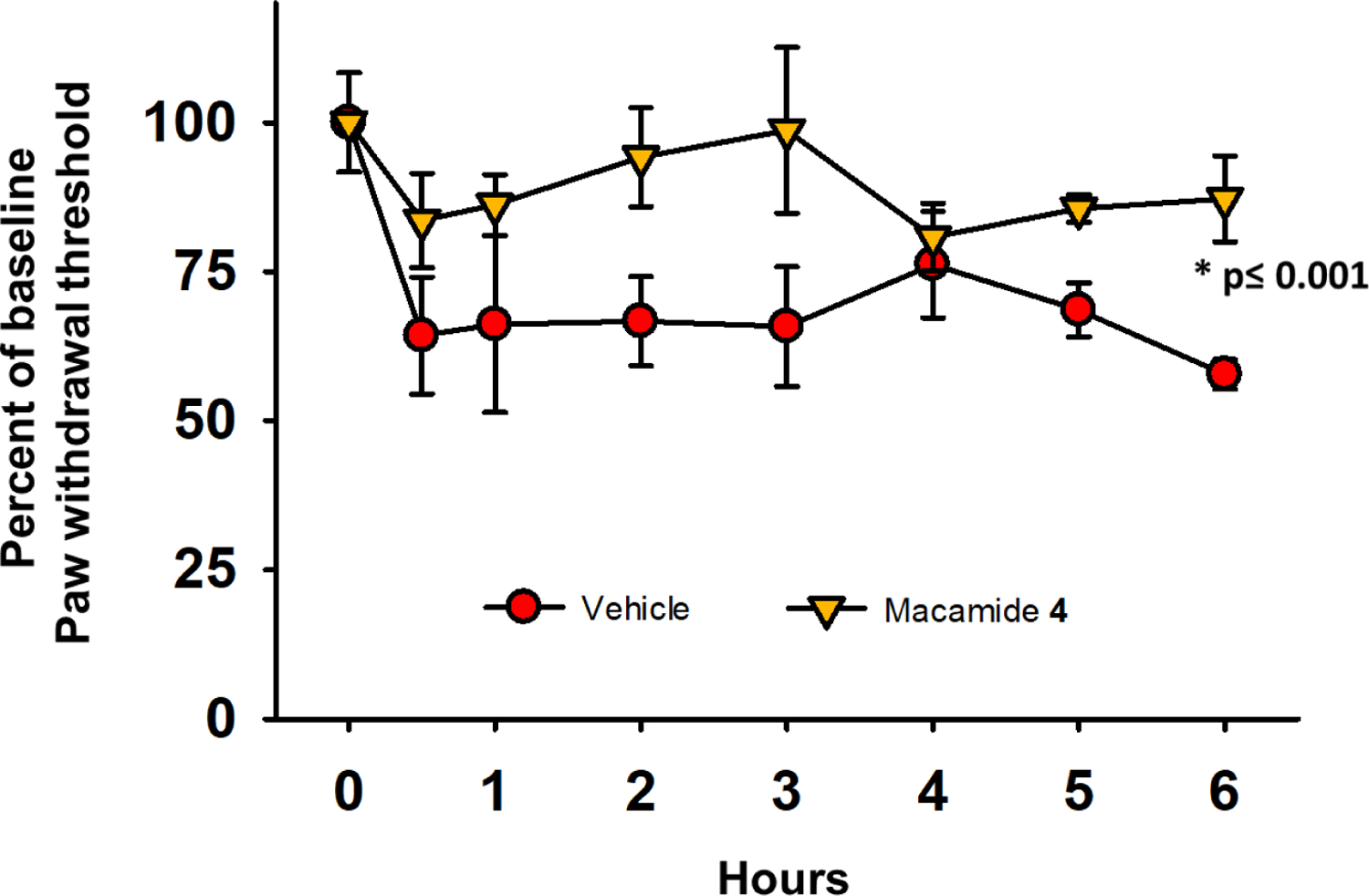
Macamide 4 (100 mg/kg) was efficacious against LPS-induced inflammatory pain in rat. Scores are the means ± SEM reported as percent of baseline (normalized to 100%), calculated as the score × 100/baseline score. The median value of efficacy was statistically significant compared to the vehicle control (NEOBEE 1053) over a 6-h time-course (Kruskal-Wallis One Way Analysis of Variance on Ranks, α=0.05, *p ≤ 0.001, n=4/group).
The discovery of macamides as sEHI can at least partially explain the vast majority of biological effects observed with L. meyenii extract or macamide treatments. Through restoration of mitochondrial health and attenuation of oxidative stress, sEHI have proven therapeutically promising using in vitro and in vivo models of Parkinson’s disease.44–46 sEHI have also demonstrated antidepressant effects in mice, through improved brain-derived neurotrophic factor signaling in a stress model.47 Mechanistically and/or functionally, the effects parallel macamide-mediated neuroprotection under these disease conditions.20–22 Similarly, protection against ROS underlies the efficacy of sEHI against seemingly distinct pathologies3 and mirrors macamide activity in dissimilar disease conditions tied together by oxidative stress.23–25 The significantly greater potency towards sEH, compared to FAAH (Table 1), also strengthens the case for sEH inhibition as the more prevalent biological mechanism of action for macamides. However, macamides could in fact act as dual inhibitors of sEH and FAAH, potentially leading to synergism of biological effects, such as analgesia,31 by facilitating both EpFA bioactivity and endocannabinoid-mediated agonism of cannabinoid (CB) receptors. The mixture of bioactive natural products in an L. meyenii extract could have further additive or synergistic effects and could provide another advantage over classic small-molecule therapeutics. Finally, macamides (such as compound 4) themselves selectively bind to the CB1 receptor,48 and hence might directly exert some of the observed analgesic effects. The cannabimimetic activity could help explain the recreational use of maca, though it suggests perhaps some caution should be exercised when considering L. meyenii for medicinal applications.
CONCLUSION
Nineteen potential macamides were synthesized, their inhibitory potency towards sEH was tested and their levels in commercial L. meyenii products were quantified. These compounds, several of which are known natural products, were found to be a novel and promising class of potent sEHI. N-Benzyl-linoleamide (4) is the most therapeutically significant macamide studied so far, based on a combination of its dominant abundance in maca products, in vitro potency, and in vivo efficacy. Two unsaturation sites in the fatty acid tail appear to provide an optimal balance between inhibitory potency and oral bioavailability, leading to significant analgesia in an inflammatory pain model. The therapeutic relevance of total macamides in maca products depends on the post-harvest treatment of L. meyenii, due to its significant influence on macamide biosynthesis. Certain samples contained levels that were nearly two orders of magnitude greater than those of sEHI found in previously identified dietary sources. Hence, careful selection and preparation of maca products is the key consideration in their potential application as nutraceuticals. In this study, analysis of macamides in L. meyenii products was conducted only using compounds with synthetic standards. However, it is probable other macamides exist and should be investigated in future studies. Current and new synthetic standards may facilitate efforts in breeding, post-harvest handling and other agronomic practices to increase the efficacy and concentration of macamides in commercial extracts.
EXPERIMENTAL SECTION
General Experimental Procedures.
Compound 1 and most reagents required for synthesis are commercially available and were purchased from one of the following commercial vendors: Nu-Chek Prep, Inc. (Elysian, MN), Sigma Aldrich Chemical Co. (Milwaukee, WI), Fisher Scientific (Houston, TX), Eanmine LLC (Monmouth Jct, NJ), Oakwood Chemical (Estill, SC), Chem-Impex Inc (Wood Dale, IL) or Combi-Blocks (San Diego, CA). All reactions were carried out in anhydrous solvents, under an atmosphere of nitrogen or argon and at room temperature. All chemicals purchased from commercial sources were used as received without further purification. NMR spectra were recorded on a 400 MHz Bruker Avance III HD Nanobay NMR spectrometer. Multiplicity is described by the abbreviations, b = broad, s = singlet, d = doublet, t = triplet, m = multiplet. Chemical shifts (δ) are expressed as ppm. 1H NMR spectra were referenced to the residual solvent peak at δ 7.26 (CDCl3) or 2.51 (DMSO-d6). 13C NMR spectra were referenced to the solvent peak at δ 77.16 (CDCl3) or 40.01 (DMSO-d6). HRESIMS were recorded on a Thermo Electron LTQ-Orbitrap XL Hybrid mass spectrometer, equipped with an electrospray ionization (ESI) source operating in the positive-ion mode. Analytical TLC was performed on Merck TLC silica gel 60 F254 plates and spots were revealed under 254 nm UV light or developed with a potassium permanganate stain. Flash chromatography was performed on silica gel (230–400 mesh) from Macherey Nagel. Commercial L. meyenii product extracts were analyzed via HPLC-MS/MS. An Agilent 1200 SL liquid chromatography series (Agilent Corporation, Palo Alto, CA, USA), utilizing a Kinetex C18 100 Å, LC 100 × 2.1 mm, 1.7 μm column was employed. It was coupled to a 4000 Q-Trap tandem mass spectrometer (Applied Biosystems Instrument Corp.), equipped with an ESI source (Turbo V) operating in the positive-ion mode. Multiple Reaction Monitoring (MRM) transitions were optimized via direct infusion of standards.
Chemistry.
General Synthetic Method for Compounds 2–19, as shown with the Representative N-Benzyloctadeca-9Z,12Z-dienamide (4). Linoleic acid (2.00 g, 7.13 mmol), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (1.71 g, 8.91 mmol) and 4-dimethylaminopyridine (catalytic amount) were stirred in dichloromethane (10 mL) for 15 min. Benzylamine (1.09 mL, 10.0 mmol) was added and the mixture was stirred overnight. After completion (confirmed by TLC), the crude mixture was concentrated under reduced pressure, redissolved in hexanes and purified by flash chromatography (ethyl acetate-hexanes 15:85 → 20:80). Yield 2.21g (84%).
N-Benzyloctadecanamide (2):
Yield 29%; white powder, mp 89–91 °C; 1H NMR (400 MHz, CDCl3) δ 7.38–7.29 (5H, m, aromatic), 5.70 (1H, bs, NH), 4.47 (2H, d, J = 5.6 Hz, benzylic), 2.23 (2H, t, J = 7.6 Hz, α-H), 1.72–1.64 (2H, m, β-H), 1.34–1.27 (28H, m, CH2), 0.90 (3H, t, J = 6.8 Hz, CH3); 13C NMR (100 MHz, CDCl3) δ 173.00 (CC(=O)N), 138.46 (C), 128.71 (CH), 127.90 (CH), 127.84 (CH), 43.59 (CH2), 36.84 (CH2), 31.94 (CH2), 29.72 (CH2), 29.70 (CH2), 29.67 (CH2), 29.63 (CH2), 29.52 (CH2), 29.38 (CH2), 29.37 (CH2), 29.34 (CH2), 25.80 (CH2), 22.71 (CH2), 14.14 (CH3); MS/MS MRM transition (Q1 → Q3) m/z 374.30 [M + H]+ → 91.00 [benzylium].
N-Benzyloctadec-9Z-enamide (3):
Yield 90%; white powder, mp 48–50 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.28 (1H, t, J = 6.0 Hz, NH), 7.33–7.21 (5H, m, aromatic), 5.37–5.29 (2H, m, vinylic), 4.25 (2H, d, J = 6.4 Hz, benzylic), 2.12 (2H, t, J = 7.6 Hz, α-H), 2.01–1.97 (4H, m, allylic), 1.53–1.50 (2H, m, β-H), 1.32–1.25 (20H, m, CH2), 0.86 (3H, t, J = 7.2 Hz, CH3); 13C NMR (100 MHz, DMSO-d6) δ 172.54 (CC(=O)N), 140.26 (C), 130.11 (CH), 128.68 (CH), 127.61 (CH), 127.12 (CH), 42.41 (CH2), 35.80 (CH2), 31.75 (CH2), 29.57 (CH2), 29.30 (CH2), 29.16 (CH2), 29.13 (CH2), 29.11 (CH2), 29.06 (CH2), 29.03 (CH2), 27.08 (CH2), 27.05 (CH2), 25.78 (CH2), 22.56 (CH2), 14.42 (CH3); MS/MS MRM transition (Q1 → Q3) m/z 372.30 [M + H]+ → 91.10 [benzylium].
N-Benzyloctadeca-9Z,12Z-dienamide (4):
Yield 84%; white powder, mp 30–32 °C; 1H NMR (400 MHz, CDCl3) δ 7.38–7.30 (5H, m, aromatic), 5.74 (1H, bs, NH), 5.44–5.32 (4H, m, vinylic), 4.47 (2H, d, J = 6.0 Hz, benzylic), 2.80 (2H, t, J = 6.4 Hz, bisallylic), 2.23 (2H, t, J = 7.2 Hz, α-H), 2.10–2.04 (4H, m, allylic), 1.72–1.64 (2H, m, β-H), 1.40–1.33 (14H, m, CH2), 0.92 (3H, t, J = 6.4 Hz, CH3); 13C NMR (100 MHz, CDCl3) δ 172.92 (CC(=O)N), 138.40 (C), 130.21 (CH), 130.03 (CH), 128.69 (CH), 128.02 (CH), 127.88 (CH), 127.81 (CH), 127.48 (CH), 43.96 (CH2), 36.78 (CH2), 31.90 (CH2), 29.98 (CH2), 29.33 (CH2), 29.27 (CH2), 29.24 (CH2), 29.12 (CH2), 27.18 (CH2), 25.74 (CH2), 25.60 (CH2), 22.95 (CH2), 14.07 (CH3); HREIMS m/z 370.3117 [M + H]+ (calcd for C25H40NO+, 370.3104); MS/MS MRM transition (Q1 → Q3) m/z 370.30 [M + H]+ → 91.00 [benzylium].
N-Benzyloctadeca-9Z,12Z,15Z-trienamide (5):
Yield 83%; colorless oil; 1H NMR (400 MHz, CDCl3) δ 7.36–7.29 (5H, m, aromatic), 5.73 (1H, bs, NH), 5.45–5.31 (6H, m, vinylic), 4.46 (2H, d, J = 5.2 Hz, benzylic), 2.83 (4H, t, J = 6.4 Hz, bisallylic), 2.22 (2H, t, J = 7.6 Hz, α-H), 2.12–2.05 (4H, m, allylic), 1.72–1.68 (2H, m, β-H), 1.39–1.33 (8H, m, CH2), 1.00 (3H, t, J = 7.2 Hz, CH3); 13C NMR (100 MHz, CDCl3) δ 173.20 (CC(=O)N), 138.61 (C), 131.96 (CH), 130.26 (CH), 128.80 (CH), 128.30 (CH), 128.26 (CH), 127.75 (CH), 127.70 (CH), 127.33 (CH), 127.14 (CH), 43.43 (CH2), 36.65 (CH2), 29.62 (CH2), 29.33 (CH2), 29.31 (CH2), 29.19 (CH2), 27.24 (CH2), 25.82 (CH2), 25.65 (CH2), 25.56 (CH2), 20.58 (CH2), 14.32 (CH3); HREIMS m/z 368.2958 [M + H]+ (calcd for C25H38NO+, 368.2948); MS/MS MRM transition (Q1 → Q3) m/z 368.30 [M + H]+ → 108.10 [benzylaminium].
N-Benzylicosa-5Z,8Z,11Z,14Z-tetraenamide (6):
Yield 91%; colorless oil; 1H NMR (400 MHz, CDCl3) δ 7.34–7.25 (5H, m, aromatic), 6.38 (1H, bs, NH), 5.45–5.33 (8H, m, vinylic), 4.39 (2H, d, J = 6.0 Hz, benzylic), 2.87–2.79 (6H, m, bisallylic), 2.23 (2H, t, J = 7.6 Hz, α-H), 2.14–2.05 (4H, m, allylic), 1.77–1.69 (2H, m, β-H), 1.42–1.29 (6H, m, CH2), 0.92 (3H, t, J = 7.2 Hz, CH3); 13C NMR (100 MHz, CDCl3) δ 172.72 (CC(=O)N), 138.46 (C), 130.53 (CH), 129.13 (CH), 128.71 (CH), 128.22 (CH), 127.84 (CH), 127.52 (CH), 43.60 (CH2), 36.06 (CH2), 34.68 (CH2), 31.61 (CH2), 29.36 (CH2), 29.09 (CH2), 27.26 (CH2), 26.73 (CH2), 25.66 (CH2), 22.66 (CH2), 20.69 (CH2), 14.12 (CH3); MS/MS MRM transition (Q1 → Q3) m/z 394.30 [M + H]+ → 91.00 [benzylium].
N-Benzylicosa-5Z,8Z,11Z,14Z,17Z-pentaenamide (7):
Yield 90%; colorless oil; 1H NMR (400 MHz, CDCl3) δ 7.33–7.24 (5H, m, aromatic), 6.41 (1H, bs, NH), 5.43–5.30 (10H, m, vinylic), 4.39 (2H, d, J = 5.6 Hz, benzylic), 2.89–2.79 (8H, m, bisallylic), 2.21 (2H, t, J = 7.6 Hz, α-H), 2.13–2.06 (4H, m, allylic), 1.76–1.69 (2H, m, β-H), 0.99 (3H, t, J = 7.6 Hz, CH3); 13C NMR (100 MHz, CDCl3) δ 172.91 (CC(=O)N), 138.55 (C), 132.04 (CH), 129.19 (CH), 128.69 (CH), 128.61 (CH), 128.60 (CH), 128.28 (CH), 128.26 (CH), 128.15 (CH), 128.11 (CH), 127.88 (CH), 127.70 (CH), 127.36 (CH), 127.04 (CH), 43.46 (CH2), 35.94 (CH2), 26.74 (CH2), 25.67 (CH2), 25.60 (CH2), 25.58 (CH2), 20.60 (CH2), 14.32 (CH3); MS/MS MRM transition (Q1 → Q3) m/z 392.30 [M + H]+ → 91.00 [benzylium].
N-(3-Methoxybenzyl)hexadecanamide (8):
Yield 36%; white powder, mp 60–62 °C; 1H NMR (400 MHz, CDCl3) δ 7.27–7.25 (1H, m, aromatic), 6.89 (1H, d, J = 7.6 Hz, aromatic), 6.84–6.83 (2H, m, aromatic), 5.75 (1H, bs, NH), 4.45 (2H, d, J = 5.6 Hz, benzylic), 3.82 (3H, s, H-C-O), 2.25 (2H, t, J = 7.2 Hz, α-H), 1.71–1.66 (2H, m, β-H), 1.34–1.28 (24H, m, CH2), 0.92 (3H, t, J = 6.8 Hz, CH3); 13C NMR (100 MHz, CDCl3) δ 172.97 (CC(=O)N), 159.92 (CC(=C)O), 140.01 (C), 129.76 (CH), 120.04 (CH), 113.39 (CH), 112.99 (CH), 55.25 (O-CH3), 43.57 (CH2), 36.87 (CH2), 31.94 (CH2), 29.71 (CH2), 29.67 (CH2), 29.62 (CH2), 29.51 (CH2), 29.37 (CH2), 29.34 (CH2), 25.79 (CH2), 22.70 (CH2), 14.13 (CH3); MS/MS MRM transition (Q1 → Q3) m/z 376.30 [M + H]+ → 121.00 [3-methoxybenzylium].
N-(3-Methoxybenzyl)octadecanamide (9):
Yield 83%; white powder, mp 77–79 °C; 1H NMR (400 MHz, CDCl3) δ 7.22–7.18 (1H, m, aromatic), 6.83 (1H, d, J = 7.6 Hz, aromatic), 6.79–6.76 (2H, m, aromatic), 6.41 (1H, bs, NH), 4.34 (2H, d, J = 5.6 Hz, benzylic), 3.76 (3H, s, H-C-O), 2.18 (2H, t, J = 7.6 Hz, α-H), 1.66–1.58 (2H, m, β-H), 1.31–1.26 (28H, m, CH2), 0.89 (3H, t, J = 7.2 Hz, CH3); 13C NMR (100 MHz, CDCl3) δ 173.25 (CC(=O)N), 159.82 (CC(=C)O), 140.22 (C), 129.98 (CH), 119.88 (CH), 113.25 (CH), 112.72 (CH), 55.10 (O-CH3), 43.35 (CH2), 36.66 (CH2), 31.95 (CH2), 29.74 (CH2), 29.72 (CH2), 29.71 (CH2), 29.69 (CH2), 29.67 (CH2), 29.57 (CH2), 29.43 (CH2), 29.39 (CH2), 25.86 (CH2), 22.71 (CH2), 14.13 (CH3); MS/MS MRM transition (Q1 → Q3) m/z 404.30 [M + H]+ → 121.00 [3-methoxybenzylium].
N-(3-Methoxybenzyl)octadec-9Z-enamide (10):
Yield 98%; colorless oil; 1H NMR (400 MHz, CDCl3) δ 7.27–7.24 (1H, m, aromatic), 6.88 (1H, d, J = 7.2 Hz, aromatic), 6.84–6.82 (2H, m, aromatic), 5.76 (1H, bs, NH), 5.41–5.32 (2H, m, vinylic), 4.44 (2H, d, J = 5.6 Hz, benzylic), 3.82 (3H, s, H-C-O), 2.23 (2H, t, J = 7.2 Hz, α-H), 2.05–2.00 (4H, m, allylic), 1.69–1.64 (2H, m, β-H), 1.36–1.29 (20H, m, CH2), 0.90 (3H, t, J = 6.8Hz, CH3); 13C NMR (100 MHz, CDCl3) δ 172.95 (CC(=O)N), 159.91 (CC(=C)O), 140.03 (C), 130.01 (CH), 129.75 (CH), 120.02 (CH), 113.39 (CH), 112.95 (CH), 55.23 (O-CH3), 43.55 (CH2), 36.82 (CH2), 31.92 (CH2), 29.78 (CH2), 29.72 (CH2), 29.54 (CH2), 29.33 (CH2), 29.32 (CH2), 29.28 (CH2), 29.16 (CH2), 27.24 (CH2), 27.19 (CH2), 25.78 (CH2), 22.70 (CH2), 14.13 (CH3); MS/MS MRM transition (Q1 → Q3) m/z 402.30 [M + H]+ → 121.00 [3-methoxybenzylium].
N-(3-Methoxybenzyl)octadeca-9Z,12Z-dienamide (11):
Yield 39%; colorless oil; 1H NMR (400 MHz, CDCl3) δ 7.28–7.24 (1H, m, aromatic), 6.89 (1H, d, J = 7.6 Hz, aromatic), 6.84–6.82 (2H, m, aromatic), 5.75 (1H, bs, NH), 5.44–5.31 (4H, m, vinylic), 4.43 (2H, d, J = 5.6 Hz, benzylic), 3.82 (3H, s, H-C-O), 2.80 (2H, t, J = 6.8 Hz, bisallylic), 2.23 (2H, t, J = 7.6 Hz, α-H), 2.09–2.04 (4H, m, allylic), 1.69–1.66 (2H, m, β-H), 1.42–1.28 (14H, m, CH2), 0.91 (3H, t, J = 6.8 Hz, CH3); 13C NMR (100 MHz, CDCl3) δ 172.91(CC(=O)N), 159.91 (CC(=C)O), 140.01 (C), 130.24 (CH), 130.06 (CH), 129.76 (CH), 128.06 (CH), 127.91 (CH), 120.03 (CH), 113.41 (CH), 112.96 (CH), 55.24 (O-CH3), 43.56 (CH2), 36.82 (CH2), 31.54 (CH2), 29.72 (CH2), 29.62 (CH2), 29.36 (CH2), 29.31 (CH2), 29.27 (CH2), 29.16 (CH2), 27.21 (CH2), 25.77 (CH2), 25.64 (CH2), 22.53 (CH2), 14.09 (CH3); MS/MS MRM transition (Q1 → Q3) m/z 400.30 [M + H]+ → 121.00 [3-methoxybenzylium].
N-(3-Methoxybenzyl)octadeca-9Z,12Z,15Z-trienamide (12):
Yield 71%; colorless oil; 1H NMR (400 MHz, CDCl3) δ 7.28–7.24 (1H, m, aromatic), 6.89 (1H, d, J = 7.2 Hz, aromatic), 6.84–6.82 (2H, m, aromatic), 5.77 (1H, bs, NH), 5.45–5.30 (6H, m, vinylic), 4.44 (2H, d, J = 5.6 Hz, benzylic), 3.82 (3H, s, H-C-O), 2.82 (4H, t, J = 6.0 Hz, bisallylic), 2.22 (2H, t, J = 7.6 Hz, α-H), 2.11–2.04 (4H, m, allylic), 1.69–1.66 (2H, m, β-H), 1.38–1.28 (8H, m, CH2), 1.00 (3H, t, J = 7.6 Hz, CH3); 13C NMR (100 MHz, CDCl3) δ 172.95 (CC(=O)N), 159.91 (CC(=C)O), 140.02 (C), 131.98 (CH), 130.28 (CH), 129.75 (CH), 128.30 (CH), 128.27 (CH), 127.74 (CH), 127.13 (CH), 120.02 (CH), 113.40 (CH), 112.94 (CH), 55.24 (O-CH3), 43.55 (CH2), 36.81 (CH2), 29.71 (CH2), 29.59 (CH2), 29.31(CH2), 29.27 (CH2), 29.15 (CH2), 27.22 (CH2), 25.77 (CH2), 25.63 (CH2), 25.54 (CH2), 20.57 (CH2), 14.29 (CH3); MS/MS MRM transition (Q1 → Q3) m/z 398.30 [M + H]+ → 121.00 [3-methoxybenzylium].
N-(3-Methoxybenzyl)icosa-5Z,8Z,11Z,14Z-tetraenamide (13):
Yield 90%; colorless oil; 1H NMR (400 MHz, CDCl3) δ 7.28–7.24 (1H, m, aromatic), 6.88 (1H, d, J = 7.2 Hz, aromatic), 6.84–6.83 (2H, m, aromatic), 5.73 (1H, bs, NH), 5.45–5.34 (8H, m, vinylic), 4.43 (2H, d, J = 6.0 Hz, benzylic), 3.82 (3H, s, H-C-O), 2.86–2.80 (6H, m, bisallylic), 2.24 (2H, t, J = 7.6 Hz, α-H), 2.17–2.04 (4H, m, allylic), 1.81–1.73 (2H, m, β-H), 1.45–1.28 (6H, m, CH2), 0.90 (3H, t, J = 6.8 Hz, CH3); 13C NMR (100 MHz, CDCl3) δ 172.68 (CC(=O)N), 159.91 (CC(=C)O), 139.90 (C), 130.54 (CH), 129.78 (CH), 129.08 (CH), 128.83 (CH), 128.25 (CH), 128.16 (CH), 127.87 (CH), 127.53 (CH), 120.08 (CH), 120.04 (CH), 113.47 (CH), 112.96 (CH), 55.24 (O-CH3), 43.60 (CH2), 36.10 (CH2), 31.52 (CH2), 29.33 (CH2), 27.23 (CH2), 26.70 (CH2), 25.65 (CH2), 25.54 (CH2), 22.58 (CH2), 14.08 (CH3); MS/MS MRM transition (Q1 → Q3) m/z 424.30 [M + H]+ → 121.00 [3-methoxybenzylium].
N-(3-Methoxybenzyl)icosa-5Z,8Z,11Z,14Z,17Z-pentaenamide (14):
Yield 97%; colorless oil; 1H NMR (400 MHz, CDCl3) δ 7.28–7.24 (1H, m, aromatic), 6.86 (1H, d, J = 7.2 Hz, aromatic), 6.83–6.81 (2H, m, aromatic), 5.78 (1H, bs, NH), 5.45–5.31 (10H, m, vinylic), 4.42 (2H, d, J = 5.6 Hz, benzylic), 3.81 (3H, s, H-C-O), 2.87–2.80 (8H, m, bisallylic), 2.24 (2H, t, J = 7.6 Hz, α-H), 2.17–2.05 (4H, m, allylic), 1.80–1.72 (2H, m, β-H), 0.99 (3H, t, J = 7.2 Hz, CH3); 13C NMR (100 MHz, CDCl3) δ 172.72 (CC(=O)N), 159.90 (CC(=C)O), 139.90 (C), 132.07 (CH), 129.77 (CH), 129.10 (CH), 129.04 (CH), 128.80 (CH), 128.60 (CH), 128.29 (CH), 128.22 (CH), 128.10 (CH), 127.87 (CH), 127.01 (CH), 120.04 (CH), 113.47 (CH), 112.93 (CH), 55.24 (O-CH3), 43.59 (CH2), 36.09 (CH2), 29.71 (CH2), 26.70 (CH2), 25.64 (CH2), 25.55 (CH2), 25.54 (CH2), 20.57 (CH2), 14.28 (CH3); MS/MS MRM transition (Q1 → Q3) m/z 422.30 [M + H]+ → 121.00 [3-methoxybenzylium].
N-(3,4-Dimethoxybenzyl)hexadecanamide (15):
Yield 33%; white, amorphous solid; 1H NMR (400 MHz, CDCl3) δ 6.84 (3H, bs, aromatic), 5.66 (1H, bs, NH), 4.41 (2H, d, J = 6.0 Hz, benzylic), 3.89 (6H, s, H-C-O), 2.22 (2H, t, J = 7.6 Hz, α-H), 1.56–1.51 (2H, m, β-H), 1.34–1.31 (24H, m, CH2), 0.86 (3H, t, J = 6.8 Hz, CH3); 13C NMR (100 MHz, CDCl3) δ 172.90 (CC(=O)N), 149.20 (CC(=C)O), 148.51 (CC(=C)O), 131.07 (C), 120.11 (CH), 111.23 (CH), 111.18 (CH), 55.96 (O-CH3), 55.89 (O-CH3), 43.45 (CH2), 36.09 (CH2), 31.65 (CH2), 29.71 (CH2), 29.69 (CH2), 29.67 (CH2), 29.63 (CH2), 29.51 (CH2), 29.37 (CH2), 29.35 (CH2), 29.07 (CH2), 25.35 (CH2), 22.66 (CH2), 14.21 (CH3); MS/MS MRM transition (Q1 → Q3) m/z 406.30 [M + H]+ → 151.10 [3,4-dimethoxybenzylium].
N-(3,4-Dimethoxybenzyl)octadeca-9Z,12Z-dienamide (16):
Yield 85%; white, amorphous solid; 1H NMR (400 MHz, CDCl3) δ 6.84 (3H, bs, aromatic), 5.68 (1H, bs, NH), 5.39–5.36 (4H, m, vinylic), 4.41 (2H, d, J = 6.0 Hz, benzylic), 3.89 (6H, s, H-C-O), 2.79 (2H, t, J = 6.4 Hz, bisallylic), 2.22 (2H, t, J = 7.8 Hz, α-H), 2.09–2.04 (4H, m, allylic), 1.79–1.72 (2H, m, β-H), 1.39–1.27 (14H, m, CH2), 0.92 (3H, t, J = 6.4 Hz, CH3); 13C NMR (100 MHz, CDCl3) δ 172.84 (CC(=O)N), 149.20 (CC(=C)O), 148.51 (CC(=C)O), 131.08 (C), 130.23 (CH), 130.01 (CH), 128.07 (CH), 127.90 (CH), 120.10 (CH), 111.24 (CH), 111.18 (CH), 55.93 (O-CH3), 55.87 (O-CH3), 43.45 (CH2), 36.86 (CH2), 31.43 (CH2), 29.62 (CH2), 29.15 (CH2), 29.07 (CH2), 27.28 (CH2), 27.21 (CH2), 25.76 (CH2), 25.28 (CH2), 22.66 (CH2), 14.10 (CH3); HREIMS m/z 430.3324 [M + H]+ (calcd for C27H44NO3+, 430.3316); MS/MS MRM transition (Q1 → Q3) m/z 430.30 [M + H]+ → 151.10 [3,4-dimethoxybenzylium].
N-(3,4-Dimethoxybenzyl)octadeca-9Z,12Z,15Z-trienamide (17):
Yield 87%; white, amorphous solid; 1H NMR (400 MHz, CDCl3) δ 6.84 (3H, bs, aromatic), 5.66 (1H, bs, NH), 5.42–5.33 (6H, m, vinylic), 4.41 (2H, d, J = 5.6 Hz, benzylic), 3.89 (6H, s, H-C-O), 2.83 (4H, t, J = 6.8 Hz, bisallylic), 2.22 (2H, t, J = 7.6 Hz, α-H), 2.12–2.05 (4H, m, allylic), 1.69–1.62 (2H, m, β-H), 1.40–1.32 (8H, m, CH2), 1.00 (3H, t, J = 7.2 Hz, CH3); 13C NMR (100 MHz, CDCl3) δ 172.83 (CC(=O)N), 149.20 (CC(=C)O), 148.52 (CC(=C)O), 131.98 (C), 131.06 (CH), 130.24 (CH), 128.31 (CH), 128.25 (CH), 127.76 (CH), 127.12 (CH), 120.11 (CH), 111.25 (CH), 111.18 (CH), 55.95 (O-CH3), 55.89 (O-CH3), 43.36 (CH2), 36.87 (CH2), 31.60 (CH2), 29.59 (CH2), 29.27 (CH2), 29.14 (CH2), 29.07 (CH2), 27.68 (CH2), 25.78 (CH2), 25.35 (CH2), 22.66 (CH2), 14.31 (CH3); HREIMS m/z 428.3165 [M + H]+ (calcd for C27H42NO3+, 428.3159); MS/MS MRM transition (Q1 → Q3) m/z 428.30 [M + H]+ → 151.10 [3,4-dimethoxybenzylium].
N-Phenethyloctadeca-9Z,12Z-dienamide (18):
Yield 82%; white powder, mp 31–33 °C; 1H NMR (400 MHz, CDCl3) δ 7.36–7.21 (5H, m, aromatic), 5.71 (1H, bs, NH), 5.44–5.32 (4H, m, vinylic), 3.57–3.52 (2H, m, H-C-N), 2.86–2.78 (4H, m, bisallylic, benzylic), 2.15–2.04 (6H, m, allylic, α-H), 1.63–1.59 (2H, m, β-H), 1.41–1.28 (14H, m, CH2), 0.91 (3H, t, J = 7.2 Hz, CH3); 13C NMR (100 MHz, CDCl3) δ 173.07 (CC(=O)N), 138.96 (C), 130.25 (CH), 130.07 (CH), 128.79 (CH), 128.65 (CH), 128.06 (CH), 127.92 (CH), 126.52 (CH), 40.48 (CH2), 36.86 (CH2), 35.74 (CH2), 31.54 (CH2), 29.63 (CH2), 29.36 (CH2), 29.27 (CH2), 29.25 (CH2), 29.15 (CH2), 27.22 (CH2), 25.74 (CH2), 25.65 (CH2), 22.59 (CH2), 14.09 (CH3); MS/MS MRM transition (Q1 → Q3) m/z 384.30 [M + H]+ → 105.10 [1-phenylethan-1-ylium].
N-Phenethyloctadeca-9Z,12Z,15Z-trienamide (19):
Yield 88%; colorless oil; 1H NMR (400 MHz, CDCl3) δ 7.35–7.20 (5H, m, aromatic), 5.73 (1H, bs, NH), 5.48–5.30 (6H, m, vinylic), 3.57–3.52 (2H, m, H-C-N), 2.85–2.81 (6H, m, bisallylic, benzylic), 2.15–2.05 (6H, m, allylic, α-H), 1.64–1.57 (2H, m, β-H), 1.39–1.28 (8H, m, CH2), 1.00 (3H, t, J = 7.8 Hz, CH3); 13C NMR (100 MHz, CDCl3) δ 173.09 (CC(=O)N), 138.97 (C), 131.98 (CH), 130.28 (CH), 128.78 (CH), 128.64 (CH), 128.30 (CH), 128.26 (CH), 127.74 (CH), 127.13 (CH), 126.51 (CH), 40.50 (CH2), 36.84 (CH2), 35.74 (CH2), 29.72 (CH2), 29.61 (CH2), 29.25 (CH2), 29.14 (CH2), 27.22 (CH2), 25.74 (CH2), 25.63 (CH2), 25.55 (CH2), 20.57 (CH2), 14.29 (CH3); HREIMS m/z 382.3115 [M + H]+ (calcd for C26H40NO+, 382.3104); MS/MS MRM transition (Q1 → Q3) m/z 382.30 [M + H]+ → 105.10 [1-phenylethan-1-ylium].
sEH Inhibition Assay.
The assay was performed as previously described.49 All IC50 values for recombinant human, mouse, and rat sEHs were determined by a fluorescence-based assay system in a 96-well, serial dilution format. Non-fluorescent cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl] carbonate (MNPC) was used as the assay substrate at a final concentration of 5 μM. MNPC is hydrolyzed by sEH to the fluorescent 6-methoxynaphthaldehyde. Formation of the product was measured by a Molecular Devices M-2 plate reader (λex = 330 nm, λem = 465 nm). All measurements were performed in triplicate and the means are reported. t-TUCB, a classic sEHI, was run in parallel and the obtained IC50 values were corroborated with reported literature values,43 to validate the experimental results (Table S4, Supporting Information).
Extraction and Analysis of Macamides from L. meyenii Products.
Products 1–7 (Table S1, Supporting Information) were first homogenized via mortar and pestle grinding. For products 1–12 (Table S1, Supporting Information), 1 g of L. meyenii root powder was extracted with a 40 mL mixture of hexanes-ethanol (7:1) at 50 °C, under ultra-sonication for 15 min. Each suspension was filtered, evaporated under reduced pressure, and reconstituted in 5 mL of ethyl acetate. Aliquots were diluted in HPLC-grade methanol and filtered through a 0.22 μm filter prior to analysis. Product 13 (Table S1, Supporting Information) was directly diluted, filtered, and analyzed. Other solvent and extraction systems were also tested, and normalized extraction efficiencies are described in the Supporting Information (Table S5). Maca extract samples were placed in an autosampler, and 10 μL aliquots were injected on the HPLC column and macamide concentrations were quantified by HPLC-MS/MS analysis using standard curves and dilution factors. The HPLC trace for the mixture of macamides detected in the extract of Product 2 is displayed in the Supporting Information (Figure S3).
Pharmacology.
All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee (IACUC) of University of California, Davis (approval nos. 21204 for Pharmacokinetics and 21509 for Inflammatory Pain Model to Prof. Bruce D. Hammock).
Pharmacokinetics.
Male Sprague−Dawley (SD) rats (n=4/group, 8 weeks old, 250–300 g), purchased from Charles River Laboratories, were used in the pharmacokinetic study of compounds 4 and 5. Each compound (100 mg/kg) was dissolved in 100% NEOBEE 1053 and administered via oral gavage. Whole blood (10 μL) was collected with a pipet from the tail vein, punctured by a lancet at 0, 0.25, 0.5, 1, 2, 4, 8, 12, 24, 48, 72 and 96 h after dosing. Each blood sample was immediately transferred to a tube containing 100 μL of water with 0.1% EDTA, vortexed and stored at −80 °C until analysis. According to a previously reported method,50 the blood samples were processed, and compound concentrations were determined.
Inflammatory Pain Model.
A von Frey assay measuring mechanical allodynia was performed, as previously described,51 in male SD rats (n=4/group). The study was conducted in a randomized and blinded manner. Mechanical withdrawal thresholds (MWT) were determined before dosing to establish a baseline score. Then, 1 mL of 100% NEOBEE 1053 vehicle, 100 mg/kg compound 4 or 100 mg/kg compound 5 was oral gavaged. Immediately following oral gavage, 50 μL of a 0.2 μg/mL solution of LPS in saline were injected intraplanarly in a hind (ipsilateral) paw. The rats were then assessed for MWT over a 6-h time-course. The ipsilateral MWT were measured three-five times per rat per time point, and scores are reported as the means of a group of rats.
Supplementary Material
ACKNOWLEDGMENTS
This study was partially supported by National Institutes of Health grants, National Institute of Environmental Health Sciences (NIEHS) RIVER Award (R35 ES030443–01), NIEHS/Superfund Research Program (P42 ES004699), and National Institute of Neurological Disorders and Stroke (R01 DK107767).
Footnotes
ASSOCIATED CONTENT
Supporting Information. Descriptions of thirteen commercial L. meyenii products, normalized extraction efficiencies, analytical method limits of quantitation, sums of abundance/IC50 ratios, IC50s of in vitro positive control, blood concentration profiles of compounds 4 and 5, analgesic efficacy of 5, HPLC trace of commercial L. meyenii product extract (E2) and 1H and 13C NMR spectra for new compounds (16, 17, and 19).
REFERENCES
- (1).Imig JD; Hammock BD Nat. Rev. Drug Discov 2009, 8, 794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Morisseau C; Hammock BD Annu. Rev. Pharmacol. Toxicol 2013, 53, 37–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Inceoglu B; Bettaieb A; Haj FG; Gomes AV; Hammock BD Prostaglandins Other Lipid Mediat 2017, 133, 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Wagner KM; McReynolds CB; Schmidt WK; Hammock BD Pharmacol. Ther 2017, 180, 62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Jo AR; Kim JH; Yan X-T; Yang SY; Kim YH J. Enzyme Inhib. Med. Chem 2016, 31, 70–78. [DOI] [PubMed] [Google Scholar]
- (6).Thao NP; Luyen BTT; Lee JS; Kim JH; Kim YH Bioorg. Med. Chem. Lett 2017, 27, 1874–1879. [DOI] [PubMed] [Google Scholar]
- (7).Thao NP; Kim JH; Thuy Luyen BT; Dat NT; Kim YH Int. J. Biol. Macromol 2017, 98, 526–534. [DOI] [PubMed] [Google Scholar]
- (8).Kim JH; Jo YD; Kim HY; Kim BR; Nam B Comput. Struct. Biotechnol. J 2018, 16, 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Kim JH; Jo YD; Jin CH Int. J. Biol. Macromol 2019, 135, 1202–1207. [DOI] [PubMed] [Google Scholar]
- (10).He X; Zhao WY; Shao B; Zhang BJ; Liu TT; Sun CP; Huang HL; Wu JR; Liang JH; Ma XC Int. J. Biol. Macromol 2020, 158, 1362–1368. [DOI] [PubMed] [Google Scholar]
- (11).Sun C-P; Zhang J; Zhao W-Y; Yi J; Yan J-K; Wang Y-L; Morisseau C; Liu Z-B; Hammock BD; Ma X-C Bioorg. Chem 2020, 96, 103637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Xuan Duy L; Le Ba V; Gao D; Hoang VD; Quoc Toan T; Yang SY; Duy Quang D; Kim YH; Cuong NM Nat. Prod. Res 2020, 1–6. (DOI: 10.1080/14786419.2020.1774759) [DOI] [PubMed] [Google Scholar]
- (13).Kitamura S; Morisseau C; Inceoglu B; Kamita SG; De Nicola GR; Nyegue M; Hammock BD PloS One 2015, 10, e0117438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Kitamura S; Morisseau C; Harris TR; Inceoglu B; Hammock BD PloS One 2017, 12, e0176571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Shin BC; Lee MS; Yang EJ; Lim HS; Ernst E BMC Complement Altern. Med 2010, 10, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Gonzales GF Evid. -Based Complement. Altern. Med 2011, 2012, 193496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Dording CM; Schettler PJ; Dalton ED; Parkin SR; Walker RS; Fehling KB; Fava M; Mischoulon D Evid. -Based Complement. Altern. Med 2015, 2015, 949036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Piacente S; Carbone V; Plaza A; Zampelli A; Pizza CJ Agric. Food Chem 2002, 50, 5621–5625. [DOI] [PubMed] [Google Scholar]
- (19).Yi F; Tan X.-l.; Yan X; Liu H-B Chinese Med 2016, 11, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Ai Z; Cheng AF; Yu YT; Yu LJ; Jin WJ Med. Food 2014, 17, 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Zhou Y; Li P; Brantner A; Wang H; Shu X; Yang J; Si N; Han L; Zhao H; Bian B Sci. Rep 2017, 7, 44660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Gugnani KS; Vu N; Rondón-Ortiz AN; Böhlke M; Maher TJ; Pino-Figueroa AJ Toxicol. Appl. Pharmacol 2018, 340, 67–76. [DOI] [PubMed] [Google Scholar]
- (23).Lin L; Huang J; Sun-Waterhouse D; Zhao M; Zhao K; Que J Int. J. Food Sci. Technol 2018, 53, 304–312. [Google Scholar]
- (24).Yang Q; Jin W; Lv X; Dai P; Ao Y; Wu M; Deng W; Yu L Pharm. Biol 2016, 54, 827–834. [DOI] [PubMed] [Google Scholar]
- (25).Qiu C; Zhu T; Lan L; Zeng Q; Du Z Braz. Arch. Biol. Technol 2016, 59, e16150462. [Google Scholar]
- (26).Zhang Y; Zhou F; Ge F BMC Complement. Altern. Med 2019, 19, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Wu H; Kelley CJ; Pino-Figueroa A; Vu HD; Maher TJ Bioorg. Med. Chem 2013, 21, 5188–5197. [DOI] [PubMed] [Google Scholar]
- (28).Alasmari M; Bӧhlke M; Kelley C; Maher T; Pino-Figueroa A Mol. Neurobiol 2019, 56, 1770–1781. [DOI] [PubMed] [Google Scholar]
- (29).Deutsch DG; Ueda N; Yamamoto S Prostaglandins Leuk. Essent. Fatty Acids 2002, 66, 201–210. [DOI] [PubMed] [Google Scholar]
- (30).Nantermet PG; Henze DA In Annual Reports in Medicinal Chemistry; Macor JE; Ed.; Academic Press: Cambridge, MA, 2011; Vol. 46, pp 19–32. [Google Scholar]
- (31).Sasso O; Wagner K; Morisseau C; Inceoglu B; Hammock BD; Piomelli D Pharmacol. Res 2015, 97, 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Morisseau C; Goodrow MH; Newman JW; Wheelock CE; Dowdy DL; Hammock BD Biochem. Pharmacol 2002, 63, 1599–1608. [DOI] [PubMed] [Google Scholar]
- (33).Kim I-H; Heirtzler FR; Morisseau C; Nishi K; Tsai H-J; Hammock BD J. Med. Chem 2005, 48, 3621–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Pecic S; Deng S-X; Morisseau C; Hammock BD; Landry DW Bioorg. Med. Chem. Lett 2012, 22, 601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Esparza E; Hadzich A; Kofer W; Mithofer A; Cosio EG Phytochemistry 2015, 116, 138–148. [DOI] [PubMed] [Google Scholar]
- (36).Chen J-J; Zhao Q-S; Liu Y.-l.; Gong P.-f.; Cao L.-l.; Wang X-D; Zhao B Int. J. Food Prop 2017, 20, 3112–3123. [Google Scholar]
- (37).Huang Y-J; Peng X-R; Qiu M-H Nat. Prod. Bioprospect 2018, 8, 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Zhang S-Z; Yang F; Shao J-L; Pu H-M; Ruan Z-Y; Yang W-L; Li H Int. J. Food Sci. Technol 2020, 55, 2428–2440 [Google Scholar]
- (39).Ohlrogge J; Browse J Plant Cell 1995, 7, 957–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Somerville C In Biochemistry and Molecular Biology of Plants; Buchanan BB, Gruissem W, Jones RL, Eds.; American Society of Plant Physiologists: Rockville, MD, 2000, pp 456–527. [Google Scholar]
- (41).Falck JR; Kodela R; Manne R; Atcha KR; Puli N; Dubasi N; Manthati VL; Capdevila JH; Yi XY; Goldman DH; Morisseau C; Hammock BD; Campbell WB J. Med. Chem 2009, 52, 5069–5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Oliw EH; Brodowsky ID; Hörnsten L; Hamberg M Arch. Biochem. Biophys 1993, 300, 434–439. [DOI] [PubMed] [Google Scholar]
- (43).Wagner K; Inceoglu B; Dong H; Yang J; Hwang SH; Jones P; Morisseau C; Hammock BD Eur. J. Pharmacol 2013, 700, 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Ren Q; Ma M; Yang J; Nonaka R; Yamaguchi A; Ishikawa KI; Kobayashi K; Murayama S; Hwang SH; Saiki S; Akamatsu W; Hattori N; Hammock BD; Hashimoto K Proc. Natl. Acad. Sci. U.S.A 2018, 115, E5815–e5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Huang HJ; Wang YT; Lin HC; Lee YH; Lin AM Mol. Neurobiol 2018, 55, 138–144. [DOI] [PubMed] [Google Scholar]
- (46).Lakkappa N; Krishnamurthy PT; Pandareesh MD; Hammock BD; Hwang SH Neurotoxicology 2019, 70, 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Ren Q; Ma M; Ishima T; Morisseau C; Yang J; Wagner KM; Zhang JC; Yang C; Yao W; Dong C; Han M; Hammock BD; Hashimoto K Proc. Natl. Acad. Sci. U.S.A 2016, 113, E1944–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Hajdu Z; Nicolussi S; Rau M; Lorántfy L; Forgo P; Hohmann J; Csupor D; Gertsch JJ Nat. Prod 2014, 77, 1663–1669. [DOI] [PubMed] [Google Scholar]
- (49).Jones PD; Wolf NM; Morisseau C; Whetstone P; Hock B; Hammock BD Anal. Biochem 2005, 343, 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Liu JY; Lin YP; Qiu H; Morisseau C; Rose TE; Hwang SH; Chiamvimonvat N; Hammock BD Eur. J. Pharm. Sci 2013, 48, 619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Inceoglu B; Jinks SL; Schmelzer KR; Waite T; Kim IH; Hammock BD Life Sci 2006, 79, 2311–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


