Abstract
Background and Purpose:
The present study was conducted to investigate the inhibitory effects of Carum carvi essential oil (EO) against ERG6 gene expression in relation to fungal growth and some important virulence factors in Candida albicans.
Materials and Methods:
The minimum inhibitory concentration (MIC) of C. carvi EO against C. albicans was determined by the Clinical and Laboratory Standards Institute M27-A4 method at a concentration range of 20-1280 μg/ml. Furthermore, the expression of ERG6 gene was studied at the 0.5× MIC concentration of C. carvi EO using real-time polymerase chain reaction. The proteinase and phospholipase activities, cell surface hydrophobicity (CSH), and cell membrane ergosterol (CME) content of C. albicans were also assessed at the 0.5× MIC concentration of the plant EO using the approved methods. In addition, fluconazole (FLC) was used as a control antifungal drug.
Results:
The results indicated that the MIC and minimum fungicidal concentration of C. carvi EO for C. albicans growth were 320 and 640 μg/ml, respectively. The expression of fungal ERG6 at an mRNA level and ergosterol content of yeast cells were significantly decreased by both C. carvi EO (640 μg/ml) and FLC (2 μg/ml). The proteinase and phospholipase activities were also reduced in C. carvi EO by 49.82% and 53.26%, respectively, while they were inhibited in FLC-treated cultures by 27.72% and 34.67%, respectively. Furthermore, the CSH was inhibited in EO- and FLC-treated cultures by 12.75% and 20.80%, respectively.
Conclusion:
Our findings revealed that C. carvi EO can be considered a potential natural compound in the development of an efficient antifungal agent against C. albicans.
Keywords: Antifungal activity, Candida albicans, Carum carvi, ERG6, Virulence factors
Introduction
The prevalence of infections related to Candida species has increased over the past three decades, especially in patients with different conditions, such as those with an immunocompromised state and AIDS, tissue transplantation, antibiotic therapy, and malignancy diseases [ 1 ]. Candida albicans is a major pathogenic agent that profoundly invades various parts of the human body by promoting hyphal switching, surface recognition molecules, phenotypic switching, and extracellular hydrolytic enzyme production attributed as virulence factors [ 2 , 3 ]. Extracellular hydrolytic enzymes, such as phospholipases and secreted proteinases, facilitate adherence, tissue penetration, and host invasion [ 2 - 4 ].
The adherence of C. albicans to host cells is an essential early step in fungal infection related to cell surface hydrophobicity (CSH) [ 2 , 5 ]. Candida albicans can also adhere to the surfaces of medical devices and form biofilms. It is well documented that there is a positive association between the degree of virulence and the ability to form infection [ 6 ]. The ERG6 has been reported to be a putative structural gene for ergosterol biosynthesis and essential for the normal functioning of the fungal cell membrane [ 7 ].
Several studies have addressed the pathogenic factors in Candida species to facilitate the diagnosis, treatment, and prevention of candidiasis [ 8 - 11 ]. Herbal agents with antifungal activity represent an ideal alternative for chemical medicines regarding the limitations of synthetic antifungal drugs with a high production cost and numerous side effects [ 8 ]. Accordingly, the antibacterial and antifungal effects of many plant species have been widely investigated during the recent two decades [ 12 - 17 ].
Carum carvi L. (caraway) is an annual herbaceous plant that belongs to the Umbelliferae family. The plant is native to some certain regions of Iran and is used in foods and herbal medicine [ 18 ]. Carum carvi have been well documented as a powerful antifungal and antimicrobial compound in both traditional and modern medicines [ 19 - 21 ]. The present study was conducted to investigate the potential effect of C. carvi essential oil (EO) on the growth, proteinase and phospholipase activities, CSH, and cell membrane ergosterol (CME) content of C. albicans. This study also involved the evaluation of the effect of C. carvi EO on ERG6 gene expression using real-time polymerase chain reaction (PCR) assay.
Materials and Methods
Fungal strain and Carum carvi essential oil
For the purpose of the study, C. albicans standard strain ATCC10231 was obtained from the Pathogenic Fungi Culture Collection of the Pasteur Institute of Iran (http://fa.pasteur.ac.ir/VisitDetails.aspx?Id=1311). The strains were cultured on Sabouraud dextrose agar (Merck, Germany) for 48 h at 37°C. In addition, C. carvi seeds were purchased from the market. Plant materials were steam distilled for 90 min in a full glass apparatus. The EO was also prepared by the hydrodistillation of sterilized seeds using a Clevenger-type apparatus over a period of 4 h. The yield of EO was about 0.25% and was kept at 4°C [ 21 ].
Antifungal susceptibility assay
Antifungal susceptibility assay was conducted according to the guidelines of the National Committee for Clinical Laboratory Standards CLSI M27-A4 method [ 22 ]. Briefly, C. albicans was adjusted to a final concentration of 0.5-2.5×103 CFU/mL in RPMI-1640 (Sigma-Aldrich, USA), using 3-(N-morpholino) propanesulfonic acid medium and then added to a 96-well plate. To prepare the stock solution, C. carvi EO was dissolved in dimethyl sulfoxide (Sigma-Aldrich, USA) to obtain a final concentration of 100 mg/ml. Subsequently, two-fold serial dilutions of the EO were prepared in RPMI to obtain the final concentrations of 20-1280 μg/ml.
For fluconazole, the serial two-fold concentrations of 0.5-256 µg/ml were prepared from a stock solution of fluconazole (Sigma-Aldrich, USA) in methanol (Merck, Germany). The plates were incubated at 35°C for 24-48 h. The minimum inhibitory concentration (MIC) of C. carvi EO and fluconazole was defined as 100% inhibition of fungal growth in 96-well microplates by visual assay. For determining minimum fungicidal concentration (MFC), 50 µl of the contents of each well over MIC with no visual fungal growth was transferred to Sabouraud dextrose agar (SDA) plates and incubated for 72 h at 35°C. The MFC was defined as the minimum concentration of EO and fluconazole which caused no growth on SDA plates. All tests were conducted in triplicate in three separate experiments.
Proteinase activity assay
The determination of proteinase activity was assessed according to Dabiri et al. [ 11 ]. Briefly, 10 µl of a 18-hour yeast suspension (106 cells/ml) was inoculated on bovine serum albumin (1%) medium which contained dextrose (2%), MgSO4 (0.05%), KH2PO4 (0.1%), and agar (2%) with 0.5× MIC concentration of C. carvi EO (160 μg/ml) and fluconazole (2 μg/ml). Petri dishes were incubated for 5 days at 35oC and fixed with trichloroacetic acid (TCA, 20%). The zone of the proteolysis around the yeast colonies was measured according to Price et al. [ 23 ].
Phospholipase activity assay
The phospholipase production was determined by the zone of precipitation around the fungal colony according to Samaranayake et al. using a slightly modified method [ 24 ]. The egg-yolk agar medium consisted of SDA (13 g; Merck, Germany), NaCl (11.7 g), CaCl2 (0.111 g), and 10% sterile egg yolk. Therefore, 10 µl of the yeast suspension (106 cells/ml) was inoculated on the egg-yolk medium with C. carvi EO (160 μg/ml) and fluconazole (2 μg/ml). The precipitation zone around the fungal colony was measured after incubation at 35ºC for 72 h.
Determination of cell surface hydrophobicity
The effect of C. carvi EO on C. albicans CSH was measured by the biphasic hydrocarbon-aqueous phase method [ 11 ]. Briefly, 10 µl of yeast suspension (106 cells/ml) was inoculated onto Sabouraud dextrose broth medium with C. carvi EO (160 μg/ml) and fluconazole (2 μg/ml) and incubated at 35°C for 48 h. Subsequently, 5 ml of cell suspension was added to sterile glass test tubes. In addition, a test and an untreated control were prepared from the suspending medium and used as blank. In the next stage, 1 ml of xylene was added to each test suspension.
The test and controls were placed in a water bath at 35°C for 10 min to equilibrate and then vortexed for 30 sec and returned to the water bath for a further 30 min. The lower aqueous phase was carefully transferred to a clean test tube. The absorbance at 520 nm was measured after mixing for 5 sec to suspend any unwanted aggregates. The suspension without xylene was used as a negative control. The hydrophobicity was described as the percentage reduction in the optical density of the test suspension, compared to that of the control. The percentage of hydrophobicity was calculated as follows:
Hydrophobicity (%)=[1−(A1/A0)] × 100
Assay of cell membrane ergosterol content
The CME content was measured according to Jahanshiri et al. [ 7 ]. Candida albicans (106 cells/ml) was inoculated onto Sabouraud dextrose broth medium with C. carvi EO (160 μg/ml) and fluconazole (2 μg/ml) and then incubated at 35ºC for 72 h. The fungal cells were harvested by centrifugation at 5,000 rpm for 5 min, washed with distilled water, dried at room temperature, and weighed. In the next stage, 3 mL of 25% alcoholic potassium hydroxide was added to each pellet and vortexed for 1 min.
The suspension was transferred to glass tubes and incubated at 90°C for 1 h in a water bath and cooled at room temperature. Afterward, 1 mL distilled water and 3 mL n-heptane were added and vortexed vigorously for 3 min. After 30 min, the heptane layer was transferred to clear glass tubes and stored at -20°C for 24 h. Then, 1 mL of sterol aliquot was diluted 5 folds in 100% ethanol and scanned at 230-300 nm using an ultraviolet-visible spectrophotometer (Shimadzu, Japan). The presence of ergosterol and late sterol intermediate 24(2*) DHE in the extracted sample resulted in a characteristic four-peak curve. The ergosterol content was calculated as the percentage of the wet weight of the cell using the following calculation:
% ergosterol + % 24(28) DHE=[(A281.5/290) × F] /fungal pellet weight
%24(28) DHE=(A230/518) × F / fungal pellet weight
% ergosterol=% ergosterol + % 24 (28) DHE − % 24 (28) DHE
where F is the factor for dilution in ethanol and 290 and 518 are the E values determined for crystalline ergosterol and 24(28) DHE, respectively.
Determination of ERG6 gene expression
In order to evaluate the effect of C. carvi EO on the expression of ERG6 in C. albicans, total RNA was extracted from the treated fungal at the 0.5× MIC concentration of C. carvi EO (160 μg/ml) and fluconazole (2 μg/ml) by the RNX-Plus kit (Sinacolon, Iran). In addition, RNA concentrations and purity were determined, and complementary DNA (cDNA) synthesis was carried out using a kit (Fermentas, USA) following a study performed by Jahanshiri et al. [ 25 ]. The primers used in this study included F: 5'GTGGTGTAGGTGGTCCTGGT 3' and R: 5' CAATGGCATAAACAGCATCG 3' (ERG), as well as F: 5' CCA GCT TTC TAC GTT TCC 3' and reverse: 5' CTG TAA CCA CGT TCA GAC 3' (ACT1) [ 26 ]. To calibrate the real-time quantitative PCR system, the standard curve of the serial dilutions (10−1 to 10−4) of C. albicans was drawn with cDNA as a template. Real-time PCR was carried out using the SYBR green master mix (Applied Biosystems) in a final volume of 20 µl reaction, including 10 µl master mix (2X), 1 µl primers (10 mM), 1 µl of total cDNA sample, and distilled water for each reaction. Each sample was normalized for the amount of the template to the level of ẞ-actin as a reference gene. The experiments were repeated in triplicate for each sample. The PCR conditions consisted of an initial incubation at 95°C for 10 min, as well as 40 cycles of 15 sec at 95°C, 1 min at 60°C, and 15 sec at 72°C by the ABI PRISM 7500 thermal cycler (Applied Biosystems). To determine the level of ERG6 expression, the differences between the threshold cycle (CT) of the samples and calibrator was calculated using the following formula:
(2−ΔΔCT)
Statistical analysis
All data were analyzed and compared in GraphPad Prism software, version 6.0 (Sandiego, CA) using the ANOVA test. A p-value less than 0.05 was considered statistically significant.
Results
Antifungal susceptibility testing
The MIC and MFC values of C. carvi EO were determined and then compared with those of fluconazole against the standard clinical isolate of C. albicans (Table 1). Based on the results, the MIC and MFC values of plant EO against C. albicans were 320 and 640 μg/ml, respectively. For fluconazole, these values were reported as 4 and 8 μg/ml, respectively.
Table 1.
Minimum inhibitory concentration and minimum fungicidal concentration values of Carum carvi essential oil and fluconazole (μg/ml) for Candida albicans
| Antifungal compound | Range | MIC | MFC |
|---|---|---|---|
| Essential oil | 20-1280 | 320 | 640 |
| Fluconazole | 0.5-256 | 4 | 8 |
MIC: minimum inhibitory concentration, MFC: minimum fungicidal concentration
Determination of proteinase and phospholipase activities
The production of proteinase and phospholipase was reduced in C. carvi EO and fluconazole-treated culture (figures 1 and 2). The proteinase and phospholipase activities were reduced in C. carvi EO by 49.82% and 53.26%, respectively, while they were inhibited in fluconazole-treated cultures by 27.72% and 34.67%, respectively (Table 2). A significant difference was shown between the treated and control groups in terms of this variable (P<0.05). The results revealed that C. carvi EO was as effective as fluconazole in the reduction of both proteinase and phospholipase production.
Figure 1.

Proteinase activity in Candida albicans treated with Carum carvi essential oil (a) and fluconazole (b) in comparison to that in the control (c)
Figure 2.
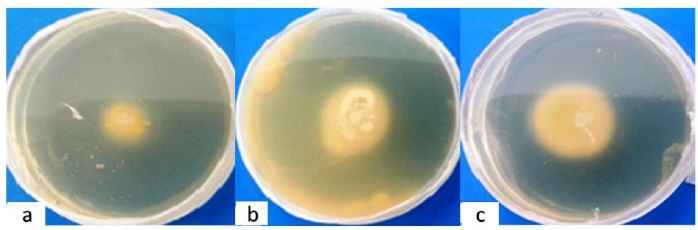
Phospholipase activity in Candida albicans treated with Carum carvi essential oil (a) and fluconazole (b) in comparison to that in the control (c)
Table 2.
Determination of proteinase and phospholipase production, cell surface hydrophobicity, and cell membrane ergosterol content in Candida albicans treated with Carum carvi essential oil and fluconazole
| Groups | Proteinase production (mm) | Proteinase inhibition (%) | Phospholipase production (mm) | Phospholipase inhibition (%) | CSH (%) | CME (µg/g) | CME Inhibition (%) |
|---|---|---|---|---|---|---|---|
| C. carvi EO -treated | 1.43±0.07 | 49.8 | 0.93±0.02 | 53.26 | 12.75 | 0.084±0.01 | 25.74 |
| Fluconazole -treated | 2.06±0.05 | 27.72 | 1.30±0.08 | 34.67 | 20.80 | 0.064±0.01 | 43.36 |
| Control (Non-treated) | 2.85± 0.15 | 00.00 | 1.99±0.10 | 00.00 | 38.98 | 0.113±0.01 | 00.00 |
Results of the mean±SD of two experiments, each in triplicate.
CSH: cell surface hydrophobicity, CME: cell membrane ergosterol
Determination of cell surface hydrophobicity
The CSH of C. albicans treated with C. carvi EO and fluconazole is shown in Table 2. According to the results, the CSH of C. albicans treated with C. carvi EO and fluconazole respectively reduced by 12.75% and 20.80% in comparison to that of the non-treated control. A significant difference was shown between the treated and control groups in this regard (P<0.05).
Determination of cell membrane ergosterol content
The CME contents of C. albicans in C. carvi EO- and fluconazole-treated cultures were measured at 0.084 and 0.064 µg/g, respectively (Table 2), indicating a significant difference between the treated and control groups (P<0.05). The CME was inhibited at the rates of 25.74% and 43.36% in C. carvi EO- and fluconazole-treated cultures, respectively. The results showed that fluconazole was more efficient than C. carvi EO in reducing the ergosterol content of C. albicans.
Determination of ERG6 gene expression
The expression of ERG6 gene, as a mediator for the biosynthesis of ergosterol, was studied by means of quantitative PCR using specific primers in C. albicans-treated with 0.5× MIC of C. carvi EO (160 μg/ml) and fluconazole (2 μg/ml). The amplification products were also analyzed by agarose gel electrophoresis (Figure 3). The relative gene expression of ERG6 was measured according to the [2−ΔΔCT] formula. The ERG6 gene expression in the C. carvi EO- and fluconazole-treated samples was reduced by around 2 folds at the mRNA level which was significant in comparison with that in the non-treated control (Figure 4; P<0.05). Therefore, both plant EO and fluconazole similarly inhibited ERG6 expression. Therefore, there was no significant difference between the EO- and fluconazole-treated samples in terms of the inhibition of ERG6 expression (P>0.05).
Figure 3.

Real-time polymerase chain reaction product electrophoresis showing the presence of complementary DNA bands; 1) Carum carvi essential oil-treated sample, 2) negative control (D.W.), 3) fluconazole-treated sample, 4) reference gene ACT1, and 5) DNA ladder (100 bp)
Figure 4.

Expression of ERG6 at the mRNA level in Candida albicans treated with Carum carvi essential oil (160 μg/ml) and fluconazole (2 μg/mL) (P<0.05)
Discussion
Candida albicans is an opportunistic fungus accounting for a growing number of life-threatening infections in immunocompromised individuals [ 1 , 2 , 27 ]. It has been shown that phospholipase and proteinase production and hydrophobicity may play significant roles in the pathogenesis and virulence of C. albicans [ 3 , 7 , 28 ]. Nowadays, many investigations are targeted toward finding new natural products with strong antifungal effects to be used as an alternative for the treatment of fungal diseases. There are several studies addressing the antifungal effects of many organic and natural compounds against C. albicans [ 13 - 17 , 20 , 29 ]. Carum carvi is known as ‘Black Zira’ in Iran and has been used for many therapeutic purposes in traditional medicine [ 12 , 18 , 19 ].
The present study was aimed to investigate the inhibitory effects of C. carvi EO against some virulence factors, such as the production of proteinase and phospholipase, CSH, cell membrane ergosterol content, and expression of ERG6 gene as a mediator for the biosynthesis of ergosterol in C. albicans. The EOs are complex natural mixtures composed of numerous constituents at quite different concentrations. Therefore, it is difficult to attribute the antifungal activity to a specific component of this product.
Carvone (44.5-95.9%) and limonene (1.5-51.3%) have been reported as the main components of C. carvi EO. The other constituents include β-myrcene (0-0.4%), trans-dihydrochalcone (0-0.5%), trans-caveolae (0-0.2%), α-pinene, sabinene, n-octanal, trans-β-ocimene, and γ-terpinene. Additionally, cuminaldehyde (22.08%), c-terpinene (17.86%), c-terpinene-7-al (15.41%), p-cymene (7.99%), myristicin, and dillapiol have been identified as the important constituents of C. carvi EO [ 21 , 30 , 31 ].
The antifungal activity of C. carvi EO was studied against a wide variety of fungi [ 20 , 32 - 37 ]. Carum carvi EO was found to possess remarkable antifungal activity against Candida krusei ATCC 6258, Candida parapsilosis ATCC 22019, Aspergillus niger, Aspergillus flavus, and Aspergillus fumigatus, as well as an effective inhibition against C. krusei growth (42-mm inhibition zone) [ 32 ]. Carum carvi reportedly acts as an inhibitor of aflatoxin production in A. parasiticus. However, it showed no effect on fungal growth even at the highest concentration (1000 μg/ml) [ 21 ].
Our results showed that C. carvi EO at a concentration of 320 μg/ml declined the growth rate of C. albicans by 50%. However, at the concentration of 640 μg/ml, this product completely inhibited fungal growth. With respect to the MIC values of fluconazole (4 μg/ml), it had a potency that was 80 times more than that of C. carvi EO, which is usual due to the fact that we compared the MIC of a pure antifungal agent with the MIC of a mixed unpurified plant EO. This means that a comparison will be more accurate if the MIC of fluconazole is compared with the MIC of the inhibitory bioactive component of C. carvi EO.
Carum carvi EO contains rich oxygenated compounds, such as carvone and limonene, affecting the integrity of the fungal cell membrane. It has been shown that free-terpene hydrocarbons containing oxygenated compounds with low molecular weights are able to penetrate through the cell membrane after combining with lipophilic molecules [ 36 ]. Furthermore, phenolic compounds with OH group and aromatic structure have been identified to contribute to the deactivation of fungal enzymes [ 36 ]. Our findings revealed that C. carvi EO could successfully inhibit extracellular proteinase and phospholipase activities in C. albicans. Moreover, based on the evidence, proteinase and phospholipase activities are also involved in adhesion and tissue invasion by C. albicans [ 4 , 10 ]. The reduction of proteinase and phospholipase activities may be regarded as a consequence of targeting the active sites of these enzymes by C. carvi EO.
Patel et al. observed that a crude extract of Dodonaea viscosa was not able to inhibit proteinase and phospholipase activities in C. albicans. However, the presence of phytosterol and tannins in the extract may have caused extensive cellular damage, including cell wall damage [ 9 ]. It was revealed that the susceptibility or resistance of a fungus to the antifungal activity of an EO depends on the capacity of their main compounds. In this study, the presence of C. carvi EO (0.5×MIC) caused a significant reduction in CSH similar to fluconazole.
Ergosterol is one of the main parts of the fungal cell membrane and is reported to be the main target for antifungal drugs. Ergosterol biosynthesis is mediated by ERG gene family among which ERG6 is an important gene. This gene encodes C-24 methyltransferase, which converts zymosterol to fecosterol in the ergosterol biosynthesis pathway. The ERG6 gene is required for the normal function of the cell membrane in C. albicans; therefore, the mutation of this gene can inhibit the antifungal activities of drugs [ 37 ]. In the current study, CME content was reduced to 25.74% at a 0.5× MIC concentration of C. carvi EO (160 μg/ml). In addition, the expression of ERG6 was decreased around 2 folds at the mRNA level at the same concentration.
Since the absence of a functional sterol methyltransferase could induce cell hypersensitivity to exogenous compounds, the inhibition of ERG6 gene expression may increase the potency of new or existing antifungals. Therefore, the natural inhibitors of ERG6 gene product, such as C. carvi EO, introduced in the present study allows for the clinical treatment of candidiasis in a safer manner at a lower dosage [ 26 ]. On the other hand, it has been shown that C. carvi EO suppresses the expression of cytochrome P450 1A1 (CYP1A1) at the transcription level [ 38 ], which is responsible for the hepatotoxic effects of azole antifungal agents [ 39 ].
Conclusion
As the findings indicated, C. carvi EO could suppress ERG6 gene as a crucial gene in ergosterol biosynthesis in C. albicans and efficiently inhibit the major virulence factors of the fungus, including exoenzymes, cell membrane hydrophobicity, and CME content. The main antifungal components of C. carvi EO may be useful as natural alternatives for antifungal drug formulations to manage fungal infections caused by C. albicans.
Acknowledgement
This study was financially supported by the Research Deputy of Tarbiat Modares University, Tehran, Iran. Author’s contribution M. S. G. managed the project and wrote the first draft of the manuscript, M. R. A. prepared the fungal strain and EO, and S. N. performed the experiments. All authors approved the final version of the manuscript.
Author’s contribution
M. S. G. managed the project and wrote the first draft of the manuscript, M. R. A. prepared the fungal strain and EO, and S. N. performed the experiments. All authors approved the final version of the manuscript.
Conflict of Interest: No conflict of interest has been declared. Financial disclosure: No financial interests related to the material of this manuscript have been declared.
Financial disclosure: No financial interests related to the material of this manuscript have been declared.
References
- 1.Costa-de-Oliveira S, Rodrigues AG. Candida albicans antifungal resistance and tolerance in bloodstream infections: the triad yeast-host-antifungal. Microorganisms. 2020; 8(2):E154. doi: 10.3390/microorganisms8020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chin VK, Lee TY, Rusliza B, Chong PP. Dissecting Candida albicans infection from the perspective of C. albicans virulence and omics approaches on host-pathogen interaction: a Review. Int J Mol Sci. 2016; 17(10):E1643. doi: 10.3390/ijms17101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sardi JC, Scorzoni L, Bernardi T, Fusco-Almeida AM, Mendes Giannini MJ. Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol. 2013; 62(Pt 1):10–24. doi: 10.1099/jmm.0.045054-0. [DOI] [PubMed] [Google Scholar]
- 4.Sadeghi G, Mousavi SF, Ebrahimi-Rad M, Ardakani E, Eslamifar A, Shams-Ghahfarokhi M, et al. In vivo and in vitro pathogenesis and virulence factors of Candida albicans strains isolated from cutaneous candidiasis. Iran Biomed J. 2020; 25:257–66. doi: 10.29252/ibj.24.5.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu Q, Zhang B, Ma F, Jia C, Xiao C, Zhang B, et al. Novel mechanisms of surfactants against Candida albicans growth and morphogenesis. Chem Biol Interact. 2015; 227:1–6. doi: 10.1016/j.cbi.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Marcos-Zambrano L, Escribano P, Bouzaa E, Guineaa J. Production of biofilm by Candida and non-Candida spp. isolates causing fungemia: comparison of biomass production and metabolic activity and development of cut-off points. Int J Med Microbiol. 2014; 304(8):1192–8. doi: 10.1016/j.ijmm.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Jahanshiri Z, Shams-Ghahfarokhi M, Asghari-Paskiabi F, Saghiri R, Razzaghi-Abyaneh M. α-Bisabolol inhibits Aspergillus fumigatus Af239 growth via affecting microsomal ∆24-sterol methyltransferase as a crucial enzyme in ergosterol biosynthesis pathway. World J Microbiol Biotechnol. 2017; 33(3):55. doi: 10.1007/s11274-017-2214-9. [DOI] [PubMed] [Google Scholar]
- 8.Khan M, Ahmad I. Antibiofilm activity of certain phytocompounds and their synergy with fluconazole against Candida albicans biofilms. J Antimicrob Chemother. 2012; 67(3):618–21. doi: 10.1093/jac/dkr512. [DOI] [PubMed] [Google Scholar]
- 9.Patel M, Gulube Z, Dutton M. The effect of Dodonaea viscosa var. angustifolia on Candida albicans proteinase and phospholipase production and adherence to oral epithelial cells. J Ethnopharmacols. 2009; 124(3):562–5. doi: 10.1016/j.jep.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Consolaro ME, Gasparetto A, Svidzinski TI, Peralta RM. Effect of pepstatin A on the virulence factors of Candida albicans strains isolated from vaginal environment of patients in three different clinical conditions. Mycopathologia. 2006; 162(2):75–82. doi: 10.1007/s11046-006-0026-9. [DOI] [PubMed] [Google Scholar]
- 11.Dabiri S, Shams-Ghahfarokhi M, Razzaghi-Abyaneh M. Comparative analysis of proteinase, phospholipase, hydrophobicity and biofilm forming ability in Candida species isolated from clinical specimens. J Mycol Med. 2018; 28(3):437–42. doi: 10.1016/j.mycmed.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Razzaghi-Abyaneh M, Shams-Ghahfarokhi M, Rai M. Antifungal plants of Iran: an insight into ecology, chemistry, and molecular biology, in Antifungal Metabolites from Plants. Berlin: Springer; 2013P. 27-57. [Google Scholar]
- 13.Shams-Ghahfarokhi M, Shokoohamiri MR, Amirrajab N, Moghadasi B, Ghajari A, Zeini F, et al. In vitro antifungal activities of Allium cepa, Allium sativum and ketoconazole against some pathogenic yeasts and dermatophytes. Fitoterapia. 2006; 77(4):321–3. doi: 10.1016/j.fitote.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Alves M, Gonçalves MJ, Zuzarte M, Alves-Silva JM, Cavaleiro C, Cruz MT, et al. Unveiling the antifungal potential of two iberian thyme essential oils: effect on C. albicans germ tube and preformed biofilms. Front Pharmacol. 2019; 10:446. doi: 10.3389/fphar.2019.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serra E, Hidalgo-Bastida LA, Verran J, Williams D, Malic S. Antifungal activity of commercial essential oils and biocides against Candida albicans. Pathogens. 2018; 7(1):E15. doi: 10.3390/pathogens7010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freire JC, Júnior JK, Silva DF, de Sousa JP, Guerra FQ, de Oliveira Lima E. Antifungal activity of essential oils against Candida albicans strains isolated from users of dental prostheses. Evid Based Complement Alternat Med. 2017; 2017:7158756. doi: 10.1155/2017/7158756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassanpour P, Shams-Ghahfarokhi M, Razzaghi-Abyaneh M. Antifungal activity of eugenol against Cryptococcus neoformans biological activity and Cxt1p gene expression. Curr Med Mycol. 2020; 6(1):9–14. doi: 10.18502/cmm.6.1.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panda H. Handbook on spices and condiments (cultivation, processing and extraction). New Delhi, India: Asia Pacific Business Press Inc; 2010. [Google Scholar]
- 19.Grigore A, Colceru-Mihul SV, Paraschiv I, Nita SU, Christof RA, Iuksel RA, et al. Chemical analysis and antimicrobial activity of indigenous medicinal species volatile oils. Roman Biotechnol Lett. 2012; 17(5):7620–7. [Google Scholar]
- 20.Nasiri S, Shams-Ghahfarokhi M, Razzaghi-Abyaneh M. Inhibitory effect of Carum carvi essential oils on growth of Candida albicans. Sci J Microb. 2014; 3(7):74–7. [Google Scholar]
- 21.Razzaghi-Abyaneh M, Shams-Ghahfarokhi M, Rezaee MB, Jaimand K, Alinezhad S, Saberi R, et al. Chemical composition and antiaflatoxigenic activity of Carum carvi L. , Thymus vulgaris and Citrus aurantifolia essential oils. Food Control. 2009; 20(11):1018–24. [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts. CLSI standard M27-A4. 4th ed Wayne, PA: Clinical and Laboratory Standards Institute; 2017. . [Google Scholar]
- 23.Price MF, Wikinson ID, Gentry LO. Plate method for detection of phospholipase activity in Candida albicans. Sabouraudia. 1982; 20(1):7–14. doi: 10.1080/00362178285380031. [DOI] [PubMed] [Google Scholar]
- 24.Samaranayake YH, Dassanayake RS, Jayatilake JA, Chgeung BP, Yau JY, Yeung KW, et al. Phospholipase B enzyme experission is not associated with other virulence attributes in Candida albicans isolates from patints with human immunodeficiency virus infection. J Med Microbiol. 2005; 54(6):583–93. doi: 10.1099/jmm.0.45762-0. [DOI] [PubMed] [Google Scholar]
- 25.Jahanshiri Z, Shams-Ghahfarokhi M, Allameh A, Razzaghi-Abyaneh M. Inhibitory effect of eugenol on aflatoxin B1 production in Aspergillus parasiticus by downregulating the expression of major genes in the toxin biosynthetic pathway. World J Microbiol Biotechnol. 2015; 31(7):1071–8. doi: 10.1007/s11274-015-1857-7. [DOI] [PubMed] [Google Scholar]
- 26.Jensen-Pergakes KL, Kennedy MA, Lees ND, Barbuch R, Koegel C, Bard M. Sequencing, disruption, and characterization of the Candida albicans sterol methyltransferase (ERG6) gene: drug susceptibility studies in ERG6 mutants. Antimicrob Agents Chemother. 1998; 42(5):1160–7. doi: 10.1128/aac.42.5.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Razzaghi-Abyaneh M, Shams-Ghahfarokhi M, Rai M. Medical mycology: current trends and future prospects. Florida: CRC Press; 2015. P. 442. [Google Scholar]
- 28.Pakshir K, Zomorodian K, Karamitalab M, Jafari M, Taraz H, Ebrahimi H. Phospholipase, esterase and hemolytic activities of Candida spp. isolated from onychomycosis and oral lichen planus lesions. J Mycol Med. 2013; 23(2):113–8. doi: 10.1016/j.mycmed.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Razzagh-parast A, Shams Ghahfarokhi, Yadegari MH, Razzaghi Abyaneh M. Antifungal effects of Allium cepa and some azoles in intact forms and in combinations to each other against pathogenic yeasts. Kowsar Med J. 2008; 13(2):103–13. [Google Scholar]
- 30.Nehiri M, Aouane EM, Tarfaoui K, Choukri S, Chaib Y, Inekach S, et al. Evaluation of the antibacterial activity of Carum Carvi L.essential oil. Am J Innovat Res Appl Sci. 2017; 5(6):67–70. [Google Scholar]
- 31.Razzaghi-Abyaneh M, Yoshinari T, Shams-Ghahfarokhi M, Rezaee MB, Nagasawa H, Sakuda S. Dillapiol and apiol as specific inhibitors of the biosynthesis of aflatoxin G1 in Aspergillus parasiticus. Biosci Biotechnol Biochem. 2007; 71(9):2329–32. doi: 10.1271/bbb.70264. [DOI] [PubMed] [Google Scholar]
- 32.Ibrahim GS, Kiki MJ. Chemical composition, antifungal and antioxidant activity of some spice essential oils. Int J Life Sci Pharma Res. 2020; 10(1):L43–50. [Google Scholar]
- 33.Iacobellis N, Cantore P, Capasso F, Felice S, Antibacterial activity of Cuminum cyminum L. and Carum carvi essential oils. J Agric Food Chem. 2005; 53(1):57–61. doi: 10.1021/jf0487351. [DOI] [PubMed] [Google Scholar]
- 34.Begum J, Bhuiyan MN, Chowdhury JU, Hoque MN, Anwar MN. Antimicrobial activity of essential oil from seeds of Carum carvi and its composition. Bangladesh J Microbiol. 2008; 25(2):85–9. [Google Scholar]
- 35.Siripornvisal S, Thawornluk P, Rungprom W. Screening for antifungal activity and active components of crude extracts from 6 Umbelliferae. Agric Sci J. 2011; 42(2):361–4. [Google Scholar]
- 36.Ben Salha G, Herrera Díaz R, Lengliz O, Abderrabba M, Labidi J. Effect of the chemical composition of free-terpene hydrocarbons essential oils on antifungal activity. Molecules. 2019; 24(19):E3532. doi: 10.3390/molecules24193532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanglard D, Ischer F, Parkinson T, Falconer D, Bille J. Candida albicans mutations in the ergosterol biosynthetic pathway and resistance to several antifungal agents. Antimicrob Agents Chemother. 2003; 47(8):2404–12. doi: 10.1128/AAC.47.8.2404-2412.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naderi-Kalali B, Allameh A, Rasaee MJ, Bach HJ, Behechti A, Doods K, et al. Suppressive effects of caraway (Carum carvi) extracts on 2, 3, 7, 8-tetrachloro-dibenzo-p-dioxin-dependent gene expression of cytochrome P450 1A1 in the rat H4IIE cells. Toxicol in Vitro. 2005; 19(3):373–7. doi: 10.1016/j.tiv.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Korashy HM, Shayeganpour A, Brocks DR, El-Kadi AO. Induction of cytochrome P450 1A1 by ketoconazole and itraconazole but not fluconazole in murine and human hepatoma cell lines. Toxicol Sci. 2007; 97(1):32–43. doi: 10.1093/toxsci/kfm012. [DOI] [PubMed] [Google Scholar]


