Abstract
Objective:
Neurological complications occur in up to 40% of adult solid organ abdominal transplant (SOT) recipients and are associated with increased mortality. Comparable pediatric data are sparse. This study describes the occurrence of neurological and behavioral complications (neurobehavioral complications) in pediatric SOT recipients. We examine the association of these complications with length of stay, mortality, and tacrolimus levels.
Design:
The electronic health record (EHR) was interrogated for inpatient readmissions of pediatric SOT recipients from 2009-2017. A computable composite definition of neurobehavioral complication, defined using structured electronic data for neurological and/or behavioral phenotypes, was created.
Setting:
Quaternary children’s hospital with an active transplant program
Patients:
Pediatric SOT recipients
Interventions:
None
Measurements and Main Results:
Computable phenotypes demonstrated a specificity 98.7% and sensitivity of 63.0% for identifying neurobehavioral complications. There were 1542 readmissions among 318 patients, with 65 (20.4%) having at least one admission with a neurobehavioral complication (total 109 admissions). Median time from transplant to admission with neurobehavioral complication was 1.2 years [IQR 0.52, 2.28]. Compared to encounters without an identified neurobehavioral complication, encounters with a neurobehavioral complication were more likely to experience intensive care unit (ICU) admission (OR 3.9 [2.41-6.64]; P<0.001), have longer ICU length of stay (LOS, median 10.3 vs. 2.2 days; P<0.001) and hospital LOS (8.9 vs. 4.3 days; P<0.001), and demonstrate higher maximum tacrolimus level (12.3 vs. 9.8 ng/ml; P=0.001). Patients with a neurobehavioral complication admission were more likely to die (OR 5.04 [1.49-17.09], P=0.009). In a multivariable analysis, type of transplant, ICU admission, and tacrolimus levels were independently associated with the presence of a neurobehavioral complication.
Conclusions:
Common EHR variables can be used to accurately identify neurobehavioral complications in the pediatric SOT population. Late neurobehavioral complications are associated with increased hospital resource utilization, mortality, and tacrolimus exposure. Additional studies are required to delineate the relationship between maximum tacrolimus level and neurobehavioral complications to guide therapeutic drug monitoring and dosing.
Keywords: pediatric solid organ transplant, abdominal, neurological complication, neurobehavioral complication, computable phenotype, tacrolimus
Introduction
It has been reported that 15-40% of adult abdominal transplant recipients experience a neurological complication in the post-transplant period, such as seizures, encephalopathy or central nervous system lymphoproliferative disease, and these morbidities have been associated with increased length of stay (LOS) and greater mortality (1–6). Most neurological complications have been reported in the first 6 months after transplant, though late complications have also been noted to occur (1–4). Although data are more limited, studies of small cohorts of pediatric transplant recipients suggest similar risks compared to adult patients. The reported prevalence of neurological complications among children receiving a solid organ transplant (SOT) is 15-35%. The most commonly reported neurological complication is seizures, typically occurring soon after transplant, and the presence of such morbidities have been associated with increased mortality (7–12). Both pediatric and adult studies have shown an association with the immunosuppressant tacrolimus and post-transplant neurological and/or behavioral complications (5, 10, 13–15).
The pediatric transplant program at University of Pittsburgh Medical Center (UPMC) Children’s Hospital of Pittsburgh (CHP) has been a leading center for pediatric transplantation over the past 30 years. The objective of this study was to describe the prevalence of neurological and/or behavioral complications among a large, single-center cohort of pediatric transplant recipients, and identify factors associated with these morbidities. We hypothesized that neurological and/or behavioral complications would be associated with increased hospitalizations, longer hospital and intensive care unit (ICU) LOS, increased mortality, and higher serum tacrolimus levels.
Methods
Study Subjects
Institutional Review Board approval was obtained from the University of Pittsburgh. All patients who received a solid organ abdominal transplant (SOT) from January 2009 – December 2016 with subsequent inpatient readmissions to Children’s Hospital of Pittsburgh (CHP) until July 2017 were included. An admission was classified as inpatient if the hospital length of stay was greater than 24 hours. CHP is a quaternary children’s hospital with an active SOT program during the study period. Solid organ recipients included patients who received a liver, intestinal, kidney, liver with kidney, liver with double lung, or a multi-visceral transplant. Multi-visceral transplant patients included those who received a stomach, duodenum, pancreas, and small intestine, as well as modifications that included liver and/or colon transplantation.
Data Variables
Data were extracted from the institution’s electronic health record (EHR)-derived (Cerner, Kansas City, Missouri, USA) clinical data warehouse using the business intelligence platform SAP BusinessObjects (SAP, Walldorf, Germany). Data extracted included demographics, hospital and ICU length of stay, disposition, neuro-imaging and electroencephalogram (EEG) reports, medication administration, biochemical serum measurements, and tacrolimus levels. Transplant specific data such as transplant date and time, reason for transplant and type of transplant were obtained from a separate institutional transplant database.
Computable Phenotypes for Neurobehavioral Complications
Computable phenotypes allow for classification of a disease using data available in the EHR (16, 17). Currently there are no available computable phenotypes to describe neurological or behavioral complications during or after a hospital admission for children. We grouped neurobehavioral complication phenotypes into three phenotypes: neurological, behavioral, or a combination of both complications (Supplementary Table 1). Neurological complications were defined as documentation of neuroimaging (brain magnetic resonance imaging (MRI) or head computerized tomography (CT)) or EEG obtained during admission. Inclusion was based on whether the test was obtained, irrespective of the test results. Behavioral complications were defined as a behavioral health consult (the study center’s inpatient mental health service) in addition to administration of an anti-psychotic medication (e.g. olanzapine or haloperidol) or dexmedetomidine during admission. Dexmedetomidine administration was included in this definition as it is commonly used at the study center to treat patients with agitated delirium (18).
Data Verification
Quality of data extraction was confirmed by two different individuals (AMA and EJY). Chart review was performed for all admissions with an identified neurobehavioral complication using the phenotype, as well as 10% of the admissions without an identified neurobehavioral complication using the phenotype. Sensitivity and specificity were then calculated. The proportion of admissions without an identified neurobehavioral complication that were reviewed for validation was determined a priori. Comparisons between the chart review results and the International Classification of Diseases (ICD) 9 and 10 diagnostic codes extracted from the clinical data warehouse were also made in a similar fashion for the same select admissions.
Phenotype computations to classify admissions were found to have 100% interrater reliability. Chart review of all admissions with a neurobehavioral complication identified by the computable phenotype showed 17 misclassified encounters without acute neurological or behavioral pathology, or false positives for a neurobehavioral complication. The most common reason for a false positive neurobehavioral complication was concern for post-transplant lymphoproliferative disorder (PTLD) with a negative brain MRI or head CT, as these patients had neuroimaging performed for disease staging, irrespective of neurologic symptoms. Of the admissions that did not meet a neurobehavioral complication phenotype definition, 155 (10%) of the admissions were screened and 6 (3.9%) were found to be incorrectly classified, or false negatives. The reasons for these false negatives included significant behavioral concerns without an identified behavioral health consultation, a headache without neuroimaging performed at the study center, and seizure without EEG ordered. Accounting for these misclassifications and extrapolating these findings, the neurobehavioral complication phenotype definitions have an estimated sensitivity of 63.0%, an estimated specificity of 98.7%, an estimated positive predictive value (PPV) of 84.4% and an estimated negative predictive value (NPV) of 96.2%. In comparison, the ICD codes demonstrated a sensitivity of 15.3%, specificity of 99.4%, positive predictive value of 93.6% and negative predictive value of 66.5% for detection of a neurobehavioral diagnosis.
Statistical Analysis
Categorical variables were described with frequencies and percentages, while median and interquartile ranges (IQRs) were used to describe continuous variables. For patient level analyses, the Chi-squared test was used for categorical variables and Wilcoxon rank-sum test was used for non-parametric continuous variables in univariate analysis. Multivariable logistic regression was used to assess the effect of demographic variables (age at time of admission, gender, race), type of transplant, need for ICU admission and medication administration (glucocorticoids and tacrolimus) on the outcome of meeting criteria for a neurobehavioral complication. Generalized estimating equations with robust standard errors, clustered by patient, were used to account for patients with multiple admissions in the multivariable model. Statistical analysis was performed using Stata, Version 15 (StataCorp, College Station, TX).
Results
A total of 401 patients received a SOT between 2009-2016, of which 318 patients had at least one inpatient admission following the initial transplant period. There were 1542 inpatient admissions that met inclusion criteria. Sixty-five patients (20.4%) had at least one identified neurobehavioral complication using the computable phenotype definitions, contributing to 109 admissions with a detected neurobehavioral complication (Figure 1). Patients with more readmissions were also more likely to have at least one admission with an identified neurobehavioral complication. Two hundred and thirty-six patients had 1-5 readmissions following the transplant period, of which 25 (10.5%) had at least one neurobehavioral complication admission. Fifty patients had 6-10 readmissions and 16 (32%) had at least one neurobehavioral complication admission. Twenty-two patients had 11-20 readmissions and 15 (68.2%) had at least one neurobehavioral complication admission. Lastly, 10 patients had over 21 readmissions (Figure 2). Supplemental Figure 1 demonstrates the types of admissions (neurobehavioral complication present versus absent) relative to the time from transplant. Of all neurobehavioral complication admissions, 55 were classified as neurological, 44 were classified as behavioral and 10 were classified as a combination of both neurological and behavioral complications.
Figure 1.
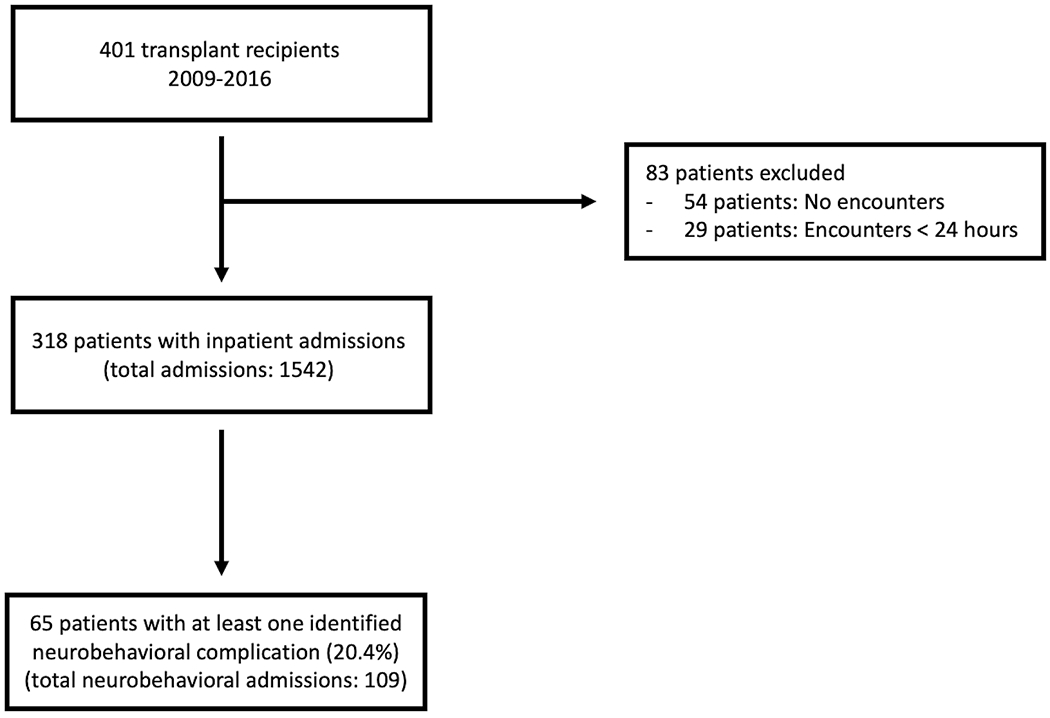
Flow diagram of the study population and included admissions
Figure 2.
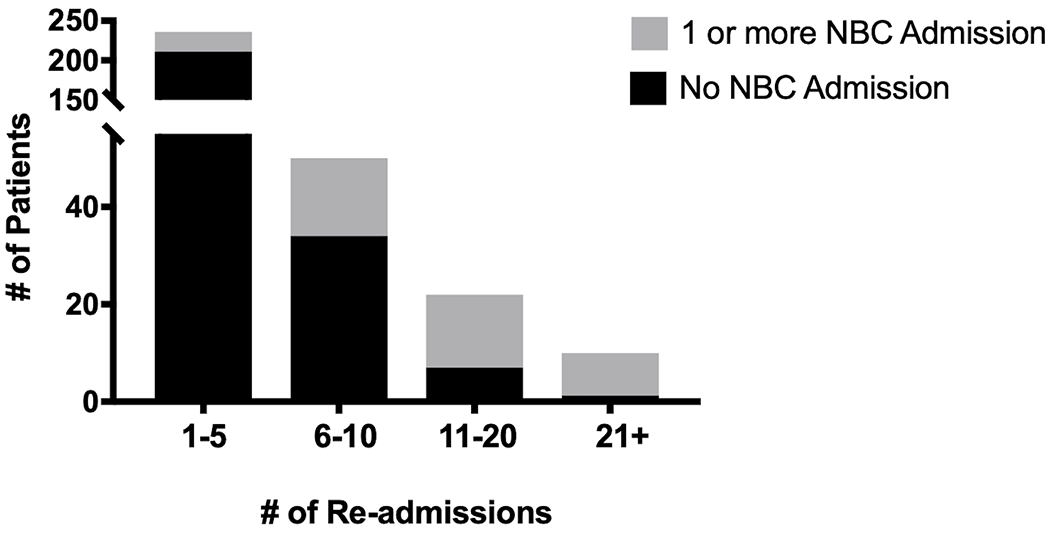
Proportion of patients with an identified neurobehavioral complication admission based on the number of readmissions. NBC = neurobehavioral complication
Demographics for the cohort are shown in Table 1. Of the 186 patients who received a liver transplant, 53 (28.5%) were grafts from living donors. Forty-four (56.4%) kidney transplant recipients received a living donor graft. Gender and race were similar between patients with and without a neurobehavioral complication admission. Patients with an identified neurobehavioral complication were older at the time of transplant and had more admissions post-transplant compared to patients who did not have an admission with a neurobehavioral complication. Table 2 shows hospital and ICU LOS by admission type. Encounters with an identified neurobehavioral complication had longer hospital LOS, were more likely to require ICU admission, and had longer ICU LOS compared to encounters not associated with a neurobehavioral complication. There were 12 deaths in the cohort and 4 (33%) occurred during an admission with an identified neurobehavioral complication. Patients with at least one neurobehavioral complication admission were more likely to die during the study period (OR 5.04 [95% CI 1.49-17.09], P=0.009).
Table 1.
Baseline Demographics of Transplant Recipients with and without an Identified Neurobehavioral Complication
| Patient Characteristic | At Least One Neurobehavioral Admission (n = 65 patients) | No Neurobehavioral Admissions (n = 253 patients) |
|---|---|---|
| Gender (n, % male) | 30 (46.2%) | 132 (52.2%) |
| Race (n, %) | ||
| White | 54 (83.1%) | 214 (84.6%) |
| Black | 6 (9.2%) | 15 (5.9%) |
| Other | 5 (7.7%) | 24 (9.5%) |
| Type of Transplant (n, %) | ||
| Liver | 23 (35.4%) | 164 (64.8%) |
| Kidney | 17 (26.2%) | 61 (24.1%) |
| Multi-visceral | 10 (15.4%) | 14 (5.5%) |
| Intestinal | 14 (21.5%) | 10 (4.0%) |
| Liver/Kidney | -- | 4 (1.6%) |
| Liver/Lung | 1 (1.5%) | -- |
| Reason for Transplant (n, %) | ||
| Structural/Anatomical | 26 (40.0%) | 72 (28.5%) |
| Primary | 19 (29.2%) | 60 (23.7%) |
| Secondary | 8 (12.3%) | 26 (10.3%) |
| Autoimmune | 7 (10.8%) | 18 (7.1%) |
| Metabolic | 3 (4.6%) | 61 (24.1%) |
| Malignancy | 2 (3.1%) | 16 (6.3 %) |
| Age at time of transplant, years (median, IQR) | 9.8 (4.8, 16.6) | 7.0 (2.4, 14.7) |
| Number admissions post-transplant (median, IQR) | 8 (3, 12) | 2 (1, 4) |
| Time to 1st post-transplant admission, days (median, IQR) | 129.1 (63.1, 475.2) | 90.2 (40.6, 374.6) |
Table 2.
Outcomes of Admissions with and without an Identified Neurobehavioral Complication
| Admission Details | Neurobehavioral Admissions (n = 109) | Non-Neurobehavioral Admissions (n = 1433) | |
|---|---|---|---|
| PICU admission, n (%) | 30 (27.5%) | 125 (8.7%) | OR 3.9 (95% CI 2.41-6.64), P<0.001 |
| PICU LOS, days (median, IQR) | 10.3 (2.9, 27.6) | 2.2 (1.1, 4.9) | P<0.001 |
| Hospital LOS, days (median, IQR) | 8.9 (4.3, 28.4) | 4.3 (2.4, 7.4) | P<0.001 |
LOS = Length of Stay
Neuroimaging was obtained in 62 (95.4%) of patients with a neurological or combined neurological and behavioral phenotype and an abnormality was detected in 22 (35.5%) and 25 (40.3%) of these patients, respectively. Head CT imaging was performed in 33 (50.7%) of admissions with a neurological or combined neurological and behavioral phenotype. This imaging was normal in 18 (54.6%), demonstrated a previously identified abnormality in 11 (33.3%), and detected a new abnormality in 4 (12.1%) admissions. Ten of the 11 (90.9%) previously identified abnormalities were volume loss. Newly identified abnormalities on CT included acute subdural hematomas, intracranial PTLD lesions, uncal herniation and mastoid opacification. Brain MRI was obtained in 43 (66.2%) admissions, detecting a previously identified abnormality in 14 (32.5%) and identifying a new abnormality in 18 (41.9%) of admissions. The remaining 11 (25.6%) MRI images were normal. The most common previous abnormality detected was volume loss in 11 (78.6%). New, non-specific lesions were found in 6 (54.5%) and posterior reversible encephalopathy syndrome (PRES) was diagnosed in 4 (36.3%) admissions. The remainder of the new findings included white matter changes, mastoiditis, basal ganglia lesions, subdural hematomas, acute hydrocephalus, and PTLD-associated lesions. In 15 admissions, both a head CT and brain MRI were performed. Study findings were consistent between both imaging modalities in 9 (60%). Five patients had a head CT that was either normal or demonstrated volume loss, but brain MRI imaging had findings of basal ganglia lesions, white matter changes and non-specific lesions, or PRES (Supplementary Table 2).
An EEG was obtained in 11 (16.9%) of the admissions with neurological complications and three (33%) with a combined neurological and behavioral complications. Seven (50%) EEGs were obtained following a seizure or abnormal spell, the remainder were obtained for persistent altered mental status or encephalopathy. Nine (64.3%) of these EEGs had a documented abnormality. All abnormal EEGs were noted to have generalized slowing. One EEG demonstrated electrographic seizures.
Maximum serum tacrolimus levels during admissions with neurobehavioral complications were significantly higher than admissions without an identified neurobehavioral complication (12.3 [IQR 7, 14.1] versus 9.8 [IQR 8.8, 19.3] ng/mL; P=0.001; Figure 3A) and were highest among the combined neurological and behavioral (20.6 [IQR 16.6, 23.7] ng/mL) and behavioral alone (14.6 [IQR 9.6, 20.6] ng/mL) phenotypes (Figure 3B). Maximum tacrolimus levels did not differ between admissions with the neurological alone phenotype and admissions without identified neurobehavioral complications (P=0.205; Figure 3B). Encounters with a neurobehavioral complication were significantly more likely to receive glucocorticoids compared to encounters without a neurobehavioral complication (OR 1.79 [95% CI 1.19 – 2.68]; P=0.005). A multivariable model identified type of transplant, ICU admission and maximum admission tacrolimus level as independently associated with a neurobehavioral complication during admission, adjusting for age at admission, gender, race and glucocorticoid administration (Table 3). Minimum serum sodium, magnesium and albumin levels and maximum serum creatinine, alanine aminotransferase and aspartate aminotransferase levels did not significantly differ between admissions with and without a neurobehavioral complication (Supplementary Table 3).
Figure 3.
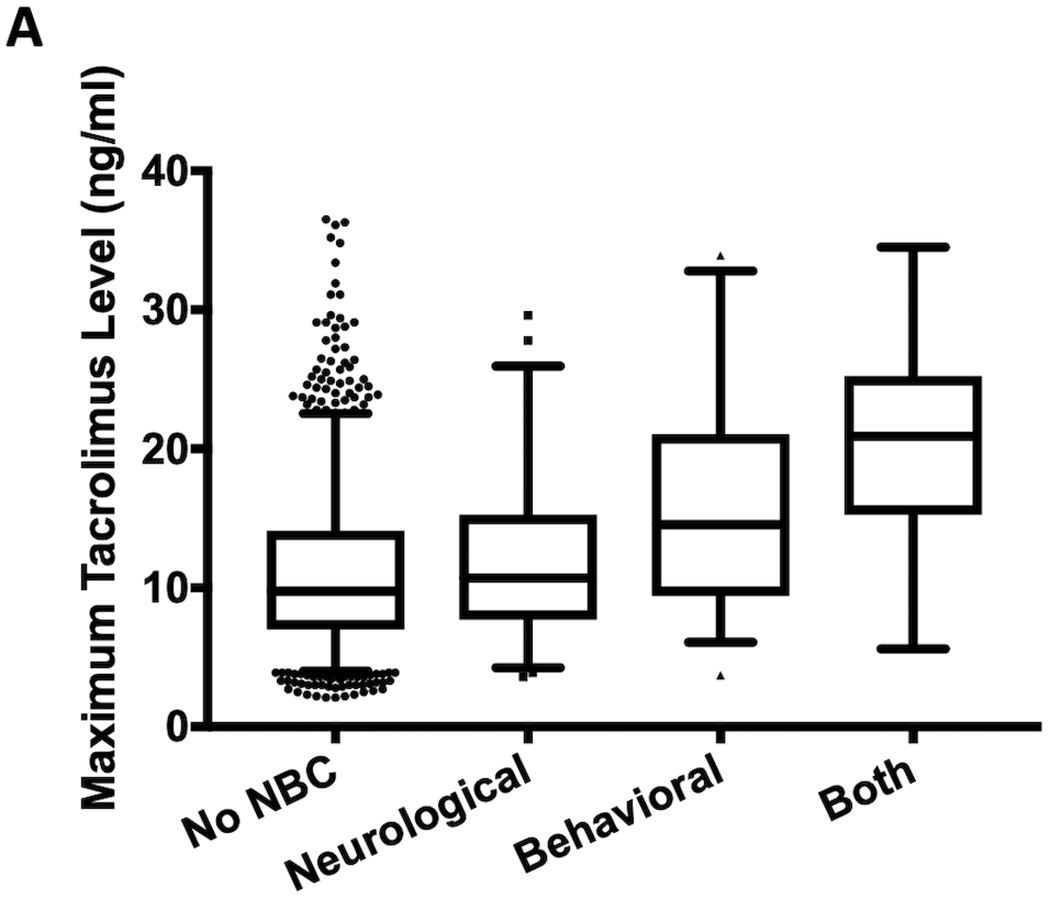
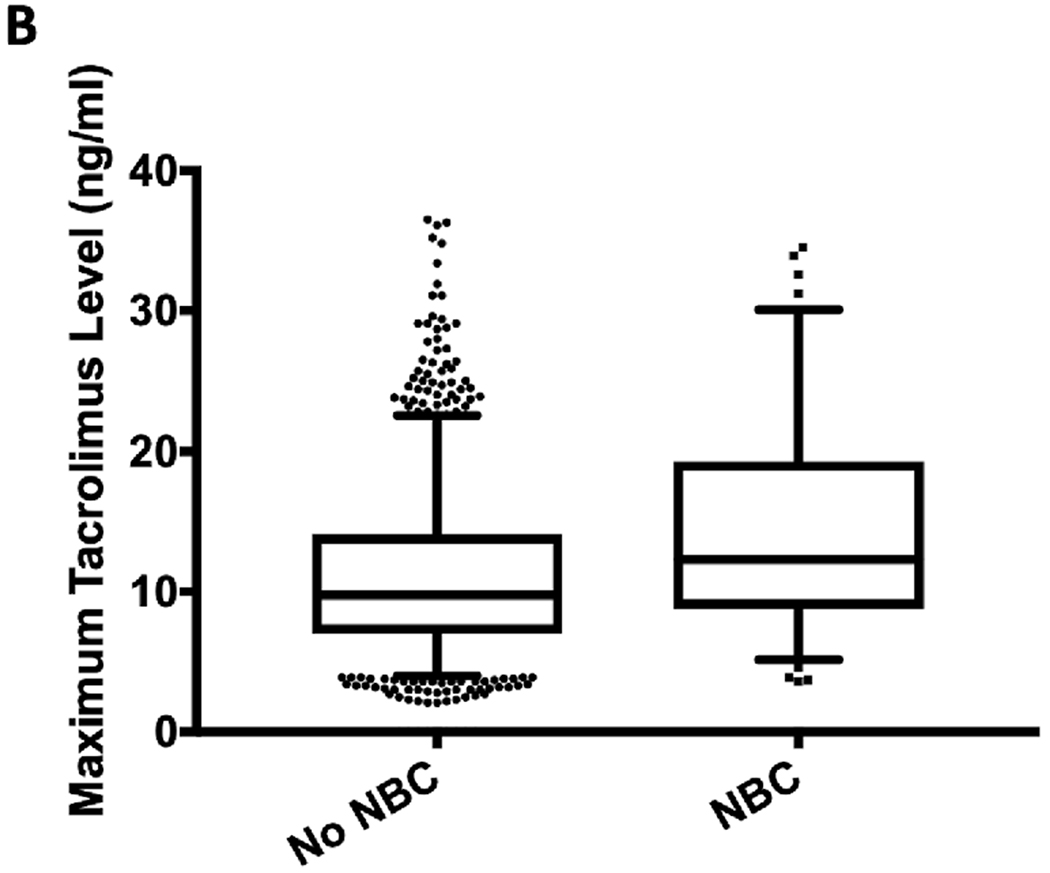
Maximum tacrolimus levels between (A) admissions with and without an identified neurobehavioral complication and (B) admissions with and without one of the three different neurobehavioral complication phenotypes. Note: There is one value of 80 ng/ml not shown for admissions without a neurobehavioral complication in both figures. *P=0.001, †P<0.001. NBC = neurobehavioral complication.
Table 3.
Multivariable Analysis of Risk Factors for an Identified Neurobehavioral Complication
| Variable | OR (95% CI) | P |
|---|---|---|
| Age at time of admission | 1.06 (1.01-1.10) | 0.075 |
| Gender | 0.471 | |
| Female | Ref | |
| Male | 0.66 (0.33-1.33) | |
| Race | 0.957 | |
| White | Ref | |
| Black | 1.07 (0.41-2.79) | |
| Other | 1.14 (0.39-3.31) | |
| Type of Transplant | <0.001 | |
| Kidney | Ref | |
| Liver | 2.41 (0.69-8.33) | |
| Intestinal | 6.31 (1.47-27.0) | |
| Multi-visceral | 10.3 (2.71-39.2) | |
| Liver Kidney | 0.00 (0.00-0.00) | |
| Steroid administration | 0.66 (0.27-1.64) | 0.229 |
| ICU Admission | 4.55 (2.08-9.92) | <0.001 |
| Max Tacrolimus Level | 1.04 (1.01-1.08) | 0.019 |
Discussion
To our knowledge, this is the largest pediatric study to evaluate the prevalence and impact of neurobehavioral complications following transplantation, and the first in a cohort of all solid organ abdominal transplant recipients. The three main findings from our single center study are: 1) Neurobehavioral complications are common in the late post-transplant period and can be accurately identified in the EHR using computable composite definitions; 2) Neurobehavioral complications are associated with increased number of hospital admissions, more frequent ICU utilization and higher risk for mortality; and 3) Maximum tacrolimus levels are independently associated with neurobehavioral complications, raising the importance of further evaluating the role of tacrolimus therapeutic drug monitoring in preventing adverse outcomes.
That 20% of our cohort demonstrated a neurobehavioral complication is consistent with the existing literature and indicates these complications are prevalent following pediatric solid organ transplantation. Additionally, our data suggest that these neurobehavioral complications may be more prevalent in the late transplant period than previously described (7, 9, 10). The median time from transplant to neurobehavioral complication readmission in our study was 1.2 years, with most patients having at least one prior admission without a neurobehavioral complication. This differs from previous studies which describe neurobehavioral complications as commonly occurring early after transplant, typically within the first 30 days (9–11, 19, 20). As this study focused specifically on readmissions following transplantation, our data may have more optimally identified late neurobehavioral complications.
Although the computable phenotype is not dependent on abnormalities detected on neuroimaging or EEG, over 75% of patients identified as having a neurological or combined neurological and behavioral phenotype had abnormalities identified, with many patients demonstrating volume loss on neuroimaging. This finding is compatible with other studies that have identified cerebral atrophy as a common finding among transplant recipients. Brain atrophy is hypothesized to be multi-factorial in etiology, resulting from both chronic organ dysfunction and medication exposures such as glucocorticoids (21, 22). Notably, non-specific white or gray matter changes were a common finding. As many patients do not have neuroimaging prior to transplantation, it is difficult to ascertain if these findings are new or acquired post-transplantation.
In this study, post-transplant neurobehavioral complications were associated with increased healthcare utilization. Patients who had multiple admissions with an identified neurobehavioral complication also experienced more overall hospital admissions than patients who never experienced a neurobehavioral complication. As this is a retrospective study, it is difficult to determine if repeat hospitalizations were a risk factor for development of a neurobehavioral complication or if it was the presence of a neurobehavioral complication that resulted in more hospitalizations. In addition, admissions with an identified neurobehavioral complication also had significantly longer ICU and hospital LOS compared to admissions without an identified neurobehavioral complication. Lastly, as seen in previous studies, patients who experienced at least one neurobehavioral complication admission had increased odds of mortality in the post-transplant period.
Interestingly, in our study we found an association between maximum serum tacrolimus level and neurobehavioral complications. The multiple types of SOTs included in this study resulted in a wide range of observed tacrolimus levels, as kidney and liver transplant recipients generally require less immunosuppression and have lower target tacrolimus levels compared with patients who receive a multi-visceral or intestinal transplants. Tacrolimus, a calcineurin inhibitor, is the most commonly used immunosuppressive medication following transplantation and has a known side effect of neurotoxicity (23, 24). The precise mechanism of neurotoxicity is not known, but there are several hypothesized processes: increased drug uptake secondary to increased low-density lipoprotein (LDL) receptors on astrocytes in the setting of hypocholesterolemia levels, excessive endothelin production resulting in endothelial disruption and cerebral vascular constriction or spasm, sympathetic alterations leading to systemic hypertension, mitochondrial dysfunction, and direct pathological cerebral changes (23–25). Several previous studies have suggested a potential link between administration of tacrolimus and presence of neurological complications following transplant (5, 10, 13–15). Some of these studies have correlated supratherapeutic drug levels to such adverse events, while others have indicated that exposure in pre-determined therapeutic levels may result in toxicity given symptoms resolve with discontinuation of the drug. The observed association between maximum serum tacrolimus level and neurobehavioral complications represents a possible intervenable finding that could lead to improved quality of life among transplant patients. In addition, the association seen with increased odds of neurobehavioral complications in multivariable analysis for intestinal and multi-visceral transplantation may be reflective of different tacrolimus goals and management strategies. Further studies are necessary to confirm this finding and determine individual risk factors for tacrolimus-related behavioral effects, optimal therapeutic drug monitoring, and perhaps more stringent therapeutic target ranges.
In addition, to the best of our knowledge, this is also the first study that uses computable phenotypes to identify neurobehavioral complications in hospitalized patients. Computable phenotypes allow for both definition of a clinical problem and identification of a population of interest for further data analysis and interpretation (16, 17). Both neurological (e.g. seizure, encephalopathy and/or altered mental status after transplant) and behavioral (e.g. depression, anxiety, post-traumatic stress) complications have been described as post-transplant complications (8, 12, 20, 26). Our computable composite definitions were highly specific for neurobehavioral complications in this pediatric SOT population and demonstrated an estimated sensivity comparable to the use of positive cultures for the detection of severe sepsis (27). We plan to verify whether these EHR-nested definitions can be used for early detection of neurobehavioral complications in a prospective, real-time analysis of both ICU SOT and non-transplant patients. Such functionality would ensure involvement of all appropriate support services (physical and occupational therapy, physiatry, behavioral health, psychiatry, etc.) and reenforce the need for neuroprotective strategies for ICU-related care.
The size of the pediatric SOT population at our institution is a strength of this study. From this single-center evaluation, our data suggests that neurobehavioral complications are a significant issue in a large proportion of SOT recipients, typically as a late morbidity, and associated with increased healthcare needs. We also demonstrated the use of a computable phenotype that can accurately identify such neurobehavioral complications specifically for this population and may have broader applications to the rest of the pediatric critical care population. However, this retrospective study has some important limitations. Factors that may change a patient’s risk for development of a neurobehavioral complications are difficult to ascertain from structured EHR data alone, such as a history of pre-transplant neurological deficits, concurrent infectious processes, and other pharmacological exposures. It is possible that we are underestimating the true incidence of neurobehavioral complications as many of our patients are referred to our institution for their transplant and return to institutions closer to their home after the initial post-operative period. This was identified during our chart review as we had some patients for whom the neurologic work-up (particularly neuroimaging) was initiated at a referring institution. Additionally, we only evaluated neurobehavioral complications in the context of hospital admission, therefore it is likely that we are underestimating the prevalence of milder neurobehavioral complications that may be diagnosed and managed as an outpatient. Strategies for capturing structured electronic data from referring institutions and outpatient settings could increase the sensitivity of the current computable definitions. Despite these limitations, this study highlights the need for further prospective investigation to improve the ability of providers to predict at-risk patients and ultimately allow for early interventions to improve neurocognitive outcomes and quality of life post-transplantation.
Conclusions
Neurobehavioral complications are found in 20% of hospitalized pediatric solid organ abdominal transplant patients. These complications are associated with a greater number of hospitalizations, more frequent admissions to the ICU, longer ICU and hospital LOS, and increased mortality. An association with maximum serum tacrolimus level and neurobehavioral complications represents a potential opportunity for intervention. As the number of pediatric transplant recipients continues to rise, pre-existing and acquired neurobehavioral complications should be a focus for the critical care provider in this unique subset of patients.
Supplementary Material
Acknowledgements:
We would like to thank Nayln Siripong, PhD and Li Wang, MS from the University of Pittsburgh Clinical and Translational Science Institute for their statistical support.
Funding: This project was supported in part by the National Institutes of Health through T32-HD40686 (AMA) and UL1-TR-001857 (statistical support), as well as the UPMC Children’s Hospital of Pittsburgh Foundation Trust Young Investigator Award (CMH).
Copyright form disclosure: Drs. Horvat, Alcamo, and Aneja received support for article research from National Institutes of Health (NIH). Dr. Horvat’s institution received funding from NIH T32-HD40686. Dr. Alcamo’s institution received funding from the NIH. Dr. Aneja received funding from UptoDate. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
Article Tweet: Neurobehavioral complications following #transplant are associated with increased #PedsICU LOS, mortality, and hospital readmissions
References
- 1.Balderramo D, Prieto J, Cárdenas A, et al. Hepatic encephalopathy and post-transplant hyponatremia predict early calcineurin inhibitor-induced neurotoxicity after liver transplantation. Transpl Int 2011; 24: 812–819. [DOI] [PubMed] [Google Scholar]
- 2.Colombari RC, de Ataíde EC, Udo EY, et al. Neurological complications prevalence and long-term survival after liver transplantation. Transplant Proc 2013; 45: 1126–1129. [DOI] [PubMed] [Google Scholar]
- 3.Kim BS, Lee SG, Hwang S, et al. Neurologic complications in adult living donor liver transplant recipients. Clin Transplant 2007; 21: 544–547. [DOI] [PubMed] [Google Scholar]
- 4.Saner FH, Gensicke J, Olde Damink SWM, et al. Neurologic complications in adult living donor liver transplant patients: an underestimated factor? Journal of Neurology 2010; 257: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rompianesi G, Montalti R, Cautero N, et al. Neurological complications after liver transplantation as a consequence of immunosuppression: univariate and multivariate analysis of risk factors. Transpl Int 2015; 28: 864–869. [DOI] [PubMed] [Google Scholar]
- 6.Otan E, Aydin C, Yönder H, et al. Evaluation of Early Postoperative Neurological Complications Following Living Donor Liver Transplantation. Noro Psikiyatr Ars 2015; 52: 15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez D, El-Azzabi TI, Jain V, et al. Neurologic problems after pediatric liver transplantation and combined liver and bowel transplantations: a single tertiary centre experience. Transplantation 2010; 90: 319–324. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh PS, Hupertz V and Ghosh D: Neurological complications following pediatric liver transplant. J Pediatr Gastroenterol Nutr 2012; 54: 540–546. [DOI] [PubMed] [Google Scholar]
- 9.Lee YJ, Yum MS, Kim EH, et al. Risk factors for neurological complications and their correlation with survival following pediatric liver transplantation. Pediatr Transplant 2014; 18: 177–184. [DOI] [PubMed] [Google Scholar]
- 10.Xie M, Rao W, Sun LY, et al. Tacrolimus-related seizure after pediatric liver transplantation--a single-center experience. Pediatr Transplant 2014; 18: 58–63. [DOI] [PubMed] [Google Scholar]
- 11.Erol I, Alehan F, Ozcay F, et al. Neurological complications of liver transplantation in pediatric patients: a single center experience. Pediatric Transplantation 2007; 11: 152–159. [DOI] [PubMed] [Google Scholar]
- 12.Gungor S, Kilic B, Arslan M, et al. Early and late neurological complications of liver transplantation in pediatric patients. Pediatr Transplant 2017; 21: [DOI] [PubMed] [Google Scholar]
- 13.Sevmis S, Karakayali H, Emiroglu R, et al. Tacrolimus-related seizure in the early postoperative period after liver transplantation. Transplantation Proceedings 2007; 39: 1211–1213. [DOI] [PubMed] [Google Scholar]
- 14.Varghese J, Reddy MS, Venugopal K, et al. Tacrolimus-related adverse effects in liver transplant recipients: its association with trough concentrations. Indian J Gastroenterol 2014; 33: 219–225. [DOI] [PubMed] [Google Scholar]
- 15.Böttiger Y, Brattström C, Tydén G, et al. Tacrolimus whole blood concentrations correlate closely to side-effects in renal transplant recipients. Br J Clin Pharmacol 1999; 48: 445–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett TD, DeWitt PE, Dixon RR, et al. Development and Prospective Validation of Tools to Accurately Identify Neurosurgical and Critical Care Events in Children With Traumatic Brain Injury. Pediatr Crit Care Med 2017; 18: 442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tasker RC: Why Everyone Should Care About “Computable Phenotypes”. Pediatr Crit Care Med 2017; 18: 489–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrasco G, Baeza N, Cabre L, et al. Dexmedetomidine for the Treatment of Hyperactive Delirium Refractory to Haloperidol in Nonintubated ICU Patients: A Nonrandomized Controlled Trial. Crit Care Med 2016; 44: 1295–1306. [DOI] [PubMed] [Google Scholar]
- 19.Gungor S, Kilic B, Arslan M, et al. Early and late neurological complications of liver transplantation in pediatric patients. Pediatric Transplantation 2017; 21: [DOI] [PubMed] [Google Scholar]
- 20.Fredericks EM, Lopez MJ, Magee JC, et al. Psychological functioning, nonadherence and health outcomes after pediatric liver transplantation. Am J Transplant 2007; 7: 1974–1983. [DOI] [PubMed] [Google Scholar]
- 21.Ozturk M, Akdulum I, Dag N, et al. Analysis of magnetic resonance imaging findings of children with neurologic complications after liver transplantation. Radiologia Medica 2017; 122: 617–622. [DOI] [PubMed] [Google Scholar]
- 22.Agildere AM, Basaran C, Cakir B, et al. Evaluation of neurologic complications by brain MRI in kidney and liver transplant recipients. Transplantation Proceedings 2006; 38: 611–618. [DOI] [PubMed] [Google Scholar]
- 23.Bechstein WO: Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transpl Int 2000; 13: 313–326. [DOI] [PubMed] [Google Scholar]
- 24.Anghel D, Tanasescu R, Campeanu A, et al. Neurotoxicity of immunosuppressive therapies in organ transplantation. Maedica (Buchar) 2013; 8: 170–175. [PMC free article] [PubMed] [Google Scholar]
- 25.Pizzolato GP, Sztajzel R, Burkhardt K, et al. Cerebral vasculitis during FK 506 treatment in a liver transplant patient. Neurology 1998; 50: 1154–1157. [DOI] [PubMed] [Google Scholar]
- 26.Erol I, Alehan F, Ozcay F, et al. Neurological complications of liver transplantation in pediatric patients: a single center experience. Pediatr Transplant 2007; 11: 152–159. [DOI] [PubMed] [Google Scholar]
- 27.Phua J, Ngerng W, See K, et al. Characteristics and outcomes of culture-negative versus culture-positive severe sepsis. Crit Care 2013; 17: R202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


