Abstract
To analyze EVs with conventional flow cytometers, most researchers will find it necessary to bind EVs to beads that are large enough to be individually resolved on the flow cytometer available in their lab or facility. Although high-resolution flow cytometers are available and are being used for EV analysis, the use of these instruments for studying EVs requires careful use and validation by experienced small-particle flow cytometrists, beyond the scope of this chapter. Shown here is a method for using streptavidin-coated beads to capture biotinylated antibodies, and stain the bead-bound EVs with directly conjugated antibodies. We find that this method is a useful tool not only on its own, without further high resolution flow cytometric analysis, but also as a means for optimizing staining methods and testing new labels for later use in high resolution, single EV flow cytometric studies. The end of the chapter includes sphere-packing calculations to quantify aspects of EV- and bead-surface geometry, as a reference for use as readers of this chapter optimize their own flow cytometry assays with EVs.
Keywords: Flow cytometry, Extracellular vesicles, Exosomes, Subsets
1. Introduction
High sensitivity flow cytometers have been reported [1], and methods for analysis of extracellular vesicles (EVs) have been reported with bead-based assays [2], imaging cytometers [3], and adaptations of commercially available flow cytometers [4, 5]. However, the methods of use of those instruments for the study of extracellular vesicles are specialized and not readily implemented by researchers without focused training or the assistance of experienced flow cytometrists.
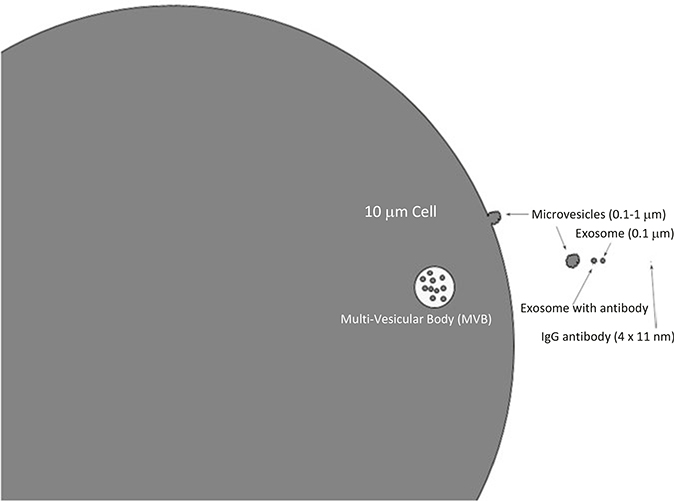
The difficulty of studying EVs with flow cytometry lies in the small size of the materials being studied (Fig. 1). EVs are so much smaller than the cells that modern flow cytometers were designed to study, that the analysis of EVs with conventional flow cytometers can be accompanied by numerous artifacts if the researcher does not take appropriate precautions to avoid swarm [6], which can be due to coincident events at the laser intercept with the sample and can be due excess event anomalies in instrument signal processing. The purpose of this chapter is to present a method that can be used and adapted by any laboratory with access to any flow cytometer that is used to study cells.
Fig. 1.
Relative sizes of EVs, cells, and antibodies. The typical size of a laser intercept at the point of flow cytometric analysis of cells, beads, or EVs is 10–20 μm, while exosomes and similar EVs are ~0.1 μm. The objects in this figure are drawn to scale, to illustrate relative sizes of relevant structures and objects
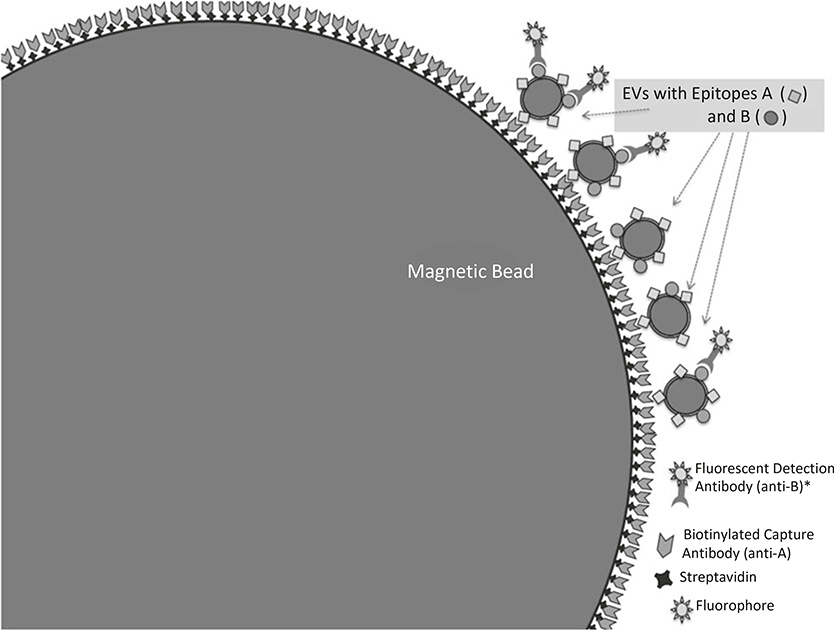
To analyze EVs with conventional flow cytometers, a broadly useful approach is to bind EVs to beads that are large enough to be reliably resolved on the flow cytometer. An early example of this approach demonstrated that 30–100 nm exosomes could be isolated from cell culture supernatants and characterized by flow cytometry, after binding the exosomes to latex beads [7]. Shown here is a method for using streptavidin-coated beads to capture biotinylated antibodies, prior to washing the beads (to remove unbound antibodies), capturing EVs, and staining the bead-bound EVs with directly conjugated antibodies. We find that the use ligands that are specific for EV populations of interest is helpful for reducing nonspecific background that can be caused by protein binding to latex or other protein-binding beads. Figure 2 illustrates the conceptual approach, while Fig. 3 sets out the basic steps for the method. Objects in Fig. 2 are not drawn to scale. Rather, they are drawn to best illustrate the conceptual assembly of the beads with ligands.
Fig. 2.
Detection of EVs and EV-associated surface molecules by binding EVs to beads. To analyze EVs with conventional flow cytometers, it is generally necessary to bind EVs to beads that are large enough to be individually resolved on the flow cytometer. The objects are not drawn to scale. Rather, they are drawn to best illustrate the conceptual assembly of the beads with ligands
Fig. 3.
Flowchart for capture and analysis of EVs by binding to beads. Shown here is a general method for using streptavidin-coated beads to capture biotinylated antibodies, prior washing the beads (to remove unbound antibodies), capturing EVs, and staining the bead-bound EVs with directly conjugated antibodies
2. Materials
Maintaining sterile conditions throughout these steps will help to reduce background and preserve EV integrity. Because precipitates or small particles of salts, proteins, or other materials can interfere with nanometric sample analysis, it is important to perform all experiments with ultrapure reagents, with low background, verified by nanoparticle tracking analysis (NTA) or other similar small particle measuring instrument (see Note 1).
PureProteome™ Streptavidin Magnetic Beads (EMDMillipore).
Anti-CD81-biotin and anti-CD9-biotin, 0.5 mg/ml (Biolegend).
PureProteome™ Magnetic Stand, 8-well (EMDMillipore).
Dulbecco’s hosphate buffered saline (dPBS), pH 7.4 (GIBCO/Invitrogen).
Tris buffered saline (TBS), pH 7.4.
Tween 20 (Bio-Rad).
Casein blocking buffer (1 % Casein in TBS).
T-TBS: 0.1 % Tween 20 in TBS.
Additional antibodies for flow cytometry, including Fc-Block. Fc-Block is Rat Anti-Mouse CD16/CD32 (BD Biosciences).
Agitation/mixing/inversion system.
DNAse/RNAse-free, sterile, low protein-binding microcentrifuge tubes.
3. Methods
There are three steps to this method. The first step is isolation and quantification of EVs, from cell culture supernatants or biofluids. Isolation of EVs can be performed in a crude manner, removing only the cells from supernatants of a cell culture, or in a more precise manner with size exclusion chromatography or with serial ultracentrifugation, as previously described [8, 9], and is not further described here. When binding EVs to beads, it is critical to know the approximate EV binding capacity of the surface of the bead, as well as the approximate EV concentration in the solution from which EVs will be captured and bound onto beads (Subheading 3.1) for staining prior to flow cytometric analysis (Subheading 3.2). Table 1 includes estimates of the relevant surface- binding capacity of cells, beads, and EVs (see Notes 2 and 3).
Table 1.
Size vs fluorophore density for cells, EVs, and beads
| Object | Typical diameter (μm) | Volume (μm3) | Surface area (μm2) | Max surface binding sites for 100 nm EVsa | Surface binding sites possible for typical IgG, short axis (5 nm)b | Surface binding sites possible for typical IgG, long axis (10 nm)c | Surface binding sites possible for PE-conjugated IgG, (∼25 nm) IgGd |
|---|---|---|---|---|---|---|---|
| Stem cells | 30–40 | 14,100–33,500 | 2800–5000 | 280,000 – 500,000 | |||
| Macrophages | 20 – 80 | 4200–268,000 | 1250–20,000 | 125,000–2,000,000 | |||
| T Lymphocyte | 7–10 | 180–520 | 150–314 | 15,000–36,000 | |||
| Microvesicles /microparticles | 0.10–1 | 0.00005–0.52 | 0.03–3 | – | 1100–130,000 | 300–34,400 | 48–5500 |
| Exosomes | 0.04–0.12 | 0.00003–0.0009 | 0.005–0.045 | – | 200–2000 | 50–500 | 6–70 |
| IgG | 4×11 nm | – | – | – | – | – | – |
| 10 μm bead | 10 | 520 | 314 | 31,300–36,000 | >12,550,000 | >3,200,000 | 500,000–580,000 |
| 4 μm bead | 4 | 33.5 | 50 | 5000–5700 | >2,000,000 | 500,000–577,000 | 79,000–92,000 |
| 10 μm bead + 100 nm EVs | 10.2 | – | – | – | 31,300×1100 = 34,430,000 | 31,300×300 = 9,930,000 | 31,300×48 = 1,502,400 |
| 4 μm bead + 100 nm EVs | 4.2 | – | – | – | 5000×1100 = 5,500,000 | 5000×300 = 1,500,000 | 5000×48 = 240,000 |
Flow cytometric detection of specific epitopes on cells or EVs requires fluorescent labels, usually in the form of fluorophore-conjugated antibodies. When considering detection of fluorescently labeled EVs, as compared to the detection of fluorescently labeled cells, the relevance of size becomes apparent, especially in terms of how many antibodies can theoretically bind to the EV, cell, or bead coated with EVs. When cells or EVs are labeled with antibodies, the extremely small size of the EVs limits the number of possible epitope density. Surface binding calculations in this chart represent maximal ligand binding density, assuming complete surface area coverage with ligands with the indicated approximate ligand diameter (100 nm estimate for EVsa). Since antibodies are asymmetric, estimates with ligand diameters correlated with longc and shortb axes of IgG were performed. Antibodies conjugated with APC (105 kDa) or PE (240 kDa) at 1:1 coupling efficiency are expected to be at least twice as large as unlabeled IgG (150 kDa)d
3.1. EV Capture on Beads
In this protocol, 10 μm magnetic beads (Millipore) are coupled with 100 μg/ml biotinylated antibody for 1 h at room temperature, under gentle agitation. EV capture is performed with rotation overnight at room temperature or at 4 °C, depending on epitope and EV-cargo stability. Beads of this size were selected due to their large EV-binding capacity and their size, which is equivalent to cells that can be visualized with standard flow cytometric methods.
This protocol is designed to prepare a minimal volume of 8 μl of beads, which we find is a minimal quantity to perform 4–5 assays, with a minimum of ~50,000 bead events to be collected for each analysis by flow cytometry (see Notes 4 and 5). Two microliters of beads can be used the capture EVs from 10 to 15 ml of tissue culture supernatants, after 300 × g and 10,000 × g centrifugation steps to remove cells and large debris. When adding 2 μl of the Millipore Streptavidin Magnetic Beads to 10 ml of tissue culture supernatant, the concentration of beads during the incubation is 2.4 × 104/ml. If purified (and concentrated, small volume) EV samples are used, ~1011 EVs at 1012 EV/ml is recommended as a starting point for this protocol (see Note 6).
To capture CD81-positive and CD9-positive EVs on beads:
Mix streptavidin-coated magnetic bead stock by inversion or gentle vortexing, to resuspend completely.
Transfer 2 μl of beads to a 2 ml sterile microfuge tube (round bottom, if available). Add 40 μl T-TBS, mix gently.
Place the tube with the beads into a magnetic stand that will support 1.5–2 ml microcentrifuge tubes (Dynal magnetic stand by Invitrogen, or PureProteome magnetic stand by EMDMillipore).
The beads will migrate to the magnet within minutes, and be visible as a dark (or ruddy) patch or stripe.
Carefully aspirate away >95 % of the liquid, being cautious not to touch the beads with the pipette tip.
Add 250 μl of T-TBS to the tube, and then remove the tube and mix the beads gently in the buffer to wash.
Place the tube back on the magnetic stand, and, again, aspirate and discard the free buffer, taking care not to disturb the beads that at the side of the tube.
Repeat steps 6–7 as a wash, twice more.
Add 2 μg of SAV-conjugated antibody (1 μg of anti-CD81 and 1 μg of anti-CD9; 2 μl of each antibody, in this example).
Add T-TBS to a final volume of 8 μl (an additional 4 μl in this example).
Incubate for 1 h at room temperature with gentle agitation. It is important to make sure that the solution is mixing, and that the beads are not stationary at the bottom of the tube.
After the incubation, place the tube back in the magnet, remove the supernatant, and repeat steps 6 and 7 three times, with 250 μl T-TBS each time, to wash away unbound antibody. To quantify the amount of residual antibody that did not bind to the beads, the first supernatant from step 12 may be set aside for analysis, rather than be discarded after aspiration.
Wash once in the magnet with phosphate buffered saline, pH 7.4.
Resuspend the antibody-coupled beads in 8 μl of PBS.
The final concentration of beads is 1.25 % w/v, or approximately 120 million beads per ml.
Combine 2 μl of these labeled beads with 10–15 ml tissue culture supernatant that contains EVs of interest. (The supernatant should be free of cells and other debris, after a 10,000 × g centrifugation, or equivalent size exclusion chromatography step.)
Incubate the beads and supernatant overnight, with constant, gentle rotation in a refrigerated room.
3.2. EV Staining on Beads for Flow Cytometric Analysis
For staining, EV-coated beads are blocked with Fc Block (Fc Block may be optional for human EVs) in a saline buffer containing 5 mg/ml casein, 25 mM Tris and 150 mM NaCl at pH 7.4, and then directly conjugated antibodies (e.g., PE-, FITC-, APC-, or other label-coupled antibodies) are added at 10 μg/ml in same buffer for 15 min (see Note 7). As with all protein-based protocols, the following protocol is best performed under refrigerated conditions (4 °C) (see Notes 8 and 9).
Centrifuge the mixture of supernatant with beads at 300 × g, for 5 min. Beads and EVs bound to beads will pellet at this step.
Aspirate supernatant (keep for later analysis, if desired) to leave the bead pellet along with ~500 μl of fluid. The bead pellet may be difficult to visualize at this step, so leaving the 500 μl of buffer is a means of being careful not to lose the beads in the aspirate.
Mix the remaining 500 μl and beads, then transfer to a microfuge tube and place in the magnetic stand.
Remove the buffer supernatant with care to not disturb the beads at the side of the tube, beside the magnet.
Add, 100 μl Casein blocking buffer + 2 μl Fc Block per tube.
Incubate for 10 min, with gentle agitation.
- Prepare in separate tubes: 100 μl of antibody in casein-blocking buffer, for each staining assay (see Note 10).
- Each staining solution should have 1 μg of antibody per tube
- Example: if the concentration of the antibody stock is 100 μg/ml, use 10 μl of antibody per 100 μl of staining solution (with 90 μl casein buffer to complete the volume) (see Note 11).
- For negative controls, isotype control antibodies as a “negative staining control” are one appropriate negative control, but another important negative control is a negative control for nonspecific binding to the beads. For this nonspecific binding control, we use beads that were coated with biotinylated antibody, but not EVs, and then stained with the same antibodies used to stain the EV-bound beads.
After 10 min blocking step with agitation, return the sample tube with beads to the magnetic stand, remove the buffer from the beads, and then add the antibody mix (see Note 12).
Incubate for 15 min in agitation.
Wash two times more with Casein-Blocking TBS buffer.
Resuspend the beads with stained EVs in 150 μl of blocking buffer, and proceed to analysis with conventional flow cytometric methods, with appropriate instrument calibration and sample compensation controls (Fig. 4).
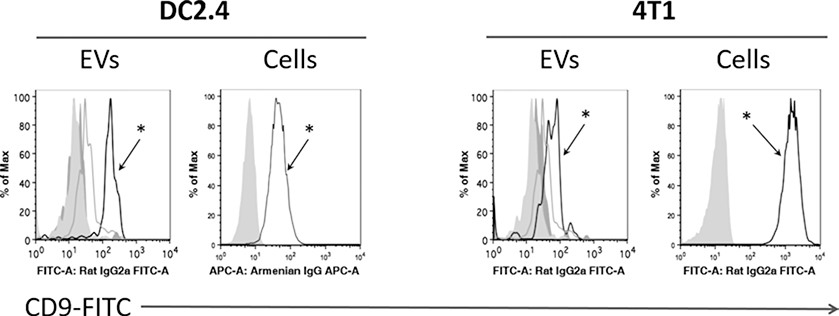
Fig. 4.
Example analysis of epitope detection EVs by binding to beads. EVs isolated from DC2.4 and 4T1 cell cultures (dark grey histograms), as well as control EV-depleted medium (light grey histograms), were incubated with anti-CD9 coated magnetic beads overnight, and subsequently labeled with anti-CD9-FITC antibodies. The same clone of anti-CD9 was used for capture and detection, to ensure EV-anchored CD9 detection and not free (soluble) CD9 detection, if any. Open histograms with (asterisk) correspond to FITC-CD9 staining profile on the surface of EVs from DC2.4 and 4T1 cell lines, while the other histograms represent isotype and nonspecific binding controls
Footnotes
Antibodies, beads, EV preparations, and all combinations thereof must be titrated for optimal results. We find that saving supernatants, rather than discarding them, at steps along the protocol, and then analyzing the supernatants with protein quantification or with gel electrophoresis can help to ascertain whether more or less material may be required in future iterations.
SI = Staining index.
MFI = Mean Fluorescence Intensity.
SD = Standard Deviation.
This bead-analysis protocol is optimized for use with cell culture supernatants that have been generated for the production of EVs. In this specific protocol, we used EVs in the range of 1011 EVs per bead-binding reaction. The concentration of the EVs produced by cell lines varies, depending on the cell type and on the conditions or stressors of the cell growth. As noted above, titration may be required to optimize conditions for different cell lines and for different specific EV populations that are being isolated from the supernatants.
Because staining intensity of the beads will depend upon number of positive EVs bound to each bead, in addition to how many epitopes are available per EV to bind to the labeled antibody, care should be taken to interpret results carefully. Brighter staining might be either due to higher levels of ligand per EV, or more EVs with the ligand bound to the bead.
Molecules of Equivalent Soluble Fluorescence (MESF) beads can be used to quantify number of fluorescent molecules per bead, but these beads must be run with each experiment to be quantified. MESF beads are only available (Bangs Labs or Spherotech) for certain fluorophores, such as FITC and PE, and the results can only produce estimates within the linear range of the standard curve produced by the beads.
IF the reader does undertake direct flow cytometric analysis of individual vesicles, additional methods may be required to remove unbound labels from the EV-bound labels. Options for this include sucrose cushions or size exclusion chromatography if dilution alone is insufficient to remove background due to the unbound label.
Fluorescent labels can undergo quenching, or diminishment of the observed fluorescence due to tight fluorophore packing. Quenching is one of the reasons that most commercial antibodies are produced to have one bound PE (phycoerythrin) molecule, rather than three or four bound PE molecules. If an antibody has too many fluorophores, the labeled antibody may appear less bright than one with an optimal coupling ratio (typically 1, or at most two PE molecules per antibody). A typical antibody is ~4 nm × 11 nm, and quenching effects that are known to be important for optimal labeling of antibody molecules should be expected to be relevant to surface labeling of 30–100 nm exosomes and other EVs as well.
For the methods specifically outlined here, we used dPBS, without calcium or magnesium. However, some EV epitopes, and their ligands, such as Annexin V, require calcium for binding. Selection of buffer, and inclusion or omission of cations such as calcium should be considered for this protocol, just as this would be considered for staining of cells.
Flow cytometer standardization with calibration beads, and appropriate compensation standards, should be performed when analyzing beads, just as with conventional flow cytometry for the analysis of cells.
1 % bovine serum albumin (BSA) and 5 % BSA in PBS can be used for blocking, but we find that casein blocking buffer is more effective, and yields lower background.
Serum and other biofluids contain biotin, so it is preferable to link the biotinylated antibodies to the streptavidin-coated beads, prior to incubating the beads in the supernatant or biofluid, where physiological biotin would compete with the biotinylated antibody for binding to streptavidin on the beads.
Proteins will denature if allowed to dry. When the beads bind to the magnetic side of the microcentrifuge tube, and the supernatant is removed, buffer needs to be added within a couple of minutes to ensure that the beads do not dry out, which would denature the antibodies and proteins of the EVs, and interfere with effective staining.
References
- 1.Zhu S et al. (2014) Light-scattering detection below the level of single fluorescent molecules for high-resolution characterization of functional nanoparticles. ACS Nano 8(10):10998–11006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arakelyan A et al. (2013) Nanoparticle-based flow virometry for the analysis of individual virions. J Clin Invest 123(9):3716–3727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erdbrugger U, Lannigan J (2016) Analytical challenges of extracellular vesicle detection: a comparison of different techniques. Cytometry A 89(2):123–134 [DOI] [PubMed] [Google Scholar]
- 4.Higginbotham JN et al. (2016) Identification and characterization of EGF receptor in individual exosomes by fluorescence-activated vesicle sorting. J Extracell Vesicles 5:29254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danielson KM et al. (2016) Diurnal variations of circulating extracellular vesicles measured by nano flow cytometry. PLoS One 11(1):e0144678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Pol E et al. (2012) Single vs. swarm detection of microparticles and exosomes by flow cytometry. J Thromb Haemost 10(5):919–930 [DOI] [PubMed] [Google Scholar]
- 7.Lasser C, Eldh M, Lotvall J (2012) Isolation and characterization of RNA-containing exosomes. J Vis Exp 59:e3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thery C et al. (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol Chapter 3:22. [DOI] [PubMed] [Google Scholar]
- 9.Lobb RJ et al. (2015) Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles 4:27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumgarth N, Bigos M (2004) Optimization of emission optics for multicolor flow cytometry. Methods Cell Biol 75:3–22 [DOI] [PubMed] [Google Scholar]
- 11.Maecker HT et al. (2004) Selecting fluorochrome conjugates for maximum sensitivity. Cytometry A 62(2):169–173 [DOI] [PubMed] [Google Scholar]