Abstract
Acetaminophen (APAP) hepatotoxicity is the most common cause of acute liver failure in the United States, and while a significant percentage of APAP overdose patients develop kidney injury, molecular mechanisms involved in APAP-induced nephrotoxicity are relatively unknown. We have shown that 4-methylpyrazole (4MP, Fomepizole) protects against APAP-induced liver injury by inhibiting reactive metabolite formation through Cyp2E1, and analysis of data from APAP overdose patients indicated that kidney dysfunction strongly correlated with severe liver injury. Since Cyp2E1 is also expressed in the kidney, this study explored protection by 4MP against APAP-induced nephrotoxicity. Male C57BL/6J mice were treated with either 300 or 600mg/kg APAP with or without 4MP for 2, 6 or 24h, followed by measurement of APAP metabolism and tissue injury. Interestingly, levels of APAP and its non-oxidative metabolites were significantly higher in kidneys when compared to the liver. APAP-protein adducts were present in both tissues within 2h, but were absent in kidney mitochondria, unlike in the liver. While GSH depletion was seen in both tissues, activation of c-jun N-terminal kinase and its translocation to the mitochondria, which is a critical feature of APAP-induced liver injury, was not detected in the kidney. Treatment with 4MP attenuated APAP oxidative metabolite generation, GSH depletion as well as kidney injury indicating its potential use in protection against APAP-induced nephrotoxicity. In conclusion, since reactive metabolite formation seems to be common in both liver and kidney, 4MP mediated inhibition of Cyp2E1 protects against APAP-induced nephrotoxicity. However, downstream mechanisms of APAP-induced nephrotoxicity seem distinct from the liver.
Keywords: Acetaminophen, nephrotoxicity, hepatotoxicity, Fomepizole, N-acetylcysteine, protein adducts
INTRODUCTION
Acetaminophen (APAP) is one of the most commonly used analgesic and anti-pyretic drugs, which is safe at therapeutic doses, but can cause severe hepatotoxicity if taken as an overdose (Fisher and Curry, 2019; Jaeschke, 2015). In fact, acetaminophen overdose is the most common cause of acute liver failure (ALF) in the United States, comprising 46% of all cases (Hodgman and Garrard, 2012; Yoon et al., 2016). While the acute liver injury caused by APAP has been extensively studied (Ramachandran and Jaeschke, 2019), leading to introduction of N-acetylcysteine (NAC) as an antidote, late presenting patients can still develop ALF as evident in the statistics above and may require liver transplants for survival (Hodgman and Garrard, 2012; Heard, 2008). Mortality due to ALF typically develops after multi-organ failure, though exact mechanisms mediating these in the context of APAP are not well understood. This is important, since it is evident that transplant recipients who had developed ALF due to APAP toxicity versus ALF from non-APAP related causes were found to suffer from a lower 30 days survival (Cooper et al., 2009). Thus, it is likely that pathological effects of an APAP overdose on target organs other than the liver could potentially contribute to the increased morbidity and mortality observed in APAP-induced ALF transplant recipients (Chopyk et al., 2019).
Acute kidney injury (AKI) is a syndrome encompassing a wide variety of etiologies and pathophysiologic processes leading to decreased kidney function (Hoste et al., 2018). This abrupt and sustained decline in renal function is common in conditions such as ALF where it contributes to increased morbidity and is strongly associated with worse prognosis (Moore et al., 2013). Recent evidence from Europe indicates that AKI is a frequent complication of ALF, with approximately 40% of patients requiring renal replacement therapy (Hadem et al., 2019). AKI in these patients correlated with remote organ damage and independently predicted outcome (Hadem et al., 2019). An US study showed that a greater proportion of patients with APAP-induced ALF had severe kidney injury than patients with other etiologies, with 34% requiring renal replacement therapy (Tujios et al., 2015). Almost 80% of patients admitted to a tertiary referral liver intensive therapy unit with APAP-induced hepatotoxicity developed AKI (O’Riordan et al., 2011), and the presence of AKI impacted the long-term outcome in these patients (Tujios et al., 2015). APAP as the cause of ALF was also shown to be an independent predictor of AKI (Leithead et al., 2009), indicating that APAP may have a direct nephrotoxic effect. This conclusion is supported by the fact that AKI can develop in APAP overdose patients without liver failure (Eguia and Materson, 1997; Jeffery et al., 1981; Cobden et al., 1982).
APAP-induced AKI has important clinical relevance, since NAC protects against APAP hepatotoxicity in the liver by replenishing depleted hepatic glutathione, but glutathione derived APAP metabolites have been implicated in APAP-induced nephrotoxicity (Stern et al., 2005). Hence, NAC did not protect against APAP-mediated nephrotoxicity while protecting against hepatotoxicity in animal experiments (Slitt et al., 2004). However, when patients were treated early with NAC, which then prevented APAP-induced liver injury, no kidney injury was detected (Prescott et al., 1979). Late presenting patients developing severe liver injury despite NAC treatment also experience severe kidney dysfunction and tubular necrosis (Davenport and Finn, 1988). Thus, mechanistic insight into APAP-induced nephrotoxicity would enable development of targeted therapeutic approaches with mechanisms distinct from NAC which could block development of AKI and prevent the cascading events leading to multi-organ failure after ALF. We have recently shown that 4-methylpyrazole (4MP), an FDA approved antidote against methanol and ethylene glycol poisoning (Brent, 2009), protects against APAP hepatotoxicity by inhibition of cytochrome P450-mediated reactive metabolite formation (Akakpo et al., 2018) and by inhibition of c-jun N-terminal kinase (JNK)-induced amplification of injury (Akakpo et al., 2019). 4MP has now been tested in human volunteers and shown efficacy in inhibiting oxidative metabolism (Kang et al., 2020). In addition, off-label use in patients with severe overdose suggest potential benefits in addition to NAC in patients with high overdoses (Rampon et al., 2020). Thus, we tested the hypothesis that 4MP inhibits the oxidative metabolism of APAP in the kidney and protects against APAP-induced nephrotoxicity.
MATERIALS AND METHOD
Animals and experimental design
All experiments involving animals were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Kansas Medical Center, conforming to National Institute of Health and the National Research Council for the care and use of laboratory animals guidelines. Male C57BL/6J mice (8 to 12 weeks old) were purchased from Jackson Labs (Bar Harbor, ME) and acclimated for at least 5 days. Then, animals were fasted overnight for 15 hours before being treated i.p. with 300 or 600mg/kg APAP (Sigma-Aldrich, St. Louis, MO) along with 50mg/kg 4MP (Sigma-Aldrich) as a co-treatment as described (Akakpo et al., 2018). Animals were euthanized under isoflurane anesthesia followed by collection of blood, kidney and liver samples.
Patient characteristics and data analysis
Clinical data from 500 patients who presented to Banner – University Medical Center Phoenix following known or suspected acute or chronic APAP overdose were studied prospectively (Curry et al., 2019). The study design and protocol were approved by the institutional review board (IRB), with regular follow-up assessment. Inclusion criteria included a reported history of APAP overdose, and/or elevated serum APAP levels, and/or unexplained abnormal liver function tests (based on ALT, AST, PT, bilirubin). All of the participants fulfilled at least 2 of these criteria. After obtaining informed consent, demographic and clinical information was collected during hospitalization, including serial laboratory studies and initial comprehensive urine toxicology screening. All patients received standard therapy with intravenous NAC. At the time of discharge, physician medical toxicologists reviewed all historical and clinical information, and classified patients into one of three groups: 1) definite APAP overdose (acute or chronic); 2) definitely not APAP overdose; and 3) indeterminate APAP overdose. Renal failure was defined as a serum creatinine concentration ≥ 2 mg/dL more than 24 hours after admission. This renal failure definition allowed exclusion of patients with transient elevated creatinine concentrations that normalized over the first 24 hours after correction of dehydration or poor cardiac output, but without intrinsic kidney injury. From this group of 500 subjects we selected only those subjects categorized as having definite APAP overdose (375 patients) and compared parameters between those with and without renal failure.
Biochemical assays
Mouse blood samples were centrifuged at 16,000×g at 4ºC for 5 min to collect plasma. Plasma urea nitrogen (BUN) was measured using a QuantiChrom™ Urea Assay kit from BioAssay Systems (Hayward, CA) to assess the extent of kidney injury. Plasma alanine aminotransferase (ALT) activity was measured with a kit purchased from Pointe Scientific (Canton, MI) to assess the extent of liver injury. Total kidney glutathione (GSH+GSSG) was measured using a modified Tietze assay as described in detail (McGill and Jaeschke, 2015).
Measurement of Cytochrome P450 enzyme activity
Cytochrome P450 enzyme activities were measured in liver or kidney homogenates using a fluorogenic substrate, 7-ethoxy-4-trifluoromethyl coumarin (7-EFC) (Invitrogen, Carlsbad, CA), which is a substrate for Cyp2E1 and Cyp1A2, as described (Buters et al., 1993; Chang et al., 2006). Briefly, a 10% homogenate of liver or kidney tissue was prepared in homogenizing buffer (25 mM 4–2-hydroxyethyl-1-piperazineethanesulfonic acid (HEPES), pH 7.5, 5 mM ethylenediaminetetraacetic acid (EDTA), 2 mM dithiothreitol, 0.1 % CHAPS, 1 μl/ml pepstatin, 0.1 μl/ml leupeptin and 0.1 μl/ml apoproteinin). Samples were spun at 16,000×g for 5 min at 4°C and the resulting supernatant diluted 1:5 and incubated in 0.1 M phosphate buffer with 0.4 mg/ml BSA and 1 mM NADPH at 37°C for 5 min. Samples were transferred to a 96 well plate and the reaction was started by addition of 7-EFC with or without 4MP to a final concentration of 50 μM each, and the increase in fluorescence monitored overtime (Excitation: 410 nm, Emission: 510 nm).
Isolation of subcellular fractions and western blotting
Mitochondria and cytosolic fractions were isolated as described (Cover et al., 2005). Briefly, aliquots of liver tissue or one of the kidneys were homogenized in ice cold isolation buffer (pH 7.4) containing 220 mM mannitol, 70 mM sucrose, 2.5 mM HEPES, 10 mM EDTA, 1 mM EGTA, and 0.1% bovine serum albumin. Mitochondria were isolated by differential centrifugation (20,000×g) and washed with 2 ml of isolation buffer. The 20,000×g supernatant was used to measure JNK activation in the cytosol by western blotting in the liver and kidney as described (Akakpo et al., 2018), with the following Cell Signaling primary antibodies: rabbit anti-JNK antibody (Cat. # 9252) or rabbit anti-phospho-JNK antibody (Cat. # 4668). Liver and kidney samples were also used for western blotting with antibodies to NGAL (Santa Cruz, Cat. #sc-515876), cytochrome P450 2E1 (Abcam rabbit polyclonal antibody, cat. # ab28146) and ß-actin (Cell Signaling, Cat. # 4967). Anti-rabbit or anti-mouse IgG horseradish peroxidase coupled secondary antibodies (SantaCruz Biotechnology) (1:5000 dilution), coupled with the ECL kit from Amersham (Piscataway, NJ) were then used for detection of protein bands by chemiluminescence on the Licor Odyssey imaging system (LICOR Biosciences).
Histopathological analysis
After kidney and liver tissue collections, samples were fixed in 10% formalin for 16 hours. Then, fixed tissue samples were embedded in paraffin. Five μm sections were cut and stained with hematoxylin & eosin (H&E) for evaluation of necrotic cell death.
APAP-protein adducts measurements
APAP protein adducts were measured as described in detail previously (Akakpo et al., 2018). Briefly, to remove low molecular weight metabolites that could interfere with detection of APAP protein adducts, liver homogenates were filtered through a Bio-Spin 6 column from Bio-Rad (Hercules, CA) (McGill et al., 2012b). Then, 8 U/ml Streptomyces griseus solution was mixed with the filtrate containing proteins with APAP bound to cysteine residues in a 1:1 ratio. The mixture was digested for 15 hours at 50°C to release the APAP-Cys adducts from the cellular proteins. Finally, APAP-Cys residues derived from proteins were filtered and analyzed by high pressure liquid chromatography (HPLC) with a Coularray electrochemical detector from ESA Biosciences (Chelmsford, MA).
LC-MS-MS measurement of APAP and its metabolites
Collected kidney and liver samples were analyzed for concentrations of APAP and its metabolites APAP-glucuronide (APAP-Gluc), APAP-sulfate (APAP-Sulf), APAP-GSH, free APAP-cysteine (APAP-CYS), and APAP-N-acetylcysteine (APAP-NAC) using LC-MS/MS, as described previously (Akakpo et al., 2018). Briefly, 1 mM of APAP and its conjugated metabolites in 50:50 water:methanol stock solutions were used to prepare a nine point calibration curve in drug free mouse tissue homogenate at final concentrations of 0, 0.25, 0.5, 0.75, 1, 2.5, 10, 25 and 75 μM. Then, 20 μl of each tissue homogenate sample was supplemented with internal standard 4-acetaminophen-d3 sulfate (Toronto Research Chemicals, Toronto, Canada) as well as 100% methanol for analyte extraction. All samples were vortexed, incubated on ice for 10 min and centrifuged for 20 min 14,000 rpm at 4°C. Then, 5 μl of the supernatant was analyzed by LC-MS/MS with a Waters Acquity Ultra-Performance Liquid Chromatography (UPLC®) system interfaced by electrospray ionization with a Waters Quattro Premier XE triple quadruple mass spectrometer (Waters Corp., Milford, MA) as described (Akakpo et al., 2018). The lower limits of quantification (LLOQ) were determined to be 0.25 μM for APAP-GSH; 0.125 μM for APAP-Sulf and APAP-Gluc; 0.063 μM for free APAP-CYS; and 0.025 μM for APAP-NAC. Analytes’ levels below the limits of quantification were assumed to be zero.
Statistical analysis
All data were analyzed to assess statistically significant differences between groups using one-way analysis of variance (ANOVA). Then, multiple comparisons were performed with The Student Newman-Keul’s test. Non-Normally distributed data were analyzed with Kruskal-Wallis test with Dunn’s multiple comparison. The software used for statistical analysis is SPSS Statistics for windows, V21.0, IBM (Corp., Armonk, NY). All data were represented as means ± SEM and P < 0.05 was considered as significant for all tests.
RESULTS
Incidence of abnormal creatinine levels in APAP overdose patients with liver injury.
To explore the clinical relevance of APAP-induced nephrotoxicity and establish the clinical need for an intervention targeting APAP-induced kidney injury, initial analysis of APAP overdose patients treated at the Banner - University Medical Center in Phoenix was carried out. Plasma creatinine elevations were used as a marker of renal injury, and as seen in Table 1, out of 375 definitive APAP overdose patients, 46 patients (12%) suffered from renal failure. There was no relevant difference in the percentage of female patients with renal failure (12.7%) compared to male patients (11.3%). In addition, both groups had similarly elevated creatinine levels and had similar liver injury (plasma ALT, AST activities). However, all patients with renal failure had more severe liver injury as indicated by the 2.5 to 3.6-fold higher ALT and AST levels (Table 1). These clinical data support the conclusion that more severe liver injury in APAP overdose patients correlates with kidney dysfunction.
Table 1.
Creatinine Levels of Hospitalized APAP Overdose Patients
| APAP Overdose Patients | ||
|---|---|---|
| Patient # Female : Male | 260 : 115 | |
| Parameters | Renal Failure | No Renal Failure |
| Patient # F:M | 33 : 13 | 227 : 102 |
| Median Age (Range) F : M | 32 (19–63) : 35 (27–43) | 31 (18–71) : 33 (18–72) |
| Creatinine (mg/dl) F : M | 3.97 ± 0.41 : 4.50 ± 0.60 | 0.90 ± 0.03 : 1.06 ± 0.04 |
| ALT (U/L) F : M | 5,656 ± 600 : 6,530 ± 1,090 | 2,055 ± 218 : 2,314 ± 350 |
| AST (U/L) F : M | 8,003 ± 1,184 : 9,935 ± 2,113 | 3,184 ± 358 : 2,745 ± 503 |
Peak values for each parameters are reported as average ± SEM.
Cyp2E1 expression in mouse kidney compared to liver.
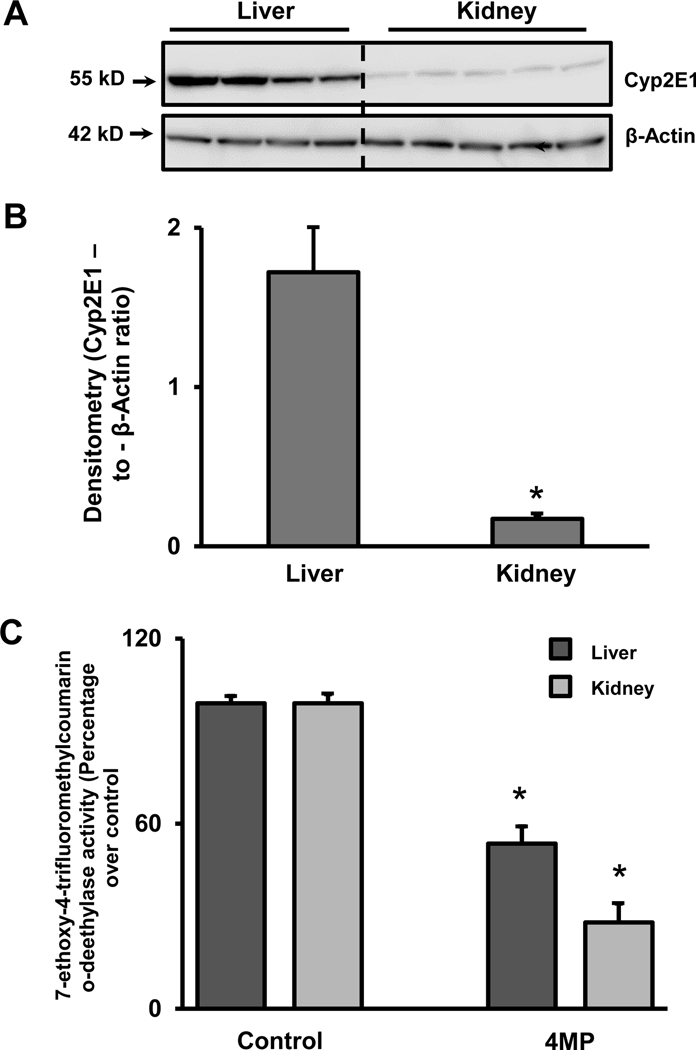
Cyp2E1 is the main cytochrome P450 enzyme responsible for the formation of the reactive metabolite NAPQI, which is critical for APAP hepatotoxicity. Therefore, Cyp2E1 protein expression was examined in the mouse kidney in comparison to the liver. At baseline (untreated animals), Cyp2E1 protein expression was significantly lower in the kidney when compared to the liver (Figure 1A,B). Cytochrome P450 activities were measured in liver and kidney homogenate with the fluorogenic compound 7-EFC, which is a preferred substrate of Cyp2E1 and Cyp1A2 (Buters et al., 1993). Consist with our previous observations in the liver (Akakpo et al., 2018), 50 μM 4MP inhibited P450 enzyme activities in liver and kidney homogenate by 46% and 72%, respectively (Figure 1C) suggesting that 4MP can act as a direct inhibitor of cytochrome P450 enzymes.
Figure 1: Renal and hepatic Cyp2E1 protein expression levels and Cyp enzyme activities.
Liver and kidney homogenates from untreated C57BL/6J mice were subjected to western blot analysis to assess baseline expression of Cyp2E1 (A). Densitometric evaluation of Cyp2E1 and β-actin. The data are expressed as the mean ± SEM of the ratio for liver and kidney of 4–5 animals per group. *P<0.05 (compared to liver) (B). Cyp enzyme activities were assayed in liver and kidney homogenate using the fluorogenic substrate, 7-ethoxy-4-trifluoromethyl coumarin (7-EFC), which detects Cyp2E1 and Cyp1A2 activities, in the presence or absence of 50 μM 4MP. The enzyme activities were 260 and 500 RFU/min/mg protein for the control liver and the kidney respectively, which were normalized to 100%. (C). Data represent means ± SEM of 3 separate measurements. *P< 0.05 (compared to the respective control).
4MP attenuated APAP metabolite levels in mouse kidney and liver.
Since Cyp2E1 is expressed in both organs, the temporal course of APAP metabolism in kidney and liver was evaluated. APAP levels in the kidney increased within 15 min after i.p. administration of 300 mg/kg APAP; these levels peaked at 30 min and rapidly declined thereafter (Figure 2A). Administration of 4MP had no significant effect on parent APAP levels in the kidney when measured at the 2 h time point (Figure 2E).
Figure 2: Formation of APAP metabolites in the kidney: effect of 4MP.
C57BL/6J mice were treated with 300 mg/kg APAP and either 50 mg/kg 4MP or 10 ml/kg saline. Renal concentrations of the parent compound APAP (A), the non-oxidative metabolites APAP-Glucuronide (B) and APAP-Sulfate (C) as well as its oxidative metabolite APAP-Cysteine (D) were measured by LC/MS/MS within the first 2 h after APAP administration. In a second experiments, renal concentrations of APAP (E), APAP-Glucuronide (F), APAP-Sulfate (G) and APAP-Cysteine (H) were measured 2 h after treatment with APAP and either 4MP or its solvent. Concentrations of all metabolites are expressed in nmol/mg protein. Data represent means ± SEM of n= 6 animals per group. *P< 0.05 (compared with APAP alone)
Glucuronidation and sulfation are the predominant routes of APAP metabolism in the liver, and these metabolites are then excreted mainly through the kidneys. Renal APAP glucuronide and sulfate levels increased within 15 min of APAP administration and then showed sustained elevations up to 2 h indicative of ongoing APAP metabolism and excretion (Figure 2B,C). As expected, APAP-glucuronide levels were much higher than sulfate levels suggesting that glucuronidation is the preferred pathway of APAP metabolism in the kidney. Interestingly, levels of oxidative APAP metabolites (APAP-Cysteine) showed a different trend, increasing rapidly within 15 min and peaking at 30 mins with a steep decline by 2 h (Figure 2D). This matched the time course seen with the parent compound (Figure 2A), indicating that oxidative metabolite formation was maximal when APAP concentrations were highest and tapers off as APAP concentrations decline. It is important to point out that the other oxidative metabolites, i.e. APAP-GSH and APAP-NAC, were below the limit of quantification (LOQ) in the kidney (data not shown). Treatment with 4MP had no significant effect on APAP-glucuronide levels at 2 h (Figure 2F), while APAP-sulfate levels were significantly elevated (Figure 2G). However, of toxicological relevance to nephrotoxicity is the significant reduction in oxidative metabolite levels in the kidney by 4MP (Figure 2H), which may also explain the elevation in APAP-sulfate due to shifting Cyp2E1-mediated metabolism to sulfation in presence of 4MP.
To evaluate how the time course of APAP metabolites in the kidney compares to that in the liver, these metabolites were measured in the liver of the same animals. The time course of the parent drug in the liver was similar to that in the kidney with a peak between 15 and 30 min (Figure 3A). Liver APAP-glucuronide showed a rapid increase through 60 min and a further increase by 120 min (Figure 3B) unlike the kidney, where rapid elevation within 15 min was obvious (Figure 2B). APAP-sulfate accumulation in the liver showed a similar trend as in the kidney (Figure 3C), though the peak levels were at 30 min rather than 15 min seen in the kidney (Figure 2C). The faster rise to peak levels in the kidney suggests that these metabolites were generated locally and not transferred from the liver. Levels of the non-oxidative metabolites were also higher in the kidney than in the liver during the measured time course when expressed per milligram cellular protein. Treatment with 4MP had no significant effect on these metabolites in the liver (Figure 3D-F). Examination of the time course of oxidative metabolites in the liver revealed that unlike the kidney, hepatic levels of APAP-CYS increased by 30 minutes and stayed elevated subsequently (Figure 4A). Hepatic levels of APAP-NAC and APAP-GSH also showed a rapid elevation until 30 minutes but then progressively decreased (Figure 4B,C). In contrast to the kidney where only APAP-CYS was detected, APAP-GSH represented >85% of the oxidative metabolites in the liver. Treatment with 4MP resulted in a significant decrease in all oxidative metabolites in the liver (Figure 4D,F).
Figure 3: Formation of non-oxidative APAP metabolites in the liver: effect of 4MP.
C57BL/6J mice were treated with 300 mg/kg APAP and either 50 mg/kg 4MP or 10 ml/kg saline. Hepatic concentrations of the parent compound APAP (A) and its non-oxidative metabolites APAP-Glucuronide (B) and APAP-Sulfate (C) were measured by LC/MS/MS within the first 2 h after APAP administration. In a second experiments, hepatic concentrations of APAP (D), APAP-Glucuronide (E) and APAP-Sulfate (F) were measured 2 h after treatment with APAP and either 4MP or its solvent. Concentrations of all metabolites are expressed in nmol/mg protein. Data represent means ± SEM of n= 6 animals per group.
Figure 4: Formation of oxidative APAP metabolites in the liver: effect of 4MP.
C57BL/6J mice were treated with 300 mg/kg APAP and either 50 mg/kg 4MP or 10 ml/kg saline. Hepatic concentrations of the oxidative metabolites APAP-Cysteine (A), APAP-N-Acetylcysteine (B) and APAP-GSH (C) were measured by LC/MS/MS within the first 2 h after APAP administration. In a second experiments, hepatic concentrations of APAP-Cysteine (D), APAP-N-Acetylcysteine (E) and APAP-GSH (F) were measured 2 h after treatment with APAP and either 4MP or its solvent. Concentrations of all metabolites are expressed in nmol/mg protein. Data represent means ± SEM of n= 6 animals per group. *P< 0.05 (compared to APAP).
APAP induces GSH depletion in the kidney and causes APAP protein adducts formation.
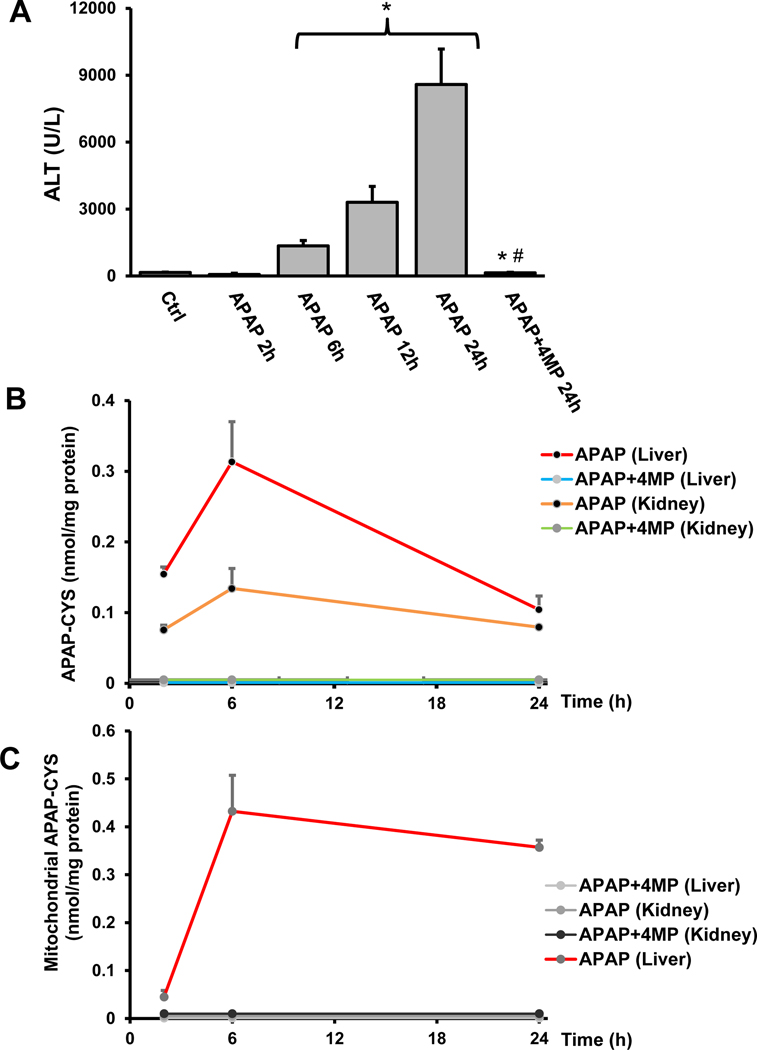
Because depletion of hepatic glutathione due to scavenging of NAPQI is a critical feature of APAP-induced hepatotoxicity, kidney GSH levels were measured. Compared to untreated controls, GSH levels in the kidney of APAP-treated animals were significantly depleted by 60% at 2 h after administration of a standard hepatotoxic dose of 300 mg/kg APAP (Figure 5A). 4MP treatment significantly attenuated renal GSH depletion (Figure 5A). Formation of cellular APAP-protein adducts, especially in the mitochondria, is a hallmark of APAP-induced liver injury (McGill et al., 2012b; Ramachandran and Jaeschke, 2019). Measurement of protein adducts (assessed as protein-derived APAP-cysteine) indicated that treatment with an APAP overdose increased protein adducts levels in the kidney at 2 h; however, the adducts in the kidney were only about 20% of the levels observed in the liver (Figure 5B). Interestingly, no protein adducts were detected in kidney mitochondria unlike the liver where it is a critical feature of hepatotoxicity (Figure 5C). APAP protein adducts formation in whole tissue was completely inhibited by 4MP treatment in both organs (Figure 5B); 4MP also prevented mitochondrial adducts formation in the liver (Figure 5C). These data demonstrate that early mitochondrial APAP protein adducts formation is absent in the kidney unlike the liver, indicating that the mechanism of toxicity may be different between the organs. 4MP, however, is a potent inhibitor of protein adducts formation in the kidney as in the liver.
Figure 5: Renal glutathione and APAP protein adducts levels: effect of 4MP.
C57BL/6J mice were treated with 300 mg/kg APAP and either 50 mg/kg 4MP or 10 ml/kg saline. Total GSH was measured in renal tissue homogenate at 2 h post-APAP (A). Concentration of protein-derived APAP-cysteine adducts (APAP-CYS) in tissue homogenate (B) as well as the mitochondrial fractions (C) were measured by HPLC-ECD at 2 h post APAP treatment. APAP-CYS concentrations are expressed in nmol/mg protein. Data represent means ± SEM of n= 4 animals per group. *P< 0.05 (compared with control). #P< 0.05 (compared to APAP)
JNK activation is not a part of APAP-induced injury mechanism in kidney.
Early activation and mitochondrial translocation of the MAP kinase JNK is another feature of APAP-induced liver injury (Ramachandran and Jaeschke, 2020). JNK is present in the cytosol in control livers and APAP caused the activation (phosphorylation) of JNK (P-JNK) in the cytosol (Figure 6A). P-JNK also translocated to the mitochondria. Both the activation and the mitochondrial translocation of JNK were eliminated by 4MP treatment (Figure 6A) consistent with our previous report (Akakpo et al., 2019). In striking contrast, an APAP overdose did neither induce JNK activation in the cytosol of kidneys nor did it trigger a translocation of JNK or P-JNK to the mitochondria (Figure 6B). This further confirms the mechanistic differences between APAP-induced hepatotoxicity and nephrotoxicity.
Figure 6: JNK activation and JNK translocation to the mitochondria in liver and kidney.
C57BL/6J mice were treated with 300 mg/kg APAP and either 50 mg/kg 4MP or 10 ml/kg saline. JNK and P-JNK were assessed in the cytosolic fraction and isolated mitochondria by western blotting. The two bands reflect JNK1 (46kD) and JNK2 (54 kD). Liver (A) and kidney (B) samples were obtained from controls (Cont) and 2 h after APAP or APAP+4MP.
A moderate APAP overdose upregulates early acute kidney injury marker but causes no histological change.
The dose of 300mg/kg APAP is a moderate overdose, which produces significant liver injury as measured by the time course of elevated plasma ALT levels between 6 and 24 h (Figure 7A). In order to assess kidney injury at these time points, the acute kidney stress marker NGAL protein levels were measured by western blotting. NGAL expression at 2 and 6 h after APAP was similar to controls (Figure 7B). However, we observed a significant up regulation of NGAL protein at 12 and 24 h after 300mg/kg APAP. Interestingly, histopathological analysis of kidney tissue indicated the lack of significant histological injury of kidney tubules compared to 2 and 6h (Figure 7C), suggesting that though this moderate overdose of APAP induces molecular mediators of cellular stress in the kidney, it does not lead to marked cell death and functional deficits up to the 24 h time point.
Figure 7: Liver and kidney injury after a moderate overdose of APAP: effect of 4MP.
C57BL/6J mice were treated with 300 mg/kg APAP and sacrificed at 2, 6, 12, or 24 h. In addition, a group was co-treated with APAP and 50 mg/kg 4MP for 24 h. Plasma ALT activities (A). Kidney homogenates were subjected to western blotting for NGAL and β-actin (B). Representative H&E-stained kidney sections of a control and 24 h post APAP (×50 magnification) (C). Data represent means ± SEM of n= 4 animals per group. *P< 0.05 (compared to control). #P< 0.05 (compared to APAP 24 h). Abbreviations: T=tubules, BC=Bowman capsule, G=Glomerulus.
Severe APAP overdose induces protein adduct formation in the kidney which is prevented by 4MP treatment.
The initially presented clinical evidence suggested that noticeable APAP-induced renal damage occurs in patients who develop severe liver injury after APAP overdose. Hence, experiments were repeated with administration of a higher dose of 600 mg/kg APAP, which results in severe liver injury in mice where liver recovery is compromised (Bhushan et al., 2014). Plasma ALT activities were significantly higher than those with the moderate overdose at 6–24 h (Figure 8A). Despite the more severe liver injury with the higher dose of APAP, 4MP co-treatment completely inhibited the elevation in plasma ALT activities (Figure 8A). Subsequent examination of protein adduct levels 2–24 h after 600 mg/kg APAP revealed substantial adduct formation, which peaked at 6 h in both liver homogenate and in liver mitochondria (Figure 8B,C). The adduct levels also peaked in the kidney between 2 to 6h after 600mg/kg APAP (Figure 8B) but no mitochondrial adducts were detectable in the kidney even with this severe APAP overdose (Figure 8C). 4MP treatment consistently blocked protein adduct formation in the kidney and the liver (Figure 8B,C). These results confirm that early mitochondrial protein adduct formation is unlikely to be a feature in APAP induced nephrotoxicity, although treatment with 4MP can prevent adduct formation in kidney even at the higher APAP overdose.
Figure 8: Liver injury and APAP protein adducts in liver and kidney after a severe overdose.
C57BL/6J mice were co-treated with 600 mg/kg APAP and 50 mg/kg 4MP or 10 ml/kg saline for 2, 6, 12, or 24 h. Time course of plasma ALT activities (A). Time course of protein-derived APAP-cysteine adducts measured in liver and kidney homogenate (B) as well as their mitochondrial fractions (C). APAP-CYS concentrations are expressed in nmol/mg protein. Data represent means ± SEM of n= 4 animals per group. *P< 0.05 (compared to untreated controls). #P< 0.05 (compared to APAP 24 h).
Early JNK activation is unlikely to be involved in APAP induced nephrotoxicity, even with severe overdose.
Though a moderate APAP overdose showed no early JNK activation in the kidney unlike the liver at 2 h after APAP, the goal of these experiments was to confirm that this lack of JNK activation was not due to the lower APAP dose or that it occurred at a different time. Thus, mice were treated with 600 mg/kg APAP and the expression of JNK protein was measured by western blotting at 2, 6 and 24 h after APAP (Figure 9). Compared to the control group, APAP treatment enhanced JNK protein activation at 2 h in the liver, which was sustained at 6 h by which time mitochondrial translocation of P-JNK was evident, with P-JNK disappearing by 24 (Figure 9). In agreement with our previous report (Akakpo et al., 2019), 4MP completely inhibited activation and mitochondrial translocation of JNK 6 h after the severe 600 mg/kg dose in the liver (Figure 9). As in the moderate APAP overdose, there was no noticeable expression of P-JNK at any time point in the kidney (Figure 9). These data further confirm that kidney injury after a severe overdose of APAP occurs independent of JNK activation.
Figure 9: JNK activation and JNK translocation to the mitochondria in liver and kidney after a severe APAP overdose.
C57BL/6J mice were treated with 600 mg/kg APAP and either 50 mg/kg 4MP or 10 ml/kg saline. JNK and P-JNK were assessed in the cytosolic fraction and isolated mitochondria by western blotting. The two bands reflect JNK1 (46kD) and JNK2 (54 kD). Liver and kidney samples were obtained from controls (Cont) and 2, 6 or 24 h after APAP or APAP+4MP.
4MP treatment improves renal dysfunction induced by a severe APAP overdose.
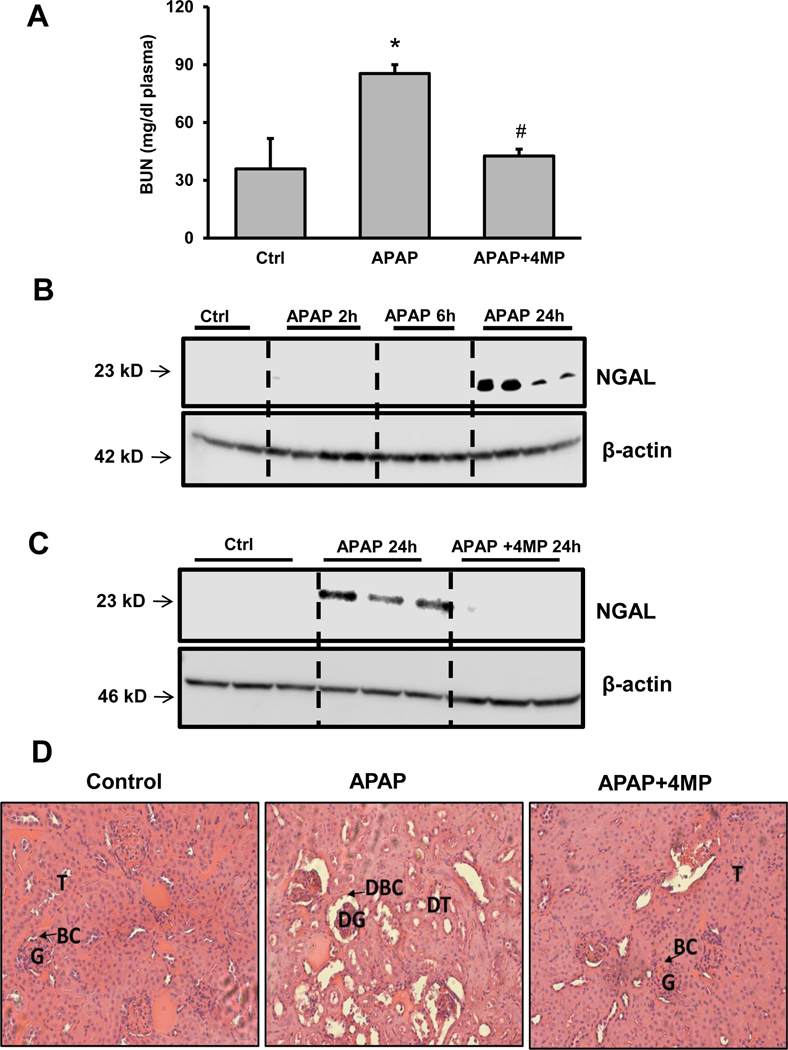
Since earlier evidence suggested that the 300mg/kg APAP dose activated acute kidney stress markers without functional kidney deficits, kidney injury was then evaluated after the severe 600mg/kg APAP dose to determine if the higher dose induced measurable kidney injury. Blood Urea Nitrogen (BUN) levels, an indicator of kidney dysfunction, were significantly increased at 24 h in animals treated with a severe APAP overdose as compared with the vehicle control group. 4MP treatment prevented the increase in BUN levels (Figure 10A). In addition, NGAL expression levels were determined, which were suggested to be a more reliable AKI biomarker than BUN in the clinic (Khawaja et al., 2019; McMahon et al., 2019). Using the samples of the time course (2, 6 and 24 h) we observed that there was substantial upregulation of NGAL protein in the kidneys of APAP-treated mice at 24 h (Figure 10B,C). Strikingly, 4MP treatment completely blocked this increase in NGAL protein expression (Figure 10C). Histological analysis of kidney sections from mice administered a severe APAP overdose indicated renal tissue damage as exhibited by severely disorganized glomeruli, dilated proximal tubules, and the presence of granular cast in the lumen of tubules (Figure 10D). 4MP co-treatment preserved the architecture of the glomeruli and the convoluted proximal tubules. At the same time, granular casting was insignificant and similar to histological sections of the control group. Together these results demonstrate the efficacy of 4MP at protecting the kidney against APAP induced nephrotoxicity.
Figure 10: Kidney injury after a severe overdose of APAP: effect of 4MP.
C57BL/6J mice were treated with 600 mg/kg APAP and sacrificed at 2, 6, or 24 h. In addition, a group was co-treated with APAP and 50 mg/kg 4MP for 24 h. Blood Urea Nitrogen (BUN) levels at 24 h (A). Kidney homogenates were subjected to western blotting for NGAL and β-actin after APAP alone (B) or in combination with 4MP (C). Representative H&E-stained kidney sections of a control, APAP and APAP+4MP at 24 h post APAP (D) (×50 magnification). Data represent means ± SEM of n= 4 animals per group. *P< 0.05 (compared to control). #P< 0.05 (compared to APAP). Abbreviations: T=tubules, BC=Bowman capsule, G=Glomerulus, DT=Damaged tubules, DBC=Damaged Bowman capsule, DG=Damaged glomerulus.
DISCUSSION
Clinical incidence of kidney dysfunction after APAP overdose
Although the liver is well established as the primary site of toxicity after APAP overdose, strong clinical evidence also suggests that APAP overdose can be nephrotoxic and lead to renal insufficiency (Kwok et al., 2004), acute kidney injury (AKI) and failure in children, adolescents and adult patients (Blakely and McDonald, 1995; Boutis and Shannon, 2001; Hengy et al., 2009; Le Vaillant et al., 2013; Ozkaya et al., 2010; Saleem et al., 2019). The importance of extrahepatic organs such as the kidney in patient prognosis after an APAP overdose is illustrated by the fact that acute liver failure can progress to multi-organ failure if the patient does not spontaneously recover or receive a liver transplant. The importance of the kidney in this regard is shown by the inclusion of kidney function parameters such as creatinine in the Model for End-Stage Liver Disease (MELD) score, which is used for liver transplant decisions (Saxena and Lai, 2015). Our data based on 375 definitive APAP overdose patients indicate that kidney dysfunction after APAP overdose strongly correlates with severe liver injury. However, preclinical data suggest that APAP can directly induce nephrotoxicity. This is relevant because extra-hepatic alterations can be consequences of the management of acute liver failure after an APAP overdose. For example, kidney injury can be caused by volume management strategies (Leventhal and Liu, 2015). However, if APAP directly causes kidney injury, as it does in the liver, subsequent insults to the kidney while managing systemic effects of acute liver failure would be a second hit on an already compromised organ.
APAP metabolism in mouse kidney and liver in vivo
Although kidney function is recognized to be affected by an APAP overdose in mice (Kennon-McGill and McGill, 2018; Placke et al., 1987), the mechanistic understanding is limited. APAP-induced hepatotoxicity is initiated by the Cyp2E1-catalyzed generation of the reactive metabolite NAPQI, which forms protein adducts to initiate downstream signaling. The presence of Cyp2E1 in the kidney, albeit at lower levels than in the liver, indicates that NAPQI can also be generated directly within the kidney. Previous data showed that human proximal tubular cells express cytochrome P450 enzymes (Cummings et al., 2000). In addition, APAP undergoes a P450-mediated metabolism, which results in NAPQI formation, GSH depletion and protein binding in the kidney (Emeigh-Hart et al., 1991; Hart et al., 1994, 1995). Immunohistochemistry studies have also demonstrated positive staining with both anti-APAP and anti-CYP2E1 antibodies in renal proximal tubules (Hart et al., 1995). Cytochrome P450-dependent nephrotoxicity and selective protein covalent binding have been observed in male CD-1 mice (Hoivik et al., 1995) and importantly, both NAPQI formation and activity of CYP2E1 have been shown in human kidney samples, which were significantly higher in males compared to females (Arzuk et al., 2018). Furthermore, histopathological analysis of renal biopsies from APAP overdose patients shows evidence of acute proximal and distal renal tubular necrosis as well as loss of tubular brush border (Bjorck et al., 1988; Kleinman et al., 1980).
Our detailed temporal analysis of APAP metabolism in the kidney in comparison to the liver unveiled some interesting findings. Significant APAP metabolism seems to occur in the kidney, evident in the rapid elevation in APAP-glucuronide, with much higher levels of the metabolite in the kidney compared to the liver. Although it cannot be completely excluded that some metabolites measured in the kidney may have been generated in the liver, the differences in the time course of these metabolites support the hypothesis that most of them were generated in the kidney. In addition, there was a substantial difference in the oxidative metabolites.
The total amount of oxidative metabolites (APAP-GSH, APAP-Cys and APAP-NAC), reflecting the total amount of NAPQI formation, was found to be significantly higher in the liver compared to the kidney. This is consistent with the higher expression of CYP2E1 in the liver. In addition, the composition of the detectable oxidative metabolites is different between the liver and the kidney. In the liver, all 3 metabolites were detectable. Because APAP-GSH is highly resistant to degradation by intrahepatic peptidases, it must be exported from hepatocytes to be metabolized (Hinchman and Ballatori, 1994). In the liver, most of the APAP-GSH is transported by the multidrug resistance-associated protein 2 (MRP2) into bile where it is degraded to APAP-Cys by γ-glutamyltransferase (γ-GT) and dipeptidase enzymes (Chen et al., 2003; Hinchman and Ballatori, 1994). Our data are consistent with this process. APAP-GSH, the primary conjugation product of NAPQI, was the dominant metabolite in the liver but both secondary oxidative metabolites APAP-Cys and APAP-NAC were clearly present but at a much lower level. In contrast, APAP-Cys was the only oxidative metabolite detectable in the kidney. Because the kidney has actually the highest expression levels of γ-GT in the body (Hinchman and Ballatori, 1994), it is not surprising that APAP-GSH is effectively degraded to APAP-Cys both in vivo and likely during homogenization of the tissue. The fact that no APAP-NAC was detectable in the kidney, despite the known presence of N-acetyltransferases in this organ (Glowinski and Weber, 1982), suggests that a potential degradation to APAP-Cys followed by its re-absorption and N-acetylation is of limited relevance in the kidney. This observation also indicates that metabolites from the liver that are excreted in urine such as APAP-NAC do not interfere with the measurements of metabolites in the kidney. This indirectly supports the argument that the metabolites detected in the kidney are generated locally and are not derived from the liver.
Mechanisms of APAP-induced cell death in the kidney versus the liver
Extensive investigations into the mechanisms of APAP-induced cell death in the liver have clearly shown the importance of CYP2E1-mediated NAPQI formation, GSH depletion and protein adduct formation as the initiating events (McGill and Jaeschke, 2013; Ramachandran and Jaeschke, 2019). It is also well established that not the general adduct formation but the adducts in the mitochondria cause a limited oxidant stress, which triggers the activation of a MAP kinase cascade ultimately resulting in JNK phosphorylation (Ramachandran and Jaeschke, 2020). P-JNK translocates to the mitochondria, amplifies the oxidative and nitrosative stress, which then causes the mitochondrial membrane permeability pore (MPTP) opening, matrix swelling with rupture of the outer membrane and release of intermembrane endonucleases triggering nuclear DNA degradation (Ramachandran and Jaeschke, 2019, 2020). Based on numerous genetic and pharmacological intervention studies, these events have been established as critical for APAP-induced hepatocellular injury in mice, primary mouse hepatocytes and primary human hepatocytes (Xie et al., 2014). Biomarkers measured in blood from APAP overdose patients also supported this mechanism (McGill et al., 2012a). Our current studies confirmed previous reports (Emeigh-Hart et al., 1991; Hart et al., 1994, 1995) that APAP can be metabolized by Cyp2E1 in the kidney forming the reactive metabolite NAPQI, which depletes GSH and form protein adducts. However, hallmarks of APAP-induced liver injury such as mitochondrial protein adduct formation, JNK activation and P-JNK translocation to the mitochondria were not detectable in the kidney at renal subtoxic and toxic doses of APAP. Although the western blot data of JNK and P-JNK for the total kidney homogenate are very clear, the fact that Cyp2E1 is expressed mainly in proximal tubules, where APAP adducts have also been localized (Hart et al., 1995), raises the possibility that limited JNK activation selectively in this area of the kidney may be occurring and not be detectable with this methodology. Future studies of APAP toxicity in isolated proximal tubules will further clarify this issue. Nevertheless, the signaling pathways of APAP-induced cell death in the kidney, although initiated by the same events as in the liver, appear to be fundamentally different between the two organs. This suggests that additional specific therapeutic targets for the kidney may be identified in future studies of the mechanisms of APAP-induced kidney injury.
4MP as a therapeutic intervention to prevent APAP-induced liver and kidney injury
NAC, the only clinically approved antidote against APAP toxicity, does not seem to be protective against renal injury in preclinical studies (Jones and Vale, 1993; Slitt et al., 2004). In contrast, our current experiments suggest that 4MP can effectively prevent APAP-induced renal dysfunction and organ damage. It is well known that the oxidative metabolism of APAP occurs mainly through Cyp2E1 (McGill and Jaeschke, 2013). In addition, 4MP has been shown to dose-dependently inhibit Cyp2E1 activity in human liver microsomes (Hazai et al., 2002) and Cyp2E1/Cyp1A2 activity in liver (Akakpo et al., 2018) and kidney homogenate (Figure 1C). In addition, molecular docking studies showed direct binding of 4MP to the substrate binding site of Cyp2E1 (Akakpo et al., 2019). 4MP has also been shown to reduce the presence of all oxidative metabolites after APAP overdose in plasma of humans (Kang et al., 2020) and in mice (Akakpo et al., 2018). Together with the demonstration that 4MP attenuated oxidative metabolism in the liver and in the kidney of mice (Figure 2–4), these data strongly suggest that APAP-induced liver and kidney injury was prevented by 4MP-mediated inhibition of Cyp2E1; however, these results do not identify 4MP as a selective inhibitor for Cyp2E1. Although 4MP can also inhibit JNK activation and mitochondrial JNK translocation (Akakpo et al., 2019), the fact that we did not detect JNK activation in the kidney after a moderate and a high overdose of APAP suggests that the protective effect in the kidney did likely not involve JNK inhibition. Thus, 4MP can prevent kidney injury after an APAP overdose by inhibiting the metabolic activation of APAP when administered early. Whether 4MP may be effective when given at later time points requires further studies into the late signaling events of renal cell death.
Gender-specific issues of APAP toxicity and protection by 4MP
We did not use female mice because of their lower susceptibility to APAP (Du et al., 2014; Masubuchi et al., 2011). Although the mechanism of injury is the same, the faster recovery of hepatic GSH after the initial depletion more effectively scavenges peroxynitrite in female mice compared to males (Du et al., 2014). Therefore, most APAP toxicity studies are performed in male mice. However, kidney injury occurs to the same percentage in male as in female patients (Table 1); the general higher percentage of female patients with APAP-induced liver injury is a reflection of the higher percentage of female overdosing on APAP (Curry et al., 2019). 4MP inhibited the oxidative metabolite formation after APAP overdose in both male and female volunteers (Kang et al., 2020), suggesting similar mechanisms of injury and protection in male and female patients.
In summary, our studies showed that the novel antidote 4MP can not only prevent APAP-induced liver injury but is equally as effective in inhibiting renal damage when administered early. The mechanism of action of 4MP seems to be only inhibiting Cyp2E1 and not JNK as there is no evidence of JNK activation in the kidney. Given the limited efficacy of NAC against APAP-induced kidney injury in animals (Jones and Vale, 1993; Slitt et al., 2004), this implies an advantage of 4MP as a potential antidote against APAP overdose. In a future randomized controlled clinical trial investigating 4MP in late presenting patients who took a very high APAP overdose, not only liver injury but also kidney dysfunction assessment needs to be included to confirm the preclinical studies.
Highlights.
APAP causes renal failure in 12% of overdose patients with severe liver injury
APAP overdose causes liver injury in mice and renal damage only at higher doses
Cyp2E1 levels and APAP protein adducts are higher in liver compared to kidney
In kidney there was no mitochondrial protein adducts or JNK activation detectable
4-methylpyrazole prevented APAP-induced kidney injury by inhibiting Cyp2E1
ACKNOWLEDGEMENTS
This work was supported by a grant from McNeil Consumer Health Care, Inc., the National Institutes of Health grant R01 DK102142, and the National Institute of General Medical Sciences (P20 GM103549 and P30 GM118247) from the National Institutes of Health. J.Y.A. was supported by a NIH Predoctoral Fellowship F31 DK120194-01.
ABBREVIATIONS
- AKI
acute kidney injury
- ALF
acute liver failure
- ALT
alanine aminotransferase
- APAP
Acetaminophen
- AST
aspartate aminotransferase
- BUN
blood urea nitrogen
- γ-GT
γ-glutamyltransferase
- 7-EFC
7-ethoxy-4-trifluoromethyl coumarin
- GSH+GSSG
total glutathione
- JNK
c-jun N-terminal kinase
- MAP kinase
mitogen activated protein kinase
- MELD score
Model for End-Stage Liver Disease score
- 4MP
4-methylpyrazole
- MRP2
multidrug resistance-associated protein-2
- NAC
N-acetylcysteine
- NAPQI
N-acetyl-p-benzoquinone imine
- NGAL
Neutrophil gelatinase-associated lipocalin
- PT
prothrombin time
Footnotes
DECLARATION OF COMPETING INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akakpo JY, Ramachandran A, Duan L, Schaich MA, Jaeschke MW, Freudenthal BD, Ding WX, Rumack BH, Jaeschke H, 2019. Delayed Treatment With 4-Methylpyrazole Protects Against Acetaminophen Hepatotoxicity in Mice by Inhibition of c-Jun N-Terminal Kinase. Toxicol. Sci 170, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akakpo JY, Ramachandran A, Kandel SE, Ni HM, Kumer SC, Rumack BH, Jaeschke H, 2018. 4-Methylpyrazole protects against acetaminophen hepatotoxicity in mice and in primary human hepatocytes. Hum. Exp. Toxicol 37, 1310–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzuk E, Turna B, Sözbilen M, Orhan H, 2018. Inter-individual and inter-organ variability in the bioactivation of paracetamol by human liver and kidney tissues. Environ. Toxicol. Pharmacol 61, 8–17. [DOI] [PubMed] [Google Scholar]
- Bhushan B, Walesky C, Manley M, Gallagher T, Borude P, Edwards G, Monga SP, Apte U, 2014. Pro-regenerative signaling after acetaminophen-induced acute liver injury in mice identified using a novel incremental dose model. Am. J. Pathol 184, 3013–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björck S, Svalander CT, Aurell M, 1988. Acute renal failure after analgesic drugs including paracetamol (acetaminophen). Nephron 49, 45–53. [DOI] [PubMed] [Google Scholar]
- Blakely P, McDonald BR, 1995. Acute renal failure due to acetaminophen ingestion: a case report and review of the literature. J. Am. Soc. Nephrol 6, 48–53. [DOI] [PubMed] [Google Scholar]
- Boutis K, Shannon M, 2001. Nephrotoxicity after acute severe acetaminophen poisoning in adolescents. J. Toxicol. Clin. Toxicol 39, 441–445. [DOI] [PubMed] [Google Scholar]
- Brent J, 2009. Fomepizole for ethylene glycol and methanol poisoning. N. Engl. J. Med 360, 2216–2223. [DOI] [PubMed] [Google Scholar]
- Buters JT, Schiller CD, Chou RC, 1993. A highly sensitive tool for the assay of cytochrome P450 enzyme activity in rat, dog and man. Direct fluorescence monitoring of the deethylation of 7-ethoxy-4-trifluoromethylcoumarin. Biochem. Pharmacol 46, 1577–1584. [DOI] [PubMed] [Google Scholar]
- Chang TK, Crespi CL, Waxman DJ, 2006. Determination of CYP2B6 component of 7-ethoxy-4-trifluoromethylcoumarin O-deethylation activity in human liver microsomes. Methods Mol. Biol 320, 97–102. [DOI] [PubMed] [Google Scholar]
- Chen C, Hennig GE, Manautou JE, 2003. Hepatobiliary excretion of acetaminophen glutathione conjugate and its derivatives in transport-deficient (TR-) hyperbilirubinemic rats. Drug Metab. Dispos 31, 798–804. [DOI] [PubMed] [Google Scholar]
- Chopyk DM, Stuart JD, Zimmerman MG, Wen J, Gumber S, Suthar MS, Thapa M, Czaja MJ, Grakoui A, 2019. Acetaminophen Intoxication Rapidly Induces Apoptosis of Intestinal Crypt Stem Cells and Enhances Intestinal Permeability. Hepatol. Commun 3, 1435–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobden I, Record CO, Ward MK, Kerr DN, 1982. Paracetamol-induced acute renal failure in the absence of fulminant liver damage. Br. Med. J. (Clin. Res. Ed). 284, 21–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SC, Aldridge RC, Shah T, Webb K, Nightingale P, Paris S, Gunson BK, Mutimer DJ, Neuberger JM, 2009. Outcomes of liver transplantation for paracetamol (acetaminophen)-induced hepatic failure. Liver Transpl. 15, 1351–1357. [DOI] [PubMed] [Google Scholar]
- Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H, 2005. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J. Pharmacol. Exp. Ther 315, 879–887. [DOI] [PubMed] [Google Scholar]
- Cummings BS, Lasker JM, Lash LH, 2000. Expression of glutathione-dependent enzymes and cytochrome P450s in freshly isolated and primary cultures of proximal tubular cells from human kidney. J. Pharmacol. Exp. Ther 293, 677–685. [PubMed] [Google Scholar]
- Curry SC, Padilla-Jones A, Ruha AM, O’Connor AD, Kang AM, Wilkins DG, Jaeschke H, Wilhelms K, Gerkin RD; Acetaminophen Adduct Study Group., 2019. The Relationship Between Circulating Acetaminophen-Protein Adduct Concentrations and Alanine Aminotransferase Activities in Patients With and Without Acetaminophen Overdose and Toxicity. J. Med. Toxicol 15, 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport A, Finn R, 1988. Paracetamol (acetaminophen) poisoning resulting in acute renal failure without hepatic coma. Nephron 50, 55–56. [DOI] [PubMed] [Google Scholar]
- Du K, Williams CD, McGill MR, Jaeschke H, 2014. Lower susceptibility of female mice to acetaminophen hepatotoxicity: Role of mitochondrial glutathione, oxidant stress and c-jun N-terminal kinase. Toxicol. Appl. Pharmacol 281, 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguia L, Materson BJ, 1997. Acetaminophen-related acute renal failure without fulminant liver failure. Pharmacotherapy 17, 363–370. [PubMed] [Google Scholar]
- Emeigh Hart SG, Beierschmitt WP, Bartolone JB, Wyand DS, Khairallah EA, Cohen SD, 1991. Evidence against deacetylation and for cytochrome P450-mediated activation in acetaminophen-induced nephrotoxicity in the CD-1 mouse. Toxicol. Appl. Pharmacol 107, 1–15. [DOI] [PubMed] [Google Scholar]
- Fisher ES, Curry SC, 2019. Evaluation and treatment of acetaminophen toxicity. Adv. Pharmacol 85, 263–272. [DOI] [PubMed] [Google Scholar]
- Glowinski IB, Weber WW, 1982. Biochemical characterization of genetically variant aromatic amine N-acetyltransferases in A/J and C57BL/6J mice. J. Biol. Chem 257, 1431–1437. [PubMed] [Google Scholar]
- Hart SG, Beierschmitt WP, Wyand DS, Khairallah EA, Cohen SD, 1994. Acetaminophen nephrotoxicity in CD-1 mice. I. Evidence of a role for in situ activation in selective covalent binding and toxicity. Toxicol Appl Pharmacol. 126, 267–275. [DOI] [PubMed] [Google Scholar]
- Hart SG, Cartun RW, Wyand DS, Khairallah EA, Cohen SD, 1995. Immunohistochemical localization of acetaminophen in target tissues of the CD-1 mouse: correspondence of covalent binding with toxicity. Fundam. Appl. Toxicol 24, 260–274. [DOI] [PubMed] [Google Scholar]
- Hadem J, Kielstein JT, Manns MP, Kümpers P, Lukasz A, 2019. Outcomes of renal dysfunction in patients with acute liver failure. United European Gastroenterol. J 7, 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazai E, Vereczkey L, Monostory K, 2002. Reduction of toxic metabolite formation of acetaminophen. Biochem. Biophys. Res. Commun 291, 1089–1094. [DOI] [PubMed] [Google Scholar]
- Heard KJ, 2008. Acetylcysteine for acetaminophen poisoning. N. Engl. J. Med 359, 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengy B, Hayi-Slayman D, Page M, Christin F, Baillon JJ, Ber CE, Allaouchiche B, Rimmelé T, 2009. [Acute renal failure after acetaminophen poisoning: report of three cases]. Can. J. Anaesth 56, 770–774. [DOI] [PubMed] [Google Scholar]
- Hinchman CA, Ballatori N, 1994. Glutathione conjugation and conversion to mercapturic acids can occur as an intrahepatic process. J. Toxicol. Environ. Health 41, 387–409. [DOI] [PubMed] [Google Scholar]
- Hodgman MJ, Garrard AR, 2012. A review of acetaminophen poisoning. Crit. Care Clin 28, 499–516. [DOI] [PubMed] [Google Scholar]
- Hoivik DJ, Manautou JE, Tveit A, Hart SG, Khairallah EA, Cohen SD, 1995. Gender-related differences in susceptibility to acetaminophen-induced protein arylation and nephrotoxicity in the CD-1 mouse. Toxicol. Appl. Pharmacol 130, 257–271. [DOI] [PubMed] [Google Scholar]
- Hoste EAJ, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM, Goldstein SL, Cerdá J, Chawla LS, 2018. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol 14, 607–625. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, 2015. Acetaminophen: Dose-Dependent Drug Hepatotoxicity and Acute Liver Failure in Patients. Dig. Dis 33, 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery WH, Lafferty WE, 1981. Acute renal failure after acetaminophen overdose: report of two cases. Am. J. Hosp. Pharm 38, 1355–1358. [PubMed] [Google Scholar]
- Jones AF, Vale JA, 1993. Paracetamol poisoning and the kidney. J. Clin. Pharm. Ther 18, 5–8. [DOI] [PubMed] [Google Scholar]
- Kang AM, Padilla-Jones A, Fisher ES, Akakpo JY, Jaeschke H, Rumack BH, Gerkin RD, Curry SC, 2020. The Effect of 4-Methylpyrazole on Oxidative Metabolism of Acetaminophen in Human Volunteers. J. Med. Toxicol 16, 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennon-McGill S, McGill MR, 2017. Extrahepatic toxicity of acetaminophen: critical evaluation of the evidence and proposed mechanisms. J. Clin. Transl. Res 3, 297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khawaja S, Jafri L, Siddiqui I, Hashmi M, Ghani F, 2019. The utility of neutrophil gelatinase-associated Lipocalin (NGAL) as a marker of acute kidney injury (AKI) in critically ill patients. Biomark. Res 7, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman JG, Breitenfield RV, Roth DA, 1980. Acute renal failure associated with acetaminophen ingestion: report of a case and review of the literature. Clin. Nephrol 14, 201–205. [PubMed] [Google Scholar]
- Kwok KL, Fu YM, Ng DK, 2004. Hepatotoxicity and persistent renal insufficiency after repeated supratherapeutic paracetamol ingestion in a Chinese boy. Hong Kong Med. J 10, 61–64. [PubMed] [Google Scholar]
- Leithead JA, Ferguson JW, Bates CM, Davidson JS, Lee A, Bathgate AJ, Hayes PC, Simpson KJ, 2009. The systemic inflammatory response syndrome is predictive of renal dysfunction in patients with non-paracetamol-induced acute liver failure. Gut 58, 443–449. [DOI] [PubMed] [Google Scholar]
- Le Vaillant J, Pellerin L, Brouard J, Eckart P, 2013. [Acetaminophen (paracetamol) causing renal failure: report on 3 pediatric cases]. Arch. Pediatr 20, 650–653. [DOI] [PubMed] [Google Scholar]
- Leventhal TM, Liu KD, 2015. What a Nephrologist Needs to Know About Acute Liver Failure. Adv. Chronic Kidney Dis 22, 376–381. [DOI] [PubMed] [Google Scholar]
- Masubuchi Y, Nakayama J, Watanabe Y, 2011. Sex difference in susceptibility to acetaminophen hepatotoxicity is reversed by buthionine sulfoximine. Toxicology. 287, 54–60. [DOI] [PubMed] [Google Scholar]
- McMahon BA, Galligan M, Redahan L, Martin T, Meaney E, Cotter EJ, Murphy N, Hannon C, Doran P, Marsh B, Nichol A, Murray PT, 2019. Biomarker Predictors of Adverse Acute Kidney Injury Outcomes in Critically Ill Patients: The Dublin Acute Biomarker Group Evaluation Study. Am. J. Nephrol 50, 19–28. [DOI] [PubMed] [Google Scholar]
- McGill MR, Jaeschke H, 2013. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm. Res 30, 2174–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Jaeschke H, 2015. A direct comparison of methods used to measure oxidized glutathione in biological samples: 2-vinylpyridine and N-ethylmaleimide. Toxicol. Mech. Methods 25, 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H, 2012a. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J. Clin. Invest 122, 1574–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Williams CD, Xie Y, Ramachandran A, Jaeschke H, 2012b. Acetaminophen-induced liver injury in rats and mice: comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol. Appl. Pharmacol 264, 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JK, Love E, Craig DG, Hayes PC, Simpson KJ, 2013. Acute kidney injury in acute liver failure: a review. Expert Rev. Gastroenterol. Hepatol 7, 701–712. [DOI] [PubMed] [Google Scholar]
- O’Riordan A, Brummell Z, Sizer E, Auzinger G, Heaton N, O’Grady JG, Bernal W, Hendry BM, Wendon JA, 2011. Acute kidney injury in patients admitted to a liver intensive therapy unit with paracetamol-induced hepatotoxicity. Nephrol. Dial. Transplant 26, 3501–3508. [DOI] [PubMed] [Google Scholar]
- Ozkaya O, Genc G, Bek K, Sullu Y, 2010. A case of acetaminophen (paracetamol) causing renal failure without liver damage in a child and review of literature. Ren. Fail 32, 1125–1127. [DOI] [PubMed] [Google Scholar]
- Placke ME, Wyand DS, Cohen SD, 1987. Extrahepatic lesions induced by acetaminophen in the mouse. Toxicol. Pathol 15, 381–387. [DOI] [PubMed] [Google Scholar]
- Prescott LF, Illingworth RN, Critchley JA, Stewart MJ, Adam RD, Proudfoot AT, 1979. Intravenous N-acetylcystine: the treatment of choice for paracetamol poisoning. Br. Med. J 2, 1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Jaeschke H, 2019. Acetaminophen Hepatotoxicity. Semin. Liver Dis 39, 221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Jaeschke H, 2020. A mitochondrial journey through acetaminophen hepatotoxicity. Food Chem. Toxicol 140, 111282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampon G, Wartman H, Osmon S, Scalzo A, 2020. Use of fomepizole as an adjunct in the treatment of acetaminophen overdose: a case series. Toxicol. Commun 4, 1–4. [Google Scholar]
- Saleem M, Iftikhar H, 2019. A Rare Case of Acetaminophen Toxicity Leading to Severe Kidney Injury. Cureus 11, e5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena V, Lai JC, 2015. Kidney Failure and Liver Allocation: Current Practices and Potential Improvements. Adv. Chronic Kidney. Dis 22, 391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slitt AL, Dominick PK, Roberts JC, Cohen SD, 2004. Standard of care may not protect against acetaminophen-induced nephrotoxicity. Basic Clin. Pharmacol. Toxicol 95, 247–248. [DOI] [PubMed] [Google Scholar]
- Stern ST, Bruno MK, Hennig GE, Horton RA, Roberts JC, Cohen SD, 2005. Contribution of acetaminophen-cysteine to acetaminophen nephrotoxicity in CD-1 mice: I. Enhancement of acetaminophen nephrotoxicity by acetaminophen-cysteine. Toxicol. Appl. Pharmacol 202, 151–159. [DOI] [PubMed] [Google Scholar]
- Tonge RP, Kelly EJ, Bruschi SA, Kalhorn T, Eaton DL, Nebert DW, Nelson SD, 1998. Role of CYP1A2 in the hepatotoxicity of acetaminophen: investigations using Cyp1a2 null mice. Toxicol. Appl. Pharmacol 153, 102–108. [DOI] [PubMed] [Google Scholar]
- Tujios SR, Hynan LS, Vazquez MA, Larson AM, Seremba E, Sanders CM, Lee WM; Acute Liver Failure Study Group., 2015. Risk factors and outcomes of acute kidney injury in patients with acute liver failure. Clin. Gastroenterol. Hepatol 13, 352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, McGill MR, Dorko K, Kumer SC, Schmitt TM, Forster J, Jaeschke H, 2014. Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol. Appl. Pharmacol 279, 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon E, Babar A, Choudhary M, Kutner M, Pyrsopoulos N, 2016. Acetaminophen-Induced Hepatotoxicity: a Comprehensive Update. J. Clin. Transl. Hepatol 4, 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]