Abstract
Beyond being the product of gene expression, RNA can also influence the regulation of chromatin. The majority of the human genome has the capacity to be transcribed and the majority of the non-protein-coding transcripts made by RNA Polymerase II are enriched in the nucleus[1]. Many chromatin regulators can bind to these ncRNAs in the nucleus[2–4]; in some cases there are clear examples of direct RNA-mediated chromatin regulation mechanisms stemming from these interactions, while others have yet to be determined. Recent studies have highlighted examples of chromatin regulation via RNA matchmaking, a term we use broadly here to describe intermolecular base-pairing interactions between one RNA molecule and an RNA or DNA match. This review provides examples of RNA matchmaking that regulates chromatin processes and summarizes the technical approaches used to capture these events.
Introduction
Definition of “RNA Matchmaking” within the scope of this review
The term “RNA matchmaking” was initially used to describe RNA base-pairing activity (annealing) that is promoted by RNA binding proteins[5, 6]. Early use of the term mainly described the activity of heterogeneous nuclear ribonucleoproteins (hnRNPs) that use multiple RNA binding domains to bring two RNA sequences together to promote RNA annealing, frequently to make loops in pre-mRNA that promote splicing[7]. Because they participate in co-transcriptional splicing, hnRNPs often perform RNA matchmaking with nascent, chromatin-bound RNA, allowing them and other splicing factors to perform additional functions in regulating the underlying chromatin[4]. Multiple other proteins have since been dubbed “RNA matchmakers”, including those that promote intermolecular RNA-RNA interactions involved in bacterial stress response, RNA editing, splicing, and rRNA processing[8–11]. A more-general use of RNA matchmaking has described the transcriptome-wide intermolecular RNA-RNA interactions recently profiled by novel genomics techniques[12]. This review uses “RNA matchmaking” broadly to describe base-pairing interactions between RNA and another nucleic acid, primarily those interactions facilitated by proteins. In describing some of the approaches and systems to study RNA matchmaking, we include in the review some examples of protein bridging of RNAs, in the absence of base-pairing. We note throughout the review that protein bridging does not fall under our definition of RNA matchmaking; however, on occasion the benefits of including some of these mechanisms outweigh the potential for mis-representation.
General Functions for RNA matchmaking on chromatin
While much is still to be learned about how RNA matchmaking influences chromatin regulation, a number of systems have been studied to date. Some of the clearest examples of RNA matchmaking-mediated chromatin regulation are of nuclear noncoding RNAs functioning to directly regulate heterochromatin[13, 14], the transcriptionally repressed chromatin structure required for organismal development and to control repetitive elements in the genome. RNA matchmaking can also influence other chromatin regulation mechanisms such as gene activation, alternative splicing, and DNA repair[15, 16]. These RNA-regulated processes can be mis-regulated in disease, with examples of RNA-mediated heterochromatin formation contributing to many cancers[17].
One example of an RNA matchmaking-mediated chromatin regulation mechanism that extends from fission yeast to human is the harnessing of small RNA base-pairing to directly target genomic loci via transcribed regions. Most well understood are the fission yeast siRNA pathway for heterochromatin formation and the piRNA pathway for transposable element silencing by heterochromatin[13]. Small RNA pathways have also been shown to directly regulate chromatin in transcriptional activation and DNA repair[16].
Long noncoding RNAs (lncRNAs), RNAs of >200 nt with little protein coding potential, can also function in genome regulation[18], frequently through heterochromatin formation. Recent work highlights that some lncRNAs perform RNA matchmaking, with another RNA molecule or with genomic DNA, that facilitates targeting to and/or direct regulation of chromatin[19].
Targets for RNA matchmaking on chromatin
Nascent RNA serves as a platform for its own processing to mRNA (Figure 1A). Some nascent RNAs coordinate additional activities by interacting with other nuclear RNAs to trigger regulation of the underlying chromatin[14]. This is most apparent for the small RNA targeting pathways of chromatin regulation where the main models include the small RNA bound via an Argonaute protein to a nascent transcript. Nascent RNA can also be targeted by lncRNAs, to facilitate chromatin regulation.
Figure 1. Mechanisms of RNA Matchmaking.
A) Noncoding RNAs such as small RNAs (piRNAs, siRNAs) and long noncoding RNAs (lncRNAs) can form base-pairing interactions, often mediated by proteins, to target the RNP to chromatin via nascent RNA. B) RNAs can interact with DNA through additional mechanisms such as mediated by protein-protein interaction (does not fall under the definition of “RNA Matchmaking”), triple helix formation, or R-loop pairing with unwound DNA.
DNA can also be the target of RNA matchmaking (Figure 1B). Models for small RNA pathways targeting DNA have been proposed and appear to determine the ability of small RNAs to promote DNA repair[20]. LncRNAs have been shown to participate in R-loops as well as RNA-DNA triple helices, fostering multiple types of chromatin regulation, depending on the context[19].
The potential of complex, repetitive genomes to produce RNA-RNA interactions
The sheer amount of repetitive sequence in the human genome gives rise to a significant potential for RNA-RNA interaction to occur within and between these regions, when transcribed in opposite directions. RNAs can harbor certain “hotspot” regions that have the potential to base-pair with other transcripts[21, 22]. The capacity for RNA-RNA interactions at these defined sequence regions appears to be facilitated through degenerate repetitive sequences in opposite orientations embedded within these RNAs. LncRNA genes frequently evolve through transposon acquisition and divergence[23], as do non-coding elements of mRNAs. These processes can lead to a strong matching sequence between two RNAs. As an example, we have studied an extensive match between HOTAIR lncRNA and the 5’UTR of the mRNA of a HOTAIR target gene, JAM2. The matching region in HOTAIR is a hotspot for energetically favorable matches with many RNAs[21, 24]. Additionally, the HOTAIR matching region occurs in a locus that is highly-conserved among vertebrates, with the potential for near-perfect base-pairing with a transcribed non-coding region of the first-identified HOTAIR target located at HOXD[25]. The HOXC locus, where HOTAIR is transcribed, and the HOXD locus, arose as duplication events that have since diverged[25, 26]; therefore the two loci bear similar relationships with transposon duplicates that have diverged.
Due to the large fraction of the human genome that is derived from repetitive elements and duplications, the capacity for RNA-RNA interactions in trans is high, especially among ncRNAs and non-coding regions of mRNAs. Antisense transcription, coincident and overlapping with sense transcription, occurs at many protein-coding genes as well and may also give rise to intermolecular dsRNAs [27]. It has been technically challenging to capture intermolecular RNA-RNA duplexes from sense/anti-sense pairs and transcribed repeats by genomic approaches, because the sequences are similar to each other and other loci, and the interactions may be transient. Therefore, the full extent of intermolecular RNA-RNA interactions that occur in the cell, and the nucleus in particular, is not known.
In addition to intermolecular RNA-RNA base-pairing interactions in the nucleus, intermolecular interactions between RNA and DNA, outside of the transcription bubble, have been found and are associated with chromatin regulation. These generally fall into two classes: 1) RNA-DNA hybrids in R-loops, presumed to be the causes of base-pairing of the RNA back to its DNA template after leaving the core RNA polymerase machinery[28]; 2) RNA-DNA triplexes formed from non-canonical base-pairing[29]. The extent and function of these RNA-DNA interactions is currently under investigation and much is still unknown about how they form and what they do.
Techniques to survey intermolecular RNA base-pairing interactions in the nucleus
Recent profiling of RNA-RNA interactions using psoralen derivatives to make intermolecular crosslinks has focused mainly on total RNA, identifying abundant interactions in trans between snoRNAs and rRNAs, as well as a small set of nuclear interactions[30–33]. A major challenge in this approach is that each RNA in an intermolecular pair could originate from a repetitive region that is difficult to uniquely map. While the full-length RNAs that interact would be from unique genomic loci, the base-pairing portions, which are the hybrid “hit” in the sequencing approach, would be highly similar, allowing them to match.
Overview of the following sections
With the emerging appreciation that RNA matchmaking interactions play roles in genome regulation, we use this review to highlight key model examples and summarize the current techniques used to capture these events.
Argonaute Based Silencing via small RNA-nascent RNA interactions
The Argonaute (AGO) proteins are part of an evolutionarily conserved pathway of small RNA based gene silencing[34, 35]. AGO is most well-known for the miRNA and siRNA pathways which use a guided small RNA targeting approach to silence specific genes by mRNA slicing or triggering degradation machinery. In addition to this post-transcriptional silencing, some family members also have a transcriptional silencing function that targets the nascent transcript or promoter regions in the nucleus [36, 37]. Using small RNAs, nuclear AGO proteins can target specific sequences for stable long-term heritable silencing through heterochromatin formation.
One of the most well characterized models of nuclear AGO function is the Ago1/siRNA system in fission yeast [38, 39]. Schizosaccharomyces pombe only has one Argonaute protein, Ago1, which is required for the establishment of pericentromeric heterochromatin. Work in fission yeast has determined that repressive histone marks, particularly the methylation of histone H3 at lysine 9 (H3K9me3) are triggered in the small RNA based transcriptional silencing pathway[40, 41]. As part of the RNA-Induced Transcriptional Silencing (RITS) complex, Ago1/siRNAs target pericentromeric repeats and recruit the Clr4 methyltransferase to add the repressive H3K9me3 mark[42, 43]. H3K9 methylation is then bound by heterochromatin protein 1 (HP1) to establish heterochromatin. These events lead to the establishment of a nucleosome dense region with low histone turnover, effectively limiting access to transcription machinery such as RNA polymerase II (Pol II)[44]. Targeting of Ago1-dependent heterochromatin absolutely requires nascent transcription, emphasizing that matchmaking of siRNA and target RNA is critical for this gene regulation mechanism[45]. In addition to the fission yeast system, a miRNA based transcriptional silencing system has been demonstrated in human cells[46]. In this system, the human slicer-competent AGO2 uses small RNAs to target transcriptional start sites to direct the SWI/SNF chromatin remodeler in order to increase nucleosome occupancy at the target loci [47].
The other branch of the AGO family are PIWI proteins which interact with a separate class of small RNAs known as piRNAs[36, 48–50]. piRNAs can target either mature transcripts or nascent RNAs in a miRNA like manner where seed sequences dictate targeting, allowing mismatches elsewhere in the piRNA [51, 52]. Although many piRNAs target transposable elements in the germline, the most abundant form of piRNAs, pachytene piRNAs, mostly map to the coding regions and 3’ UTRs of mRNAs[53]. This evidence suggests that there may be other targets regulated by the PIWI pathway in addition to transposable elements.
The PIWI family is generally comprised of three functionally distinct members from fly to human, two cytoplasmic and one nuclear PIWI. The nuclear PIWI is MIWI2 in mice and Piwi in flies[54–57]. These nuclear PIWIs are either slicer activity-deficient or this activity is not necessary for function, suggesting that initiation of chromatin-based transcriptional silencing is their main mechanism of action[58–60]. Upon knockdown or knockout, MIWI2 and Piwi-deficient tissues have a reduction in H3K9me3 marks at transposable element target sites, resulting in increased transcription[51, 61]. PIWIs act in a multi-protein complex to direct heterochromatic marks. Many proteins have been found in the PIWI pathway in fly that, upon depletion, affect transposable element expression and fertility [61–63]. For instance, Silencio/Panoramix is thought to be an adaptor protein downstream of Piwi as protein-DNA tethering experiments using Silencio have shown H3K9me3 formation at the targeted loci[61, 62]. This contrasts with direct fly Piwi tethering experiments where gene expression remains unchanged, suggesting either piRNA loading or nascent RNA target recognition, RNA matchmaking, induces a conformational change in PIWI that is able to promote methyltransferase activity[61, 62, 64]. Similar proteomic studies have been done with mouse PIWIs[58, 59, 65, 66]. Studies have shown pathway conservation between fly and mammals, however the human PIWI pathway has only begun to be explored [67] and differences exist, even between mouse and human. C. elegans and Oxytrichia piRNA pathways demonstrate further diversity of molecular mechanism targeting both RNA and DNA[20, 68].
LncRNA matchmaking interactions and their functions
There are various other types of RNAs that form RNA-RNA and RNA-DNA interactions to guide their regulatory functions. One primary example are lnRNAs that can recognize nascent transcripts or genomic DNA through direct hybridization or mediated by proteins, facilitating localization to specific genomic loci for regulation of chromatin and transcription. The size and structured nature of these RNAs enables interactions with multiple molecules simultaneously, including chromatin regulatory complexes, RNA processing proteins, and transcription factors.
RNA-RNA matchmaking by LncRNAs
Many lncRNAs accumulate in the nucleus where they act in different chromatin regulation pathways. In some cases, intermolecular RNA-RNA interaction between the lncRNA and another transcript contribute to lncRNA function. The following are examples of these mechanisms.
The Epstein-Barr virus produces an abundant nuclear noncoding RNA called EBER2 that is required for lytic replication through the localization of host transcription factor PAX5 to the terminal repeats of the Epstein-Barr virus genome. The EBER2-PAX5 complex is recruited via RNA-RNA interaction between EBER2 and viral nascent transcripts from the terminal repeat locus[69]. Additionally, EBER2 forms RNA-RNA interactions with host nascent transcripts on chromatin, including both functional noncoding RNAs as well as three putative enhancer RNAs, though the function of these interactions has yet to be uncovered[70].
The heterochromatin histone methyltransferase Polycomb Repressive Complex 2 (PRC2) has been associated with RNA-mediated chromatin regulation for some time, though the nature of the relationship is intensely debated to date[71, 72]. PRC2 can associate broadly with many nuclear RNAs through a high affinity for tracts of guanines [73–77]. Though RNA has often been presumed to work productively with PRC2, association with RNA actually inhibits PRC2 histone methyltransferase activity, suggesting additional contexts are necessary to activate PRC2. Recently, we and others have identified mechanisms that connect RNA matchmaking with PRC2 activity [24, 78–80]. The nuclear lncRNA HOTAIR promotes the activity of PRC2 at the developmental HOXD locus[81], though mouse HOTAIR is not essential for developmental patterning[82]. When aberrantly overexpressed in multiple cancers, HOTAIR promotes mis-regulation of cancer-related genes, promoting aggressive tumor characteristics in cancer cells [17, 83]. HOTAIR can repress transcription in the absence of PRC2 activity[84], suggesting that its first actions occur upstream of PRC2 action. HOTAIR interacts with the transcripts of certain target genes through a conserved sequence [25] predicted to form thermodynamically favorable RNA-RNA interactions with many RNAs when surveyed across the human transcriptome[21, 78]. Genome-wide HOTAIR-dependent PRC2 activity occurs at genes whose transcripts can make these favorable RNA interactions with HOTAIR[24]. Matchmaking interactions between HOTAIR and a target with base-pairing potential are promoted by the RNA binding protein hnRNP B1[78], which can bind to RNA molecules in anti-parallel orientation[85]. This RNA matchmaker protein is required for HOTAIR-mediated PRC2 activity in cells overexpressing HOTAIR[78]. Matchmaking RNA-RNA interactions facilitate structural changes within HOTAIR and relieve the inhibitory effects of single RNAs bound to PRC2 [24]. PRC2 associates with many nuclear RNAs through a high affinity for tracts of guanines which are inhibitory to PRC2 catalytic activity [73–77]. RNA has recently been found essential for PRC2 chromatin occupancy and function in human iPSCs [86], suggesting that an interplay similar to HOTAIR-PRC2 may be more widespread.
The lncRNA SPRIGHTLY binds to the intronic regions of a set of cancer-related pre-mRNAs (SOX5, SMYD3, SND1, MEOX2, DCTN6, and RASAL2), where SPRIGHTLY deletion reduces expression levels resulting in decreased tumor growth[87]. These pre-mRNAs interact with multiple regions within the lncRNA, corresponding to a core pseudoknot domain. Functional analysis of these pre-mRNAs indicated that they constitute a connected network of functionally related genes. The authors suggested that SPRIGHTLY may coordinate the spatial organization of chromatin at these genes for co-transcriptional regulation. However, the exact mechanisms of co-regulation need to be further investigated.
Nuclear RNA-RNA interactions occur with the lncRNA MALAT1, which is involved in the formation of nuclear speckles, alternative splicing, and transcriptional regulation and is mis-regulated in disease. MALAT1 interacts directly with the U1 snRNA which can mediate chromatin localization[88]. MALAT1 also interacts with nascent transcripts of alternatively spliced pre-mRNAs at actively transcribed genes, which is mediated by protein factors [89]. Furthermore, MALAT1 associates with multiple chromatin modifiers and transcription factors, where it is thought to promote activation or repression of genes by facilitating the recruitment of these specific regulators to target genes via Malat1-RNA interactions [90, 91].
A conserved lncRNA that overlaps the 5S rDNA loci, termed 5S-OT, was found to contain a primate-specific transposable Alu element that interacts with the splicing factor U2AF65. In human cells, 5S-OT RNA can modulate alternative splicing via Alu/anti-Alu RNA-RNA pairing with target genes and subsequent recruitment of U2AF65 to these locations [92], which suggests some generalizable principles for splicing regulation[93].
RNA-DNA triplex matchmaking by LncRNAs
In addition to RNA-RNA interactions that influence chromatin regulation, lncRNAs are known to make triplex RNA-DNA interactions through Hoogsteen base-pairing with the underlying DNA at target loci, where a sequence specific third strand inserts into the duplex structure. Triplex interactions form at homopurine-homopyrimidine sequences via Hoogsteen hydrogen bonding between the purine-rich duplex DNA strand and either a pyrimidine-rich or purine-rich third RNA strand[94, 95]. Third strand interactions that are pyrimidine-rich are stabilized by T•AT, and C+•GC Hoogsteen bonds, whereas purine-rich interaction are stabilized by A•AT and G•GC reverse-Hoogsteen bonds.
The trans-acting lncRNA MEG3 is expressed at significantly lower levels in various breast cancer vs normal breast tissue [17]. In this context, knockdown of MEG3 and the PRC2 enzymatic component EZH2 results in deregulation of a subset of overlapping TGF-β pathway genes[96]. MEG3 is targeted to chromatin through RNA–DNA triplex formation via GA-rich sequences residing in the distal regulatory elements of target genes. Upon MEG3 knockdown, these distal regulatory elements displayed enhancer activity, interacted with the downstream promoter, and had decreased enrichment of EZH2 and H3K27me3. Together, this suggests a mechanism where MEG3 regulates TGF-β pathway genes through triplex formation via GA-rich sequences within distal regulatory elements promoting EZH2 association with these targets for repression. MEG3 may directly recruit PRC2 to chromatin or the lncRNA may contribute indirectly to promote PRC2 activity at these loci.
PARTICLE is a cis acting lncRNA that represses the tumor suppressor gene MAT2A upon irradiation[97]. PARTICLE is proposed to recruit the chromatin repressive proteins G9a and SUZ12 to the MAT2A promoter via RNA-DNA triplex formation. The mouse lncRNA Fendrr is essential for proper heart and body wall development [98].
Fendrr functions in cis and trans by modifying chromatin at specific differentiation genes. Fendrr binds both PRC2 and TrxG/MLL complexes and is thought to be recruited to chromatin through triplex formation at specific target promoters [99].
R-loop formation
Another type of RNA-DNA hybrid that impacts chromatin regulation are R-loops. R-loops are three-stranded nucleic acid structures that form when RNA hybridizes to dsDNA, displacing one DNA strand, leaving it single-stranded. The most well-known example of this is during transcription, when the 3’ end of nascent RNA base-pairs with the template stand. The transcription bubble R-loop is usually quite small, ~8–9 bp, and nearly surrounded by the polymerase, shielding it to any potential recognition by other proteins. The bubble is transient for any one DNA sequence, as the RNA is produced and displaced from pairing to DNA during transcription elongation [100]. RNA can re-pair with template DNA after polymerase has passed, potentially trapping the RNA in an R-loop, promoted by G-rich nascent RNA or negative supercoiling of the DNA [101, 102].
While the formation of R-loops can have deleterious effects on genome integrity through improper DNA-damage response, DNA recombination and double-stranded breaks, it has become apparent that R-loops can also function in the regulation of gene expression [103]. R-loops were found to form over a subset of Polycomb repressed target genes in mice, where loss of R-loops leads to transcriptional activation of these targets [79]. In Drosophila, Polycomb complexes are recruited to target genes via DNA elements called Polycomb Response Elements (PRE). R-loops were recently found to form at many PREs, where PRC1 and PRC2 can recognize R-loops and the ssDNA bubble[80]. Interestingly, PRC2 was found to drive the formation of RNA-DNA hybrids leading to R-loop formation, though at present the strand exchange mechanism has yet to be determined.
In Arabidopsis thaliana, the promoter of the lncRNA COOLAIR is repressed by an R-loop via binding of the transcription factor AtNDX on the displaced ssDNA strand. AtNDX binding stabilizes the R-loop, thereby inhibiting COOLAIR transcription, which in turn modifies the flowering gene expression pathway [104]. Regulatory R-loops can often be generated by antisense lncRNAs, which recruit specific chromatin regulators. The lncRNA GATA3-AS1, which is transcribed divergently from the GATA3 promoter, upregulates GATA3 expression through the formation of an R-loop upstream of the GATA3 transcriptional start site [105]. GATA3-AS1 binds components of the H3K4 methyltransferase complex MLL, recruiting MLL to the GATA3 locus and promoting transcription. GATA3-AS1 expression from a plasmid is also able to activate GATA3, indicating the R-loop can form with trans acting RNA. Antisense lncRNA ANRASSF1 is transcribed from the opposite strand of the tumor-suppressor gene RASSF1A [106]. ANRASSF1 forms an R-loop and recruits PRC2 to the RASSF1A promoter leading to increased H3K27me3 and RASSF1A silencing. These examples demonstrate that R-loops can function as both positive or negative regulators of transcription depending upon the specific interactions and contexts. [102].
Future work will be required to determine the pervasiveness of these RNA-RNA and RNA-DNA interactions. Recently, many tools and techniques have been developed to identify and dissect the mechanisms of RNA intermolecular interactions and how they function in RNA localization to chromatin and transcriptional regulation.
Techniques to investigate RNA matchmaking interactions
Multiple techniques have been developed to profile how and where RNA interacts with chromatin[107] (Table 1). These methods can be subdivided based on which component of chromatin the RNA associates with, including 1) direct interactions with DNA (Figure 2), 2) direct RNA:RNA interactions with nascent RNA, and 3) indirect associations mediated through RNA binding proteins (RBPs) (Figure 3).
Table 1.
List of techniques to investigate RNA matchmaking interactions.
| Direct Interactions with DNA | ||
| Method | Concept | Reference |
| ChOP | Modified ChIP assay that uses biotinylated antisense oligonucleotide to affinity purify the target RNA and associated chromatin (PCR is used to identify binding at specific regions of the genome) | Mariner PD et al., 2008 |
| ChIRP | Uses multiple tiling affinity-tagged antisense oligos and glutaraldehyde (DSG) crosslinking to pulldown a targeted RNA with its associated DNA (chromatin is then subjected to deep sequencing) | Chu C et al., 2011 |
| CHART | Similar to ChIRP, uses formaldehyde instead of DSG and only uses RNase H (as opposed to the cocktail of RNase A and H used in ChIRP) to specifically elute RNA interacting with chromatin | Simon, MD et al., 2011 |
| RAP | Similar to ChIRP and CHART, uses formaldehyde and omits the RNase treatment in favor of DNase to digest the genomic DNA to ∼150bp sized fragments which provides high-res mapping of binding sites | Engreitz JM et al., 2013 |
| CHIRT-seq | Similar to ChIRP and CHART, but only uses a single oligo probe to allow for profiling of more repetitive RNAs | Chu HP et al., 2017 |
| MARGI-seq | Genome-wide approach that captures all RNA:DNA interactions by coupling proximity ligation with a unique linker sequence that allows the RNA and DNA components to be differentiated | Sridhar B et al., 2017 |
| GRID-seq | Similar to MARGI-seq, but uses 2-step crosslinking to capture longer-range RNA:DNA interactions and a restriction enzyme step to create identical size-selected fragments | Li X et al., 2017 |
| ChAR-seq | Similar to MARGI-seq and ChAR-seq, uses long single-end reads to accurately capture the polarity of the bridge linker and performs the proximity ligation on intact nuclei to reduce spurious events | Bell JC et al., 2018 |
| Hi-ChIRP | Combines ChIRP-seq with Hi-C chromatin conformation capture to characterize specific RNAs involved in mediating inter-chromosomal interactions | Mumbach MR et al., 2019 |
| RADICL-seq | Similar to GRID-seq, but uses fewer cells, omits the 2-step crosslinking, utilizes DNase 1 instead of restriction enzyme to partially digest chromatin, and generates longer fragments to increase mapping efficiency | Bonetti A et al., 2020 |
| RD-SPRITE | Modification of the split-and-pool based SPRITE technique that couples crosslinking with a unique barcoding strategy to examine the three-dimensional organization of interacting RNA and DNA within the nucleus | Quinodoz S et al., 2020 |
|
RNA-RNA Interactions on Chromatin | ||
| Method | Concept | Reference |
| RPL | Uses proximity ligation followed by deep sequencing to profile global RNA:RNA interactions, though without crosslinking is largely limited to profiling intra-molecular RNA interactions | Ramani V et al., 2015 |
| RAP-RNA | Modified RAP method, uses affinity-tagged antisense oligos and UV crosslinking of the psoralen-based crosslinker aminomethyltrioxalen (AMT) to directly capture interactions mediated by a target RNA | Engreitz JM et al., 2014 |
| LIGR-seq | Uses in vivo crosslinking with AMT, proximity ligation, and 2D purification of RNA duplexes to generate genome-wide maps of RNA:RNA interactions | Sharma E et al., 2016 |
| PARIS | AMT crosslinking coupled with proximity ligation, uses partial RNase digestion to ensure identified crosslinks are limited to direct base-paired RNAs and 2D electrophoresis to purify only crosslinked fragments | Lu Z et al., 2016 |
| SPLASH | Use biotinylated psoralen to crosslink RNA before undergoing proximity ligation and deep sequencing | Aw JG et al., 2016 |
| COMRADES | Uses in vivo crosslinking of an azide-modified psoralen derivative and biotinylated DNA oligos to selectively capture interactions between a target RNA and proximal RNA molecules | Ziv O et al., 2018 |
| Proximity RNA-seq | Uses a water-in-oil emulsion barcoding technique followed by high-throughput sequencing to profile the subcellular localization of RNA interactions within the nucleus | Morf J et al., 2019 |
|
RNA Interactions Mediated by RNA Binding Proteins | ||
| Method | Concept | Reference |
| CLASH | Uses UV crosslinking, ligation, and immunoprecipitation to enrich for interacting RNA molecules mediated by a specific RNA binding protein (RBP) and sequencing the resulting hybrids | Helwak A et al., 2013 |
| hiCLIP | Similar to CLASH, identifies RNA duplexes by ligating two interacting RNAs but incorporates an adaptor between the two strands to increase ligation efficiency and the ability to identify the respective molecules | Sugimoto Y et al., 2015 |
| MARIO | Global profiling of RNA:RNA interactions via UV crosslinking of RNAs to biotinylated RBPs followed by proximity ligation to link RNA molecules that are associated with the same protein | Nguyen TC et al., 2016 |
| RIC-seq | Uses a biotinylated cytidine phosphate (pCp-biotin) linker to label RNAs and then captures RNA:RNA interactions via proximity ligation at single-nucleotide resolution) | Cai Z et al., 2020 |
Figure 2. Strategies for capturing RNA-DNA interactions.
Fragmented chromatin is crosslinked directory or indirectly to RNA. Specific RNAs can be enriched by oligo capture prior to sequencing of associated DNA. Specific RNA-DNA structures can be enriched by antibodies recognizing the structure (R-loop, triplex), irrespective of the sequence. All RNA-DNA interactions can be profiled by ligation of the RNA to the DNA and subsequent hybrid sequencing.
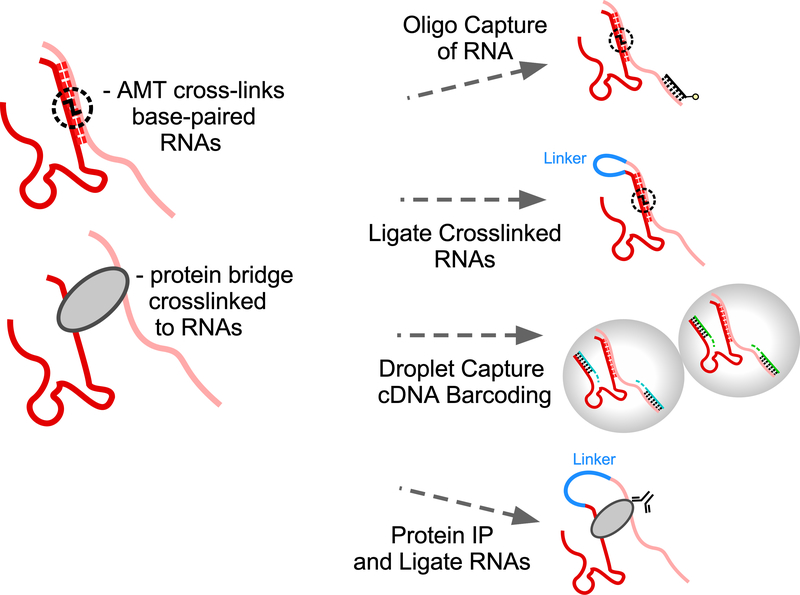
Figure 3. Strategies for capturing RNA-RNA interactions.
Base-paired RNAs can be crosslinked directly with psoralen derivatives such as AMT or indirectly via bridging proteins. Associated RNAs can be identified for a specific RNA bait using oligo capture and subsequent RNA sequencing. All base-pairing RNA interactions can be profiled by ligating AMT-crosslinked RNAs and hybrid sequencing. Droplet barcoding of crosslinked RNAs is an alternative strategy to identify associated pairs. Specific bridging proteins can be isolated to identify which RNAs are being bridged via ligation and hybrid RNA sequencing. Note: protein bridging in the absence of base-pairing does not fall under this review’s definition of “RNA Matchmaking”.
Identifying RNA interactions with DNA
Initial methods to map how RNA associates with genomic DNA relied on targeting a specific RNA with capture oligonucleotides in a manner largely analogous to chromatin immunoprecipitation (ChIP)[108, 109], but isolating specific RNAs instead, by antisense oligonucleotide capture. ChOP (chromatin oligo-affinity precipitation), used biotinylated antisense oligonucleotides to target and capture Alu RNA from formaldehyde fixed cells[110]. Similarly, RAP[111] (RNA antisense purification), ChIRP[112] (Chromatin isolation by RNA purification), and CHART[113] (Capture Hybridization Analysis of RNA Targets) all couple the use of affinity-tagged antisense oligo probes (complimentary to a specific RNA) with next-generation sequencing to achieve high-resolution mapping of chromatin regions associated with a specific target RNA.
Each technique follows a similar protocol: following chemical fixation of cells and isolation/lysis of nuclei, sheared chromatin is incubated with biotinylated probes to anneal to RNA and subsequent capture via streptavidin-labeled magnetic beads that enables isolation of the bound complex of chromatin and associated RNA. The genomic DNA is then purified and used to prepare a sequencing library that allows for the identification of genomic regions that are bound by the target RNA (genomic locations with significant read density indicate that the RNA of interest had bound to that region). These methods are principally similar in their approach, with subtle differences in the chemical used to fix the cells (Disuccinimidyl glutarate (DSG) and formaldehyde for RAP, formaldehyde for CHART, glutaraldehyde for ChIRP). Elution from the annealed oligo bound to beads is accomplished by either RNAase A and H (ChIRP), RNase H only (CHART), or DNase (RAP). A key feature of all these sequencing approaches is the use of multiple oligonucleotides that are designed to be complementary to different regions of the target RNA. In contrast, a similar method termed CHIRT-seq[114], only uses a single oligonucleotide to allow for profiling of more repetitive RNAs. Lastly, a recent method termed HiChIRP[115] combines ChIRP-seq with Hi-C chromatin conformation capture to characterize specific RNAs involved in mediating inter-chromosomal interactions.
In contrast to the methods surveyed above where a specific RNA of interest is targeted, there are a number of newly developed genome-wide approaches that capture all RNA-DNA interactions to generate global binding profiles of RNA to chromatin. These methods, all coupled to high-throughput sequencing, include: ChAR-seq[116] (Chromatin-Associated RNA), GRID-seq[117] (Global RNA Interactions with DNA), and MARGI-seq[118] (Mapping RNA-genome interactions). The principle approach of these methods is to ligate chromatin-associated RNAs with the genomic DNA they are proximally associated with using a specially designed linker sequence that allows the RNA and DNA components of the chimeric fragment to be accurately differentiated after sequencing. While similar in technique (cells are cross-linked, permeabilized, and then the RNA and genomic DNA are partially digested to allow for proximity ligation), each approach has unique features. For instance, whereas the proximity ligations in MARGI-seq are performed on beads, ChAR-seq performs the ligations on intact nuclei which likely leads to a reduction in spurious events. Additionally, while all approaches use formaldehyde crosslinking, GRID-seq implements a second crosslinker, DSG, that can capture longer-range RNA:DNA interactions. There are also differences in the sequencing and library preparation; ChAR-seq uses long single-end reads to accurately capture the polarity of the bridge linker, whereas GRID-seq and MARGI-seq use paired-end reads, with MARGI-seq incorporating specific primers on either end of the fragment (allowing the DNA and RNA sides to be uniquely associated with a respective mate from the pair), and GRID-seq implementing a restriction enzyme step that results in identical size-selected fragments (which likely reduces the number of uninformative molecules that are sequenced, although the regions representing DNA and RNA are significantly shorter, i.e.19 to 23 bp, and thus potentially harder to accurately map). More recently, a new method, RD-SPRITE (RNA & DNA – Split-Pool Recognition of Interactions by Tag Extension) is a modification of a previous crosslinking-based approach that has been successfully used to generate 3D spatial maps of DNA:DNA interactions within the nucleus[119]. The RD-SPRITE method uses a split-and-pool barcoding strategy to uniquely tag all DNA and RNA components contained within the crosslinked complex allowing for accurate profiling of the 3D organization of these molecules within the nucleus. Because this method does not rely on proximity ligation, it can be used to detect higher-order structures of interacting RNA and DNA that might be overlooked by previous strategies.
Antibodies that recognize R-loops and RNA-DNA triplexes have also been used to isolate genomic regions forming these structures[95, 102]. In these ChIP experiments, the DNA is detected and the RNA molecule bound at a specific DNA locus is not definitively identified, precluding the distinction between R-loops that form through re-pairing with template DNA versus those that form with an RNA transcribed from a different DNA template.
RNA-RNA interactions with nascent RNA on chromatin
In addition to interacting with chromatin DNA, RNA molecules can also form intermolecular RNA-RNA duplexes with the nascent RNA being actively transcribed on chromatin. Such RNA complexes are used by many noncoding RNAs (ncRNAs) to regulate gene expression. Initial methods to profile intermolecular RNA-RNA interactions such as CLASH[120] (cross-linking, ligation, and sequencing of hybrids) and Hi-CLIP[121] relied on the RNA of interest being associated with a specific RNA binding protein. While these approaches can be useful for profiling specific functions of an RBP of interest, they are limited to interactions mediated by one RBP and they cannot distinguish between direct and indirect RNA-RNA interactions. More recently, a proximity ligation-based approach, RPL[122] (RNA proximity ligation) has been employed to profile global RNA-RNA interactions without the need for any ‘bait’ protein or RNA. Similar to 3C and Hi-C methods for DNA conformation, RPL uses digestion and re-ligation of RNA followed by deep sequencing to yield chimeric reads with ligation junctions in the vicinity of structurally proximate bases. However, as this method does not utilize any crosslinking, it is mainly limited to profiling intra-molecular RNA interactions as opposed to interactions between distinct RNA molecules.
For direct capture of intermolecular RNA interactions, the RAP method described above has been modified (RAP-RNA[89]) to allow for mapping of RNA-RNA interactions by coupling the use of biotinylated antisense oligos with aminomethyltrioxalen (AMT), a psoralen-derivative crosslinker. AMT generates reversible inter-strand crosslinks between uridine bases in base-paired RNA upon 365nm UV irradiation. AMT does not react with proteins, making it an ideal solution to identify direct RNA-RNA interactions at high-resolution in de-proteinized samples. The RAP-RNA technique is limited to profiling interactions with a single target RNA and is therefore unable to characterize the entire RNA:RNA interactome on a genome wide scale. A number of methods have expanded the use of psoralen crosslinking and coupled it with proximity ligation to generate global RNA:RNA interaction profiles. LIGR-seq[31] (LIGation of interacting RNA followed by high-throughput sequencing), SPLASH[32] (sequencing of psoralen crosslinked, ligated, and selected hybrids), and PARIS[30] (psoralen analysis of RNA interactions and structures) all use similar strategies (in vivo crosslinking with AMT, proximity ligation, and 2D purification of RNA duplexes) to generate genome-wide maps of RNA:RNA interactions. While these methods have been extremely useful in profiling RNA-RNA associations within cells, the question of where these RNAs are specifically localized when they form contacts remains unanswered. To address this, recently a non-psoralen-based RNA capture method, Proximity RNA-seq [123], was developed to profile the subcellular localization of interacting RNAs in 3D space. The technique uses a water-in-oil emulsion with beads that uniquely barcode cDNAs in millions of subnuclear particles with high-throughput sequencing to allow for accurate reconstruction of associations between chromatin-associated, nascent RNA, and non-coding RNAs within nuclei.
RNA Interactions Mediated by RNA Binding Proteins
While direct RNA-DNA and RNA-RNA interactions are widely prevalent, it has been repeatedly observed that such associations are often mediated by RNA-binding proteins (RBPs). Over 1,500 such proteins have been identified in mammalian cells [124], with coordinating roles that traverse all aspects of cellular regulatory function. Numerous CLIP-based techniques have been developed to profile RNA interactions with a specific protein of interest, and several of the methods outlined above have been coupled with mass-spectrometry to identify novel proteins that interact with RNA.
Strict focus on stable RNA duplexes that are captured through AMT misses the opportunity to broadly profile RBP-mediated interactions between RNAs. To address this challenge, MARIO[33] (Mapping RNA-RNA interactome and RNA structure in vivo) was developed to massively reveal RNA-RNA interactions on a global scale. MARIO can identify both protein-assisted inter and intra-molecular RNA interactions by crosslinking the RNAs to their bound proteins and then using proximity ligation to link RNA molecules that are associated with the same protein. This chimeric RNA molecule is then captured by streptavidin-coated magnetic beads and subjected to paired-end sequencing, with each resulting read reflecting a unique molecular interaction.
Most recently a technique termed RIC-seq[125] (RNA in situ conformation sequencing) uses a biotinylated cytidine phosphate (pCp-biotin) linker to capture RNA-RNA interactions. Briefly, instead of linking RNA strands with AMT, the RNAs are randomly digested with micrococcal nuclease and dephosphorylated at their 3′ overhangs; these open 3′ ends are then labelled with the pCp-biotin and subsequently ligated to other RNA fragments in close proximity. After extracting and purifying the biotin-containing RNA, the fragments are converted into strand-specific libraries for sequencing. While other techniques are also able to generate three-dimensional interaction maps of RNA, the pCp-biotin labeling employed by RIC-seq adds utility via its ability to profile such interactions at single-nucleotide resolution.
Conclusion
The ability of RNA to interact with chromatin or chromatin-associated RNA provides allows for multiple mechanisms of genome regulation. RNA matchmaking of lncRNAs or small RNAs harnesses perfect and imperfect base-pairing sequences, as well as interactions with proteins, to achieve targeting and RNA remodeling for these mechanisms. The wealth of techniques for studying these interactions will facilitate the exploration of RNA matchmaking into many aspects of biological function.
Perspectives.
Noncoding RNAs are abundant in the nucleus. Their ability to interact with chromatin, via RNA matchmaking by base-pairing to RNA/DNA or bridged by protein, allows them to target specific genomic loci for multiple types of regulatory mechanisms.
Long noncoding RNAs and small RNAs are both able to use nucleotide sequence in RNA matchmaking, often to nascent RNA, to directly influence chromatin regulation or by recruiting additional regulatory proteins.
Techniques to profile RNA matchmaking interactions on chromatin have only recently been developed and offer a path to identifying and characterizing novel regulatory mechanisms.
Acknowledgements
We thank Allison Porman for helpful comments on the manuscript. This work was supported by NIH grants F31CA247343 (J.T), T32GM008730 (J.T.), and R35GM119575 (A.M.J).
Footnotes
Competing Interests
The authors declare that they have no competing interests relating to the content of this manuscript.
References
- 1.Djebali S, et al. , Landscape of transcription in human cells. Nature. 489(7414): p. 101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalil AM, et al. , Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A, 2009. 106(28): p. 11667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He C, et al. , High-Resolution Mapping of RNA-Binding Regions in the Nuclear Proteome of Embryonic Stem Cells. Mol Cell, 2016. 64(2): p. 416–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X and Fu XD, Chromatin-associated RNAs as facilitators of functional genomic interactions. Nat Rev Genet, 2019. 20(9): p. 503–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Portman DS and Dreyfuss G, RNA annealing activities in HeLa nuclei. EMBO J, 1994. 13(1): p. 213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weighardt F, Biamonti G, and Riva S, The roles of heterogeneous nuclear ribonucleoproteins (hnRNP) in RNA metabolism. Bioessays, 1996. 18(9): p. 747–56. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Contreras R, et al. , hnRNP proteins and splicing control. Adv Exp Med Biol, 2007. 623: p. 123–47. [DOI] [PubMed] [Google Scholar]
- 8.Updegrove TB, Zhang A, and Storz G, Hfq: the flexible RNA matchmaker. Curr Opin Microbiol, 2016. 30: p. 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schumacher MA, et al. , Crystal structures of T. brucei MRP1/MRP2 guide-RNA binding complex reveal RNA matchmaking mechanism. Cell, 2006. 126(4): p. 701–11. [DOI] [PubMed] [Google Scholar]
- 10.Bae E, et al. , Structure and interactions of the first three RNA recognition motifs of splicing factor prp24. J Mol Biol, 2007. 367(5): p. 1447–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah BN, Liu X, and Correll CC, Imp3 unfolds stem structures in pre-rRNA and U3 snoRNA to form a duplex essential for small subunit processing. RNA, 2013. 19(10): p. 1372–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graveley BR, RNA Matchmaking: Finding Cellular Pairing Partners. Mol Cell, 2016. 63(2): p. 186–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holoch D and Moazed D, RNA-mediated epigenetic regulation of gene expression. Nat Rev Genet, 2015. 16(2): p. 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson WL and Straight AF, RNA-mediated regulation of heterochromatin. Curr Opin Cell Biol, 2017. 46: p. 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rinn JL and Chang HY, Long Noncoding RNAs: Molecular Modalities to Organismal Functions. Annu Rev Biochem, 2020. 89: p. 283–308. [DOI] [PubMed] [Google Scholar]
- 16.Meister G, Argonaute proteins: functional insights and emerging roles. Nat Rev Genet, 2013. 14(7): p. 447–59. [DOI] [PubMed] [Google Scholar]
- 17.Balas M.M.a.J., A. M., Exploring the mechanisms behind long noncoding RNAs and cancer. Non-coding RNA Research, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonasio R and Shiekhattar R, Regulation of transcription by long noncoding RNAs. Annu Rev Genet, 2014. 48: p. 433–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khelifi G and Hussein SMI, A New View of Genome Organization Through RNA Directed Interactions. Front Cell Dev Biol, 2020. 8: p. 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khanduja JS, et al. , Nuclear Noncoding RNAs and Genome Stability. Mol Cell, 2016. 63(1): p. 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen ED, et al. , Global profiling of hnRNP A2/B1-RNA binding on chromatin highlights LncRNA interactions. RNA Biol, 2018: p. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kretz M, et al. , Control of somatic tissue differentiation by the long non-coding RNA TINCR. Nature, 2013. 493(7431): p. 231–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson R and Guigo R, The RIDL hypothesis: transposable elements as functional domains of long noncoding RNAs. RNA, 2014. 20(7): p. 959–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balas MM, et al. , RNA matchmaking remodels lncRNA structure and promotes PRC2 activity. bioRxiv, 2020: p. 2020.04.13.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nepal C, et al. , Ancestrally Duplicated Conserved Noncoding Element Suggests Dual Regulatory Roles of HOTAIR in cis and trans. iScience, 2020. 23(4): p. 101008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lappin TR, et al. , HOX genes: seductive science, mysterious mechanisms. Ulster Med J, 2006. 75(1): p. 23–31. [PMC free article] [PubMed] [Google Scholar]
- 27.Pelechano V and Steinmetz LM, Gene regulation by antisense transcription. Nat Rev Genet, 2013. 14(12): p. 880–93. [DOI] [PubMed] [Google Scholar]
- 28.Sanz LA, et al. , Prevalent, Dynamic, and Conserved R-Loop Structures Associate with Specific Epigenomic Signatures in Mammals. Mol Cell, 2016. 63(1): p. 167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Postepska-Igielska A, Blank-Giwojna A, and Grummt I, Analysis of RNA-DNA Triplex Structures In Vitro and In Vivo. Methods Mol Biol, 2020. 2161: p. 229–246. [DOI] [PubMed] [Google Scholar]
- 30.Lu Z, et al. , RNA Duplex Map in Living Cells Reveals Higher-Order Transcriptome Structure. Cell, 2016. 165(5): p. 1267–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma E, et al. , Global Mapping of Human RNA-RNA Interactions. Mol Cell, 2016. 62(4): p. 618–26. [DOI] [PubMed] [Google Scholar]
- 32.Aw JG, et al. , In Vivo Mapping of Eukaryotic RNA Interactomes Reveals Principles of Higher-Order Organization and Regulation. Mol Cell, 2016. 62(4): p. 603–17. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen TC, et al. , Mapping RNA-RNA interactome and RNA structure in vivo by MARIO. Nat Commun, 2016. 7: p. 12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carmell MA, et al. , The Argonaute family: tentacles that reach into RNAi, developmental control, stem cell maintenance, and tumorigenesis. Genes Dev, 2002. 16(21): p. 2733–42. [DOI] [PubMed] [Google Scholar]
- 35.Joshua-Tor L and Hannon GJ, Ancestral roles of small RNAs: an Ago-centric perspective. Cold Spring Harb Perspect Biol, 2011. 3(10): p. a003772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Czech B and Hannon GJ, One Loop to Rule Them All: The Ping-Pong Cycle and piRNA-Guided Silencing. Trends Biochem Sci, 2016. 41(4): p. 324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalantari R, Chiang CM, and Corey DR, Regulation of mammalian transcription and splicing by Nuclear RNAi. Nucleic Acids Res, 2016. 44(2): p. 524–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alper BJ, Lowe BR, and Partridge JF, Centromeric heterochromatin assembly in fission yeast--balancing transcription, RNA interference and chromatin modification. Chromosome Res, 2012. 20(5): p. 521–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martienssen R and Moazed D, RNAi and heterochromatin assembly. Cold Spring Harb Perspect Biol, 2015. 7(8): p. a019323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakayama J, et al. , Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science, 2001. 292(5514): p. 110–3. [DOI] [PubMed] [Google Scholar]
- 41.Yamada T, et al. , The nucleation and maintenance of heterochromatin by a histone deacetylase in fission yeast. Mol Cell, 2005. 20(2): p. 173–85. [DOI] [PubMed] [Google Scholar]
- 42.Volpe TA, et al. , Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science, 2002. 297(5588): p. 1833–7. [DOI] [PubMed] [Google Scholar]
- 43.Buhler M, Verdel A, and Moazed D, Tethering RITS to a nascent transcript initiates RNAi- and heterochromatin-dependent gene silencing. Cell, 2006. 125(5): p. 873–86. [DOI] [PubMed] [Google Scholar]
- 44.Aygun O, Mehta S, and Grewal SI, HDAC-mediated suppression of histone turnover promotes epigenetic stability of heterochromatin. Nat Struct Mol Biol, 2013. 20(5): p. 547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shimada Y, Mohn F, and Buhler M, The RNA-induced transcriptional silencing complex targets chromatin exclusively via interacting with nascent transcripts. Genes Dev, 2016. 30(23): p. 2571–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Younger ST and Corey DR, Transcriptional gene silencing in mammalian cells by miRNA mimics that target gene promoters. Nucleic Acids Res, 2011. 39(13): p. 5682–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carissimi C, et al. , ARGONAUTE2 cooperates with SWI/SNF complex to determine nucleosome occupancy at human Transcription Start Sites. Nucleic Acids Res, 2015. 43(3): p. 1498–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aravin A, et al. , A novel class of small RNAs bind to MILI protein in mouse testes. Nature, 2006. 442(7099): p. 203–7. [DOI] [PubMed] [Google Scholar]
- 49.Vagin VV, et al. , A distinct small RNA pathway silences selfish genetic elements in the germline. Science, 2006. 313(5785): p. 320–4. [DOI] [PubMed] [Google Scholar]
- 50.Czech B, et al. , piRNA-Guided Genome Defense: From Biogenesis to Silencing. Annu Rev Genet, 2018. 52: p. 131–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pezic D, et al. , piRNA pathway targets active LINE1 elements to establish the repressive H3K9me3 mark in germ cells. Genes Dev, 2014. 28(13): p. 1410–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagamori I, et al. , Relationship between PIWIL4-Mediated H3K4me2 Demethylation and piRNA-Dependent DNA Methylation. Cell Rep, 2018. 25(2): p. 350–356. [DOI] [PubMed] [Google Scholar]
- 53.Li XZ, et al. , An ancient transcription factor initiates the burst of piRNA production during early meiosis in mouse testes. Mol Cell, 2013. 50(1): p. 67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shoji M, et al. , The TDRD9-MIWI2 complex is essential for piRNA-mediated retrotransposon silencing in the mouse male germline. Dev Cell, 2009. 17(6): p. 775–87. [DOI] [PubMed] [Google Scholar]
- 55.Klenov MS, et al. , Impact of nuclear Piwi elimination on chromatin state in Drosophila melanogaster ovaries. Nucleic Acids Res, 2014. 42(10): p. 6208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalmykova AI, Klenov MS, and Gvozdev VA, Argonaute protein PIWI controls mobilization of retrotransposons in the Drosophila male germline. Nucleic Acids Res, 2005. 33(6): p. 2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carmell MA, et al. , MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev Cell, 2007. 12(4): p. 503–14. [DOI] [PubMed] [Google Scholar]
- 58.De Fazio S, et al. , The endonuclease activity of Mili fuels piRNA amplification that silences LINE1 elements. Nature, 2011. 480(7376): p. 259–63. [DOI] [PubMed] [Google Scholar]
- 59.Reuter M, et al. , Miwi catalysis is required for piRNA amplification-independent LINE1 transposon silencing. Nature, 2011. 480(7376): p. 264–7. [DOI] [PubMed] [Google Scholar]
- 60.Yamaguchi S, et al. , Crystal structure of Drosophila Piwi. Nat Commun, 2020. 11(1): p. 858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sienski G, et al. , Silencio/CG9754 connects the Piwi-piRNA complex to the cellular heterochromatin machinery. Genes Dev, 2015. 29(21): p. 2258–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu Y, et al. , Panoramix enforces piRNA-dependent cotranscriptional silencing. Science, 2015. 350(6258): p. 339–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Donertas D, Sienski G, and Brennecke J, Drosophila Gtsf1 is an essential component of the Piwi-mediated transcriptional silencing complex. Genes Dev, 2013. 27(15): p. 1693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Post C, et al. , The capacity of target silencing by Drosophila PIWI and piRNAs. RNA, 2014. 20(12): p. 1977–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vagin VV, et al. , Proteomic analysis of murine Piwi proteins reveals a role for arginine methylation in specifying interaction with Tudor family members. Genes Dev, 2009. 23(15): p. 1749–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zoch A, et al. , SPOCD1 is an essential executor of piRNA-directed de novo DNA methylation. Nature, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roovers EF, et al. , Piwi proteins and piRNAs in mammalian oocytes and early embryos. Cell Rep, 2015. 10(12): p. 2069–82. [DOI] [PubMed] [Google Scholar]
- 68.Ozata DM, et al. , PIWI-interacting RNAs: small RNAs with big functions. Nat Rev Genet, 2019. 20(2): p. 89–108. [DOI] [PubMed] [Google Scholar]
- 69.Lee N, et al. , EBV noncoding RNA binds nascent RNA to drive host PAX5 to viral DNA. Cell, 2015. 160(4): p. 607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nanni AV and Lee N, Identification of host RNAs that interact with EBV noncoding RNA EBER2. RNA Biol, 2018. 15(9): p. 1181–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Davidovich C and Cech TR, The recruitment of chromatin modifiers by long noncoding RNAs: lessons from PRC2. RNA, 2015. 21(12): p. 2007–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Almeida M, Bowness JS, and Brockdorff N, The many faces of Polycomb regulation by RNA. Curr Opin Genet Dev, 2020. 61: p. 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Davidovich C, et al. , Promiscuous RNA binding by Polycomb repressive complex 2. Nat Struct Mol Biol, 2013. 20(11): p. 1250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang X, et al. , Targeting of Polycomb Repressive Complex 2 to RNA by Short Repeats of Consecutive Guanines. Mol Cell, 2017. 65(6): p. 1056–1067 e5. [DOI] [PubMed] [Google Scholar]
- 75.Wang X, et al. , Molecular analysis of PRC2 recruitment to DNA in chromatin and its inhibition by RNA. Nat Struct Mol Biol, 2017. 24(12): p. 1028–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaneko S, et al. , Nascent RNA interaction keeps PRC2 activity poised and in check. Genes Dev, 2014. 28(18): p. 1983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beltran M, et al. , G-tract RNA removes Polycomb repressive complex 2 from genes. Nat Struct Mol Biol, 2019. 26(10): p. 899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meredith EK, et al. , An RNA matchmaker protein regulates the activity of the long noncoding RNA HOTAIR. RNA, 2016. 22(7): p. 995–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Skourti-Stathaki K, et al. , R-Loops Enhance Polycomb Repression at a Subset of Developmental Regulator Genes. Mol Cell, 2019. 73(5): p. 930–945 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alecki C, et al. , RNA-DNA strand exchange by the Drosophila Polycomb complex PRC2. Nat Commun, 2020. 11(1): p. 1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rinn JL, et al. , Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell, 2007. 129(7): p. 1311–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Amandio AR, et al. , Hotair Is Dispensible for Mouse Development. PLoS Genet, 2016. 12(12): p. e1006232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gupta RA, et al. , Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature, 2010. 464(7291): p. 1071–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Portoso M, et al. , PRC2 is dispensable for HOTAIR-mediated transcriptional repression. EMBO J, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu B, et al. , Molecular basis for the specific and multivariant recognitions of RNA substrates by human hnRNP A2/B1. Nat Commun, 2018. 9(1): p. 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Long Y, et al. , RNA is essential for PRC2 chromatin occupancy and function in human pluripotent stem cells. Nat Genet, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee B, et al. , The long noncoding RNA SPRIGHTLY acts as an intranuclear organizing hub for pre-mRNA molecules. Sci Adv, 2017. 3(5): p. e1602505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yin Y, et al. , U1 snRNP regulates chromatin retention of noncoding RNAs. Nature, 2020. 580(7801): p. 147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Engreitz JM, et al. , RNA-RNA interactions enable specific targeting of noncoding RNAs to nascent Pre-mRNAs and chromatin sites. Cell, 2014. 159(1): p. 188–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gutschner T, Hammerle M, and Diederichs S, MALAT1 -- a paradigm for long noncoding RNA function in cancer. J Mol Med (Berl), 2013. 91(7): p. 791–801. [DOI] [PubMed] [Google Scholar]
- 91.Sun Q, Hao Q, and Prasanth KV, Nuclear Long Noncoding RNAs: Key Regulators of Gene Expression. Trends Genet, 2018. 34(2): p. 142–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hu S, Wang X, and Shan G, Insertion of an Alu element in a lncRNA leads to primate-specific modulation of alternative splicing. Nat Struct Mol Biol, 2016. 23(11): p. 1011–1019. [DOI] [PubMed] [Google Scholar]
- 93.Chen H and Shan G, The physiological function of long-noncoding RNAs. Noncoding RNA Res, 2020. 5(4): p. 178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bacolla A, Wang G, and Vasquez KM, New Perspectives on DNA and RNA Triplexes As Effectors of Biological Activity. PLoS Genet, 2015. 11(12): p. e1005696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li Y, Syed J, and Sugiyama H, RNA-DNA Triplex Formation by Long Noncoding RNAs. Cell Chem Biol, 2016. 23(11): p. 1325–1333. [DOI] [PubMed] [Google Scholar]
- 96.Mondal T, et al. , MEG3 long noncoding RNA regulates the TGF-beta pathway genes through formation of RNA-DNA triplex structures. Nat Commun, 2015. 6: p. 7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O’Leary VB, et al. , Long non-coding RNA PARTICLE bridges histone and DNA methylation. Sci Rep, 2017. 7(1): p. 1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grote P, et al. , The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev Cell, 2013. 24(2): p. 206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grote P and Herrmann BG, The long non-coding RNA Fendrr links epigenetic control mechanisms to gene regulatory networks in mammalian embryogenesis. RNA Biol, 2013. 10(10): p. 1579–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cheung AC, Sainsbury S, and Cramer P, Structural basis of initial RNA polymerase II transcription. EMBO J, 2011. 30(23): p. 4755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chedin F, Nascent Connections: R-Loops and Chromatin Patterning. Trends Genet, 2016. 32(12): p. 828–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Niehrs C and Luke B, Regulatory R-loops as facilitators of gene expression and genome stability. Nat Rev Mol Cell Biol, 2020. 21(3): p. 167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Skourti-Stathaki K and Proudfoot NJ, A double-edged sword: R loops as threats to genome integrity and powerful regulators of gene expression. Genes Dev, 2014. 28(13): p. 1384–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sun Q, et al. , R-loop stabilization represses antisense transcription at the Arabidopsis FLC locus. Science, 2013. 340(6132): p. 619–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gibbons HR, et al. , Divergent lncRNA GATA3-AS1 Regulates GATA3 Transcription in T-Helper 2 Cells. Front Immunol, 2018. 9: p. 2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Beckedorff FC, et al. , The intronic long noncoding RNA ANRASSF1 recruits PRC2 to the RASSF1A promoter, reducing the expression of RASSF1A and increasing cell proliferation. PLoS Genet, 2013. 9(8): p. e1003705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guh CY, Hsieh YH, and Chu HP, Functions and properties of nuclear lncRNAs-from systematically mapping the interactomes of lncRNAs. J Biomed Sci, 2020. 27(1): p. 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Johnson DS, et al. , Genome-wide mapping of in vivo protein-DNA interactions. Science, 2007. 316(5830): p. 1497–502. [DOI] [PubMed] [Google Scholar]
- 109.Kim TH and Ren B, Genome-wide analysis of protein-DNA interactions. Annu Rev Genomics Hum Genet, 2006. 7: p. 81–102. [DOI] [PubMed] [Google Scholar]
- 110.Mariner PD, et al. , Human Alu RNA is a modular transacting repressor of mRNA transcription during heat shock. Mol Cell, 2008. 29(4): p. 499–509. [DOI] [PubMed] [Google Scholar]
- 111.Engreitz JM, et al. , The Xist lncRNA exploits three-dimensional genome architecture to spread across the X chromosome. Science, 2013. 341(6147): p. 1237973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chu C, et al. , Genomic Maps of Long Noncoding RNA Occupancy Reveal Principles of RNA-Chromatin Interactions. Mol Cell, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Simon MD, et al. , The genomic binding sites of a noncoding RNA. Proc Natl Acad Sci U S A, 2011. 108(51): p. 20497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chu HP, et al. , TERRA RNA Antagonizes ATRX and Protects Telomeres. Cell, 2017. 170(1): p. 86–101 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mumbach MR, et al. , HiChIRP reveals RNA-associated chromosome conformation. Nat Methods, 2019. 16(6): p. 489–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bell JC, et al. , Chromatin-associated RNA sequencing (ChAR-seq) maps genome-wide RNA-to-DNA contacts. Elife, 2018. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li X, et al. , GRID-seq reveals the global RNA-chromatin interactome. Nat Biotechnol, 2017. 35(10): p. 940–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sridhar B, et al. , Systematic Mapping of RNA-Chromatin Interactions In Vivo. Curr Biol, 2017. 27(4): p. 610–612. [DOI] [PubMed] [Google Scholar]
- 119.Quinodoz SA, et al. , RNA promotes the formation of spatial compartments in the nucleus. bioRxiv, 2020: p. 2020.08.25.267435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Helwak A, et al. , Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell, 2013. 153(3): p. 654–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sugimoto Y, et al. , hiCLIP reveals the in vivo atlas of mRNA secondary structures recognized by Staufen 1. Nature, 2015. 519(7544): p. 491–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ramani V, Qiu R, and Shendure J, High-throughput determination of RNA structure by proximity ligation. Nat Biotechnol, 2015. 33(9): p. 980–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Morf J, et al. , RNA proximity sequencing reveals the spatial organization of the transcriptome in the nucleus. Nat Biotechnol, 2019. 37(7): p. 793–802. [DOI] [PubMed] [Google Scholar]
- 124.Gerstberger S, Hafner M, and Tuschl T, A census of human RNA-binding proteins. Nat Rev Genet, 2014. 15(12): p. 829–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cai Z, et al. , RIC-seq for global in situ profiling of RNA-RNA spatial interactions. Nature, 2020. 582(7812): p. 432–437. [DOI] [PubMed] [Google Scholar]